Ecotoxicity Assessment of the Water Extracts from Metal-Contaminated and Hydrocarbon-Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sites and Soil Characterization
2.2. Water Extraction Procedure
2.3. Bioassays
2.3.1. Plants
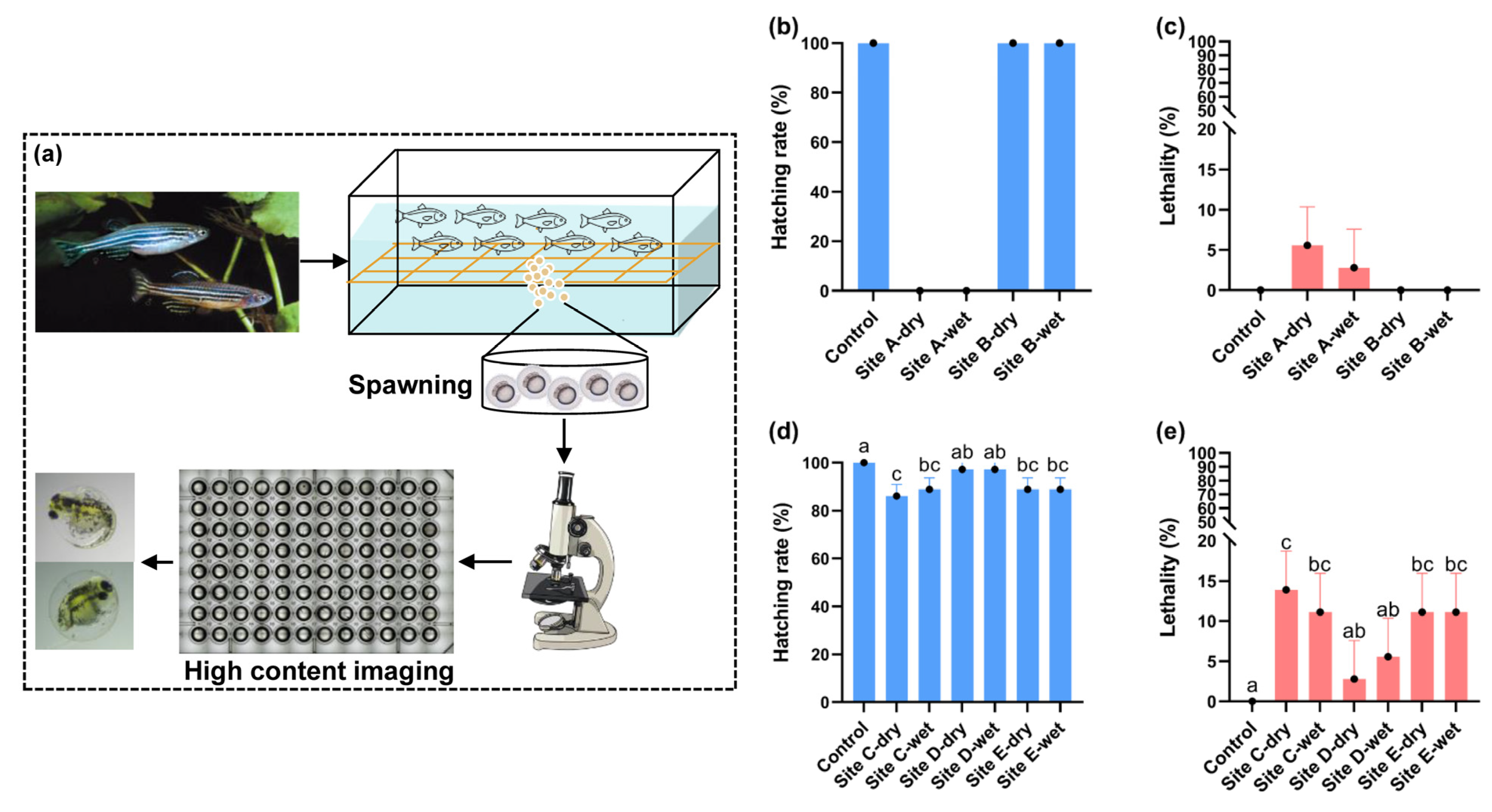
2.3.2. Zebrafish (Danio rerio)
2.4. Data Analysis
3. Results
3.1. Chemical Analysis of Contaminated Soils
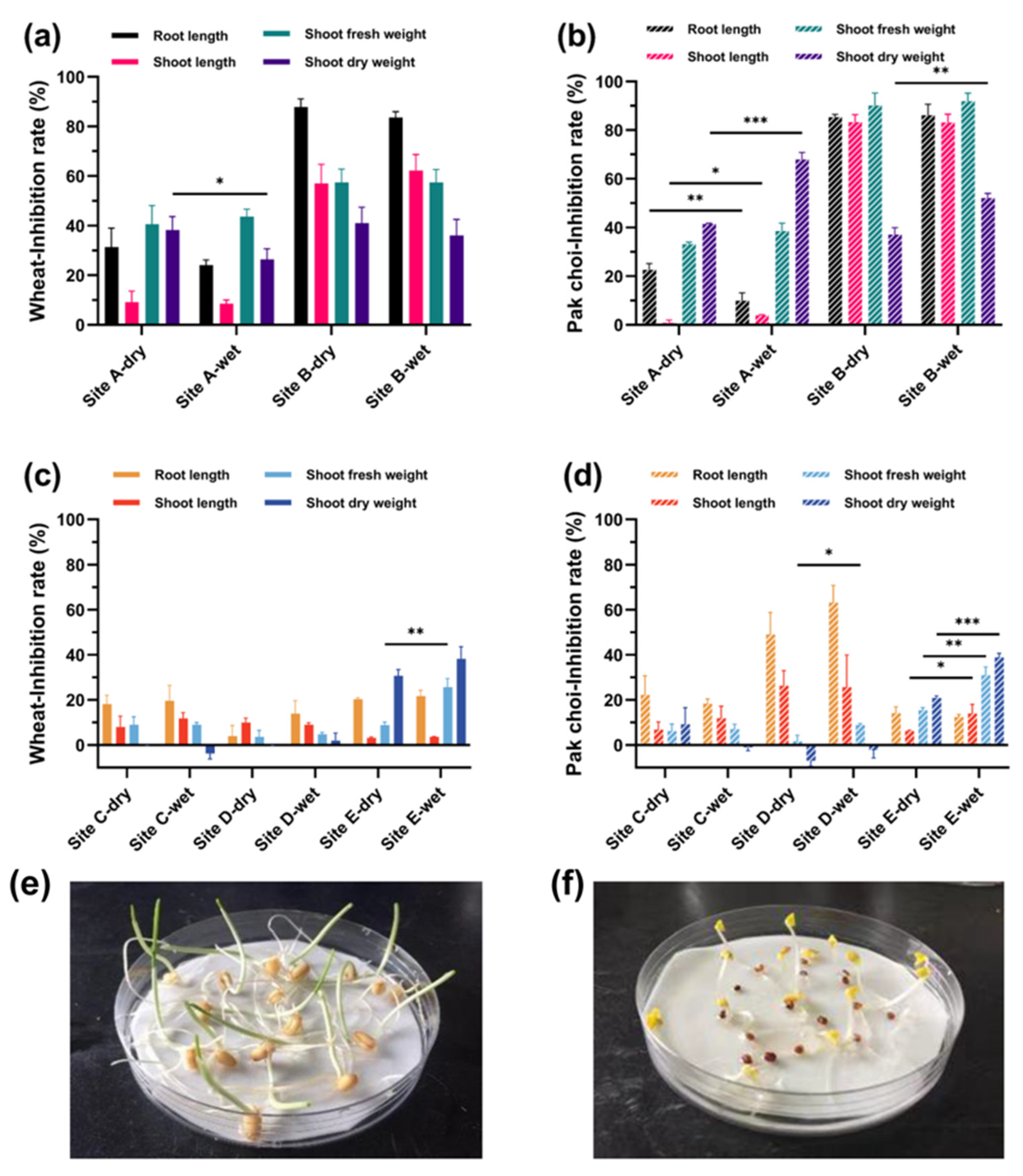
3.2. Plants Bioassays on Metal-Contaminated Soils
3.3. Plants Bioassays on Hydrocarbon-Contaminated Soils
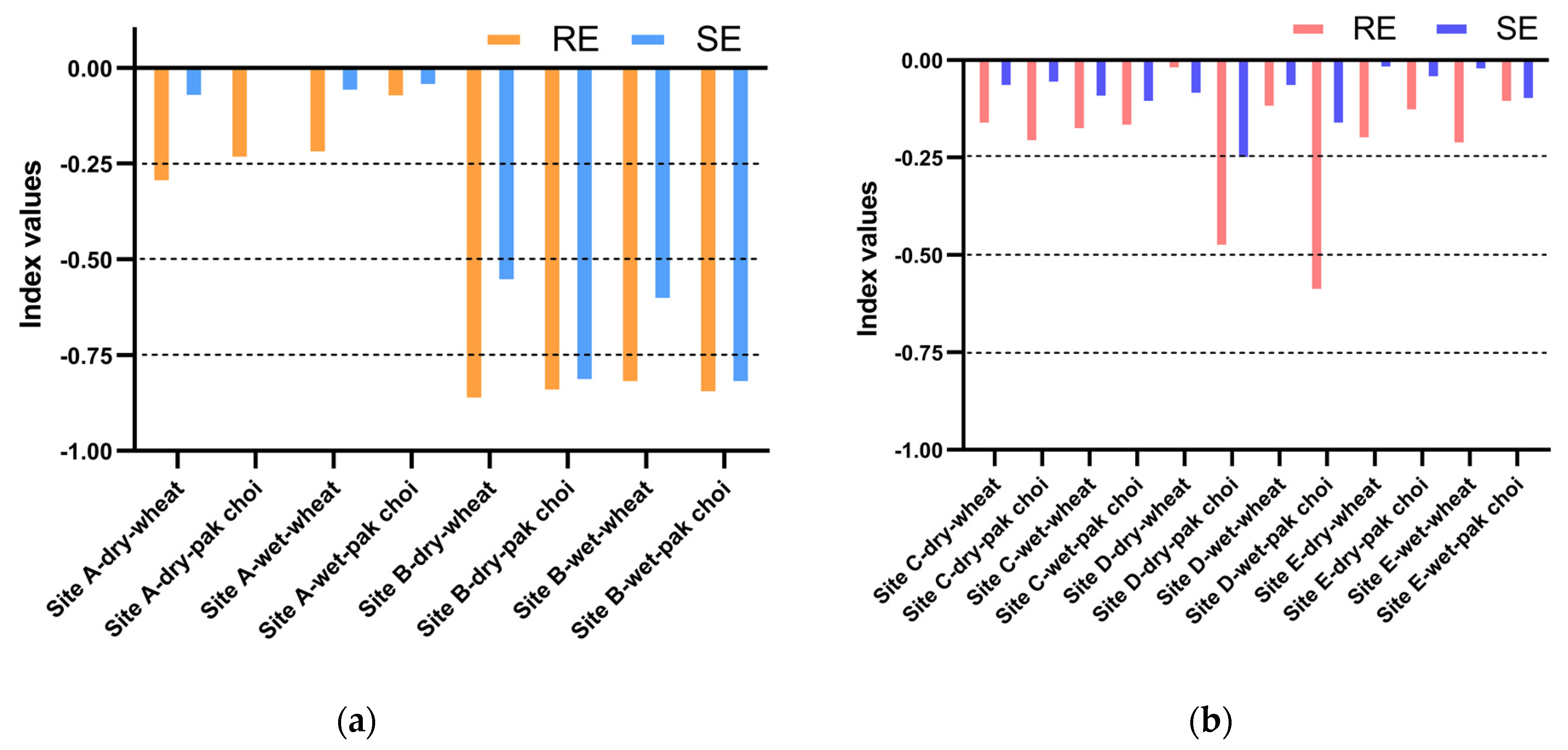
3.4. Phytotoxicity Evaluation of Soil Water Extracts
3.5. Toxicity Evaluation of Soil Water Extracts by Zebrafish Embryos
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Long, Z.J.; Zhu, H.; Bing, H.J.; Tian, X.; Wang, Z.G.; Wang, X.F.; Wu, Y.H. Contamination, sources and health risk of heavy metals in soils and dust from different functional areas in an industrial city of Panzhihua City, Southwest China. J. Hazard. Mater. 2021, 420, 126638. [Google Scholar] [CrossRef]
- Zhang, H.; Li, A.Y.; Wei, Y.Q.; Miao, Q.C.; Xu, W.X.; Zhao, B.; Guo, Y.; Sheng, Y.Z.; Yang, Y. Development of a new methodology for multifaceted assessment, analysis, and characterization of soil contamination. J. Hazard. Mater. 2022, 438, 129542. [Google Scholar] [CrossRef]
- Rocha, L.; Rodrigues, S.M.; Lopes, I.; Soares, A.M.V.M.; Duarte, A.C.; Pereira, E. The water-soluble fraction of potentially toxic elements in contaminated soils: Relationships between ecotoxicity, solubility and geochemical reactivity. Chemosphere 2011, 84, 1495–1505. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, F.H.; Wan, J.Z.; He, J.; Li, Q.; Chen, Q.; Gao, J.; Lin, Y.S.; Zhang, S.T. Ecotoxicological bioassays of sediment leachates in a river bed flanked by decommissioned pesticide plants in Nantong City, East China. Environ. Sci. Pollut. R. 2017, 24, 8541–8550. [Google Scholar] [CrossRef]
- Santos, E.S.; Abreu, M.M.; de Varennes, A.; Macías, F.; Leitão, S.; Cerejeira, M.J. Evaluation of chemical parameters and ecotoxicity of a soil developed on gossan following application of polyacrylates and growth of Spergularia purpurea. Sci. Total Environ. 2013, 461–462, 360–370. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, A.; Asensio, V.; Zaldibar, B.; Soto, M.; Marigómez, I. Toxicity assessment through multiple endpoint bioassays in soils posing environmental risk according to regulatory screening values. Environ. Sci. Pollut. Res. 2014, 21, 9689–9708. [Google Scholar] [CrossRef]
- Tang, J.C.; Wang, M.; Wang, F.; Sun, Q.; Zhou, Q.X. Eco-toxicity of petroleum hydrocarbon contaminated soil. J. Environ. Sci. 2011, 23, 845–851. [Google Scholar] [CrossRef]
- ISO 15799; Soil Quality—Guidance on the Ecotoxicological Characterization of Soils and Soil Materials. International Organization for Standardization: Genéve, Switzerland, 2019.
- ISO 15175; Soil Quality—Characterization of Soil Related to Groundwater Protection. International Organization for Standardization: Genéve, Switzerland, 2018.
- ISO 17512-1; Soil Quality—Avoidance Test for Testing the Quality of Soils and the Toxicity of Chemicals—Test with Earthworms (Eisenia fetida). International Organization for Standardization: Genéve, Switzerland, 2008.
- OECD. Earthworm, Acute Toxicity Tests. In OECD-Guideline for Testing of Chemicals No. 207; OECD: Paris, France, 1984. [Google Scholar]
- OECD. Collembolan Reproduction Test in Soil; Test No. 232; OECD: Paris, France, 2009. [Google Scholar]
- ISO 11267; Soil Quality-Inhibition of Reproduction of Collembola (Folsomia candida) by Soil Pollutants. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 11269-2; Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Part 2: Effects of Contaminated Soil on the Emergence and Early Growth of Higher Plants. ISO: Geneva, Switzerland, 2012.
- ISO 11269-1; Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Part 1: Method for the Measurement of Inhibition of Root Growth. ISO: Geneva, Switzerland, 2012.
- van Gestel, C.A.M.; van der Waarde, J.J.; Derksen, G.M.; van der Hoek, E.E.; Veul, M.F.X.W.; Bouwens, S.; Rusch, B.; Kronenburg, R.; Stokman, G.N.M. The use of acute and chronic bioassays to determine the ecological risk and bioremediation efficiency of oil-polluted soils. Environ. Toxicol. Chem. 2001, 20, 1438–1449. [Google Scholar]
- Kočí, V.; Mocová, K.; Kulovaná, M.; Vosáhlová, S. Phytotoxicity tests of solid wastes and contaminated soils in the Czech Republic. Environ. Sci. Pollut. Res. 2010, 17, 611–623. [Google Scholar] [CrossRef]
- Balestri, E.; Menicagli, V.; Ligorini, V.; Fulignati, S.; Galletti, A.M.R.; Lardicci, C. Phytotoxicity assessment of conventional and biodegradable plastic bags using seed germination test. Ecol. Indic. 2019, 102, 569–580. [Google Scholar] [CrossRef]
- He, M.M.; Li, W.H.; Liang, X.Q.; Wu, D.L.; Tian, G.M. Effect of composting process on phytotoxicity and speciation of copper, zinc and lead in sewage sludge and swine manure. Waste. Manag. 2009, 29, 590–597. [Google Scholar]
- OECD. Terrestrial plant test: Seedling emergence and seedling growth. In Guidelines for the Testing of Chemicals; Test Guideline 208; OECD: Paris, France, 2006. [Google Scholar]
- Abbas, S.; Kousar, S.; Khan, M.S. The role of climate change in food security; empirical evidence over Punjab regions, Pakistan. Environ. Sci. Pollut. R. 2022, 29, 53718–53736. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Wang, Y.H.; Liu, Z.Y.; Li, C.Y.; Feng, H. Assessment of a stay-green mutant for variety improvement in pakchoi (Brassica campestris L. spp. chinensis). Sci. Hortic. 2020, 266, 109261. [Google Scholar] [CrossRef]
- Loureiro, S.; Ferreira, A.L.G.; Soares, A.M.V.M.; Nogueira, A.J.A. Evaluation of the toxicity of two soils from Jales Mine (Portugal) using aquatic bioassays. Chemosphere 2005, 61, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.N.; Gonçalves, S.F.; Silva, A.R.R.; Soares, A.M.V.M.; Wrona, F.J.; Loureiro, S. Ecotoxicological effects of fluvial eroded bitumen sediments from the Alberta oil sands to model aquatic species. Sci. Total Environ. 2023, 862, 160592. [Google Scholar] [CrossRef] [PubMed]
- OECD. Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals, Section 2; Test Guideline 236; OECD: Paris, France, 2013. [Google Scholar]
- Hollert, H.; Keiter, S.; Koening, N.; Rudolf, M.; Ulrich, M.; Braunbeck, T. A new sediment contact assay to assess particle-bound pollutants using zebrafish (Danio rerio) embryos. J. Soils. Sediments. 2003, 3, 197–207. [Google Scholar] [CrossRef]
- Hallare, A.V.; Kosmehl, T.; Schulze, T.; Hollert, H.; Kohler, H.R.; Triebskorn, R. Assessing contamination levels of Laguna Lake sediments (Philippines) using a contact assay with zebrafish (Danio rerio) embryos. Sci. Total Environ. 2005, 347, 254–271. [Google Scholar] [CrossRef]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Legler, J.; van Velzen, M.; Cenijn, P.H.; Houtman, C.J.; Lamoree, M.H.; Wegener, J.W. Effect-directed analysis of municipal landfill soil reveals novel developmental toxicants in the zebrafish Danio rerio. Environ. Sci. Technol. 2011, 45, 8552–8558. [Google Scholar] [CrossRef]
- Chibwe, L.; Geier, M.C.; Nakamura, J.; Tanguay, R.L.; Aitken, M.D.; Simonich, S.L.M. Aerobic bioremediation of PAH contaminated soil results in increased genotoxicity and developmental toxicity. Environ. Sci. Technol. 2015, 49, 13889–13898. [Google Scholar] [CrossRef]
- Wincent, E.; Jönsson, M.E.; Bottai, M.; Lundstedt, S.; Dreij, K. Aryl hydrocarbon receptor activation and developmental toxicity in zebrafish in response to soil extracts containing unsubstituted and oxygenated PAHs. Environ. Sci. Technol. 2015, 49, 3869–3877. [Google Scholar] [CrossRef] [PubMed]
- Trine, L.S.D.; Davis, E.L.; Roper, C.; Truong, L.; Tanguay, R.L.; Simonich, S.L.M. Formation of PAH derivatives and increased developmental toxicity during steam enhanced extraction remediation of creosote contaminated superfund soil. Environ. Sci. Technol. 2019, 53, 4460–4469. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, J.; Marques, S.; Carvalho, F.P.; Oliveira, J.; Malta, M.; Santos, M.; Gonçalves, F.; Pereira, R.; Mendo, S. Uranium mining wastes: The use of the Fish Embryo Acute Toxicity Test (FET) test to evaluate toxicity and risk of environmental discharge. Sci. Total Environ. 2017, 605–606, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.; Venâncio, C.; Sousa, J.P.; Leston, S.; Ramos, F.; Soares, A.M.V.M.; Lopes, I. Ecotoxicity of eluates obtained from Basamid® contaminated soils is pH dependent: A study with Hydra viridissima, Xenopus laevis and Danio rerio. Sci. Total Environ. 2023, 868, 161640. [Google Scholar] [CrossRef]
- Yang, F.L.; Yun, Y.; Li, G.K.; Sang, N. Heavy metals in soil from gangue stacking areas increase children health risk and causes developmental neurotoxicity in zebrafish larvae. Sci. Total Environ. 2021, 794, 148629. [Google Scholar] [CrossRef]
- Zhao, M.L.; Wang, H.J.; Sun, J.X.; Tang, R.; Cai, B.Y.; Song, X.Y.; Huang, X.M.; Huang, J.; Fan, Z.Q. Spatio-temporal characteristics of soil Cd pollution and its influencing factors: A geographically and temporally weighed regression (GTWR) method. J. Hazard. Mater. 2023, 446, 130613. [Google Scholar] [CrossRef]
- Li, M.; Chen, Q.; Yang, L.; Zhang, Y.; Jiang, J.L.; Deng, S.P.; Wan, J.Z.; Fan, T.T.; Long, T.; Zhang, S.T.; et al. Contaminant characterization at pesticide production sites in the Yangtze River Delta: Residue, distribution and environmental risk. Sci. Total Environ. 2023, 860, 160156. [Google Scholar] [CrossRef]
- Deng, S.Q.; Ke, T.; Wu, Y.F.; Zhang, C.; Hu, Z.Q.; Yin, H.M.; Guo, L.M.; Chen, L.Z.; Zhang, D.Y. Heavy metal exposure alters the uptake behavior of 16 priority polycyclic aromatic hydrocarbons (PAHs) by pak choi (Brassica chinensis L.). Environ. Sci. Technol. 2018, 52, 13457–13468. [Google Scholar] [CrossRef]
- Huang, X.P.; Yang, S.; Li, B.X.; Wang, A.P.; Li, H.; Li, X.H.; Luo, J.; Liu, F.; Wu, M. Comparative toxicity of multiple exposure routes of pyraclostrobin in adult zebrafish (Danio rerio). Sci. Total Environ. 2021, 777, 145957. [Google Scholar] [CrossRef]
- Cao, Y.; Li, X.; Qian, X.Y.; Gu, H.R.; Li, J.W.; Chen, X.H.; Shen, G.X. Soil health assessment in the Yangtze River Delta of China: Method development and application in orchards. Agr. Ecosyst. Environ. 2023, 341, 108190. [Google Scholar] [CrossRef]
- Li, Q.; Wang, M.; Duan, L.; Qiu, Y.L.; Ma, T.W.; Chen, L.; Breitholtz, M.; Bergman, Å.; Zhao, J.F.; Hecker, M.; et al. Multiple biomarker responses in caged benthic gastropods Bellamya aeruginosa after in situ exposure to Taihu Lake in China. Environ. Sci. Eur. 2018, 30, 34. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Y.N.; Wen, D.D.; Fu, R.B.; Feng, L.Y. Application of alkyl polyglycosides for enhanced bioremediation of petroleum hydrocarbon-contaminated soil using Sphingomonas changbaiensis and Pseudomonas stutzeri. Sci. Total Environ. 2020, 719, 137456. [Google Scholar] [CrossRef]
- DIN 38414-S4; Determination of Leachability by Water (S4). German Standard Methods for the Examination of Water, Wastewater and Sludge. DIN: Berlin, Germany, 1984.
- ISO/TS 21268-2; Soil Quality—Leaching Procedures for Subsequent Chemical and Ecotoxicological Testing of Soil and Soil Materials. Part 2. Batch Test Using a Liquid to Solid Ratio of 10 L/kg Dry Matter. ISO: Geneva, Switzerland, 2007.
- Ministry of Ecology and Environment of People’s Republic of China. HJ 557-2010 Solid Waste-Extraction Procedure for Leaching Toxicity-Horizontal Vibration Method; Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2010.
- GB 36600-2018; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Development Land. National Soil Quality Standardization Technical Committee: Nanjing, China, 2018.
- Lin, X.L.; Sun, Z.J.; Zhao, L.; Ma, J.; Li, X.; He, F.; Hou, H. Toxicity of exogenous hexavalent chromium to soil-dwelling springtail Folsomia candida in relation to soil properties and aging time. Chemosphere 2019, 224, 734–742. [Google Scholar] [CrossRef]
- Li, M.F.; Sun, K.L.; Fang, Y.S.; Zheng, M.; Xie, X.Y.; Tang, J.C.; Liu, R.T. Toxic effects of acetone, 2-pentanone, and 2-hexanone on physiological indices of wheat (Triticum aestivum L.) germination and seedlings. Environ. Sci. Pollut. Res. 2021, 28, 64552–64560. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.J.; Luo, F.; Lomheim, L.; Mack, E.E.; Ye, S.J.; Wu, J.C.; Edwards, E.A. A dehalogenimonas population respires 1,2,4-trichlorobenzene and dichlorobenzenes. Environ. Sci. Technol. 2018, 52, 13391–13398. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.T.; Yang, M.; Li, Q.; Zhou, Y.; Xia, F.Y.; Chen, Y.; Yang, L.; Ding, D.; Zhang, S.T.; Zhang, X.D.; et al. A new insight into the influencing factors of natural attenuation of chlorinated hydrocarbons contaminated groundwater: A long-term field study of a retired pesticide site. J. Hazard. Mater. 2022, 439, 129595. [Google Scholar] [CrossRef]
- Bagur-González, M.G.; Estepa-Molina, C.; Martín-Peinado, F.; Morales-Ruano, S. Toxicity assessment using Lactuca sativa L. bioassay of the metal(loid)s As, Cu, Mn, Pb and Zn in soluble-in-water saturated soil extracts from an abandoned mining site. J. Soil. Sediment. 2011, 11, 281–289. [Google Scholar] [CrossRef]
- Lors, C.; Ponge, J.F.; Aldaya, M.M.; Damidot, D. Comparison of solid and liquid-phase bioassays using ecoscores to assess contaminated soils. Environ. Pollut. 2011, 159, 2974–2981. [Google Scholar] [CrossRef]
- Leitgib, L.; Kálmán, J.; Gruiz, K. Comparison of bioassays by testing whole soil and their water extract from contaminated sites. Chemosphere 2007, 66, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Pablos, M.V.; Fernández, C.; Babín, M.M.; Navas, J.M.; Carbonell, G.; Martini, F.; García-Hortigüela, P.; Tarazona, J.V. Use of a novel battery of bioassays for the biological characterization of hazardous wastes. Ecotox. Environ. Saf. 2009, 72, 1594–1600. [Google Scholar] [CrossRef]
- Sheppard, S.C.; Evenden, W.G.; Abboud, S.A.; Stephenson, M. A plant life-cycle bioassay for contaminated soil, with comparison to other bioassays: Mercury and zinc. Arch. Environ. Contam. Toxicol. 1993, 25, 27–35. [Google Scholar] [CrossRef]
- Capela, R.; Garric, J.; Castro, L.F.C.; Santos, M.M. Embryo bioassays with aquatic animals for toxicity testing and hazard assessment of emerging pollutants: A review. Sci. Total Environ. 2020, 705, 135740. [Google Scholar] [CrossRef]
- Löv, Å.; Larsbo, M.; Sjöstedt, C.; Cornelis, G.; Gustafsson, J.P.; Kleja, D.B. Evaluating the ability of standardised leaching tests to predict metal(loid) leaching from intact soil columns using size-based elemental fractionation. Chemosphere 2019, 222, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.W.; Bhatta, D. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—A case study: Mahanadi basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef]
- Furman, O.; Strawn, D.G.; McGeehan, S. Sample drying effects on lead bioaccessibility in reduced soil. J. Environ. Qual. 2007, 36, 899–903. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, B.; Zhang, Y.H.; Zhu, F.F.; Wang, H.; Wang, J.W.; Fu, X.M. The function of “Cambi® thermal hydrolysis + anaerobic digestion” on heavy metal behavior and risks in a full-scale sludge treatment plant based on four seasons investigation. J. Hazard. Mater. 2023, 445, 130579. [Google Scholar] [CrossRef]
- Ding, J.L.; Liu, L.; Wang, C.; Shi, L.; Xu, F.S.; Cai, H.M. High level of zinc triggers phosphorus starvation by inhibiting root-to-shoot translocation and preferential distribution of phosphorus in rice plants. Environ. Pollut. 2021, 277, 116778. [Google Scholar] [CrossRef]
- Yao, Y.; Tong, L.Z.; Zhao, R.L.; Wang, Q.H.; Qiu, J.L.; Wang, F.H.; Li, J.N.; Yan, Y.F.; He, Y.; Li, S.Q. Leaching of heavy metal(loid)s from historical Pb–Zn mining tailing in abandoned tailing deposit: Up-flow column and batch tests. J. Environ. Manag. 2023, 325, 116572. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Wei, X.L.; Lu, J.; You, J.; Wang, W.R.; Shi, R.X. Lead-induced phytotoxicity mechanism involved in seed germination and seedling growth of wheat (Triticum aestivum L.). Ecotox. Environ. Saf. 2010, 73, 1982–1987. [Google Scholar] [CrossRef]
- Salvatore, M.D.; Carafa, A.M.; Carratù, G. Assessment of heavy metals phytotoxicity using seed germination and root elongation tests: A comparison of two growth substrates. Chemosphere 2008, 73, 1461–1464. [Google Scholar] [CrossRef]
- Israr, M.; Jewell, A.; Kumar, D.; Sahi, S.V. Interactive effects of lead, copper, nickel and zinc on growth, metal uptake and antioxidative metabolism of Sesbania drummondii. J. Hazard. Mater. 2011, 186, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.L.; Nan, Z.R.; Ma, J.M.; Zang, F.; Chen, Y.Z.; Li, Y.P.; Zhang, Q. Effects of Ni stress on the uptake and translocation of Ni and other mineral nutrition elements in mature wheat grown in sierozems from northwest of China. Environ. Sci. Pollut. Res. 2015, 22, 19756–19763. [Google Scholar] [CrossRef]
- Wei, C.; Jiao, Q.J.; Agathokleous, E.; Liu, H.T.; Li, G.Z.; Zhang, J.J.; Fahad, S.; Jiang, Y. Hormetic effects of zinc on growth and antioxidant defense system of wheat plants. Sci. Total Environ. 2022, 807, 150992. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Yang, J.Y.; Peng, Q.Q.; Liang, X.P.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef]
- Hua, J.; Peijnenburg, W.J.G.M.; Vijver, M.G. TiO2 nanoparticles reduce the effects of ZnO nanoparticles and Zn ions on zebrafish embryos (Danio rerio). NanoImpact 2016, 2, 45–53. [Google Scholar] [CrossRef]
- Brun, N.R.; Lenz, M.; Wehrli, B.; Fent, K. Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleutheroembryos: Importance of zinc ions. Sci. Total Environ. 2014, 476–477, 657–666. [Google Scholar] [CrossRef]
- Lin, S.J.; Zhao, Y.; Ji, Z.X.; Ear, J.; Chang, C.H.; Zhang, H.Y.; Low-Kam, C.; Yamada, K.; Meng, H.; Wang, X.; et al. Zebrafish high-throughput screening to study the impact of dissolvable metal oxide nanoparticles on the hatching enzyme, ZHE1. Small 2013, 9, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Nabinger, D.D.; Altenhofen, S.; Bitencourt, P.E.R.; Nery, L.R.; Leite, C.E.; Vianna, M.R.M.R.; Bonan, C.D. Nickel exposure alters behavioral parameters in larval and adult zebrafish. Sci. Total Environ. 2018, 624, 1623–1633. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Das, H.; Meena, V.D. Assessment of chromium efficacy on germination, root elongation, and coleoptile growth of wheat (Triticum aestivum L.) at different growth periods. Environ. Monit. Assess 2014, 186, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Tian, X.Y.; Liang, J.L.; Chen, X.L.; Ye, J.Y.; Liu, Y.S.; Liu, Y.Y.; Wei, Y.M. Remediation of hexavalent chromium in contaminated soil using amorphous iron pyrite: Effect on leachability, bioaccessibility, phytotoxicity and long-term stability. Environ. Pollut. 2020, 264, 114804. [Google Scholar] [CrossRef]
- Hou, J.; Liu, G.N.; Xue, W.; Fu, W.J.; Liang, B.C.; Liu, X.H. Seed germination, root elongation, root-tip mitosis, and micronucleus induction of five crop plants exposed to chromium in fluvo-aquic soil. Environ. Toxicol. Chem. 2013, 33, 671–676. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Rasheed, R.; Hussain, I.; Iqbal, M.; Farooq, M.U.; Saleem, M.H.; Ali, S. Taurine modulates dynamics of oxidative defense, secondary metabolism, and nutrient relation to mitigate boron and chromium toxicity in Triticum aestivum L. plants. Environ. Sci. Pollut. R. 2022, 29, 45527–45548. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ali, S.; Refay, Y.; Rizwan, M.; Alhammad, B.A.; El-hendawy, S.W. Chromium resistant microbes and melatonin reduced Cr uptake and toxicity, improved physio-biochemical traits and yield of wheat in contaminated soil. Chemosphere 2020, 250, 126239. [Google Scholar] [CrossRef]
- Askari, S.H.; Ashraf, M.A.; Ali, S.; Rizwan, M.; Rasheed, R. Menadione sodium bisulfite alleviated chromium effects on wheat by regulating oxidative defense, chromium speciation, and ion homeostasis. Environ. Sci. Pollut. R. 2021, 28, 36205–36225. [Google Scholar] [CrossRef]
- Kumpiene, J.; Bert, V.; Dimitriou, I.; Eriksson, J.; Friesl-Hanl, W.; Galazka, R.; Herzig, R.; Janssen, J.; Kidd, P.; Mench, M.; et al. Selecting chemical and ecotoxicological test batteries for risk assessment of trace element-contaminated soils (phyto)managed by gentle remediation options (GRO). Sci. Total Environ. 2014, 496, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Pang, A.; Rutter, A.; Bordenave, S.; Gainer, A.; Haack, E.; Zeeb, B. Assessment of the toxicity of weathered petroleum hydrocarbon impacted soils to native plants from a site in the Canadian Subarctic. Ecotoxicology 2022, 31, 1287–1298. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Formicola, F.; Sbaffoni, S.; Prasad, S.; Milanese, C.; della Cuna, F.S.R.; Franzetti, A.; Vaccari, M. Treatment of petroleum hydrocarbon contaminated soil by combination of electro-Fenton and biosurfactant-assisted bioslurry process. Chemosphere 2023, 319, 138013. [Google Scholar] [CrossRef] [PubMed]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Inno. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Varjani, S.J.; Gnansounou, E.; Pandey, A. Comprehensive review on toxicity of persistent organic pollutants from petroleum refinery waste and their degradation by microorganisms. Chemosphere 2017, 188, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Prasad, S.; Gomez, F.H.; Franzetti, A.; Vaccari, M. Remediation of soil polluted with petroleum hydrocarbons and its reuse for agriculture: Recent progress, challenges, and perspectives. Chemosphere 2022, 293, 133572. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Jiang, Y.M.; Zhao, J.H.; Li, S.S.; Schulz, S.; Deng, L. BTEX biodegradation is linked to bacterial community assembly patterns in contaminated groundwater ecosystem. J. Hazard. Mater. 2021, 419, 126205. [Google Scholar] [CrossRef] [PubMed]
- Płaza, G.; Nałęcz-Jawecki, G.; Ulfig, K.; Brigmon, R.L. The application of bioassays as indicators of petroleum-contaminated soil remediation. Chemosphere 2005, 59, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Umar, H.A.; Khanan, M.F.A.; Shiru, M.S.; Ahmad, A.; Rahman, M.Z.A.; Md Din, A.H. An integrated investigation of hydrocarbon pollution in Ahoada area, Niger Delta Region, Nigeria. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef]
- Dorn, P.B.; Salanitro, J.P. Temporal ecological assessment of oil contaminated soils before and after bioremediation. Chemosphere 2000, 40, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Luo, M.Y.; Deng, S.P.; Long, T.; Sun, L.W.; Yu, R. Field study of microbial community structure and dichlorination activity in a multi-solvents co-contaminated site undergoing natural attenuation. J. Hazard. Mater. 2022, 423, 127010. [Google Scholar] [CrossRef]
- Chaudhry, Q.; Schroder, P.; Werck-Reichhart, D.; Grajek, W.; Marecik, R. Prospects and limitations of phytoremediation for the removal of persistent pesticides in the environment. Environ. Sci. Pollut. Res. 2002, 9, 4–17. [Google Scholar] [CrossRef]
- Yan, J.C.; Hu, L.C.; Gao, W.G.; Yang, L.; Qian, L.B.; Han, L.; Chen, M.F. Remediation of 1,2-dichlorobenzene contaminated soil by activated persulfate using green synthesized nanoscale zero valent iron: Activation mechanism and degradation pathways. J. Soil. Sediment. 2022, 22, 1135–1144. [Google Scholar] [CrossRef]
- Monferran, M.V.; Pesce, S.F.; Cazenave, J.; Wunderlin, D.A. Detoxification and antioxidant responses in diverse organs of Jenynsia multidentata experimentally exposed to 1,2- and 1,4-dichlorobenzene. Environ. Toxicol. 2008, 23, 184–192. [Google Scholar] [CrossRef]
- Kampfraath, A.A.; Giesen, D.; van Gestel, C.A.M.; Lann, C.L. Pesticide stress on plants negatively affects parasitoid fitness through a bypass of their phytophage hosts. Ecotoxicology 2017, 26, 383–395. [Google Scholar] [CrossRef]
- Miguel, A.S.; Faure, M.; Ravanel, P.; Raveton, M. Biological responses of maize (Zea mays) plants exposed to chlorobenzene. Case study of monochloro-, 1.4-dichloro- and 1,2,4-trichloro-benzene. Ecotoxicology 2012, 21, 315–324. [Google Scholar] [CrossRef]
- Inckot, R.C.; Santos, G.O.; Souza, L.Z.; Bona, C. Germination and development of Mimosa pilulifera in petroleum-contaminated soil and bioremediated soil. Flora 2011, 206, 261–266. [Google Scholar] [CrossRef]
- Alvarenga, P.; Palma, P.; de Varennes, A.; Cunha-Queda, A.C. A contribution towards the risk assessment of soils from the São Domingos Mine (Portugal): Chemical, microbial and ecotoxicological indicators. Environ. Pollut. 2012, 161, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Kołtowski, M.; Charmsa, B.; Skubiszewska-Zięba, J.; Oleszczuk, P. Effect of biochar activation by different methods on toxicity of soil contaminated by industrial activity. Ecotox. Environ. Safe. 2017, 136, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.; Januszewski, B.; Chen, T.F.; Delgado, A.G.; Westerhoff, P.; Rittmann, B. Using radish (Raphanus lativus L.) germination to establish a benchmark dose for the toxicity of ozonated-petroleum byproducts in soil. Chemosphere 2023, 313, 137382. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, F.L.; Shao, Z.G.; Wu, B.; Guo, S.H. Effects of thermal desorption on ecotoxicological characteristics of heavy petroleum-contaminated soil. Sci. Total Environ. 2023, 857, 159405. [Google Scholar] [CrossRef]
- Domínguez, C.M.; Ventura, P.; Checa-Fernández, A.; Santos, A. Comprehensive study of acute toxicity using Microtox® bioassay in soils contaminated by lindane wastes. Sci. Total Environ. 2023, 856, 159146. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, B.J.; Qu, X.Z.; Gu, X.Y. Are Ni-Cd toxicity models derived from simple bioassay applicable to natural soils? A bioassay-MSMs coupling approach. J. Hazard. Mater. 2022, 440, 129830. [Google Scholar] [CrossRef]
- Sivaram, A.K.; Logeshwaran, P.; Lockington, R.; Naidu, R.; Megharaj, M. Phytoremediation efficacy assessment of polycyclic aromatic hydrocarbons contaminated soils using garden pea (Pisum sativum) and earthworms (Eisenia fetida). Chemosphere 2019, 229, 227–235. [Google Scholar] [CrossRef]
- Delerue, F.; Masfaraud, J.F.; Lascourrèges, J.F.; Atteia, O. A multi-site approach to investigate the role of toxicity and confounding factors on plant bioassay results. Chemosphere 2019, 219, 482–492. [Google Scholar] [CrossRef]
- Wong, J.W.C.; Li, K.; Fang, M.; Su, D.C. Toxicity evaluation of sewage sludges in Hong Kong. Environ. Int. 2001, 27, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.L.; Li, G.K.; Sang, N. Embryonic exposure to soil samples from a gangue stacking area induces thyroid hormone disruption in zebrafish. Chemosphere 2019, 236, 124337. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Gao, R.; Yue, H.F.; Liu, X.F.; Li, G.K.; Sang, N. Polycyclic aromatic hydrocarbon (PAH)-containing soils from coal gangue stacking areas contribute to epithelial to mesenchymal transition (EMT) modulation on cancer cell metastasis. Sci. Total Environ. 2017, 580, 632–640. [Google Scholar] [CrossRef] [PubMed]
| Chemicals | Element Concentration (mg/kg) | Risk Screening Values for Soil Contamination of Development Land (mg/kg) 1 | ||
|---|---|---|---|---|
| Site A | Site B | Class I | Class II | |
| Cr6+ | <0.2 | 25 | 3.0 | 5.7 |
| Cd | 0.593 | 0.87 | 20 | 65 |
| Ni | 254 | 49.08 | 150 | 900 |
| As | 17.2 | 1.81 | 20 | 60 |
| Zn | 3580 | 136.12 | - | - |
| Pb | 939 | 38.34 | 400 | 800 |
| Cu | 1380 | 20.19 | 2000 | 18,000 |
| Chemicals | Chemical Concentration (mg/kg) | Risk Screening Values for Soil Contamination of Development Land (mg/kg) 1 | ||
| Site C | Site D | Class I | Class II | |
| Total petroleum hydrocarbons (C6-C9) | 17.9 | 30.7 | - | - |
| Total petroleum hydrocarbons (C10-C40) | 150 | 180 | 826 | 4500 |
| Benzene | <0.05 | 0.06 | 1 | 4 |
| Methylbenzene | 0.08 | 0.53 | 1200 | 1200 |
| Ethylbenzene | 0.21 | 1.02 | 7.2 | 28 |
| M-xylene and p-xylene | 0.38 | 1.15 | 163 | 570 |
| O-xylene | 0.14 | 0.49 | 222 | 640 |
| Naphthalene | <0.10 | 0.10 | 25 | 70 |
| Phenanthrene | <0.10 | 0.22 | - | - |
| Fluoranthene | <0.10 | 0.26 | - | - |
| Pyrene | <0.10 | 0.23 | - | - |
| Benzanthracene | <0.10 | 0.10 | 5.5 | 15 |
| Chrysene | <0.10 | 0.13 | 490 | 1293 |
| Benzofluoranthene | <0.10 | 0.15 | 5.5 | 15 |
| Chemicals | Chemical Elements Concentrations (mg/kg) | Risk Screening Values for Soil Contamination of Development Land (mg/kg) 1 | ||
| Site E | Class I | Class II | ||
| Chlorobenzene | 0.19 | 68 | 270 | |
| Bromobenzene | <0.05 | - | - | |
| 2-chlorotoluene | 0.12 | - | - | |
| 4-chlorotoluene | 0.08 | - | - | |
| 1,2-dichlorobenzene | 403 | 560 | 560 | |
| 1,3-dichlorobenzene | 5.29 | - | - | |
| 1,4-dichlorobenzene | 21.7 | 5.6 | 20 | |
| 1,2,4-dichlorobenzene | 0.81 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Yin, J.; Wu, L.; Fu, R.; Chen, L. Ecotoxicity Assessment of the Water Extracts from Metal-Contaminated and Hydrocarbon-Contaminated Soils. Water 2023, 15, 4061. https://doi.org/10.3390/w15234061
Li Q, Yin J, Wu L, Fu R, Chen L. Ecotoxicity Assessment of the Water Extracts from Metal-Contaminated and Hydrocarbon-Contaminated Soils. Water. 2023; 15(23):4061. https://doi.org/10.3390/w15234061
Chicago/Turabian StyleLi, Qian, Juan Yin, Lingling Wu, Rongbing Fu, and Ling Chen. 2023. "Ecotoxicity Assessment of the Water Extracts from Metal-Contaminated and Hydrocarbon-Contaminated Soils" Water 15, no. 23: 4061. https://doi.org/10.3390/w15234061
APA StyleLi, Q., Yin, J., Wu, L., Fu, R., & Chen, L. (2023). Ecotoxicity Assessment of the Water Extracts from Metal-Contaminated and Hydrocarbon-Contaminated Soils. Water, 15(23), 4061. https://doi.org/10.3390/w15234061







