Arsenic Contamination in Indian Groundwater: From Origin to Mitigation Approaches for a Sustainable Future
Abstract
1. Introduction
2. Occurrence of Arsenic in Indian Groundwater
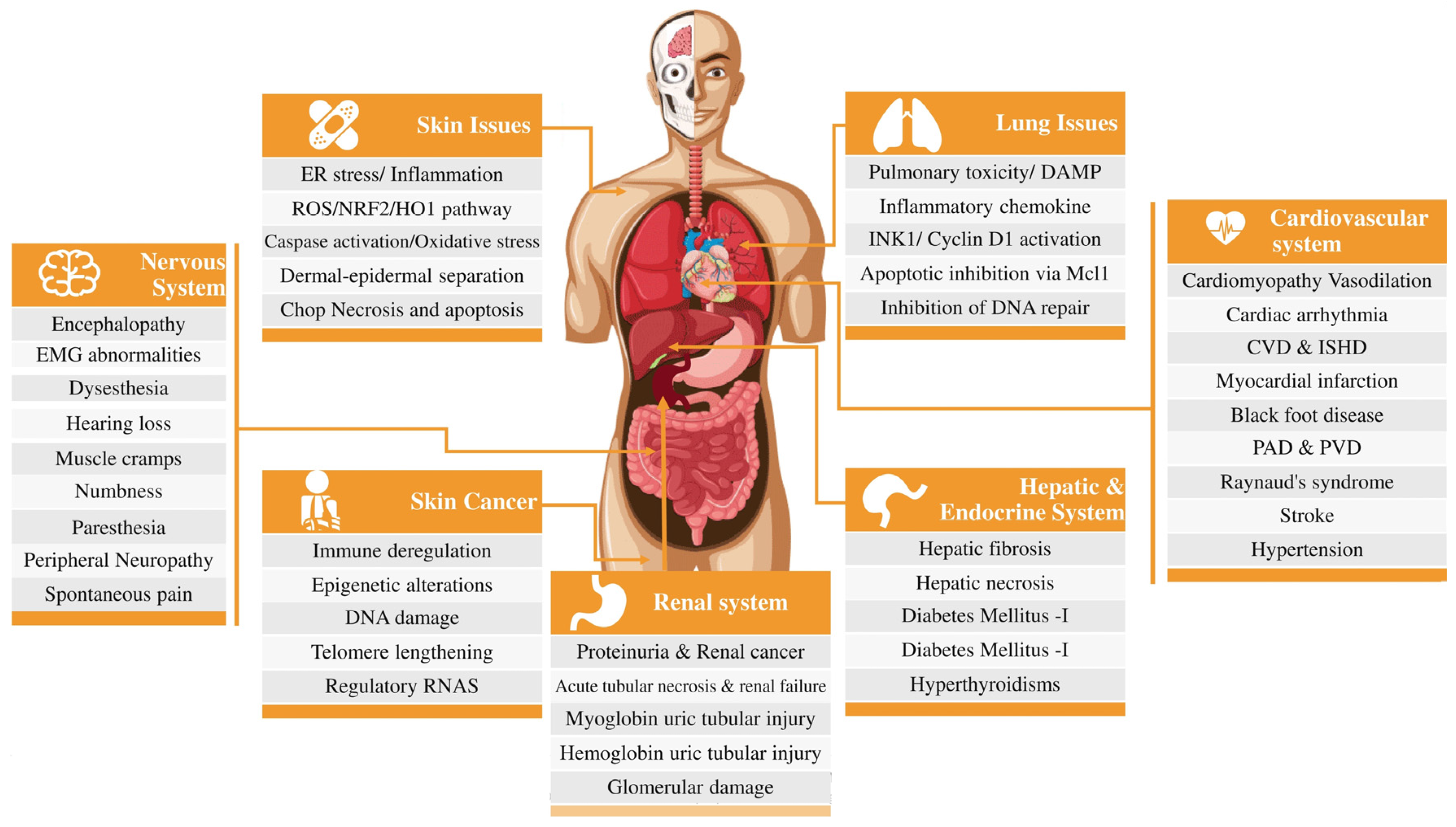
3. Health Implications of Arsenic Contamination
| State | District | Range of Concentration of Arsenic (μg/L) | Probable Mechanism | References |
|---|---|---|---|---|
| West Bengal | 24 Parganas (North and South) | 0.77–69.65 | Dissolution of Arsenopyrite mineral | [53] |
| Kolkata | 0–825 | Reductive dissolution of FeOOH faciliated in presence of high organic carbon | [54] | |
| Hooghly | 0–481 | Carbonate dissolution and Fe-oxyhydroxide reduction | [55] | |
| Howrah | 3–100 | Reductive dissolution of FeOOH faciliated in presence of high organic carbon | [56] | |
| Burdwan | 5–138 | Reductive dissolution and sedimentation | [57] | |
| Malda | 0–800 | Reductive dissolution of FeOOH faciliated in presence of high organic carbon | [58] | |
| Murshidabad | 10–4622 | Pleistocene/Holocene sediments, reductive dissolution | [59] | |
| Nadia | 3–206 | oxidation of arsenic-bearing minerals | [60] | |
| Bihar | West Champaran | 0–397 | -- | [61] |
| Siwan | 0–150 | Ferric arsenate hydrolysis of arsenopyrite. | [53] | |
| Shahpur | 0–500 | -- | [62] | |
| Samastipur | 0.19–135 | Organic matter oxidation and iron oxyhydroxide reduction | [63] | |
| Patna | 5–300 | Dissolution of Arsenopyrite mineral | [64] | |
| Bhagalpur | 3–143 | -- | [65] | |
| Buxer | 10–550 | Reductive dissolution of FeOOH faciliated in presence of high organic carbon | [66] | |
| Bhojpur | 10–1805 | Carbonate dissolution and Fe-oxyhydroxide reduction | [67] | |
| Begusarai | 21.5–94.3 | Carbonate dissolution and Fe-oxyhydroxide reduction | [68] | |
| Uttar Pradesh | Moradabad | 0–224 | Oxidation of Arsenic-rich Sulfide Minerals | [69] |
| Lakhimpur Kheri | 10–510 | Oxidation of Arsenic-rich Sulfide Minerals | [70] | |
| Ghazipur district | 10–96 | arsenolite minral dissolution | [71] | |
| Ballia | 0–300 | Fe Oxyhydroxide Reduction | [72] | |
| Unnao | 151–448 | Industries and agriculture input | [41] | |
| Ballia | 4.18–75.60 | Reductive dissolution of Fe Oxyhydroxide | [73] | |
| Jharkhand | Sahebgunj | 7–115 | Phosphate adsorption | [74] |
| Punjab | Ropar | 2–11 | Reductive dissolution of Arsenopyrite | [75] |
| Malva | 61–187 | Phosphate adsorption | [76] | |
| Amritsar | 11.4–688 | Oxidation of Arsenic-rich Sulfide Minerals | [77] | |
| Firozpur | 0–255.6 | Carbonate dissolution and Fe-oxyhydroxide reduction | [78] | |
| Pathankot | 4.35–23.25 | Phosphate adsorption | [79] | |
| Assam | Sonitpur | 0–11.15 | Arsenic bearing minerals dissolution | [46] |
| Darrang | 10.1–93.05 | Transport after Kushiara river | [80] | |
| Karimganj | 1.3–16.4 | Dissolution of Arsenic-bearing Minerals | [81] | |
| Dhemaji | 0.1–569 | Reductive dissolution of FeOOH faciliated in presence of high organic carbon | [82] | |
| Barpeta | 0–36.88 | Reductive dissolution of FeOOH faciliated in presence of high organic carbon | [83] | |
| Chhattisgarh | Rajnandgaon | 148–985 | Reductive dissolution of FeOOH faciliated in presence of high organic carbon ductive dissolution | [84] |
| Korba | 36–154 | Reductive dissolution of FeOOH faciliated in presence of high organic carbon | [85] | |
| Karnataka | Raichur | 0.19–10.55 | Phosphate adsorption | [86] |
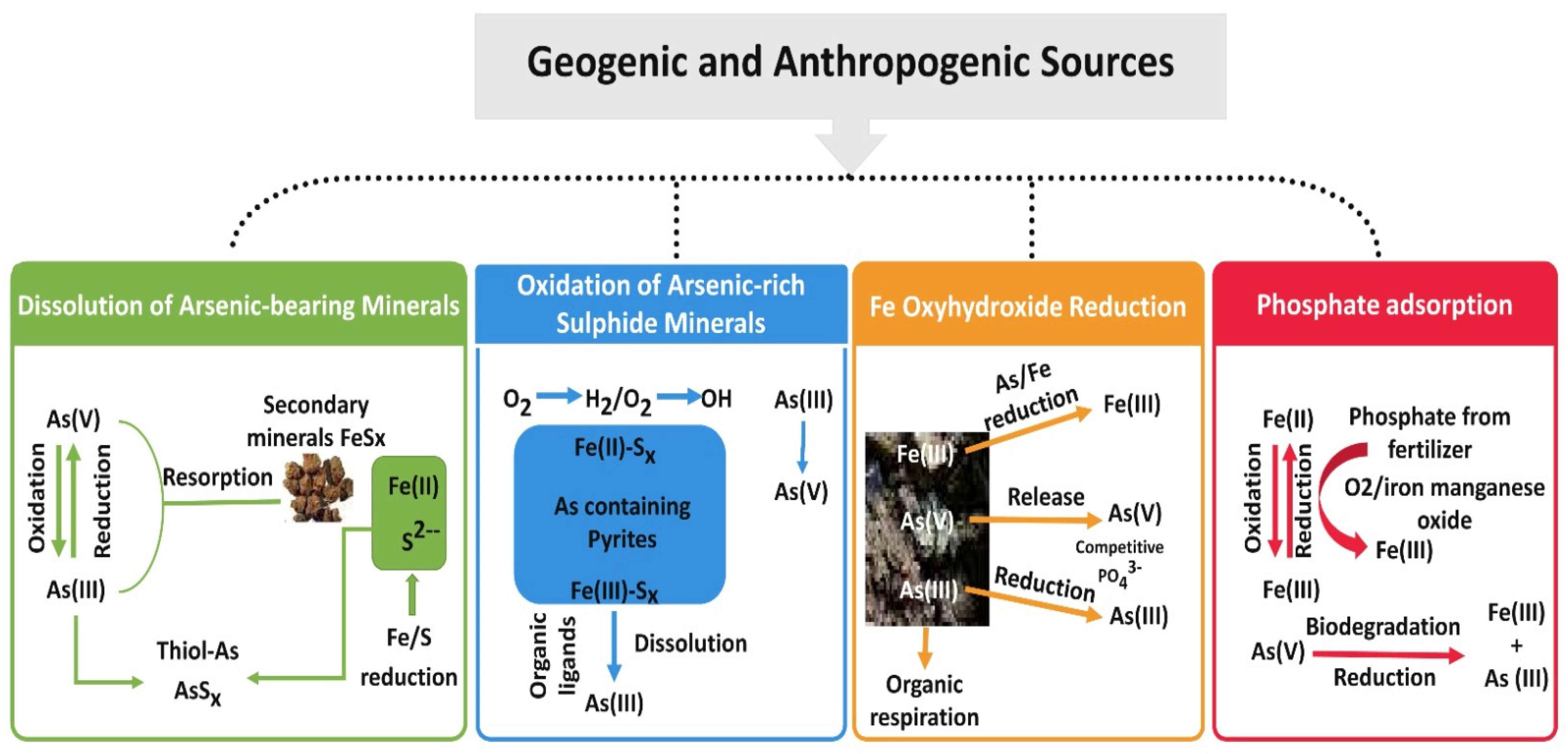
4. Fate and Transport of Arsenic in Groundwater
4.1. Dissolution of Arsenic-Bearing Minerals
4.2. Oxidation of Arsenic-Rich Sulfide Minerals
4.3. Fe Oxyhydroxide Reduction
4.4. Phosphate Adsorption
5. Factors Influencing Arsenic Mobility and Transport
5.1. pH
5.2. Redox Conditions
5.3. Desorption of Arsenic in the Alkaline Environment
5.4. Organic Matter
5.5. Microbial Activity
5.5.1. Microbial Reduction
5.5.2. Microbial Oxidation
5.5.3. Sorption and Sequestration
5.6. Competition for Electron Acceptors
5.7. Co-Existing Ions
5.8. Temporal, Seasonal, and Spatial Trends
6. Modeling Arsenic Enrichment in Indian Groundwater
6.1. Hydrogeological Modeling
6.2. Transport Modeling
6.3. Geochemical Modeling
6.4. Risk Assessment Models
7. Mitigation Strategies for Arsenic Contamination
7.1. Adsorption
7.2. Oxidation
7.3. Coagulation
7.4. Biological Treatment
7.5. Ion Exchange and Membrane Filtration
8. Community-Based Mitigation Initiatives and Their Effectiveness
9. Policy and Regulatory Measures to Address Arsenic Contamination
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Lu, L.; Zhao, Q.; Hu, S. Impact of Inorganic Solutes’ Release in Groundwater during Oil Shale In Situ Exploitation. Water 2022, 15, 172. [Google Scholar] [CrossRef]
- UNESCO World Water Assessment Programme. The United Nations World Water Development Report 2022: Groundwater: Making the Invisible Visible; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2022; pp. 1–225. [Google Scholar]
- WHO International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Report of the Advisory Group to Recommend Priorities for IARC Monographs during 2015–2019; WHO International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Agency for Toxic Substances and Disease (ATSDR). Registry Support Document to the 2019 Substance Priority List; Agency for Toxic Substances and Disease Registry (ATSDR): Atlanta, GA, USA, 2020; pp. 1–9.
- Tabassum, R.A.; Shahid, M.; Dumat, C.; Niazi, N.K.; Khalid, S.; Shah, N.S.; Imran, M.; Khalid, S. Health Risk Assessment of Drinking Arsenic-Containing Groundwater in Hasilpur, Pakistan: Effect of Sampling Area, Depth, and Source. Environ. Sci. Pollut. Res. Int. 2019, 26, 20018–20029. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Lieke, T.; Saul, N.; Pu, Y.; Yin, L.; Kochan, C.; Putschew, A.; Baberschke, N.; Steinberg, C.E.W. Neurotoxic Evaluation of Two Organobromine Model Compounds and Natural AOBr-Containing Surface Water Samples by a Caenorhabditis Elegans Test. Ecotoxicol. Environ. Saf. 2014, 104, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Mukherjee, A.; Ahmed, K.M. A Review of Groundwater Arsenic in the Bengal Basin, Bangladesh and India: From Source to Sink. Curr. Pollut. Reports 2015, 1, 220–247. [Google Scholar] [CrossRef]
- Liu, C.W.; Wu, M.Z. Geochemical, Mineralogical and Statistical Characteristics of Arsenic in Groundwater of the Lanyang Plain, Taiwan. J. Hydrol. 2019, 577, 123975. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, X.; Tang, J.; Liu, W.; Yang, H. Review of Arsenic Geochemical Characteristics and Its Significance on Arsenic Pollution Studies in Karst Groundwater, Southwest China. Appl. Geochem. 2017, 77, 80–88. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, H.; Liu, S.; Weng, H.; Han, S.; Gao, Z. Mechanisms of Groundwater Arsenic Variations Induced by Extraction in the Western Hetao Basin, Inner Mongolia, China. J. Hydrol. 2020, 583, 124599. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Kaya, S.; Vo, D.-V.N.; Sharma, A. Suppressing Inhibitory Compounds by Nanomaterials for Highly Efficient Biofuel Production: A Review. Fuel 2022, 312, 122934. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Y.; Shi, P.; Jia, W.; Zhou, J.; Liu, Y.; Ma, X.; Pan, H.; Zhang, Y.; Zhang, Z.; et al. Stable Water Isotope Monitoring Network of Different Water Bodies in Shiyang River Basin, a Typical Arid River in China. Earth Syst. Sci. Data 2022, 14, 3773–3789. [Google Scholar] [CrossRef]
- Dai, Z.; Ma, Z.; Zhang, X.; Chen, J.; Ershadnia, R.; Luan, X.; Soltanian, M.R. An Integrated Experimental Design Framework for Optimizing Solute Transport Monitoring Locations in Heterogeneous Sedimentary Media. J. Hydrol. 2022, 614, 128541. [Google Scholar] [CrossRef]
- Mhanna, R.; Naveau, A.; Bueno, M.; Shmeit, M.; Ismail, F.; Fontaine, C.; Porel, G.; Bassil, J.; Caner, L. Concomitant Behavior of Arsenic and Selenium from the Karst Infillings Materials of the Fractured Carbonate Dogger Aquifer (Hydrogeological Experimental Site, Poitiers, France). Chemosphere 2021, 275, 129935. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Engel, B.; Yeum, C.M. Integrated Environmental Modeling for Efficient Aquifer Vulnerability Assessment Using Machine Learning. Environ. Model. Softw. 2020, 124, 104602. [Google Scholar] [CrossRef]
- El Bilali, A.; Taleb, A.; Brouziyne, Y. Groundwater Quality Forecasting Using Machine Learning Algorithms for Irrigation Purposes. Agric. Water Manag. 2021, 245, 106625. [Google Scholar] [CrossRef]
- Zhao, Z.; Meng, Y.; Yuan, Q.; Wang, Y.; Lin, L.; Liu, W.; Luan, F. Microbial Mobilization of Arsenic from Iron-Bearing Clay Mineral through Iron, Arsenate, and Simultaneous Iron-Arsenate Reduction Pathways. Sci. Total Environ. 2021, 763, 144613. [Google Scholar] [CrossRef] [PubMed]
- Masue, Y.; Loeppert, R.H.; Kramer, T.A. Arsenate and Arsenite Adsorption and Desorption Behavior on Coprecipitated Aluminum:Iron Hydroxides. Environ. Sci. Technol. 2007, 41, 837–842. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Zhao, X.; Wen, M.; Cao, S.; Li, Y.; Liu, Y.; Zhang, Z.; Zhao, X.; Wen, M.; et al. Arsenic Contamination Caused by Roxarsone Transformation with Spatiotemporal Variation of Microbial Community Structure in a Column Experiment. J. Groundw. Sci. Eng. 2021, 9, 304–316. [Google Scholar] [CrossRef]
- Yadav, M.K.; Saidulu, D.; Gupta, A.K.; Ghosal, P.S.; Mukherjee, A. Status and Management of Arsenic Pollution in Groundwater: A Comprehensive Appraisal of Recent Global Scenario, Human Health Impacts, Sustainable Field-Scale Treatment Technologies. J. Environ. Chem. Eng. 2021, 9, 105203. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B. Evaluation of the Performance of Zirconia-Multiwalled Carbon Nanotube Nanoheterostructures in Adsorbing As(III) from Potable Water from the Perspective of Physical Chemistry and Chemical Physics with a Special Emphasis on Approximate Site Energy Distribut. Chemosphere 2020, 242, 125234. [Google Scholar] [CrossRef]
- Raju, N.J. Arsenic in the Geo-Environment: A Review of Sources, Geochemical Processes, Toxicity and Removal Technologies. Environ. Res. 2022, 203, 111782. [Google Scholar] [CrossRef]
- Jha, P.K.; Tripathi, P. Arsenic and Fluoride Contamination in Groundwater: A Review of Global Scenarios with Special Reference to India. Groundw. Sustain. Dev. 2021, 13, 100576. [Google Scholar] [CrossRef]
- Biswas, T.; Chandra Pal, S.; Saha, A.; Ruidas, D. Arsenic and Fluoride Exposure in Drinking Water Caused Human Health Risk in Coastal Groundwater Aquifers. Environ. Res. 2023, 238, 117257. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, J.; Wu, R.; Chakravorty, B.; Polya, D.A. Groundwater Arsenic Distribution in India by Machine Learning Geospatial Modeling. Int. J. Environ. Res. Public Health 2020, 17, 7119. [Google Scholar] [CrossRef] [PubMed]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic Contamination of Groundwater: A Global Synopsis with Focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Das, A.; Mondal, S. Geomorphic Controls on Shallow Groundwater Arsenic Contamination in Bengal Basin, India. Environ. Sci. Pollut. Res. Int. 2021, 28, 42177–42195. [Google Scholar] [CrossRef] [PubMed]
- IS 10500; Drinking Water Specification (Second Revision). Bureau of Indian Standards: New Delhi, India, 2012.
- Bhowmick, S.; Pramanik, S.; Singh, P.; Mondal, P.; Chatterjee, D.; Nriagu, J. Arsenic in Groundwater of West Bengal, India: A Review of Human Health Risks and Assessment of Possible Intervention Options. Sci. Total Environ. 2018, 612, 148–169. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Banerjee, A.; Roy, A. Hydrogeochemical Contrast between Two Study Areas of Bengal Delta, India: A Comparative Insight to Understand Arsenic Mobilization Process in Shallow Aquifers. Geochemistry 2021, 81, 125680. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B.; Darbha, G.K. The Groundwater Arsenic Contamination in the Bengal Basin—A Review in Brief. Chemosphere 2022, 299, 134369. [Google Scholar] [CrossRef]
- Saha, A.; Pal, S.C.; Chowdhuri, I.; Roy, P.; Chakrabortty, R. Effect of Hydrogeochemical Behavior on Groundwater Resources in Holocene Aquifers of Moribund Ganges Delta, India: Infusing Data-Driven Algorithms. Environ. Pollut. 2022, 314, 120203. [Google Scholar] [CrossRef]
- Chakraborti, D.; Das, B.; Rahman, M.M.; Chowdhury, U.K.; Biswas, B.; Goswami, A.B.; Nayak, B.; Pal, A.; Sengupta, M.K.; Ahamed, S.; et al. Status of Groundwater Arsenic Contamination in the State of West Bengal, India: A 20-Year Study Report. Mol. Nutr. Food Res. 2009, 53, 542–551. [Google Scholar] [CrossRef]
- Richards, L.A.; Kumari, R.; Parashar, N.; Kumar, A.; Lu, C.; Wilson, G.; Lapworth, D.; Niasar, V.J.; Ghosh, A.; Chakravorty, B.; et al. Environmental Tracers and Groundwater Residence Time Indicators Reveal Controls of Arsenic Accumulation Rates beneath a Rapidly Developing Urban Area in Patna, India. J. Contam. Hydrol. 2022, 249, 104043. [Google Scholar] [CrossRef]
- Datta, S. Hydrological Aspects of Arsenic Contamination of Groundwater in Eastern India; Elsevier: Amsterdam, The Netherlands, 2015; Volume 132, ISBN 9780128021354. [Google Scholar]
- Kumar, V.; Maity, A.; Kumar, A.; Saha, S.; Kay, P.; Singh, B.; Mukherjee, T. Critical Review on Uranium and Arsenic Content and Their Chemical Mobilization in Groundwater: A Case Study of the Malwa Region Punjab, India. Sci. Total Environ. 2023, 885, 163885. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ali, M.; Kumar, R.; Rahman, M.S.; Srivastava, A.; Chayal, N.K.; Sagar, V.; Kumari, R.; Parween, S.; Kumar, R.; et al. High Arsenic Concentration in Blood Samples of People of Village Gyaspur Mahaji, Patna, Bihar Drinking Arsenic-Contaminated Water. Expo. Heal. 2020, 12, 131–140. [Google Scholar] [CrossRef]
- Chakraborti, D.; Singh, S.K.; Rahman, M.M.; Dutta, R.N.; Mukherjee, S.C.; Pati, S.; Kar, P.B. Groundwater Arsenic Contamination in the Ganga River Basin: A Future Health Danger. Int. J. Environ. Res. Public Health 2018, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Kumar, A.; Bindal, S. Arsenic Contamination in Rapti River Basin, Terai Region of India. J. Geochem. Explor. 2018, 192, 120–131. [Google Scholar] [CrossRef]
- Dwivedi, S.; Mishra, S.; Kumar, V.; Agnihotri, R.; Sharma, P.; Tiwari, R.K.; Gupta, A.; Singh, A.P.; Kumar, S.; Sinam, G. A Comprehensive Review on Spatial and Temporal Variation of Arsenic Contamination in Ghaghara Basin and Its Relation to Probable Incremental Life Time Cancer Risk in the Local Population. J. Trace Elem. Med. Biol. 2023, 80, 127308. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.S.; Yunus, M.; Sankararamakrishnan, N. Geochemistry and Mobilization of Arsenic in Shuklaganj Area of Kanpur–Unnao District, Uttar Pradesh, India. Environ. Monit. Assess. 2012, 184, 4889–4901. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ali, M.; Kumar, R.; Kumar, M.; Sagar, P.; Pandey, R.K.; Akhouri, V.; Kumar, V.; Anand, G.; Niraj, P.K.; et al. Arsenic Exposure in Indo Gangetic Plains of Bihar Causing Increased Cancer Risk. Sci. Rep. 2021, 11, 2376. [Google Scholar] [CrossRef]
- Nickson, R.; Sengupta, C.; Mitra, P.; Dave, S.N.; Banerjee, A.K.; Bhattacharya, A.; Basu, S.; Kakoti, N.; Moorthy, N.S.; Wasuja, M.; et al. Current Knowledge on the Distribution of Arsenic in Groundwater in Five States of India. J. Environ. Sci. Heal. Part A 2007, 42, 1707–1718. [Google Scholar] [CrossRef]
- Central Ground Water Board. Ground Water Quality in Shallow Aquifers of India; Central Ground Water Board: Faridabad, India, 2018; pp. 18–21.
- Hundal, H.S.; Singh, K.; Singh, D. Arsenic Content in Ground and Canal Waters of Punjab, North–West India. Environ. Monit. Assess. 2009, 154, 393–400. [Google Scholar] [CrossRef]
- Borah, K.K.; Bhuyan, B.; Sarma, H.P. Lead, Arsenic, Fluoride, and Iron Contamination of Drinking Water in the Tea Garden Belt of Darrang District, Assam, India. Environ. Monit. Assess. 2010, 169, 347–352. [Google Scholar] [CrossRef]
- Patel, A.K.; Singh, A.; Das, N.; Kumar, M. Health Risk Associated with Consumption of Arsenic Contaminated Groundwater in the Ganga and the Brahmaputra Floodplain of India. Case Stud. Chem. Environ. Eng. 2021, 3, 100103. [Google Scholar] [CrossRef]
- Hebbar, A.; Janardhana, M.R. Arsenic Contamination in Groundwater of the Areas Surrounding Ingaldhal, Chitradurga District, Karnataka State. Int. J. Geol. Earth Environ. 2016, 3, 1–7. [Google Scholar]
- Uppal, J.S.; Zheng, Q.; Le, X.C. Arsenic in Drinking Water—Recent Examples and Updates from Southeast Asia. Curr. Opin. Environ. Sci. Health 2019, 7, 126–135. [Google Scholar] [CrossRef]
- Halder, A. Premature Greying of Hairs, Premature Ageing and Predisposition to Cancer in Jajjal, Punjab: A Preliminary Observation. J. Clin. Diagn. Res. 2007, 6, 577–580. [Google Scholar]
- Bindal, S.; Singh, C.K. Predicting Groundwater Arsenic Contamination: Regions at Risk in Highest Populated State of India. Water Res. 2019, 159, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Xu, L.; Polya, D.A. Groundwater Arsenic-Attributable Cardiovascular Disease (CVD) Mortality Risks in India. Water 2021, 13, 2232. [Google Scholar] [CrossRef]
- Singh, S.P. Spatial Relationship of Various Parameters in Drinking Water in Siwan Town of Bihar (India) with Special Emphasis on Arsenic Contamination in Groundwater. J. Chem. Sci. Rev. Lett. 2014, 2, 588–595. [Google Scholar]
- Chakraborti, D.; Das, B.; Rahman, M.M.; Nayak, B.; Pal, A.; Sengupta, M.K.; Ahamed, S.; Hossain, M.A.; Chowdhury, U.K.; Biswas, B.K.; et al. Arsenic in Groundwater of the Kolkata Municipal Corporation (KMC), India: Critical Review and Modes of Mitigation. Chemosphere 2017, 180, 437–447. [Google Scholar] [CrossRef]
- Bhowmick, S.; Nath, B.; Halder, D.; Biswas, A.; Majumder, S.; Mondal, P.; Chakraborty, S.; Nriagu, J.; Bhattacharya, P.; Iglesias, M.; et al. Arsenic Mobilization in the Aquifers of Three Physiographic Settings of West Bengal, India: Understanding Geogenic and Anthropogenic Influences. J. Hazard. Mater. 2013, 262, 915–923. [Google Scholar] [CrossRef]
- Sikdar, P.K.; Sarkar, S.S.; Palchoudhury, S. Geochemical Evolution of Groundwater in the Quaternary Aquifer of Calcutta and Howrah, India. J. Asian Earth Sci. 2001, 19, 579–594. [Google Scholar] [CrossRef]
- Biswas, G. Asphyxiants. In Review of Forensic Medicine and Toxicology; Jaypee Brothers Medical Publishers Ltd.: New Delhi, India, 2010; p. 419. [Google Scholar]
- Purkait, B. Application of Artificial Neural Network Model to Study Arsenic Contamination in Groundwater of Malda District, Eastern India. J. Environ. Inform. 2008, 12, 140–149. [Google Scholar] [CrossRef]
- Sankar, M.S.; Vega, M.A.; Defoe, P.P.; Kibria, M.G.; Ford, S.; Telfeyan, K.; Neal, A.; Mohajerin, T.J.; Hettiarachchi, G.M.; Barua, S.; et al. Elevated Arsenic and Manganese in Groundwaters of Murshidabad, West Bengal, India. Sci. Total Environ. 2014, 488–489, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Das, S.S.; Chowdhury, N.R.; Joardar, M.; Ghosh, B.; Roychowdhury, T. Quality and Health Risk Evaluation for Groundwater in Nadia District, West Bengal: An Approach on Its Suitability for Drinking and Domestic Purpose. Groundw. Sustain. Dev. 2020, 10, 100351. [Google Scholar] [CrossRef]
- Bhatia, S.; Balamurugan, G.; Baranwa, A. High Arsenic Contamination in Drinking Water Hand-Pumps in Khap Tola, West Champaran, Bihar, India. Front. Environ. Sci. 2014, 2, 49. [Google Scholar] [CrossRef][Green Version]
- Thakur, B.K.; Gupta, V.; Bhattacharya, P.; Chakraborty, T. Impact of Socioeconomic Factors on Households’ Willingness to Pay for Arsenic-Free Safe Drinking Water—A Case Study of Bihar, India. Groundw. Sustain. Dev. 2022, 19, 100837. [Google Scholar] [CrossRef]
- Kumar, M.; Ramanathan, A.; Bhattacharya, P. Evaluation of Arsenic and Its Controlling Factors in Aquifer Sands of District Samastipur, Bihar, India. In One Century of the Discovery of Arsenicosis in Latin America (1914–2014) As 2014; CRC Press/Taylor and Francis Group: London, UK, 2014; pp. 108–109. [Google Scholar]
- Singh, S.K.; Brachfeld, S.A.; Taylor, R.W. Evaluating Hydrogeological and Topographic Controls on Groundwater Arsenic Contamination in the Middle-Ganga Plain in India: Towards Developing Sustainable Arsenic Mitigation Models BT. In Emerging Issues in Groundwater Resources, Advances in Water Security; Fares, A., Ed.; Springer: Cham, Switzerland, 2016; pp. 263–287. ISBN 978-3-319-32008-3. [Google Scholar]
- Singh, N.; Singh, R.P.; Mukherjee, S.; McDonald, K.; Reddy, K.J. Hydrogeological Processes Controlling the Release of Arsenic in Parts of 24 Parganas District, West Bengal. Environ. Earth Sci. 2014, 72, 111–118. [Google Scholar] [CrossRef]
- Shah, B.A. Groundwater Arsenic Contamination Affecting Different Geological Domains in India: Its Relation to Fluvial Geomorphology and Quaternary Stratigraphy BT. In Geostatistical and Geospatial Approaches for the Characterization of Natural Resources in the Environment: Challenges, Processes and Strategies; Raju, N.J., Ed.; Springer: Cham, Switzerland, 2016; pp. 267–273. [Google Scholar]
- Chakraborti, D.; Rahman, M.M.; Ahamed, S.; Dutta, R.N.; Pati, S.; Mukherjee, S.C. Arsenic Contamination of Groundwater and Its Induced Health Effects in Shahpur Block, Bhojpur District, Bihar State, India: Risk Evaluation. Environ. Sci. Pollut. Res. 2016, 23, 9492–9504. [Google Scholar] [CrossRef]
- Agrawal, D.; Kumar, P.; Avtar, R.; Ramanathan, A.L. Multivariate Statistical Approach to Deduce Hydrogeochemical Processes in the Groundwater Environment of Begusarai District, Bihar. Water Qual. Expo. Health 2011, 3, 119–126. [Google Scholar] [CrossRef]
- Pathak, V.K. Hydrochemistry of Groundwater with Special Reference to Arsenic in Lakhimpur Kheri District, Uttar Pradesh, India. IOSR J. Appl. Chem. 2013, 6, 61–68. [Google Scholar] [CrossRef]
- Shah, B.A. Groundwater Arsenic Contamination from Parts of the Ghaghara Basin, India: Influence of Fluvial Geomorphology and Quaternary Morphostratigraphy. Appl. Water Sci. 2017, 7, 2587–2595. [Google Scholar] [CrossRef]
- Namrata, P.; Alok, L.; Mehrotra, S. Arsenic Pollution Scenario in Eastern UP, India: A Review. Int. Res. J. Environ. Sci. Int. Sci. Congr. Assoc. 2015, 4, 83–86. [Google Scholar]
- Ahamed, S.; Kumar Sengupta, M.; Mukherjee, A.; Amir Hossain, M.; Das, B.; Nayak, B.; Pal, A.; Chandra Mukherjee, S.; Pati, S.; Nath Dutta, R.; et al. Arsenic Groundwater Contamination and Its Health Effects in the State of Uttar Pradesh (UP) in Upper and Middle Ganga Plain, India: A Severe Danger. Sci. Total Environ. 2006, 370, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.L.; Singh, V.K. Assessment of Groundwater Quality of Ballia District, Uttar Pradesh, India, with Reference to Arsenic Contamination Using Multivariate Statistical Analysis. Appl. Water Sci. 2018, 8, 95. [Google Scholar] [CrossRef]
- Alam, M.O.; Shaikh, W.A.; Chakraborty, S.; Avishek, K.; Bhattacharya, T. Groundwater Arsenic Contamination and Potential Health Risk Assessment of Gangetic Plains of Jharkhand, India. Expo. Health 2016, 8, 125–142. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, I.; Nagpal, A.K. Estimation of Arsenic, Manganese and Iron in Mustard Seeds, Maize Grains, Groundwater and Associated Human Health Risks in Ropar Wetland, Punjab, India, and Its Adjoining Areas. Environ. Monit. Assess. 2018, 190, 385. [Google Scholar] [CrossRef]
- Virk, H.S. A Survey Report on Groundwater Contamination of Malwa Belt of Punjab Due to Heavy Metal Arsenic. Int. J. Sci. Res. 2019, 8, 1721–1726. [Google Scholar]
- Hundal, H.S.; Kumar, R.; Singh, K.; Singh, D. Occurrence and Geochemistry of Arsenic in Groundwater of Punjab, Northwest India. Commun. Soil Sci. Plant Anal. 2007, 38, 2257–2277. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, C.K. Arsenic Enrichment in Groundwater and Associated Health Risk in Bari Doab Region of Indus Basin, Punjab, India. Environ. Pollut. 2020, 256, 113324. [Google Scholar] [CrossRef]
- Kaur, T.; Bhardwaj, R.; Arora, S. Assessment of Groundwater Quality for Drinking and Irrigation Purposes Using Hydrochemical Studies in Malwa Region, Southwestern Part of Punjab, India. Appl. Water Sci. 2017, 7, 3301–3316. [Google Scholar] [CrossRef]
- Purkayastha, S.P.; Choudhury, M.; Deb, D.; Paul, C. Arsenic Contamination in Ground Water Is a Serious Threat in the North Karimganj Block of Karimganj District, Southern Part of Assam, India. J. Chem. Pharm. Res. 2015, 7, 371–378. [Google Scholar]
- Buragohain, M.; Bhuyan, B.; Sarma, H.P. Seasonal Variations of Lead, Arsenic, Cadmium and Aluminium Contamination of Groundwater in Dhemaji District, Assam, India. Environ. Monit. Assess. 2010, 170, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K.; Sharma, S.K.; Singh, S. Physico-Chemical Characteristics and Hydrogeological Mechanisms in Groundwater with Special Reference to Arsenic Contamination in Barpeta District, Assam (India). Environ. Monit. Assess. 2018, 190, 417. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, M.; Nathan, D.S. Chemometric Tool to Study the Mechanism of Arsenic Contamination in Groundwater of Puducherry Region, South East Coast of India. Chemosphere 2018, 208, 303–315. [Google Scholar] [CrossRef]
- Patel, K.S.; Sahu, B.L.; Dahariya, N.S.; Bhatia, A.; Patel, R.K.; Matini, L.; Sracek, O.; Bhattacharya, P. Groundwater Arsenic and Fluoride in Rajnandgaon District, Chhattisgarh, Northeastern India. Appl. Water Sci. 2017, 7, 1817–1826. [Google Scholar] [CrossRef]
- Sharma, R.; Patel, K.S.; Lata, L.; Milos, H. Contamination of Pond Water and Sediment in Coal Burning Area. J. Environ. Prot. 2017, 08, 358–379. [Google Scholar] [CrossRef]
- Ravichandran, K.; Bhange, S.A.; Lalitha, B.H.; Dhayamalar, D. Geochemical behaviour of uranium with other contaminants in groundwater of raichur district. J. Indian Water Resour. Soc. 2022, 42. [Google Scholar]
- Shah, B. Arsenic-Contaminated Groundwater in Holocene Sediments from Parts of Middle Ganga Plain, Uttar Pradesh, India. Curr. Sci. 2010, 98, 1359–1365. [Google Scholar]
- Wang, Z.; Guo, H.M.; Liu, H.Y.; Zhang, W.M. Source, Migration, Distribution, Toxicological Effects and Remediation Technologies of Arsenic in Groundwater in China. China Geol. 2023, 6, 476–493. [Google Scholar] [CrossRef]
- Wallis, I.; Pichler, T. Generating False Negatives and False Positives for As and Mo Concentrations in Groundwater Due to Well Installation. Sci. Total Environ. 2018, 631–632, 723–732. [Google Scholar] [CrossRef]
- Dong, Y.; Ma, T.; Li, J.; Liu, Y. Arsenic Releasing from Poly-Metallic Sulfide Deposits at Hetao Plain, China. Geochem. Int. 2018, 56, 1179–1188. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Singh, A.P.; Singh, S.K.; Barman, A.; Patra, A.; Mondal, B.P.; Banerjee, K. Spatial variability of arsenic in indo-Gangetic basin of Varanasi and its cancer risk assessment. Chemosphere 2020, 238, 124623. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Das, N.; Tripathi, S.; Verma, A.; Jha, P.K.; Bhattacharya, P.; Mahlknecht, J. Global Co-Occurrences of Multi-(Emerging)-Contaminants in the Hotspots of Arsenic Polluted Groundwater: A Pattern of Menace. Curr. Opin. Environ. Sci. Health 2023, 34, 100483. [Google Scholar] [CrossRef]
- Ahmed, K.M.; Bhattacharya, P.; Hasan, M.A.; Akhter, S.H.; Alam, S.M.M.; Bhuyian, M.A.H.; Imam, M.B.; Khan, A.A.; Sracek, O. Arsenic Enrichment in Groundwater of the Alluvial Aquifers in Bangladesh: An Overview. Appl. Geochem. 2004, 19, 181–200. [Google Scholar] [CrossRef]
- Kumar, S.; Pati, J. Assessment of Groundwater Arsenic Contamination Level in Jharkhand, India Using Machine Learning. J. Comput. Sci. 2022, 63, 101779. [Google Scholar] [CrossRef]
- Mukherjee, A.; Scanlon, B.R.; Fryar, A.E.; Saha, D.; Ghosh, A.; Chowdhuri, S.; Mishra, R. Solute Chemistry and Arsenic Fate in Aquifers between the Himalayan Foothills and Indian Craton (Including Central Gangetic Plain): Influence of Geology and Geomorphology. Geochim. Cosmochim. Acta 2012, 90, 283–302. [Google Scholar] [CrossRef]
- Dowling, C.B.; Poreda, R.J.; Basu, A.R.; Peters, S.L.; Aggarwal, P.K.; Dowling, C.; Poreda, R.J.; Basu, A.R.; Peters, S.L.; Aggarwal, P.K. Geochemical Study of Arsenic Release Mechanisms in the Bengal Basin Groundwater. Water Resour. Res. 2002, 38, 12-1–12-18. [Google Scholar] [CrossRef]
- Goswami, R.; Neog, N.; Bhagat, C.; Hdeib, R.; Mahlknecht, J.; Kumar, M. Arsenic in the Groundwater of the Upper Brahmaputra Floodplain: Variability, Health Risks and Potential Impacts. Chemosphere 2022, 306, 135621. [Google Scholar] [CrossRef]
- Manning, B.A.; Goldberg, S. Adsorption and Stability of Arsenic(III) at the Clay Mineral–Water Interface. Environ. Sci. Technol. 1997, 31, 2005–2011. [Google Scholar] [CrossRef]
- Anawar, H.M.; Akai, J.; Sakugawa, H. Mobilization of Arsenic from Subsurface Sediments by Effect of Bicarbonate Ions in Groundwater. Chemosphere 2004, 54, 753–762. [Google Scholar] [CrossRef]
- Qiao, J.T.; Li, X.M.; Li, F.B. Roles of Different Active Metal-Reducing Bacteria in Arsenic Release from Arsenic-Contaminated Paddy Soil Amended with Biochar. J. Hazard. Mater. 2018, 344, 958–967. [Google Scholar] [CrossRef]
- Vega, M.A.; Kulkarni, H.V.; Mladenov, N.; Johannesson, K.; Hettiarachchi, G.M.; Bhattacharya, P.; Kumar, N.; Weeks, J.; Galkaduwa, M.; Datta, S. Biogeochemical Controls on the Release and Accumulation of Mn and As in Shallow Aquifers, West Bengal, India. Front. Environ. Sci. 2017, 5, 267730. [Google Scholar] [CrossRef]
- Kumar, M.; Patel, A.K.; Singh, A. Anthropogenic Dominance on Geogenic Arsenic Problem of the Groundwater in the Ganga-Brahmaputra Floodplain: A Paradox of Origin and Mobilization. Sci. Total Environ. 2022, 807, 151461. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Kumar, M.; Biyani, N.; Shea, P.J. Arsenic Exposure and Perception of Health Risk Due to Groundwater Contamination in Majuli (River Island), Assam, India. Environ. Geochem. Health 2020, 42, 443–460. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.M.; Ravenscroft, P.; Safiulla, S.; Thirlwall, M.F. Arsenic in Groundwater: Testing Pollution Mechanisms for Sedimentary Aquifers in Bangladesh. Water Resour. Res. 2001, 37, 109–117. [Google Scholar] [CrossRef]
- Rowland, H.A.L.; Polya, D.A.; Lloyd, J.R.; Pancost, R.D. Characterisation of Organic Matter in a Shallow, Reducing, Arsenic-Rich Aquifer, West Bengal. Org. Geochem. 2006, 37, 1101–1114. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Effect of Natural Organic Matter on Arsenic Release from Soils and Sediments into Groundwater. Environ. Geochem. Health 2006, 28, 197–214. [Google Scholar] [CrossRef]
- Farooq, S.H.; Chandrasekharam, D.; Abbt-Braun, G.; Berner, Z.; Norra, S.; Stüben, D.; Farooq, S.H.; Chandrasekharam, D.; Abbt-Braun, G.; Berner, Z.; et al. Dissolved Organic Carbon from the Traditional Jute Processing Technique and Its Potential Influence on Arsenic Enrichment in the Bengal Delta. Appl. Geochem. 2012, 27, 292–303. [Google Scholar] [CrossRef]
- Mohapatra, B.; Saha, A.; Chowdhury, A.N.; Kar, A.; Kazy, S.K.; Sar, P. Geochemical, Metagenomic, and Physiological Characterization of the Multifaceted Interaction between Microbiome of an Arsenic Contaminated Groundwater and Aquifer Sediment. J. Hazard. Mater. 2021, 412, 125099. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, X.; Yin, H.; Zhang, X. Current Knowledge on Molecular Mechanisms of Microorganism-Mediated Bioremediation for Arsenic Contamination: A Review. Microbiol. Res. 2022, 258, 126990. [Google Scholar] [CrossRef]
- Pratush, A.; Kumar, A.; Hu, Z. Adverse Effect of Heavy Metals (As, Pb, Hg, and Cr) on Health and Their Bioremediation Strategies: A Review. Int. Microbiol. 2018, 21, 97–106. [Google Scholar] [CrossRef]
- Saha, A.; Mohapatra, B.; Kazy, S.K.; Sar, P. Variable Response of Arsenic Contaminated Groundwater Microbial Community to Electron Acceptor Regime Revealed by Microcosm Based High-Throughput Sequencing Approach. J. Environ. Sci. Health Part A 2021, 56, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.C. An Overview of Arsenic Removal by Pressure-Drivenmembrane Processes. Desalination 2005, 172, 85–97. [Google Scholar] [CrossRef]
- Chatterjee, D.; Kundu, A.; Saha, D.; Barman, S.; Mandal, U. Groundwater Arsenic in the Bengal Delta Plain: Geochemical and Geomorphological Perspectives. Procedia Earth Planet. Sci. 2017, 17, 622–625. [Google Scholar] [CrossRef]
- Sathe, S.S.; Mahanta, C. Groundwater Flow and Arsenic Contamination Transport Modeling for a Multi Aquifer Terrain: Assessment and Mitigation Strategies. J. Environ. Manag. 2019, 231, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Modeling of Arsenic Transport in Groundwater Using MODFLOW: A Case Study. Available online: https://www.researchgate.net/publication/309398014_Modeling_of_arsenic_transport_in_groundwater_using_MODFLOW_A_case_study (accessed on 17 November 2023).
- Chowdhury, A.; Rahnuma, M. Groundwater Contaminant Transport Modeling Using MODFLOW and MT3DMS: A Case Study in Rajshahi City. Water Pract. Technol. 2023, 18, 1255–1272. [Google Scholar] [CrossRef]
- Mishra, D.; Das, B.S.; Sinha, T.; Hoque, J.M.; Reynolds, C.; Rafiqul Islam, M.; Hossain, M.; Sar, P.; Menon, M. Living with Arsenic in the Environment: An Examination of Current Awareness of Farmers in the Bengal Basin Using Hybrid Feature Selection and Machine Learning. Environ. Int. 2021, 153, 106529. [Google Scholar] [CrossRef]
- Dummer, T.J.B.; Yu, Z.M.; Nauta, L.; Murimboh, J.D.; Parker, L. Geostatistical Modelling of Arsenic in Drinking Water Wells and Related Toenail Arsenic Concentrations across Nova Scotia, Canada. Sci. Total Environ. 2015, 505, 1248–1258. [Google Scholar] [CrossRef]
- Morrissey, M.B.; Ruxton, G.D. Multiple Regression Is Not Multiple Regressions: The Meaning of Multiple Regression and the Non-Problem of Collinearity. Philos. Theory Pract. Biol. 2018, 10. [Google Scholar] [CrossRef]
- Golia, E.E.; Diakoloukas, V. Soil Parameters Affecting the Levels of Potentially Harmful Metals in Thessaly Area, Greece: A Robust Quadratic Regression Approach of Soil Pollution Prediction. Environ. Sci. Pollut. Res. 2022, 29, 29544–29561. [Google Scholar] [CrossRef]
- Singh, S.K.; Taylor, R.W.; Pradhan, B.; Shirzadi, A.; Pham, B.T. Predicting Sustainable Arsenic Mitigation Using Machine Learning Techniques. Ecotoxicol. Environ. Saf. 2022, 232, 113271. [Google Scholar] [CrossRef]
- Nafouanti, M.B.; Li, J.; Nyakilla, E.E.; Mwakipunda, G.C.; Mulashani, A. A Novel Hybrid Random Forest Linear Model Approach for Forecasting Groundwater Fluoride Contamination. Environ. Sci. Pollut. Res. 2023, 30, 50661–50674. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, A.; Iqbal, J. Spatial Distribution and Mobility Assessment of Carcinogenic Heavy Metals in Soil Profiles Using Geostatistics and Random Forest, Boruta Algorithm. Sustainability 2018, 10, 799. [Google Scholar] [CrossRef]
- Khan, M.U.; Musahib, M.; Vishwakarma, R.; Rai, N.; Jahan, A. Hydrochemical Characterization, Mechanism of Mobilization, and Natural Background Level Evaluation of Arsenic in the Aquifers of Upper Gangetic Plain, India. Geochemistry 2023, 83, 125952. [Google Scholar] [CrossRef]
- Marghade, D.; Pethe, R.M.; Patil, P.D.; Tiwari, M.S. A Unified Multivariate Statistical Approach for the Assessment of Deep Groundwater Quality of Rapidly Growing City of Maharashtra Province, India, with Potential Health Risk. Environ. Monit. Assess. 2022, 194, 891. [Google Scholar] [CrossRef] [PubMed]
- Marghade, D.; Malpe, D.B.; Duraisamy, K.; Patil, P.D.; Li, P. Hydrogeochemical Evaluation, Suitability, and Health Risk Assessment of Groundwater in the Watershed of Godavari Basin, Maharashtra, Central India. Environ. Sci. Pollut. Res. 2021, 28, 18471–18494. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Rahman, M.M.; Murrill, M.; Sarma, K.P.; Thakur, R.; Chakraborti, D. Arsenic in the Groundwater of Majuli—The Largest River Island of the Brahmaputra: Magnitude of Occurrence and Human Exposure. J. Hydrol. 2014, 518, 354–362. [Google Scholar] [CrossRef]
- Khanikar, L.; Gogoi, R.R.; Sarma, K.P. Hydrogeochemical Investigation and Health Perspective of Arsenic in the Mid-Brahmaputra Floodplain of Assam, India. In Emerging Issues in the Water Environment during Anthropocene; Springer: Singapore, 2020; pp. 143–158. [Google Scholar] [CrossRef]
- Banerjee, K.; Amy, G.L.; Prevost, M.; Nour, S.; Jekel, M.; Gallagher, P.M.; Blumenschein, C.D. Kinetic and Thermodynamic Aspects of Adsorption of Arsenic onto Granular Ferric Hydroxide (GFH). Water Res. 2008, 42, 3371–3378. [Google Scholar] [CrossRef]
- Singh, T.S.; Pant, K.K. Equilibrium, Kinetics and Thermodynamic Studies for Adsorption of As(III) on Activated Alumina. Sep. Purif. Technol. 2004, 36, 139–147. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Westerhoff, P.; Knappe, D.R.U. Intraparticle Diffusion and Adsorption of Arsenate onto Granular Ferric Hydroxide (GFH). Water Res. 2004, 38, 4002–4012. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic Removal from Water/Wastewater Using Adsorbents—A Critical Review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Goswami, D.; Das, A.K. Removal of Arsenic from Drinking Water Using Modified Fly-Ash Bed. Int. J. Water 2000, 1, 61–70. [Google Scholar] [CrossRef]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D.; Haasch, R. Removal of Arsenic (III) and Arsenic (V) from Aqueous Medium Using Chitosan-Coated Biosorbent. Water Res. 2008, 42, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Khosa, M.A.; Wu, J.; Ullah, A. Chemical Modification, Characterization, and Application of Chicken Feathers as Novel Biosorbents. RSC Adv. 2013, 3, 20800–20810. [Google Scholar] [CrossRef]
- Martinson, C.A.; Reddy, K.J. Adsorption of Arsenic(III) and Arsenic(V) by Cupric Oxide Nanoparticles. J. Colloid Interface Sci. 2009, 336, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Samadder, S.R. Removal of Arsenic from Water Using Nano Adsorbents and Challenges: A Review. J. Environ. Manag. 2016, 166, 387–406. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B. The Global Menace of Arsenic and Its Conventional Remediation—A Critical Review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Anbalagan, K.; Andal, N.M. Adsorption Dynamics and Equilibrium Studies of Zn (II) onto Chitosan. J. Chem. Sci. 2004, 116, 119–127. [Google Scholar] [CrossRef]
- Gupta, A.; Yunus, M.; Sankararamakrishnan, N. Zerovalent Iron Encapsulated Chitosan Nanospheres—A Novel Adsorbent for the Removal of Total Inorganic Arsenic from Aqueous Systems. Chemosphere 2012, 86, 150–155. [Google Scholar] [CrossRef]
- He, J.; Bardelli, F.; Gehin, A.; Silvester, E.; Charlet, L. Novel Chitosan Goethite Bionanocomposite Beads for Arsenic Remediation. Water Res. 2016, 101, 1–9. [Google Scholar] [CrossRef]
- Anderson, M.A.; Ferguson, J.F.; Gavis, J. Arsenate Adsorption on Amorphous Aluminum Hydroxide. J. Colloid Interface Sci. 1976, 54, 391–399. [Google Scholar] [CrossRef]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Adsorption of As(III) and As(V) by Fe Coated Biochars and Biochars Produced from Empty Fruit Bunch and Rice Husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar] [CrossRef]
- Budinova, T.; Savova, D.; Tsyntsarski, B.; Ania, C.O.; Cabal, B.; Parra, J.B.; Petrov, N. Biomass Waste-Derived Activated Carbon for the Removal of Arsenic and Manganese Ions from Aqueous Solutions. Appl. Surf. Sci. 2009, 255, 4650–4657. [Google Scholar] [CrossRef]
- Tuna, A.Ö.A.; Özdemir, E.; Şimşek, E.B.; Beker, U. Removal of As(V) from Aqueous Solution by Activated Carbon-Based Hybrid Adsorbents: Impact of Experimental Conditions. Chem. Eng. J. 2013, 223, 116–128. [Google Scholar] [CrossRef]
- Arcibar-Orozco, J.A.; Josue, D.-B.; Rios-Hurtado, J.C.; Rangel-Mendez, J.R. Influence of Iron Content, Surface Area and Charge Distribution in the Arsenic Removal by Activated Carbons. Chem. Eng. J. 2014, 249, 201–209. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S.; Sasai, R. Arsenate Removal from Water Using Fe3O4-Loaded Activated Carbon Prepared from Waste Biomass. Chem. Eng. J. 2010, 160, 57–62. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Y.; Wu, X.; Wang, H. Removal of Arsenic from Water by Supported Nano Zero-Valent Iron on Activated Carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef]
- Mercer, K.L.; Tobiason, J.E. Removal of Arsenic from High Ionic Strength Solutions: Effects of Ionic Strength, PH, and Preformed versus in Situ Formed HFO. Environ. Sci. Technol. 2008, 42, 3797–3802. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H.; Kandasamy, J.; Choi, H.C. Arsenic Removal by Photo-Catalysis Hybrid System. Sep. Purif. Technol. 2008, 61, 44–50. [Google Scholar] [CrossRef]
- Fu, D.; Kurniawan, T.A.; Gui, H.; Li, H.; Feng, S.; Li, Q.; Wang, Y. Role of CuxO-Anchored Pyrolyzed Hydrochars on H2O2-Activated Degradation of Tetracycline: Effects of Pyrolysis Temperature and PH. Ind. Eng. Chem. Res. 2022, 61, 8847–8857. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.; Chan, G.Y.S. Radicals-Catalyzed Oxidation Reactions for Degradation of Recalcitrant Compounds from Landfill Leachate. Chem. Eng. J. 2006, 125, 35–57. [Google Scholar] [CrossRef]
- Tahaikt, M.; El Habbani, R.; Ait Haddou, A.; Achary, I.; Amor, Z.; Taky, M.; Alami, A.; Boughriba, A.; Hafsi, M.; Elmidaoui, A. Fluoride Removal from Groundwater by Nanofiltration. Desalination 2007, 212, 46–53. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Avtar, R.; Singh, D.; Xue, W.; Dzarfan Othman, M.H.; Hwang, G.H.; Iswanto, I.; Albadarin, A.B.; Kern, A.O. Reforming MSWM in Sukunan (Yogjakarta, Indonesia): A Case-Study of Applying a Zero-Waste Approach Based on Circular Economy Paradigm. J. Clean. Prod. 2021, 284, 124775. [Google Scholar] [CrossRef] [PubMed]
- Choong, T.S.Y.; Chuah, T.G.; Robiah, Y.; Gregory Koay, F.L.; Azni, I. Arsenic Toxicity, Health Hazards and Removal Techniques from Water: An Overview. Desalination 2007, 217, 139–166. [Google Scholar] [CrossRef]
- Meng, X.; Korfiatis, G.P.; Bang, S.; Bang, K.W. Combined Effects of Anions on Arsenic Removal by Iron Hydroxides. Toxicol. Lett. 2002, 133, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, S.R.; Han, B.; Zimbron, J.; Shen, Z.; Karim, M.N. Arsenic Removal by Coagulation and Filtration: Comparison of Groundwaters from the United States and Bangladesh. Desalination 2004, 169, 231–244. [Google Scholar] [CrossRef]
- Zhu, F.; Yang, M.; Luo, Z.; Yu, R.; Hu, G.; Yan, Y. Bioaccumulation and Biotransformation of Arsenic in Leptolyngbya Boryana. Environ. Sci. Pollut. Res. 2020, 27, 29993–30000. [Google Scholar] [CrossRef]
- Zhaojuan, Z.; Binbin, S.; Cuiqing, M.; Haiwei, Z.; Chao, G.; Fei, S.; Ping, X. Relative Catalytic Efficiency of LdhL- and LdhD-Encoded Products Is Crucial for Optical Purity of Lactic Acid Produced by Lactobacillus Strains. Appl. Environ. Microbiol. 2012, 78, 3480–3483. [Google Scholar] [CrossRef]
- Ye, J.; Rensing, C.; Rosen, B.P.; Zhu, Y.-G. Arsenic Biomethylation by Photosynthetic Organisms. Trends Plant Sci. 2012, 17, 155–162. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xu, P.; Liu, C.; Liu, M.; Wang, Y.; Wang, C.; Zhang, C.; Ge, Y. Review of Arsenic Speciation, Toxicity and Metabolism in Microalgae. Rev. Environ. Sci. Bio/Technol. 2015, 14, 427–451. [Google Scholar] [CrossRef]
- Xue, X.-M.; Yan, Y.; Xiong, C.; Raber, G.; Francesconi, K.; Pan, T.; Ye, J.; Zhu, Y.-G. Arsenic Biotransformation by a Cyanobacterium Nostoc Sp. PCC 7120. Environ. Pollut. 2017, 228, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Vaaramaa, K.; Lehto, J. Removal of Metals and Anions from Drinking Water by Ion Exchange. Desalination 2003, 155, 157–170. [Google Scholar] [CrossRef]
- Ng, K.-S.; Ujang, Z.; Le-Clech, P. Arsenic Removal Technologies for Drinking Water Treatment. Rev. Environ. Sci. Biotechnol. 2004, 3, 43–53. [Google Scholar] [CrossRef]
- Košutić, K.; Furač, L.; Sipos, L.; Kunst, B. Removal of Arsenic and Pesticides from Drinking Water by Nanofiltration Membranes. Sep. Purif. Technol. 2005, 42, 137–144. [Google Scholar] [CrossRef]
- Rahmani, M.; Kaykhaii, M.; Sasani, M. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Application of Taguchi L16 Design Method for Comparative Study of Ability of 3A Zeolite in Removal of Rhodamine B and Malachite Green from Environmental Water Samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-D.; Li, X.-X.; Qu, C.-T. A Global Proteomic Change in Petroleum Hydrocarbon-Degrading Pseudomonas Aeruginosa in Response to High and Low Concentrations of Petroleum Hydrocarbons. Curr. Microbiol. 2019, 76, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Elmobarak, W.F.; Hameed, B.H.; Almomani, F.; Abdullah, A.Z. A Review on the Treatment of Petroleum Refinery Wastewater Using Advanced Oxidation Processes. Catalysts 2021, 11, 782. [Google Scholar] [CrossRef]
- Vicente, J.; Rosal, R.; Díaz, M. Noncatalytic Oxidation of Phenol in Aqueous Solutions. Ind. Eng. Chem. Res. 2002, 41, 46–51. [Google Scholar] [CrossRef]
- Ghurye, G.; Clifford, D.; Tripp, A. Iron Coagulation and Direct Microfiltration to Remove Arsenic from Groundwater. J. AWWA 2004, 96, 143–152. [Google Scholar] [CrossRef]
- Kalaimurugan, D.; Sivasankar, P.; Durairaj, K.; Lakshmanamoorthy, M.; Ali Alharbi, S.; Al Yousef, S.A.; Chinnathambi, A.; Venkatesan, S. Novel Strategy for Biodegradation of 4-Nitrophenol by the Immobilized Cells of Pseudomonas Sp. YPS3 with Acacia Gum. Saudi J. Biol. Sci. 2021, 28, 833–839. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-Up and Its Role in Future Energy Generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From Theory to Practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Shelare, S.; Kumar, R.; Gajbhiye, T.; Kanchan, S. Role of Geothermal Energy in Sustainable Water Desalination—A Review on Current Status, Parameters, and Challenges. Energies 2023, 16, 2901. [Google Scholar] [CrossRef]
- Thirunavukkarasu, O.S.; Viraraghavan, T.; Subramanian, K.S. Removal of Arsenic in Drinking Water by Iron Oxide-Coated Sand and Ferrihydrite—Batch Studies. Water Qual. Res. J. 2001, 36, 55–70. [Google Scholar] [CrossRef]
- Vilve, M.; Vilhunen, S.; Vepsäläinen, M.; Kurniawan, T.A.; Lehtonen, N.; Isomäki, H.; Sillanpää, M. Degradation of 1,2-Dichloroethane from Wash Water of Ion-Exchange Resin Using Fenton’s Oxidation. Environ. Sci. Pollut. Res. 2010, 17, 875–884. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Solar Powered Desalination—Technology, Energy and Future Outlook. Desalination 2019, 453, 54–76. [Google Scholar] [CrossRef]
- Hernandez, D.; Boden, K.; Paul, P.; Bandaru, S.; Mypati, S.; Roy, A.; Amrose, S.; Roy, J.; Gadgil, A. Strategies for Successful Field Deployment in a Resource-Poor Region: Arsenic Remediation Technology for Drinking Water. Dev. Eng. 2019, 4, 100045. [Google Scholar] [CrossRef]
- Robles, D. Indigenous Water Governance in the Anthropocene: Non-Conventional Hydrosocial Relations Among the Wayuu of the Guajira Peninsula in Northern Colombia. Ph.D. Thesis, Florida International University, Miami, FL, USA, 2020. [Google Scholar]
- Scott, I.S.P.C.; Scott, F.; McCarty, T.; Penn, C.J. Techno-Economic Analysis of Phosphorus Removal Structures. Environ. Sci. Technol. 2023, 57, 12858–12868. [Google Scholar] [CrossRef] [PubMed]
- López-Guzmán, M.; Alarcón-Herrera, M.T.; Irigoyen-Campuzano, J.R.; Torres-Castañón, L.A.; Reynoso-Cuevas, L. Simultaneous Removal of Fluoride and Arsenic from Well Water by Electrocoagulation. Sci. Total Environ. 2019, 678, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Roy, M.B.; Roy, P.K.; Wallace, J.M. Assessment of Arsenic Removal Units in Arsenic-Prone Rural Area in Uttar Pradesh, India. J. Inst. Eng. Ser. A 2019, 100, 253–259. [Google Scholar] [CrossRef]
- Raimondi, A.; Quinn, R.; Abhijith, G.R.; Becciu, G.; Ostfeld, A. Rainwater Harvesting and Treatment: State of the Art and Perspectives. Water 2023, 15, 1518. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Abedin, M.A.; Shaw, R. Community-Level Arsenicmitigation Practices in Southwestern Part of Bangladesh. In Water Insecurity: A Social Dilemma; Community, Environment and Disaster Risk Management; Emerald Group Publishing Ltd.: Bingley, UK, 2014; Volume 13, pp. 51–73. ISBN 978-1-78190-882-2. [Google Scholar]
- Martin, R.; Dowling, K.; Pearce, D.; Sillitoe, J.; Florentine, S. Health Effects Associated with Inhalation of Airborne Arsenic Arising from Mining Operations. Geosciences 2014, 4, 128–175. [Google Scholar] [CrossRef]


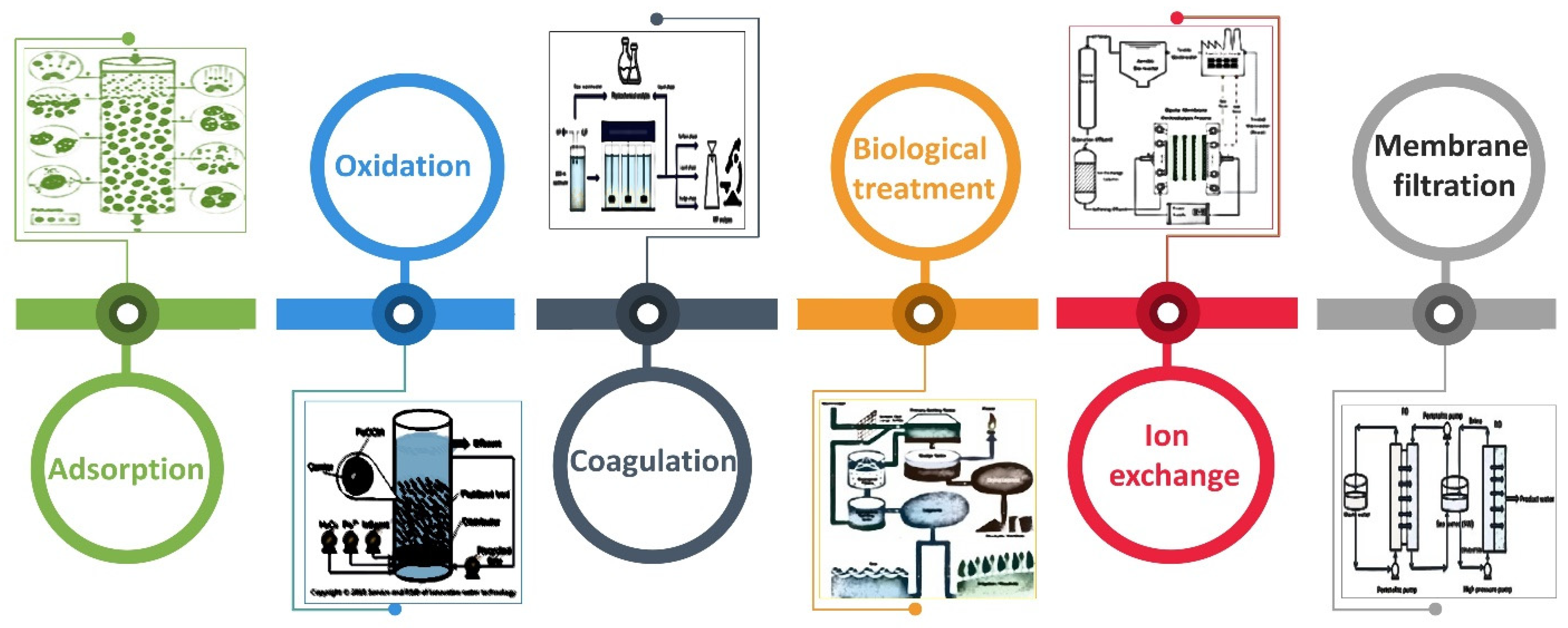
| Reference | Technology | Overview | Description | Significance | Limitations |
|---|---|---|---|---|---|
| [138,156,165,166,167] | Adsorption | Adsorbents bind Arsenic. | Activated alumina, iron-modified zeolites, and biochar. |
|
|
| [168,169,170] | Oxidation | Arsenate (As(V)) is easier to treat | Exposure to air (aeration) or chemical oxidants such as potassium permanganate, hydrogen peroxide, or chlorine. |
|
|
| [132,158,171] | Coagulation | Chemical coagulants create Arsenic-binding precipitates. Filters remove these precipitates. | Ferric chloride, ferric sulfate, and alum. |
|
|
| [172,173,174] | Biological Methods | Microorganisms can convert or absorb Arsenic. | Use Arsenic-respiring algae or bacteria. |
|
|
| [175,176,177] | Ion Exchange | Specific resins exchange Arsenic ions for non-hazardous ones. | Anion exchange resins. |
|
|
| [156,165,178] | Membrane Technologies | These methods remove Arsenic from water using semipermeable membranes. | Reverse osmosis and nanofiltration. |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marghade, D.; Mehta, G.; Shelare, S.; Jadhav, G.; Nikam, K.C. Arsenic Contamination in Indian Groundwater: From Origin to Mitigation Approaches for a Sustainable Future. Water 2023, 15, 4125. https://doi.org/10.3390/w15234125
Marghade D, Mehta G, Shelare S, Jadhav G, Nikam KC. Arsenic Contamination in Indian Groundwater: From Origin to Mitigation Approaches for a Sustainable Future. Water. 2023; 15(23):4125. https://doi.org/10.3390/w15234125
Chicago/Turabian StyleMarghade, Deepali, Girish Mehta, Sagar Shelare, Ganesh Jadhav, and Keval Chandrakant Nikam. 2023. "Arsenic Contamination in Indian Groundwater: From Origin to Mitigation Approaches for a Sustainable Future" Water 15, no. 23: 4125. https://doi.org/10.3390/w15234125
APA StyleMarghade, D., Mehta, G., Shelare, S., Jadhav, G., & Nikam, K. C. (2023). Arsenic Contamination in Indian Groundwater: From Origin to Mitigation Approaches for a Sustainable Future. Water, 15(23), 4125. https://doi.org/10.3390/w15234125






