Potential of Laccase as a Tool for Biodegradation of Wastewater Micropollutants
Abstract
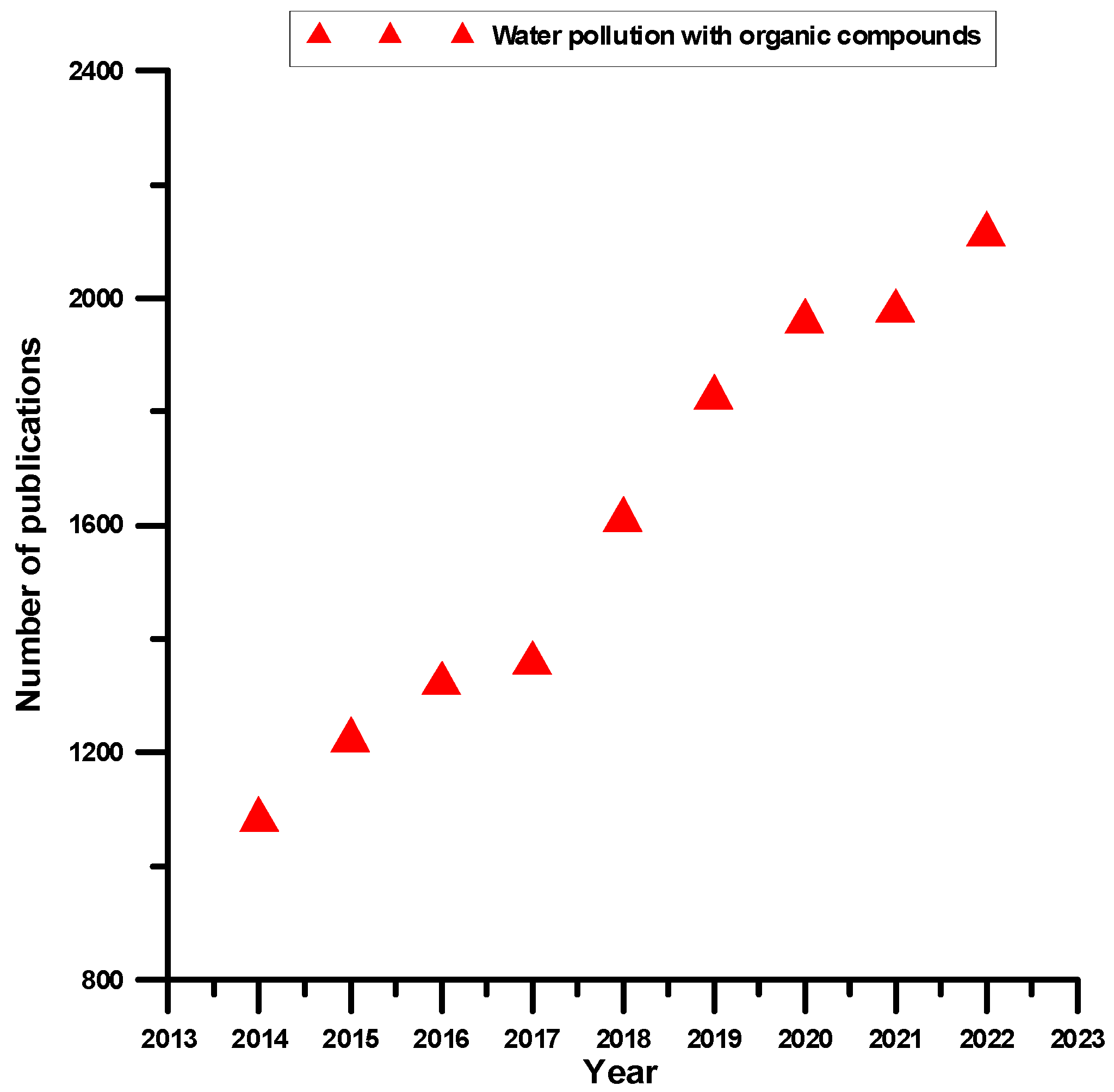
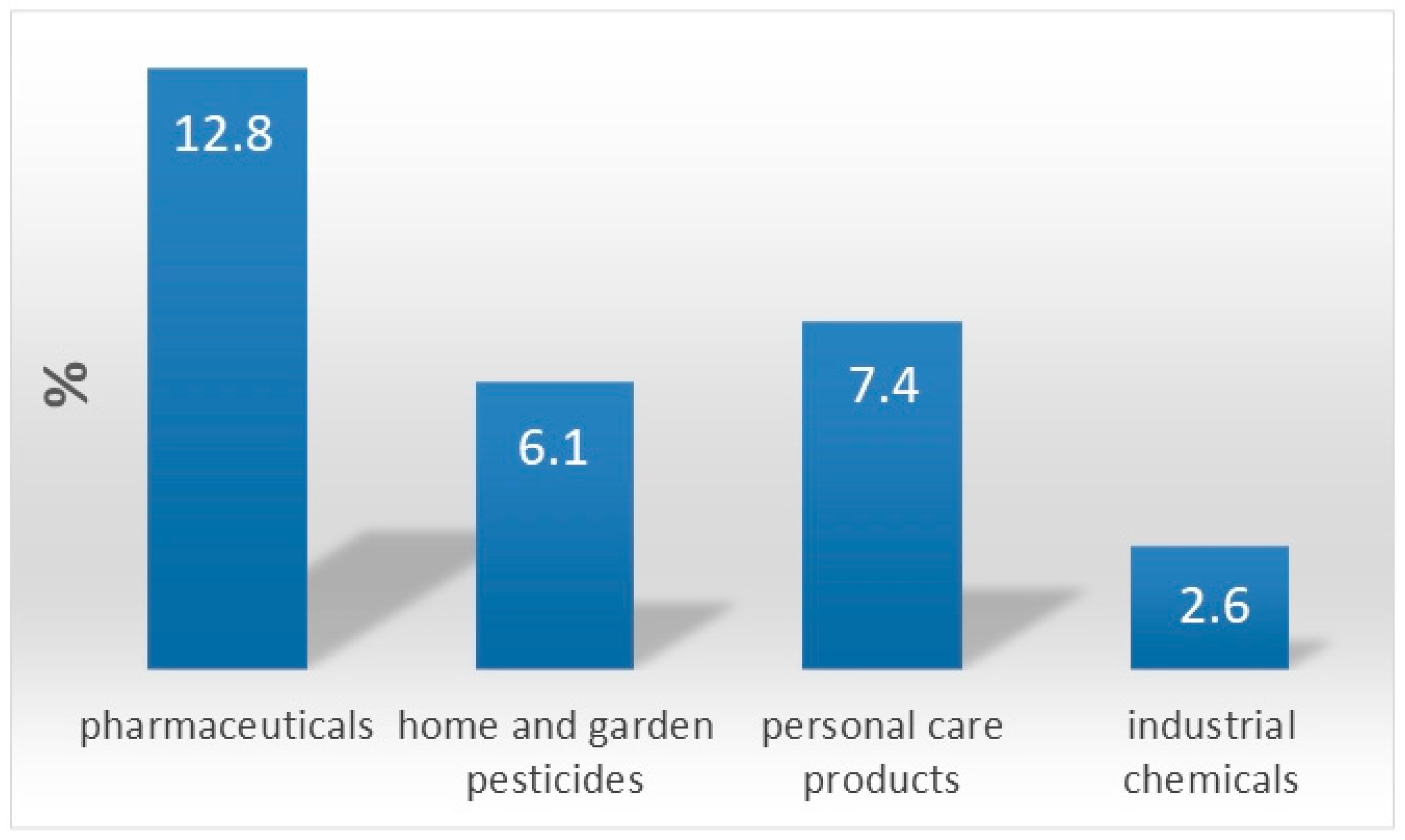
:1. Chemistry of Water Pollution
2. Methods for Removing Organic Pollutants from Wastewater
3. Laccase Characteristics as a Waste Removal Enzyme
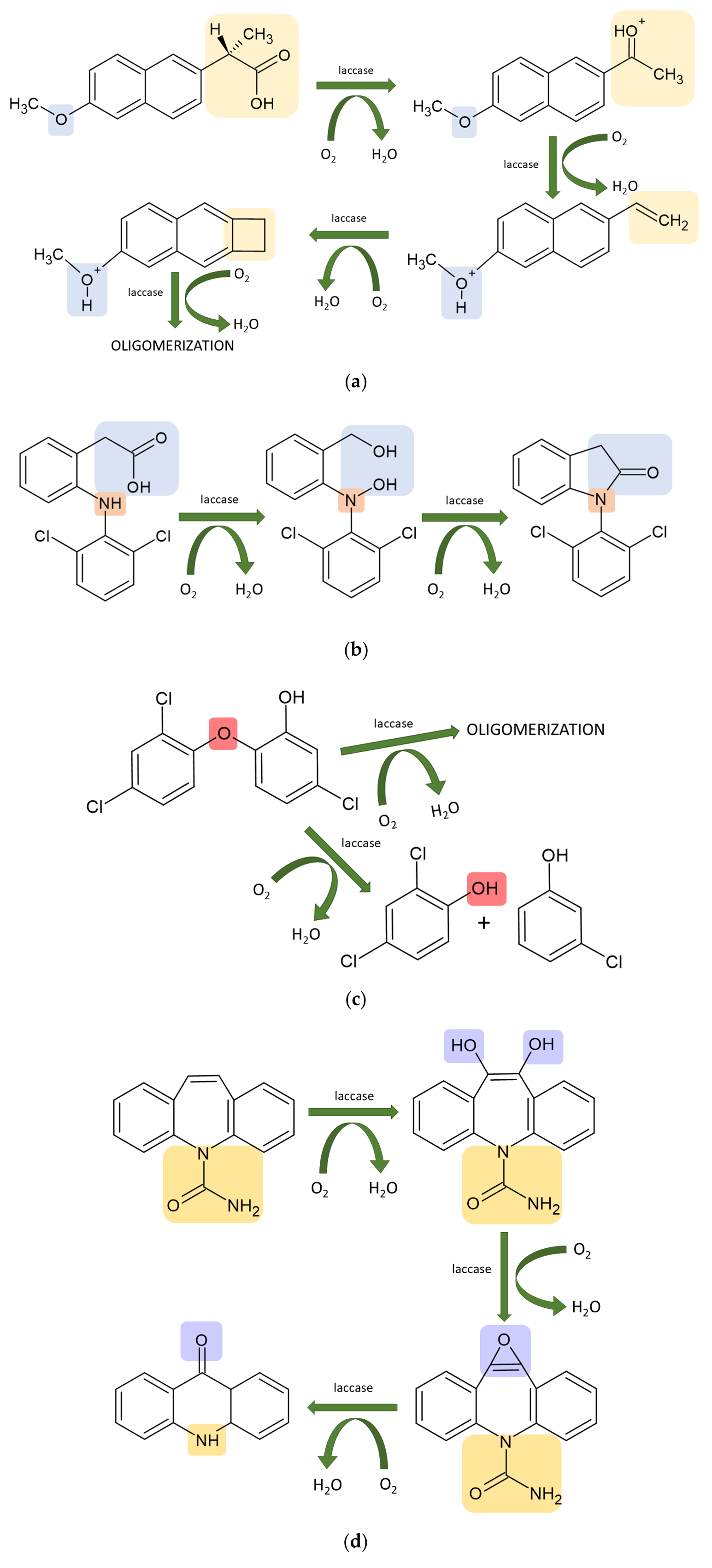
4. Laccase-Assisted Biodegradation of Water Micropollutants
| Laccase Source | Micropollutant (Substrate) | Mediator | Transformation Efficiency | Product Toxicity | Reference |
|---|---|---|---|---|---|
| T. versicolor | carbamazepine | CA, AS, SA | over 65% for p-coumaric acid in the membrane hybrid reactor with biocatalytic TiO2 | reduced (algae Chattonella marina and Microcystis aeruginosa viability test with AlamarBlue) | [117] |
| Pleurotus ostreatus | triclosan | NA | over 89.82% | reduced (green algae Chlamydomonas reinhardtii and Scenedesmus obliquus growth inhibition studies) | [116] |
| Aspergillus flavus | polyethylene microplastics | NA | 3.9% mass loss | NR | [103] |
| B. adusta | acetaminophen, bisphenol A, carbamazepine, sulfamethoxazole | NA | 100% for bisphenol A and aminophene; about 20% for sulfamethoxazole and carbamazepine | NR | [100] |
| T. versicolor | metronidazole, salicylic acid, primidone, amitriptyline, carbamazepine, ketoprofen, naproxen, ibuprofen, gemfibrozil, diclofenac, triclosan, propoxur, fenoprop, clofibric acid, atrazine, ametryn, pentachlorophenol, 4-tert-butylphenol, bisphenol A, 4-tert-octolphenol, estriol, estrone, 17α-ethinylestradiol, 17β-estradiol, 17β-estradiol acetate, enterolactone, formononetin, benzophenone, oxybenzone, octocrylene | HBT | 0–99% | slightly decreased for crude laccase; increased for laccase–HBT system (bioluminescence inhibition in Photobacterium leiognathi with ToxScreen3 assay) | [102] |
| Pleurotus dryinus | carbendazim, thiabendazole, pyrimethanil, kresoxim methyl, pyraclostrobin, trifloxystrobin, boscalid, iprodione, fludioxonil, diuron, simazine, monolinuron, atrazine, hexazinone, pendimethalin, metolachlor, imazethapyr, metobromuron, bentazon, aldicarb, carbofuran, acetamiprid, parathion, azinphos-methyl, chlorpyrifos, malathion, coumaphos, chlorfenvinphos, spinosad | NA | up to 100% | NR | [99] |
| T. versicolor | sulfomethoxazole, isoproturon | ABTS, SA, AS | complete transformation of both in presence of ABTS, as well as for sulfomethoxazole in presence of AS or SA | reduced (green alga P. subcapitata growth inhibition assay) | [119] |
| T. versicolor | ketoconazole | HBT | up to 100% | reduced (P. subcapitata, Candida albicans, Cryptococcus neoformans, and Saccharomyces cerevisiae micro-toxicity study) | [130] |
| T. versicolor | carbamazepine | ABTS | over 94% | reduced (estrogenicity tests using yeast estrogen screen (YES) assay) | [121] |
| T. versicolor | naproxen, diclofenac | NA | over 90% | reduced for encapsulated enzyme; increased for adsorbed laccase (ecotoxicity test against Artemia salina) | [115] |
| T. versicolor | triclosan | ABTS | up to 100% | reduced for crude laccase; increased with ABTS (Microtox assay with Photobacterium phosphoreum) | [131] |
| T. hirsuta | 17β-estradiol | NA | up to 99.3% | NR | [129] |
| P. ostreatus | naproxen, atrazine, oxybenzone, pentachlorophenol | HBT, SA, VA, HPI, TEMPO, ABTS, VA, vanilin | 15–23% for free laccase, ca. 10–98% for LMS | NR | [122] |
| T. versicolor | amoxicillin, ampicillin, cloxacillin, oxacillin, penicillin G, V, ciprofloxacin, enrofloxacin, sulfamethoxazole, sulfabenzamide, sulfadiazine, sulfamerazine, sulfamethoxypyridazine, sulfadimethoxine, ofloxacin, sulfamethizole, sulfanitran, sulfapyridine, sulfathiazole, sulfisomidin, sulfisoxazole, fluoroquinolones, danofloxacin, difloxacin, enoxacin, orbifloxacin, marbofloxacin, flumequine, norfloxacin, cinoxacin, nalidixic acid, oxolinic acid, pipemidic acid, tetracycline, chlorotetracycline, doxycycline, oxytetracycline, metronidazole, trimethoprim | SA | 32 antibiotics were degraded by >50% after 24 h with SA | increased (a growth inhibition assay against Bacillus subtilis and the Microtox assay using Aliivibrio fischerii) | [120] |
| T. pubescens | municipal WWTP containing mainly bis(2-ethylhexyl) phthalate, ketoprofen, diethyl phthalate | NA | over 60% | reduced ecotoxicity (tests with Raphidocelis subcapitata and Lepidium sativum); reduced estrogenic activity (MELN assay and in vitro E-screen test) | [107] |
| Cerrena unicolor | oxytetracycline, tetracycline, ampicillin, sulfamethoxazole, erythromycin, chloramphenicol, trimethoprim | ABTS | 80% for oxytetracycline and tetracycline | reduced (Escherichia coli and Bacillus licheniformis) | [132] |
| Streptomyces ipomea | ciprofloxacin, norfloxacin | NA | up to 90% | reduced (P. subcapitata) | [133] |
| M. thermophila | diclofenac, bisphenol A | SA | >95% for bisphenol A and >80% for diclofenac | NR | [134] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dojlido, J. Chemistry of Water; Wydawnictwo Arkady: Warszawa, Poland, 1987. [Google Scholar]
- Singh, J.; Yadav, P.; Pal, A.K.; Mishra, V. Water pollutants: Origin and status. In Sensors in Water Pollutants Monitoring: Role of Material; Pooja, D., Kumar, P., Singh, P., Patil, S., Eds.; Springer: Singapore, 2020; pp. 5–20. [Google Scholar] [CrossRef]
- Speight, J.G. (Ed.) Sources of water pollution. In Natural Water Remediation; Butterworth-Heinemann: Oxford, UK, 2020; pp. 165–198. [Google Scholar] [CrossRef]
- Posthuma, L.; Brack, W.; van Gils, J.; Focks, A.; Müller, C.; de Zwart, D.; Birk, S. Mixtures of chemicals are important drivers of impacts on ecological status in European surface waters. Environ. Sci. Eur. 2019, 31, 71. [Google Scholar] [CrossRef]
- Hossni, Y.A. Safety of sewage water in the irrigation of plants. Egypt. J. Hort. 1997, 24, 261–270. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology: Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar] [CrossRef]
- Kumar, A.; Schreiter, I.J.; Wefer-Roehl, A.; Tsechansky, L.; Schüth, C.; Graber, E.R. Production and utilization of biochar from organic wastes for pollutant control on contaminated sites. In Environmental Materials and Waste; Prasad, M.N.V., Shih, K., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 91–116. [Google Scholar] [CrossRef]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy metal pollution from gold mines: Environmental effects and bacterial strategies for resistance. Int. J. Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy metals and human health: Possible exposure pathways and the competition for protein binding sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef] [PubMed]
- Wasim Aktar, M.; Paramasivam, M.; Ganguly, M.; Purkait, S.; Sengupta, D. Assessment and occurrence of various heavy metals in surface water of Ganga river around Kolkata: A study for toxicity and ecological impact. Environ. Monit. Assess. 2010, 160, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.Q.; Cottee, M.; Hobbs, C. Multilateral cooperation and the prevention of nuclear terrorism: Pragmatism over idealism. Int. Aff. 2012, 88, 349–368. [Google Scholar] [CrossRef]
- Ma, R.; Zheng, C.; Liu, C. Groundwater impacts of radioactive wastes and associated environmental modeling assessment. In Environmental Geology; LaMoreaux, J.W., Ed.; Springer: New York, NY, USA, 2019; pp. 101–111. [Google Scholar] [CrossRef]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent organic pollutants in food: Contamination sources, health effects and detection methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef] [PubMed]
- Zacharia, J.T. Degradation pathways of persistent organic pollutants (POPs) in the environment. In Persistent Organic Pollutants; Stephen Kudom, D., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 3. [Google Scholar] [CrossRef]
- Ahamad, A.; Siddiqui, S.I.; Singh, P. Contamination of Water: Health Risk Assessment and Treatment Strategies, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 1–612. [Google Scholar]
- Rossi, L.; Queloz, P.; Brovelli, A.; Margot, J.; Barry, D.A. Enhancement of Micropollutant Degradation at the Outlet of Small Wastewater Treatment Plants. PLoS ONE 2013, 8, e58864. [Google Scholar] [CrossRef]
- Gautam, K.; Anbumani, S. Ecotoxicological effects of organic micro-pollutants on the environment. In Current Developments in Biotechnology and Bioengineering; Varjani, S., Pandey, A., Tyagi, R.D., Ngo, H.H., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 481–501. [Google Scholar] [CrossRef]
- Margot, J.; Rossi, L.; Barry, D.A.; Holliger, C. A review of the fate of micropollutants in wastewater treatment plants. WIREs Water 2015, 2, 457–487. [Google Scholar] [CrossRef]
- Tzanakakis, V.A.; Paranychianakis, N.V.; Angelakis, A.N. Water supply and water scarcity. Water 2020, 12, 2347. [Google Scholar] [CrossRef]
- Suraj, S.; Onkar, S. Enzymes Market Research, Global Opportunity Analysis and Industry Forecast, 2021–2031. Available online: https://www.alliedmarketresearch.com/enzymes-market (accessed on 9 December 2022).
- Woodard & Curran Inc. (Ed.) Methods for treating wastewaters from industry. In Industrial Waste Treatment Handbook, 2nd ed.; Butterworth-Heinemann: Burlington, NJ, USA, 2006; pp. 149–334. [Google Scholar] [CrossRef]
- Feng, S.; Hao Ngo, H.; Guo, W.; Woong Chang, S.; Duc Nguyen, D.; Cheng, D.; Varjani, S.; Lei, Z.; Liu, Y. Roles and applications of enzymes for resistant pollutants removal in wastewater treatment. Bioresour. Technol. 2021, 335, 125278. [Google Scholar] [CrossRef]
- Liew, Y.X.; Chan, Y.J.; Manickam, S.; Chong, M.F.; Chong, S.; Tiong, T.J.; Lim, J.W.; Pan, G.T. Enzymatic pretreatment to enhance anaerobic bioconversion of high strength wastewater to biogas: A review. Sci. Total Environ. 2020, 713, 136373. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.O.; Omotola, E.O.; Olatunji, O.S. Pharmaceuticals and personal care products in water and wastewater: A review of treatment processes and use of photocatalyst immobilized on functionalized carbon in AOP degradation. BMC Chem. 2020, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Ba, S.; Haroune, L.; Soumano, L.; Bellenger, J.P.; Jones, J.P.; Cabana, H. A hybrid bioreactor based on insolubilized tyrosinase and laccase catalysis and microfiltration membrane remove pharmaceuticals from wastewater. Chemosphere 2018, 201, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geissen, S.U. In vitro degradation of carbamazepine and diclofenac by crude lignin peroxidase. J. Hazard. Mater. 2010, 176, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Touahar, I.E.; Haroune, L.; Ba, S.; Bellenger, J.-P.; Cabana, H. Characterization of combined cross-linked enzyme aggregates from laccase, versatile peroxidase and glucose oxidase, and their utilization for the elimination of pharmaceuticals. Sci. Total Environ. 2014, 481, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Pylypchuk, I.V.; Daniel, G.; Kessler, V.G.; Seisenbaeva, G.A. Removal of diclofenac, paracetamol, and carbamazepine from model aqueous solutions by magnetic sol–gel encapsulated horseradish peroxidase and lignin peroxidase composites. Nanomaterials 2020, 10, 282. [Google Scholar] [CrossRef]
- Falade, A.O.; Mabinya, L.V.; Okoh, A.I.; Nwodo, U.U. Ligninolytic enzymes: Versatile biocatalysts for the elimination of endocrine-disrupting chemicals in wastewater. MicrobiologyOpen 2018, 7, e00722. [Google Scholar] [CrossRef]
- Yoshida, H. LXIII.—Chemistry of lacquer (Urushi). Part I. Communication from the Chemical Society of Tokio. J. Chem. Soc. Trans. 1883, 43, 472–486. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Goradia, B.; Saxena, A. Bacterial laccase: Recent update on production, properties and industrial applications. Biotech 2017, 7, 323. [Google Scholar] [CrossRef]
- Rodríguez-Couto, S. Fungal laccase: A versatile enzyme for biotechnological applications. In Recent Advancement in White Biotechnology Through Fungi: Volume 1: Diversity and Enzymes Perspectives; Yadav, A.N., Mishra, S., Singh, S., Gupta, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 429–457. [Google Scholar] [CrossRef]
- Bhatt, P.; Tiwari, M.; Parmarick, P.; Bhatt, K.; Gangola, S.; Adnan, M.; Singh, Y.; Bilal, M.; Ahmed, S.; Chen, S. Insights into the catalytic mechanism of ligninolytic peroxidase and laccase in lignin degradation. Bioremed. J. 2022, 26, 281–291. [Google Scholar] [CrossRef]
- Luna-Acosta, A.; Rosenfeld, E.; Amari, M.; Fruitier-Arnaudin, I.; Bustamante, P.; Thomas-Guyon, H. First evidence of laccase activity in the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 2010, 28, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, A.; Pezzella, C.; Giardina, P.; Faraco, V.; Giovanni, S. Heterologous laccase production and its role in industrial applications. Bioeng. Bugs 2010, 1, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Assadi, G.; Vesterlund, L.; Bonfiglio, F.; Mazzurana, L.; Cordeddu, L.; Schepis, D.; Mjösberg, J.; Ruhrmann, S.; Fabbri, A.; Vukojevic, V.; et al. Functional analyses of the Crohn’s disease risk gene LACC1. PLoS ONE 2016, 11, e0168276. [Google Scholar] [CrossRef] [PubMed]
- Bassanini, I.; Ferrandi, E.E.; Riva, S.; Monti, D. Biocatalysis with Laccases: An Updated Overview. Catalysts 2021, 11, 26. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczynski, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Swiderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkolazka, A.; Paszczynski, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Kang, D.H.; Ahn, M.Y.; Hyun, S.; Banks, M.K. Influence of a soil enzyme on iron-cyanide complex speciation and mineral adsorption. Chemosphere 2008, 70, 1044–1051. [Google Scholar] [CrossRef]
- Zille, A.; Munteanu, F.D.; Gubitz, G.M.; Cavaco-Paulo, A. Laccase kinetics of degradation and coupling reactions. J. Mol. Catal. B Enzym. 2005, 33, 23–28. [Google Scholar] [CrossRef]
- Garcia-Ruiz, E.; Mate, D.M.; Gonzalez-Perez, D.; Molina-Espeja, P.; Camarero, S.; Martínez, A.T.; Ballesteros, A.O.; Alcalde, M. Directed evolution of ligninolytic oxidoreductases: From functional expression to stabilization and beyond. In Cascade Biocatalysis; Wiley: Hoboken, NJ, USA, 2014; pp. 1–22. [Google Scholar] [CrossRef]
- Siroosi, M.; Amoozegar, M.A.; Khajeh, K. Purification and characterization of an alkaline chloride-tolerant laccase from a halotolerant bacterium, Bacillus sp strain WT. J. Mol. Catal. B Enzym. 2016, 134, 89–97. [Google Scholar] [CrossRef]
- Hajdok, S.; Conrad, R.; Leutbecher, H.; Strobel, S.; Schleid, T.; Beifuss, U. The laccase-catalyzed domino reaction between catechols and heterocyclic 1,3-dicarbonyls and the unambiguous structure elucidation of the products by NMR spectroscopy and X-ray crystal structure analysis. J. Org. Chem. 2009, 74, 7230–7237. [Google Scholar] [CrossRef]
- Saito, T.; Hong, P.; Kato, K.; Okazaki, M.; Inagaki, H.; Maeda, S.; Yokogawa, Y. Purification and characterization of an extracellular laccase of a fungus (family Chaetomiaceae) isolated from soil. Enzym. Microb. Technol. 2003, 33, 520–526. [Google Scholar] [CrossRef]
- Xiao, Y.Z.; Tu, X.M.; Wang, J.; Zhang, M.; Cheng, Q.; Zeng, W.Y.; Shi, Y.Y. Purification, molecular characterization and reactivity with aromatic compounds of a laccase from basidiomycete Trametes sp strain AH28-2. Appl. Microbiol. Biotechnol. 2003, 60, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Solanki, V.S.; Gacem, A.; Hasan, M.A.; Pare, B.; Srivastava, A.; Singh, A.; Yadav, V.K.; Yadav, K.K.; Lee, C.; et al. Bacterial Laccases as Biocatalysts for the Remediation of Environmental Toxic Pollutants: A Green and Eco-Friendly Approach—A Review. Water 2022, 14, 4068. [Google Scholar] [CrossRef]
- Niladevi, K.N.; Jacob, N.; Prema, P. Evidence for a halotolerant-alkaline laccase in Streptomyces psammoticus: Purification and characterization. Process Biochem. 2008, 43, 654–660. [Google Scholar] [CrossRef]
- Shuttleworth, K.L.; Bollag, J.M. Soluble and immobilized laccase as catalysts for the transformation of substituted phenols. Enzym. Microb. Technol. 1986, 8, 171–177. [Google Scholar] [CrossRef]
- Frasconi, M.; Favero, G.; Boer, H.; Koivula, A.; Mazzei, F. Kinetic and biochemical properties of high and low redox potential laccases from fungal and plant origin. Biochim. Biophys. Acta 2010, 1804, 899–908. [Google Scholar] [CrossRef]
- Murugesan, K.; Arulmani, M.; Nam, I.H.; Kim, Y.M.; Chang, Y.S.; Kalaichelvan, P.T. Purification and characterization of laccase produced by a white rot fungus Pleurotus sajor-caju under submerged culture condition and its potential in decolorization of azo dyes. Appl. Microbiol. Biotechnol. 2006, 72, 939–946. [Google Scholar] [CrossRef]
- Bao, S.Y.; Teng, Z.; Ding, S.J. Heterologous expression and characterization of a novel laccase isoenzyme with dyes decolorization potential from Coprinus comatus. Mol. Biol. Rep. 2013, 40, 1927–1936. [Google Scholar] [CrossRef]
- Kataoka, K.; Kogi, H.; Tsujimura, S.; Sakurai, T. Modifications of laccase activities of copper efflux oxidase, CueO by synergistic mutations in the first and second coordination spheres of the type I copper center. Biochem. Bioph. Res. Commun. 2013, 431, 393–397. [Google Scholar] [CrossRef]
- Tonin, F.; Rosini, E.; Piubelli, L.; Sanchez-Amat, A.; Pollegioni, L. Different recombinant forms of polyphenol oxidase A, a laccase from Marinomonas mediterranea. Protein Expr. Purif. 2016, 123, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Zouari, N.; Romette, J.-L.; Thomas, D. Purification and properties of two laccase isoenzymes produced by Botrytis cinerea. Appl. Biochem. Biotechnol. 1987, 15, 213–225. [Google Scholar] [CrossRef]
- Haibo, Z.; Yinglong, Z.; Feng, H.; Peiji, G.; Jiachuan, C. Purification and characterization of a thermostable laccase with unique oxidative characteristics from Trametes hirsuta. Biotechnol. Lett. 2009, 31, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Daassi, D.; Zouari-Mechichi, H.; Prieto, A.; Martinez, M.J.; Nasri, M.; Mechichi, T. Purification and biochemical characterization of a new alkali-stable laccase from Trametes sp. isolated in Tunisia: Role of the enzyme in olive mill waste water treatment. World J. Microbiol. Biotechnol. 2013, 29, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Reiss, R.; Ihssen, J.; Thony-Meyer, L. Bacillus pumilus laccase: A heat stable enzyme with a wide substrate spectrum. BMC Biotechnol. 2011, 11, 9. [Google Scholar] [CrossRef]
- Mayolo-Deloisa, K.; Gonzalez-Gonzalez, M.; Rito-Palomares, M. Laccases in food industry: Bioprocessing, potential industrial and biotechnological applications. Front. Bioeng. Biotech. 2020, 8, 222. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcalde, M. Laccase: A multi-purpose biocatalyst at the forefront of biotechnology. Microb. Biotechnol. 2017, 10, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.N.; Srikumar, K. Characterization of xerophytic thermophilic laccase exhibiting metal ion-dependent dye decolorization potential. Appl. Biochem. Biotechnol. 2012, 167, 662–676. [Google Scholar] [CrossRef]
- Dube, E.; Shareck, F.; Hurtubise, Y.; Daneault, C.; Beauregard, M. Homologous cloning, expression, and characterisation of a laccase from Streptomyces coelicolor and enzymatic decolourisation of an indigo dye. Appl. Microbiol. Biotechnol. 2008, 79, 597–603. [Google Scholar] [CrossRef]
- Shao, X.H.; Gao, Y.H.; Jiang, M.T.; Li, L. Deletion and site-directed mutagenesis of laccase from Shigella dysenteriae results in enhanced enzymatic activity and thermostability. Enzym. Microb. Technol. 2009, 44, 274–280. [Google Scholar] [CrossRef]
- Sulistyaningdyah, W.T.; Ogawa, J.; Tanaka, H.; Maeda, C.; Shimizu, S. Characterization of alkaliphilic laccase activity in the culture supernatant of Myrothecium verrucaria 24G-4 in comparison with bilirubin oxidase. FEMS Microbiol. Lett. 2004, 230, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Escribano, D.; Pliego-Magán, R.; de Salas, F.; Aza, P.; Gentili, P.; Ihalainen, P.; Levée, T.; Meyer, V.; Petit-Conil, M.; Tapin-Lingua, S.; et al. Tailor-made alkaliphilic and thermostable fungal laccases for industrial wood processing. Biotechnol. Biofuels Bioprod. 2022, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.-L.; Hu, B.-Y.; Chen, Q.-J.; Yang, A.-Z.; Wang, Q.-Y.; Li, X.-F.; Zhang, J.-Y.; Zhang, G.-Q.; Zhao, Y.-C. Purification and Characterization of a Thermo- and pH-Stable Laccase From the Litter-Decomposing Fungus Gymnopus luxurians and Laccase Mediator Systems for Dye Decolorization. Front. Microbiol. 2021, 12, 672620. [Google Scholar] [CrossRef]
- Gomare, S.S.; Jadhav, J.P.; Govindwar, S.P. Degradation of sulfonated azo dyes by the purified lignin peroxidase from Brevibacillus laterosporus MTCC 2298. Biotechnol. Bioproc. Eng. 2008, 13, 136–143. [Google Scholar] [CrossRef]
- Hupert-Kocurek, K.; Stawicka, A.; Wojcieszynska, D.; Guzik, U. Cloning and mutagenesis of catechol 2,3-dioxygenase gene from the Gram-positive Planococcus sp strain S5. J. Mol. Microb. Biotechnol. 2013, 23, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Gao, Y.; Wu, G.; Li, M.X.; Xu, J.H.; He, J.; Li, S.P.; Hong, Q. Cloning of three 2,3-dihydroxybiphenyl-1,2-dioxygenase genes from Achromobacter sp BP3 and the analysis of their roles in the biodegradation of biphenyl. J. Hazard. Mater. 2013, 261, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Park, N.; Park, S.S. Purification and characterization of a novel laccase from Fomitopsis pinicola mycelia. Int. J. Biol. Macromol. 2014, 70, 583–589. [Google Scholar] [CrossRef]
- Ko, E.M.; Leem, Y.E.; Choi, H.T. Purification and characterization of laccase isozymes from the white-rot basidiomycete Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2001, 57, 98–102. [Google Scholar]
- Miyazaki, K. A hyperthermophilic laccase from Thermus thermophilus HB27. Extremophiles 2005, 9, 415–425. [Google Scholar] [CrossRef]
- Wang, Z.X.; Cai, Y.J.; Liao, X.R.; Zhang, F.; Zhang, D.B.; Li, Z.L. Production and characterization of a novel laccase with cold adaptation and high thermal stability from an isolated fungus. Appl. Biochem. Biotechnol. 2010, 162, 280–294. [Google Scholar] [CrossRef]
- Vasdev, K.; Dhawan, S.; Kapoor, R.K.; Kuhad, R.C. Biochemical characterization and molecular evidence of a laccase from the bird’s nest fungus Cyathus bulleri. Fungal Genet. Biol. 2005, 42, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.M.; Mahmoud, M.; Abdel Karim, G.S.A.; Abdelraof, M.; Othman, A.M. Purification and biochemical characterization of two laccase isoenzymes isolated from Trichoderma harzianum S7113 and its application for bisphenol A degradation. Microb. Cell Factories 2023, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Garcia, T.A.; Santiago, M.F.; Ulhoa, C.J. Studies on the Pycnoporus sanguineus CCT-4518 laccase purified by hydrophobic interaction chromatography. Appl. Microbiol. Biotechnol. 2007, 75, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Chakraborty, T.K.; Mukherjee, M. Purification and characterization of a growth-regulating laccase from Pleurotus florida. J. Basic Microbiol. 2001, 41, 261–267. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Purification of a thermostable laccase from Leucaena leucocephala using a copper alginate entrapment approach and the application of the laccase in dye decolorization. Process Biochem. 2014, 49, 1196–1204. [Google Scholar] [CrossRef]
- Li, J.; Xie, Y.A.; Wang, R.; Fang, Z.M.; Fang, W.; Zhang, X.C.; Xiao, Y.Z. Mechanism of salt-induced activity enhancement of a marine-derived laccase, Lac15. Eur. Biophys. J. 2018, 47, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Ausec, L.; Berini, F.; Casciello, C.; Cretoiu, M.S.; van Elsas, J.D.; Marinelli, F.; Mandic-Mulec, I. The first acidobacterial laccase-like multicopper oxidase revealed by metagenomics shows high salt and thermo-tolerance. Appl. Microbiol. Biotechnol. 2017, 101, 6261–6276. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Peng, F.; Cui, B.K. Purification, biochemical characterization and dye decolorization capacity of an alkali-resistant and metal-tolerant laccase from Trametes pubescens. Biores. Technol. 2013, 128, 49–57. [Google Scholar] [CrossRef]
- Brander, S.; Mikkelsen, J.D.; Kepp, K.P. Characterization of an alkali- and halide-resistant laccase expressed in E-coli: CotA from Bacillus clausii. PLoS ONE 2014, 9, e99402. [Google Scholar] [CrossRef]
- Shafiei, M.; Afzali, F.; Karkhane, A.A.; Ebrahimi, S.M.; Haghbeen, K.; Aminzadeh, S. Cohnella sp. A01 laccase: Thermostable, detergent resistant, anti-environmental and industrial pollutants enzyme. Heliyon 2019, 5, e02543. [Google Scholar] [CrossRef]
- Wu, M.H.; Lin, M.C.; Lee, C.C.; Yu, S.M.; Wang, A.H.; Ho, T.D. Enhancement of laccase activity by pre-incubation with organic solvents. Sci. Rep. 2019, 9, 9754. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.B.; Song, C.M.; Zhang, N.; Zhou, W.; Xu, C.W.; Zhou, L.X.; Zhao, H.; Cai, Y.J.; Liao, X.R. Overexpression, characterization, and dye-decolorizing ability of a thermostable, pH-stable, and organic solvent-tolerant laccase from Bacillus pumilus W3. J. Mol. Catal. B Enzym. 2014, 101, 1–6. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, M.; Wang, T.N.; Zhao, L.Y.; Du, M.H.; Li, T.L.; Li, D.B. Characterization and dye decolorization ability of an alkaline resistant and organic solvents tolerant laccase from Bacillus licheniformis LS04. Biores. Technol. 2012, 115, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.L.; Kermasha, S.; Gao, J.M.; Borges, R.M.; Yu, X.Z. Laccase-catalyzed oxidation of phenolic compounds in organic media. J. Mol. Catal. B Enzym. 2009, 57, 89–95. [Google Scholar] [CrossRef]
- Lugaro, G.; Carrea, G.; Cremonesi, P.; Casellato, M.M.; Antonini, E. The oxidation of steroid hormones by fungal laccase in emulsion of water and organic solvents. Arch. Biochem. Biophys. 1973, 159, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rodakiewicz-Nowak, J.; Jarosz-Wilkołazka, A. Catalytic activity of Cerrena unicolor laccase in aqueous solutions of water-miscible organic solvents—Experimental and numerical description. J. Mol. Catal. B Enzym. 2007, 44, 53–59. [Google Scholar] [CrossRef]
- Wan, Y.Y.; Lu, R.; Xiao, L.; Du, Y.M.; Miyakoshi, T.; Chen, C.L.; Knill, C.J.; Kennedy, J.F. Effects of organic solvents on the activity of free and immobilised laccase from Rhus vernicifera. Int. J. Biol. Macromol. 2010, 47, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y.I. Laccase-mediator systems and their applications: A review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Baiocco, P.; Barreca, A.M.; Fabbrini, M.; Galli, C.; Gentili, P. Promoting laccase activity towards non-phenolic substrates: A mechanistic investigation with some laccase-mediator systems. Org. Biomol. Chem. 2003, 1, 191–197. [Google Scholar] [CrossRef]
- Lin, H.; Yu, Z.; Wang, Q.; Liu, Y.; Jiang, L.; Xu, C.; Xian, M. Application of Laccase Catalysis in Bond Formation and Breakage: A Review. Catalysts 2023, 13, 750. [Google Scholar] [CrossRef]
- Gu, Y.; Yuan, L.; Jia, L.; Xue, P.; Yao, H. Recent developments of a co-immobilized laccase-mediator system: A review. RSC Adv. 2021, 11, 29498–29506. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, W.; Ng, T.B.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, expression regulation, and applications in pharmaceutical biodegradation. Front. Microbiol. 2017, 8, 832. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.; Chopra, N.K.; Kumar, A.; Naveen, G. Laccase: A Green Solution for Environmental Problems. Adv. Environ. Eng. Res. 2023, 4, 30. [Google Scholar] [CrossRef]
- Dong, C.-D.; Tiwari, A.; Anisha, G.S.; Chen, C.-W.; Singh, A.; Haldar, D.; Patel, A.K.; Singhania, R.R. Laccase: A potential biocatalyst for pollutant degradation. Environ. Pollut. 2023, 319, 120999. [Google Scholar] [CrossRef] [PubMed]
- Vaithyanathan, V.K.; Vaidyanathan, V.K.; Cabana, H. Laccase-driven transformation of high priority pesticides without redox mediators: Towards bioremediation of contaminated wastewaters. Front. Bioeng. Biotechnol. 2022, 9, 770435. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.R.; Kim, S.Y.; Kang, M.; Lee, T.K. Removal of pharmaceuticals and personal care products using native fungal enzymes extracted during the ligninolytic process. Environ. Res. 2021, 195, 110878. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.B.; Hai, F.I.; Kang, J.; van de Merwe, J.P.; Leusch, F.D.L.; Price, W.E.; Nghiem, L.D. Biocatalytic degradation of pharmaceuticals, personal care products, industrial chemicals, steroid hormones and pesticides in a membrane distillation-enzymatic bioreactor. Bioresour. Technol. 2018, 247, 528–536. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Yang, S.; Kang, J.; Leusch, F.D.L.; Roddick, F.; Price, W.E.; Nghiem, L.D. Removal of pharmaceuticals, steroid hormones, phytoestrogens, UV-filters, industrial chemicals and pesticides by Trametes versicolor: Role of biosorption and biodegradation. Int. Biodeterior. Biodegrad. 2014, 88, 169–175. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef]
- Sá, H.; Michelin, M.; Tavares, T.; Silva, B. Current Challenges for Biological Treatment of Pharmaceutical-Based Contaminants with Oxidoreductase Enzymes: Immobilization Processes, Real Aqueous Matrices and Hybrid Techniques. Biomolecules 2022, 12, 1489. [Google Scholar] [CrossRef] [PubMed]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera de los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Factories 2019, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- El Yagoubi, Y.; Lemieux, B.; Segura, P.A.; Cabana, H. Characterization of laccases from Trametes hirsuta in the context of bioremediation of wastewater treatment plant effluent. Enzym. Microb. Technol. 2023, 171, 110308. [Google Scholar] [CrossRef] [PubMed]
- Spina, F.; Gea, M.; Bicchi, C.; Cordero, C.; Schilirò, T.; Varese, G.C. Ecofriendly laccases treatment to challenge micropollutants issue in municipal wastewaters. Environ. Pollut. 2020, 257, 113579. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Huang, Q. Removal of acetaminophen using enzyme-mediated oxidative coupling processes: II. cross-coupling with natural organic matter. Environ. Sci. Technol. 2009, 43, 7068–7073. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Persistence and impact of steroidal estrogens on the environment and their laccase-assisted removal. Sci. Total Environ. 2019, 690, 447–459. [Google Scholar] [CrossRef]
- Asif, M.B.; Hai, F.I.; Hou, J.; Price, W.E.; Nghiem, L.D. Impact of wastewater derived dissolved interfering compounds on growth, enzymatic activity and trace organic contaminant removal of white rot fungi—A critical review. J. Environ. Manag. 2017, 201, 89–109. [Google Scholar] [CrossRef] [PubMed]
- Zofair, S.F.F.; Ahmad, S.; Hashmi, M.A.; Khan, S.H.; Khan, M.A.; Younus, H. Catalytic roles, immobilization and management of recalcitrant environmental pollutants by laccases: Significance in sustainable green chemistry. J. Environ. Manag. 2022, 309, 114676. [Google Scholar] [CrossRef]
- Margot, J.; Bennati-Granier, C.; Maillard, J.; Blánquez, P.; Barry, D.A.; Holliger, C. Bacterial versus fungal laccase: Potential for micropollutant degradation. AMB Express 2013, 3, 63. [Google Scholar] [CrossRef]
- Zimmermann, Y.-S.; Shahgaldian, P.; Corvini, P.F.X.; Hommes, G. Sorption-assisted surface conjugation: A way to stabilize laccase enzyme. Appl. Microbiol. Biotechnol. 2011, 92, 169–178. [Google Scholar] [CrossRef]
- Jahangiri, E.; Thomas, I.; Schulze, A.; Seiwert, B.; Cabana, H.; Schlosser, D. Characterisation of electron beam irradiation-immobilised laccase for application in wastewater treatment. Sci. Total Environ. 2018, 624, 309–322. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Wyszowska, M.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Pinelo, M.; Meyer, A.S.; Moszyński, D.; Jesionowski, T. Robust biodegradation of naproxen and diclofenac by laccase immobilized using electrospun nanofibers with enhanced stability and reusability. Mater. Sci. Eng. C 2019, 103, 109789. [Google Scholar] [CrossRef]
- Sun, K.; Kang, F.; Waigi, M.G.; Gao, Y.; Huang, Q. Laccase-mediated transformation of triclosan in aqueous solution with metal cations and humic acid. Environ. Pollut. 2017, 220, 105–111. [Google Scholar] [CrossRef]
- Ji, C.; Hou, J.; Wang, K.; Zhang, Y.; Chen, V. Biocatalytic degradation of carbamazepine with immobilized laccase-mediator membrane hybrid reactor. J. Membr. Sci. 2016, 502, 11–20. [Google Scholar] [CrossRef]
- Margot, J.; Maillard, J.; Rossi, L.; Barry, D.A.; Holliger, C. Influence of treatment conditions on the oxidation of micropollutants by Trametes versicolor laccase. New Biotechnol. 2013, 30, 803–813. [Google Scholar] [CrossRef]
- Margot, J.; Copin, P.-J.; von Gunten, U.; Barry, D.A.; Holliger, C. Sulfamethoxazole and isoproturon degradation and detoxification by a laccase-mediator system: Influence of treatment conditions and mechanistic aspects. Biochem. Eng. J. 2015, 103, 47–59. [Google Scholar] [CrossRef]
- Becker, D.; Varela Della Giustina, S.; Rodriguez-Mozaz, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.-P.; Sanchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase—Degradation of compounds does not always eliminate toxicity. Biores. Technol. 2016, 219, 500–509. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R.Y. Biotransformation of carbamazepine by laccase-mediator system: Kinetics, by-products and toxicity assessment. Process Biochem. 2018, 67, 147–154. [Google Scholar] [CrossRef]
- Ashe, B.; Nguyen, L.N.; Hai, F.I.; Lee, D.-J.; van de Merwe, J.P.; Leusch, F.D.L.; Price, W.E.; Nghiem, L.D. Impacts of redox-mediator type on trace organic contaminants degradation by laccase: Degradation efficiency, laccase stability and effluent toxicity. Int. Biodeterior. Biodegrad. 2016, 113, 169–176. [Google Scholar] [CrossRef]
- Lueangjaroenkit, P.; Teerapatsakul, C.; Sakka, K.; Sakka, M.; Kimura, T.; Kunitake, E.; Chitradon, L. Two manganese peroxidases and a laccase of Trametes polyzona KU-RNW027 with novel properties for dye and pharmaceutical product degradation in redox mediator-free system. Mycobiology 2019, 47, 217–229. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcalde, M. Laccase engineering: From rational design to directed evolution. Biotechnol. Adv. 2015, 33, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; van de Merwe, J.P.; Hai, F.I.; Leusch, F.D.L.; Kang, J.; Price, W.E.; Roddick, F.; Magram, S.F.; Nghiem, L.D. Laccase–syringaldehyde-mediated degradation of trace organic contaminants in an enzymatic membrane reactor: Removal efficiency and effluent toxicity. Biores. Technol. 2016, 200, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bai, R.; Zhang, Y.; Wang, Q.; Fan, X.; Yuan, J.; Cui, L.; Wang, P. Laccase-Catalyzed Oxidative Polymerization of Phenolic Compounds. Appl. Biochem. Biotechnol. 2013, 171, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Cheng, X.; Yu, J.; Chen, L.; Wei, J.; Chen, W.; Wang, J.; Li, S.; Liu, Q.; Si, Y. Isolation of Trametes hirsuta La-7 with high laccase-productivity and its application in metabolism of 17β-estradiol. Environ. Pollut. 2020, 263, 114381. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Waigi, M.G.; Li, S.; Sun, K.; Si, Y. Fungal laccase-mediated humification of estrogens in aquatic ecosystems. Water Res. 2019, 166, 115040. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Berry, E.; Duke, S.; Milliken, A.; Patterson, H.; Prewett, D.L.; Rae, T.C.; Sridhar, V.; Wendland, N.; Gregory, B.W.; et al. Characterization of Trametes versicolor laccase-catalyzed degradation of estrogenic pollutants: Substrate limitation and product identification. Int. Biodeterior. Biodegrad. 2018, 127, 146–159. [Google Scholar] [CrossRef]
- Yousefi-Ahmadipour, A.; Bozorgi-Koshalshahi, M.; Mogharabi, M.; Amini, M.; Ghazi-Khansari, M.; Faramarzi, M.A. Laccase-catalyzed treatment of ketoconazole, identification of biotransformed metabolites, determination of kinetic parameters, and evaluation of micro-toxicity. J. Mol. Catal. B Enzym. 2016, 133, 77–84. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Nicell, J.A. Laccase-catalysed oxidation of aqueous triclosan. J. Chem. Technol. Biotechnol. 2006, 81, 1344–1352. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Y.; Yang, X.; Ng, T.B.; Ye, X.; Lin, J. Degradation of tetracycline by immobilized laccase and the proposed transformation pathway. J. Hazard. Mater. 2017, 322, 525–531. [Google Scholar] [CrossRef]
- Blánquez, A.; Guillén, F.; Rodríguez, J.; Arias, M.E.; Hernández, M. The degradation of two fluoroquinolone based antimicrobials by SilA, an alkaline laccase from Streptomyces ipomoeae. World J. Microbiol. Biotechnol. 2016, 32, 52. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Price, W.E.; Leusch, F.D.L.; Roddick, F.; McAdam, E.J.; Magram, S.F.; Nghiem, L.D. Continuous biotransformation of bisphenol A and diclofenac by laccase in an enzymatic membrane reactor. Int. Biodeterior. Biodegrad. 2014, 95, 25–32. [Google Scholar] [CrossRef]

| Manufacturer/Supplier | Product Description | Cost/Price |
|---|---|---|
| Mega Pacific Technology, Inc., Arcadia, CA, USA | Laccase Q10, purity 100%, for indigo bleaching | 1–5 USD per 1 kg |
| Xi’an International Healthcare Factory Co., Ltd., Xi’an, China | 99% min. laccase | 15–25 USD per 1 kg |
| Xi’an Multihealth Biotech Co., Ltd., Xi’an, China | 98%, food grade | 40–60 USD per 1 kg |
| Sunson Industry Group Co., Ltd., Beijing, China | Purity 99.9%, for paper chemicals | 16–22 USD per 1 kg |
| Sunson Industry Group Co., Ltd., Beijing, China | Activity of 10,000 U/g, from Aspergillus oryzae for wastewater, papermaking, textile, juice clarification, baking | 35–55 USD per 1 kg |
| Qingdao Cemo Technology Develop Co., Ltd., Shandong, China | Purity 99%, Novozyme 809, food additive | 1–10 USD per 1 kg |
| Botanical Cube Inc., Xi’an, China | MW 119.12, nutrition enhancer | 21–38 USD per 1 kg |
| Sinobios (Shanghai) Imp. & Exp. Co., Ltd., Shanghai, China | LAC8L, liquid, activity ≥ 5000 U/mL, from white-rot fungi, textile auxiliary agent | 1–5 USD per 1 kg |
| Novozymes, Denmark (from Merck, Darmstadt, Germany) | Novozym® 51003, Activity 1000 LAMU (laccase unit)/g, liquid, from A. oryzae | 450 EUR per 250 dm3 |
| Hebei Mojin Biotechnology Co., Ltd., Shijiazhuang, China | Purity 99% | 100 USD per 25 kg |
| Career Henan Chemical Co., Zhengzhou, China | Purity 98% (HPLC) | 1 USD per 1 kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janusz, G.; Skwarek, E.; Pawlik, A. Potential of Laccase as a Tool for Biodegradation of Wastewater Micropollutants. Water 2023, 15, 3770. https://doi.org/10.3390/w15213770
Janusz G, Skwarek E, Pawlik A. Potential of Laccase as a Tool for Biodegradation of Wastewater Micropollutants. Water. 2023; 15(21):3770. https://doi.org/10.3390/w15213770
Chicago/Turabian StyleJanusz, Grzegorz, Ewa Skwarek, and Anna Pawlik. 2023. "Potential of Laccase as a Tool for Biodegradation of Wastewater Micropollutants" Water 15, no. 21: 3770. https://doi.org/10.3390/w15213770
APA StyleJanusz, G., Skwarek, E., & Pawlik, A. (2023). Potential of Laccase as a Tool for Biodegradation of Wastewater Micropollutants. Water, 15(21), 3770. https://doi.org/10.3390/w15213770









