1. Introduction
The presence of
Cryptosporidium oocysts in drinking water represents a potential risk for public health. Since the first records of outbreaks associated with the contamination of water supplies by these pathogens occurred in the 1980s and 1990s, concerns about removing them from drinking water have been recurring. Cryptosporidiosis, a disease caused by the ingestion of
Cryptosporidium oocysts, has been associated with the occurrence of watery diarrhea and can get quite serious, particularly in immunocompromised patients, and lead to death [
1,
2,
3]. This pathogen is found in nature in the form of oocysts, which are structures highly resistant to different environmental conditions and to chlorination, the disinfection process most commonly used in water treatment plants (WTP) in Brazil and all over the world. Therefore, efforts to remove these structures are more focused on using disinfectants with greater inactivation power and/or optimizing the filtration process, which must be efficient in retaining oocysts [
4].
Direct filtration, also known as contact filtration, involves coagulation, sometimes flocculation, directly followed by filtration, without a settling process which is commonly used in conventional treatment. Therefore, in the direct filtration water treatment train, the rapid granular filter is the only process for the removal of suspended particles, including
Cryptosporidium oocysts. Direct filtration is recommended to treat source water with relatively low turbidity and color and can be arranged with a downflow or an upflow filter. Due to the lower costs and lower complexity associated with the installation and maintenance of the units [
4], direct filtration has been used in Brazil as well as other developing countries. The filtration efficiency is highly dependent on the destabilization of particles, promoted by chemical coagulation, as there must be a balance between the adhesion and detachment mechanisms of grains in the filtering medium [
5].
As a potential risk to public health,
Cryptosporidium oocysts, a pathogenic protozoan, should be part of the quality parameters systematically monitored at a WTP. However, due to the complexity and high costs related to the analytical routine for detecting and quantifying these pathogens, consistent and regular monitoring is almost impractical, particularly in developing countries. Furthermore, as
Cryptosporidium oocysts are mostly present at low concentrations in raw water, significant removal cannot be quantified in different processes of a water treatment plant [
6,
7]. Thus, several surrogates have been investigated by the scientific community and many of them have shown good correlations with the removal of these pathogens, such as aerobic spore-forming bacteria (ASFB) and oocyst-sized polystyrene microspheres [
5,
6,
8,
9,
10,
11,
12,
13]. Based on some of these studies, the LT2ESWTR Toolbox Guidance Manual [
7] recognizes ASFB and microspheres as suitable surrogates to demonstrate the performance of water treatment plants and a unit process.
Spores are dormant, extremely resistant structures in which some bacterial species spend part of their life cycle. Sporulation is a survival strategy that has been mainly related to the presence of unfavorable metabolic conditions. The spores have great longevity and inhabit the most diverse parts of the planet, due to both their metabolic characteristics and their high dispersion capacity through wind, water, and hosts [
14].
As described by Headd and Bradford [
11], aerobic bacterial spores can be considered promising surrogates because they meet some requirements such as non-pathogenicity, low laboratory analysis costs and complexity, persistence and abundance in the environment, and remaining unchanged during transport, sampling, and laboratory analysis [
7,
11]. Furthermore, there is consistent evidence that the removal of ASFB constitutes a conservative indicator of the efficiency of removing
Cryptosporidium oocysts through granular media filtration in drinking water treatment [
6,
12,
13].
In this context, Brazilian legislation that establishes drinking water quality guidelines, as well as monitoring and surveillance rules, Ordinance Nº 888/2021 of the Ministry of Health [
15], recommends the use of ASFB removal efficiency in water treatment plants (WTP) as a strategy to evaluate the efficiency of treatment (excluding disinfection) regarding the removal efficiency of
Cryptosporidium oocysts. This strategy is only applied when the annual geometric mean of
Escherichia coli at the point of withdrawal is above 1000/100 mL. If the ASFB removal efficiency is less than 2.5 log (99.7%), it is mandatory to monitor
Giardia cysts and
Cryptosporidium oocysts in the withdrawal point for 12 months to assess the need to reduce turbidity from 0.5 NTU to 0.3 NTU in all filtered effluent water. The incorporation of ASFB removal efficiency as an indicator of water treatment performance represented an advance in Brazilian legislation since agencies such as the United States Environment Protection Agency (USEPA) have recognized ASFB as good indicators for over ten years [
16].
In the literature, there are examples of works that investigated the removal of
Cryptosporidium oocysts and/or their surrogates through a direct filtration process, either using a downflow filter or an upflow filter [
5,
6,
8,
9,
10,
12,
13,
17,
18,
19]. However, no study was found comparing these two types of filters operating under similar conditions and assessing the removal efficiency of turbidity and oocyst surrogates. In this scenario, the present study aimed to compare, on a pilot scale, the performance of direct downflow filtration and direct upflow filtration on the removal of aerobic spore-forming bacteria and oocyst-sized fluorescent polystyrene microspheres as surrogates for
Cryptosporidium oocyst removal.
2. Materials and Methods
A pilot-scale investigation was performed with source water collected from Lake Paranoá (Brasília, Brazil) which typically has low turbidity (<10 NTU). In some filtration experiments, Paranoá Lake water was spiked with fluorescent polystyrene microspheres (Polyscience Incorporation—Warrington, PA, USA) to a concentration of 105/L. The high initial concentration of microspheres in raw water is a strategy used in various studies to ensure detection in the filtered water and allow evaluation of the removal efficiency.
Before the pilot scale investigation, preliminary jar tests, adapted for direct filtration, were carried out to identify the best range of alum dose and coagulation pH to be adopted. Coagulant doses varying from 0 to 12 mg/L of Al
2(SO
4)
3 were tested in a pH range of 5.0 to 7.5. The operational parameters of the jar tests are described in
Table 1. Results from the jar tests were used to plot the coagulation diagram, which is a graphic with pH coagulation on the X axis, coagulant dose on the Y axis, and turbidity removal (after filtration) on the Z axis. Based on the information provided by this diagram, the region of highest turbidity removal was identified and the coagulation conditions for filtration experiments were selected.
2.1. Pilot Plant Apparatus
Filtration experiments were conducted in a pilot installation comprising a raw water tank, a feed pump (ProMinent Sigma, models GALA0232 and SICAHMI2050—Heidelberg, Germany), a dosing pump (Miniplus 3, Gilson—Middleton, WI, USA), a coagulant tank, a hydraulic rapid-mix device, piezometers; and downflow and upflow filter columns (
Figure 1). Upflow and downflow filter runs were carried out in series mode, i.e., only one filter was operated during each experiment.
The upflow pilot filter (UF) consisted of an acrylic tube 4.0 m long with a 12.3 cm inside diameter, with a sand medium depth of 1.86 m over a 0.85 m support layer. Sand medium sub-layers (based on Sens et al. [
20]) are shown in
Table 2. The overall effective size of the upflow filter media was 0.70 mm and the uniformity coefficient was <2. The filter was operated at a constant filtration rate and rising feed level.
The downflow pilot filter (DF) consisted of an acrylic tube with a 2.70 m height and an inside diameter of 8.5 cm. The filter media was composed of 1.10 m of sand over 0.10 m of support layer. The effective size of the filter media was 1.00 mm and the uniformity coefficient was 1.15. The filter was operated at a constant filtration rate and constant feed level.
2.2. Operational Conditions
To compare filter performance, two operational conditions were investigated. In the first operational condition (OC-1), UF and DF were operated at the same filtration rate of 5.0 m/h, to allow evaluation of filter media impact. In the second operational condition (OC-2), UF and DF were operated at different filtration rates, but with the same flow rate of 59.41 L/h, in order to examine the behavior and water production of the filter under more realistic design filtration rates. Thus, at OC-2 the downflow filter operated with a filtration rate of about (10.5 m/h), twice the filtration rate of the upflow filter (5.0 m/h). The difference in filtration rates, with the same flow rate, was possible due to the different surface areas of the pilot filters. After each experiment, the filtration medium was backwashed with pumped tap water for 30 min at 30% medium expansion. All filtration experiments were carried out at near-neutral pH values and with optimal alum dose based on jar test results.
2.3. Sampling and Analysis
Throughout the filtration experiments, turbidity, apparent color, pH, total coliforms, and ASFB were monitored in both raw and filtered water (see sampling points SP1, SP2, and SP3 in
Figure 1). The raw water was collected from Paranoá Lake on the day of each filtration experiment. Before each filtration run, jar tests were carried out to select the optimal dose to be used.
Twenty filtration experiments were performed: ten with the upflow filter and ten with the downflow filter. In eight of these experiments, oocyst-sized polystyrene microspheres were spiked into the raw water. It should be highlighted that filtration experiments with upflow and downflow filters were carried out on consecutive days in order to minimize the influence of the raw water quality on the results.
Filtration experiments were originally planned to last 8 h. Samples of total coliforms, ASFB, and polystyrene microspheres were collected at the beginning of the filtration run (ripening period—40 min after the wash water in the filtration media had been displaced) and after 6 h of operation (stable operation). However, the experiments with the downflow filter did not reach the planned 8 h due to head loss development, and in operational condition 2 (OC-2), the sample of stable operation was collected just before the end of the filtration run, around 3.5 h. The analyzed water quality characteristics, methods, equipment, and sampling frequency are described in
Table 3. It is important to mention that total coliforms and apparent color data are not presented in this article.
The protocol of 9218-B of the Standard Methods basically involves a membrane filtration procedure preceded by a heating step that inactivates vegetative cells, leaving heat-resistant spores to be plated and counted after incubation [
12,
21]. Thus, it is a fairly simple and low-cost analytical method, as previously mentioned.
2.4. Statistical Analysis
Initially, data obtained from the filtration experiments were subjected to descriptive statistics. As data obtained did not present a normal distribution, non-parametric statistical techniques were used to compare the filtration efficiency of upflow and downflow filters under different operational conditions, as well as at different periods of filtration. The Wilcoxon test was used to compare two paired groups and the Mann–Whitney test was used to compare non-paired groups. Statistical analyses were performed using the GraphPad Prism software, version 8.0.1.
3. Results and Discussion
Raw water characteristics are presented in
Table 4. As mentioned, 20 filtration experiments were carried out: 10 with the downflow filter and the other 10 with the upflow filter.
Paranoá Lake water had low turbidity, 3.69 NTU on average, compatible with the application of a direct filtration treatment train [
23]. Alkalinity and pH values did not fluctuate much, with mean values of 29.4 mg CaCO
3/L and 7.1, respectively. Microbiological parameters results showed a wide range of values. The most probable number of total coliforms (MPN/100 mL) in raw water ranged from approximately 3.7 × 10
3 to 7.8 × 10
4. Aerobic spore-forming bacteria (ASFB) ranged from 7 × 10
2 to 5.5 × 10
3 CFU/100 mL. This quantification of ASFB in raw water is in the same magnitude as the average values reported by Nieminski et al. [
24] of 1820 CFU/100 mL, based on analysis of environmental samples from different regions of the United States. Other authors such as Rice et al. [
25], Dugan et al. [
9], and Oliveira et al. [
13] reported similar counts.
Results from the preliminary jar test (coagulation diagram) indicated that the highest turbidity removals (above 90%) were obtained within a wide range of pH values, 5 to 7.5, with alum doses ranging from 5 to 7 mg/L. Considering the natural pH of the raw water (7.1 ± 0.3) and preliminary jar test results, it was decided that pilot filtration experiments would be carried out without pH adjustment. However, it is important to point out that before each filtration experiment, a jar test was carried out in order to define the alum dose to be used in such an experiment.
3.1. Turbidity and Head Loss Development
Table 5 summarizes the average values of turbidity, as well as removal efficiencies, obtained during upflow and downflow filtration experiments.
Figure 2 shows the results of residual turbidity obtained from each operational condition tested.
Filtration experiments 1, 2, 5, 8, and 9 were carried out with operational condition 1 (same filtration rate), and experiments 3, 4, 6, 7, and 10 were carried out with operational condition 2 (same flow rate—different filtration rates), as indicated in
Table 5.
As can be seen in
Table 5 and
Figure 2, the average residual turbidity remained consistently below 0.32 NTU in all experiments, a value that is below the limits established for filtered water by the Brazilian drinking water guidelines, less than or equal to 0.5 NTU in 95% of measurements [
15]. Similar results were reported by Fagundes [
26], Fernandes [
27], Nascimento [
28], and Méndez [
29], authors who conducted their studies using raw water from the same source (Paranoá Lake) and similar filters. With turbidity values less than or equal to 0.3 NTU in 95% of measurements of filtered water, at least a 2.5 log removal of
Cryptosporidium can be obtained in direct filtration plants [
7,
16].
A comparison of the overall removal efficiencies of turbidity can be seen in
Table 6. These removal efficiencies were calculated based on the average turbidity of raw and filtered water in each experiment, and then using these values to estimate the overall log removal efficiencies.
According to
Table 6, the downflow filter exhibited a slightly higher removal efficiency than the downflow filter. The downflow filter showed an overall removal efficiency of turbidity of 1.17 log, while in the upflow filter, the removal efficiency was 1.11 log. A similar tendency is observed when operational conditions are considered. Despite that,
Figure 2 shows that the upflow filter presented less variation in filtered water turbidity.
Mann–Whitney’s nonparametric test was carried out using all the removal efficiency data of the filters. The test indicated that differences between filters were not statistically significant at 95% confidence (
p-value = 0.5661). This means that upflow and downflow filters presented a similar ability to remove turbidity. Similar behavior is reported by Teixeira et al. [
19]. The authors operated two identical pilot filters, one upflow and the other downflow, under different filtration rates, to treat water with low turbidity. Direct upflow filtration and direct downflow filtration yielded values of residual turbidity that were statistically similar across the different filtration rates evaluated.
A clear advantage of the upflow filter was concerning head loss development (
Figure 3). All the experiments carried out with the upflow filter reached 8 h as pre-established for this study, whereas the downflow filter experiments did not. Filtration runs of the DF carried out in operating condition 1 (filtration rate of 5 m/h) lasted 6 h and in operational condition 2 (filtration rate of 10.5 m/h), they lasted 3.5 h on average. Over the 8 h filter runs of the UF, a slower head loss development, not exceeding 50 cm, was observed, as can be seen in
Figure 3.
The considerably lower head loss development in the upflow filter is related to the configuration of filter media, in addition to the support layer participation in the treatment process. In this filter, coarse to fine sand grain filtration occurs, allowing the utilization of the full media depth, and, as a substantial amount of coagulated particles is removed in the coarse portion of the filter bed, the head loss development is slow. In contrast, the downflow filter’s finer sand grain sizes promoted predominant retention and accumulation of particles in the top few centimeters of the filter media, as observed from head loss monitoring data using piezometers installed along the filter media.
3.2. Aerobic Spore-Forming Bacteria (ASFB)
Aerobic spore-forming bacteria data and their removal efficiencies are presented in
Table 7 and
Table 8 for upflow and downflow filters, respectively. The quantification of ASFB in each sample collected during filtration experiments was performed in triplicate, after previous dilution of the sample. Therefore, the values presented in
Table 7 and
Table 8 are averaged numbers and some of them result in fractional numbers.
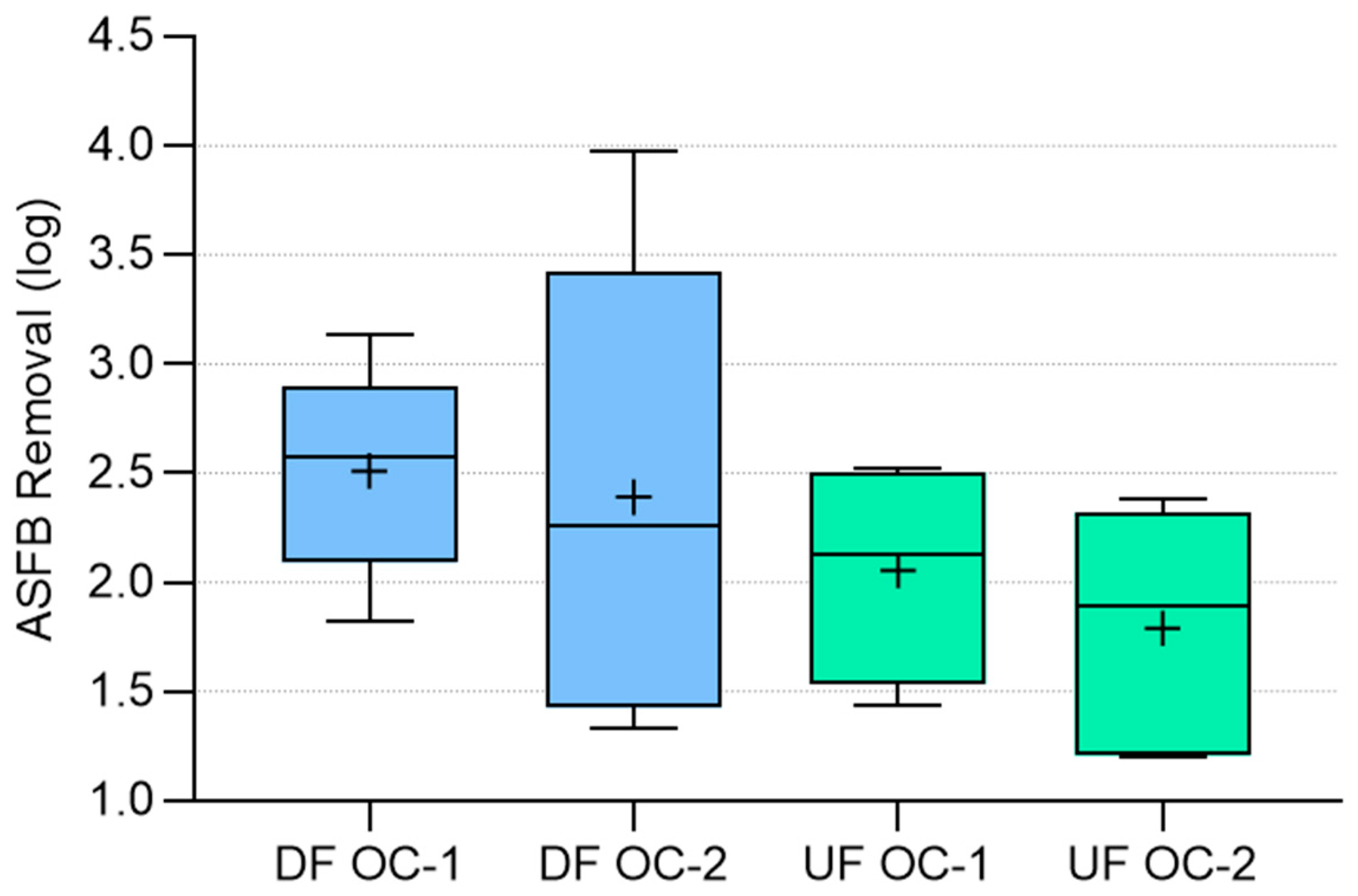
The average ASFB removal efficiency for each operational condition tested is presented in
Table 9. The UF-1 experiment result was not included in the mean calculation because it was considered an outlier.
ASFB removal efficiency varied from 1.20 to 2.52 log in the upflow filter, and from 1.33 to 3.97 log in the downflow filter (
Table 7 and
Table 8). Comparing the overall average removal efficiency of the two filters (
Table 9), it is observed that the downflow filter exhibited a higher ASFB removal independently of the operational conditions adopted. Overall (OC-1 + OC-2), during stable operation, ASFB removal in the downflow filter was 2.45 log, while the upflow filter was 1.91 log. It is worth noting that, despite being operated at twice the filtration rate at OC-2, in absolute mean values, the downflow filter still performed better than the upflow filter, both in the ripening period and the stable operation period. This unexpected behavior may be due to the accumulation of particles in the top layer of the filter and the very short filtration run.
Figure 4 illustrates the removal efficiencies of ASFB for DF and UF during stable operation in each operational condition evaluated. Comparing the behavior of the filters, it is observed that the median values of log removals are closer than the average log removal, suggesting some similarities.
Table 10, which shows the
p-values of the Mann–Whitney test (at 95% confidence) used for the comparison of UF and DF removal efficiencies, confirmed the statistical similarity of the filters under stable operation.
However, when both ripening and stable operation are considered, the Mann–Whitney test yielded a statistically significant difference (at 95% confidence) between the upflow and downflow filters operated at OC-2. This difference could be attributed to the fact that in CO-2, the DF and UF were operated at different filtration rates.
Table 11 shows that the ripening period produced statistically different results of ASFB removal for the upflow filter (
p-value = 0.0156), but not for the downflow filter. This represents that ripening is a vulnerable period within the filter cycle, as also reported by Nascimento et al. [
17]. Liu et al. [
23] suggested that longer filter ripening times are associated with large media grains, such as in the case of the bottom layers of the upflow filter.
ASFB removal efficiencies in ranges similar to those obtained in this study are reported in the literature. In downflow filters operated as part of a pilot-scale conventional treatment plant, at optimal coagulation, Dugan et al. [
9] observed that ASFB removal efficiency varied from 0.73 to 3.4 log, with an average removal of 2.0 log, whereas the average
Cryptosporidium oocyst removal was >3.7 log, in a range of 2.9 to >4.4 log. Rice et al. [
25] and Oliveira et al. [
13] reported ASFB removal efficiencies of 1.69–2.57 and 1.71 log, respectively, also in downflow filters from full-scale water treatment plants. Unfortunately, we were unable to find a study that analyzed the removal of aerobic spore-forming bacteria by upflow filter for comparison purposes.
The conservative nature of the removal of ASFB in comparison to
Cryptosporidium oocyst removal reported by Dugan et al. [
9] was also observed by Huck et al. [
30] in two pilot plants, one in Canada and the other in the USA.
B. subtilis spore removal was always lower than
Cryptosporidium oocyst removal, even in the suboptimal coagulant dose condition and during breakthrough. However, the authors [
30] emphasize that the difference between removal in stable operation and ripening was greater for
B. subtilis than for
Cryptosporidium oocysts and suggest that
B. subtilis spores are not a good quantitative surrogate for microsphere removal by filtration.
An aspect not evaluated in this work, nor in other studies that evaluated ASFB removal in water treatment processes, is the potential bactericidal effect of alum. This issue must be addressed in future investigations, as the antibacterial effect of aluminum salts has been reported in the medical area [
31] as well as its viral inactivation ability in water treatment [
32].
3.3. Fluorescent Microspheres
In addition to using aerobic spore-forming bacteria removal as an indicator of
Cryptosporidium oocyst removal, fluorescent polystyrene microspheres were also added to the raw water in this study. The use of fluorescent microspheres as oocyst surrogates has been recognized and employed in studies for over a decade.
Table 12 and
Table 13 show the counts of microspheres in raw and filtered water, as well as removal efficiencies for the upflow and downflow filters, respectively.
The filters yielded microsphere removal efficiencies ranging from 3.00 to over 5.00 log. It is worth noting that only in the FD-9 experiment did the downflow filter present a considerably higher removal efficiency than the upflow filter, while in other experiments, the upflow filter showed similar or higher efficiency than the downflow filter. No statistical comparison of microsphere removal efficiencies between upflow and downflow filters was made due to the reduced number of experiments carried out.
The median microsphere removal efficiency achieved in this study is closer to the reported by Brown and Emelko [
33], 4.00 log, and slightly lower than that reported by Emelko and Hulk [
10], around 4.60 log, during stable operation. These authors [
10,
33] investigated the removal of
Cryptosporidium oocysts and polystyrene microspheres using in-line pilot-scale dual-media downflow filters. Cerqueira [
22], however, reported a 1.44–1.46 log microsphere removal in a direct downflow filtration pilot plant. The difference between the removal efficiencies reported by different authors can be explained, in addition to the specific characteristics of each filter, by the difference in the number of microspheres that each author spiked into the raw water. Cerqueira [
22] used about 10
3 microspheres/L in the raw water, Brown and Emelko [
33] used 10
7 microspheres/L, whereas 10
5 microspheres/L were used in this study.
In-line filtration pilot experiments with multi-layer downflow filters yielded a 1.5 to 5.4 log removal of
Cryptosporidium oocysts and 0.4 to 5.1 log removal of microspheres when a wide range of operational conditions of the dual media filter was evaluated (stable operation, suboptimal coagulation, hydraulics steps) [
10]. Slightly higher values were obtained by using trimedia filtration. Positive and statically representative relationships between oocysts and microspheres were obtained when all operational conditions were considered, but a poor correlation was observed when one specific operational condition was focused on. Under the conditions investigated, the authors [
10] concluded that oocyst-sized polystyrene microspheres appeared to be reliable quantitative indicators of
C. parvum removal by filtration when data were available over a large span of oocyst and microsphere removals corresponding to a wide range of operating conditions. The majority of these findings were further confirmed using different coagulants [
33].
Regarding upflow filtration, Méndez et al. [
18], using chitosan as a coagulant, reported a microsphere removal efficiency of 4.66 log, on average, therefore higher than that obtained in this work. Nascimento et al. [
17] obtained
Cryptosporidium oocyst removal efficiencies ranging from 2.80 to 4.20 log. These two authors [
17,
18] operated an upflow pilot-scale filter with a similar configuration to the one used in the present work.
The removal efficiencies of microspheres presented in this study were, in general, superior to the removal efficiencies of ASFB and turbidity, as well as total coliforms and apparent color, whose data is not presented in this article.
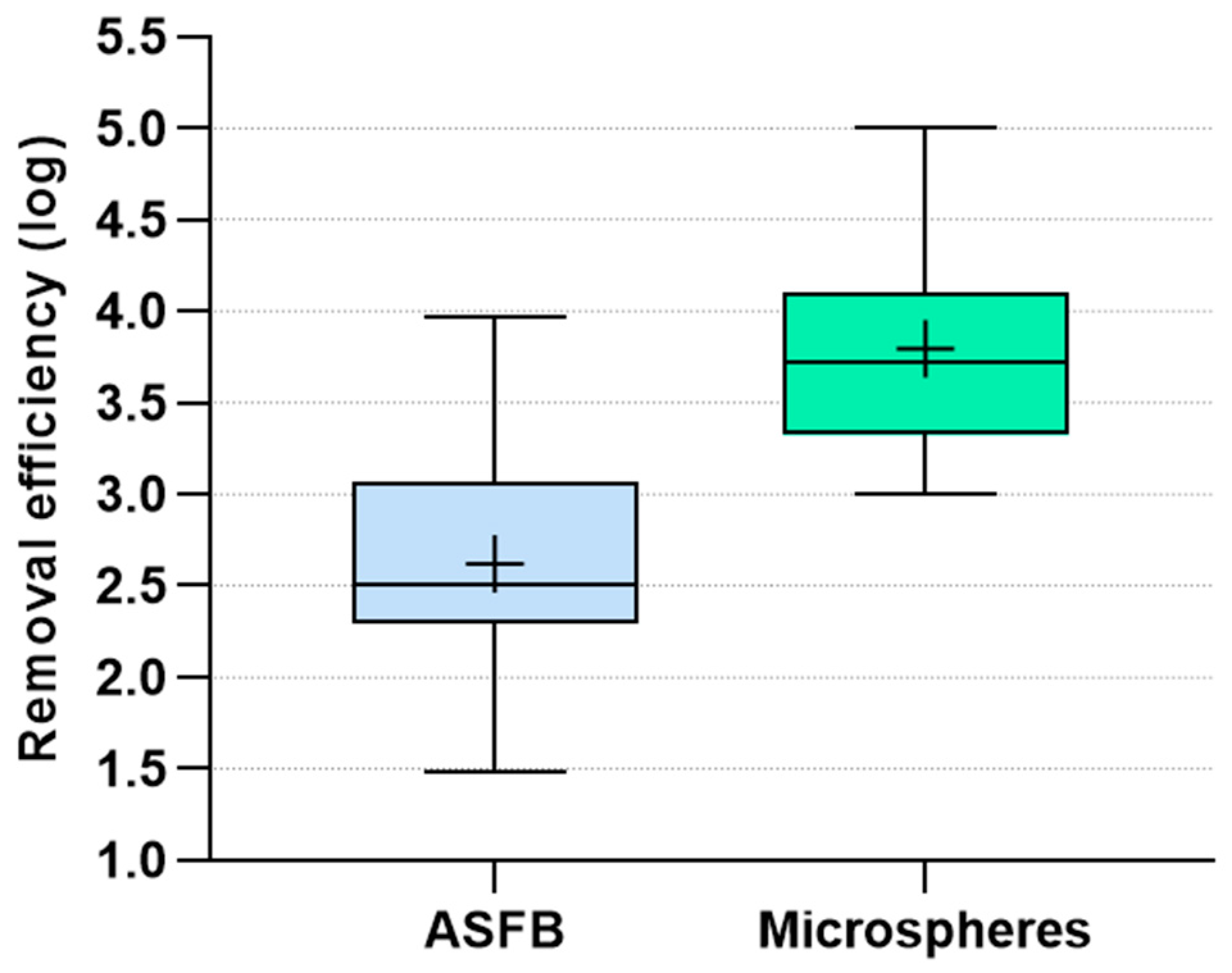
A comparison of log removals of ASFB and polystyrene microspheres, independent of filter flow direction and operational condition, is presented in
Figure 5.
It is evident from
Figure 5 that microsphere removal was considerably superior to ASFB removal (1.25 log higher in median values). Considering the literature data that shows higher removal of oocyst-sized polystyrene microspheres than
Cryptosporidium oocysts under a wide range of filter media and operation conditions, and in agreement with previous studies, our data confirmed that ASFB removal is a conservative indicator for
Cryptosporidium oocyst removal in direct filtration as well as in conventional water treatment plants. However, estimating the log removal of
Cryptosporidium oocysts based on the log removal of ASFB is still a challenge.
4. Summary
This article assessed the removal of Cryptosporidium oocyst surrogates using two different pilot-scale direct filters: a downflow sand media filter and an upflow filter with deeper and stratified sand media. The performance and removal efficiencies of the filters were compared under two operational conditions: both filters operating at the same filtration rate of 5.0 m/h; and filters operating at the same flow rate of 59.41 L/h and different filtration rates (upflow—5.0 m/h; downflow—10.5 m/h). In general, the results of removal efficiencies were slightly higher in the downflow filter, but statistical non-parametric tests showed that the differences were not statistically significant, indicating similar efficiencies of both filters. On the other hand, shorter filtration runs were obtained in the downflow filter due to its rapid head loss development.
Regarding turbidity, the downflow and upflow filters presented satisfactory removal efficiencies. The average residual turbidity was always below 0.32 NTU, values that comply with Brazilian drinking water guidelines. At the condition investigated, both upflow and downflow filters appear to be able to achieve a 2.5 log removal of Cryptosporidium oocysts.
When operated at the same filtration rate, the average ASFB removal was 1.98 and 2.35 log and median values were 2.13 and 2.27 log for upflow and downflow filters, respectively. But the Mann–Whitney non-parametrical test (95% confidence) indicated that these different results were not statistically significant.
When operated at the same flow rate and different filtration rates (10.5 m/h at DF and 5 m/h at UF), the average ASFB removal was higher in DF (2.33 log) than in UF (1.60 log). Median values were 2.35 and 1.56 log for DF and UF, respectively, and were statistically different. This unexpected result may be related to the predominant retention and accumulation of particles in the top few centimeters of the downflow filter media and its very short filtration run.
The removal efficiency of fluorescent polystyrene microspheres ranged from 3.00 to >5.00 log in the experiments performed, therefore higher than removal of ASFB, confirming that ASFB removal is a conservative surrogate for Cryptosporidium oocyst removal, once the literature recognizes oocyst-sized microsphere removal as a conservative surrogate.
Under the experimental condition evaluated, ASFB log removal efficiency by direct filtration was, in some cases, below the reference log value (2.5 log) indicated by Ordinance 888/2021 of the Ministry of Health of Brazil to consider that a water treatment plant that produces filtered water with turbidity up to 0.5 NTU is operating adequately regarding removal of Cryptosporidium oocysts. However, considering the ability of the filters to produce turbidity below 0.3 NTU most of the time and to remove 2 log of ASFB, it is suggested that direct filtration, either upflow or downflow, may be used to treat water with low concentrations of Cryptosporidium oocysts.
Although the data obtained in this work emphasize that ASFB removal can be a good tool to help with the daily monitoring of water treatment plant efficiency, the number of experiments and operational conditions investigated was still limited and more studies are needed to set the log value of removal of ASFB necessary to produce safe water with reference to protozoa (oo)cysts in granular filtration. Ideally, the studies should be performed assessing the removal of Cryptosporidium oocysts and ASFB in parallel. However, cost, difficulty, and health risks associated with working with oocysts (naturally present or spiked into water) make the direct comparison of the removal of Cryptosporidium oocysts with ASFB removal almost impractical. Thus, it is suggested to use oocyst-sized fluorescent polystyrene microspheres as model Cryptosporidium oocysts in these future studies.