Green Synthesis of Surface Modified Biochar for Simultaneous Removal of Steroidal Hormones and Heavy Metals from Wastewater: Optimisation by Central Composite Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorbent Preparation
2.3. Adsorbent Characterisation
2.4. HPLC Separation
2.5. Batch Adsorption Experiment
2.6. Optimisation by Central Composite Design (CCD)
2.7. Regeneration Experiment
3. Discussion of Results
3.1. Characterisation
3.1.1. BET of the Pristine and Modified Biochar
3.1.2. XRD Analysis
3.1.3. FTIR Spectroscopy Analysis
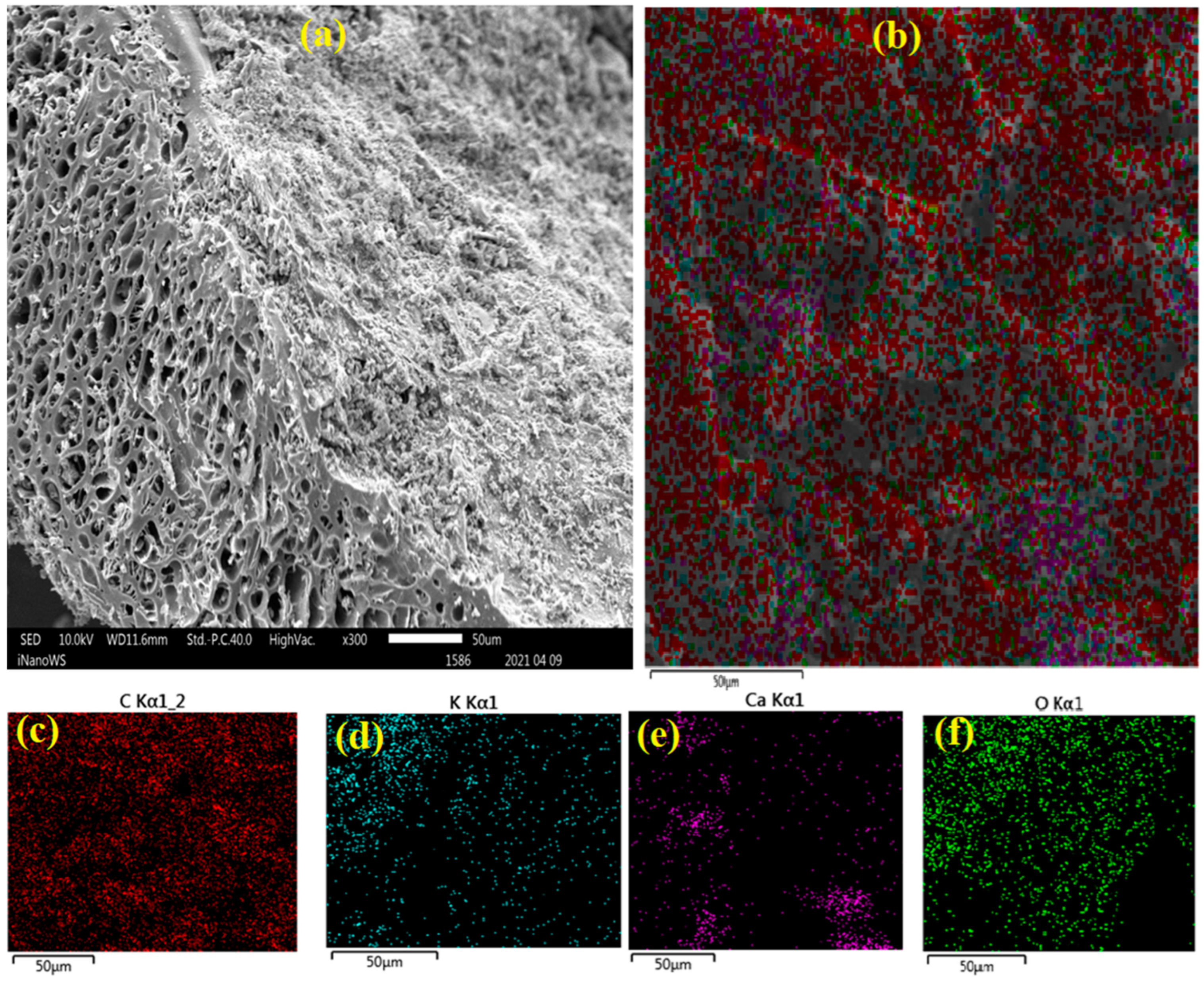
3.1.4. SEM and EDS Mapping
3.1.5. TGA Studies
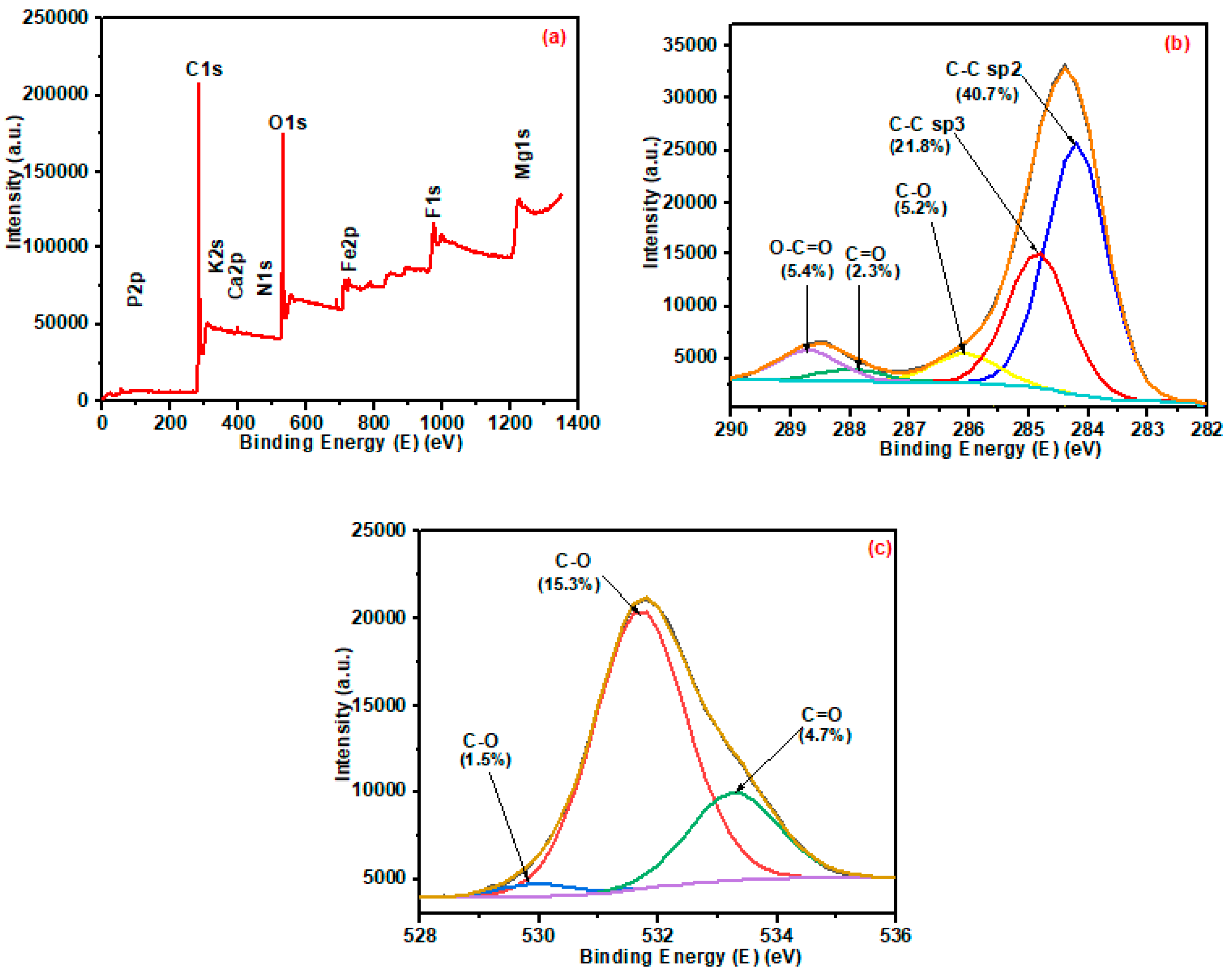
3.1.6. XPS Analysis of Pristine and Ball-Milled Biochar
3.1.7. Raman Spectroscopy Analysis
3.2. Adsorption Experiments
Adsorption Isotherm
3.3. CCD Modelling
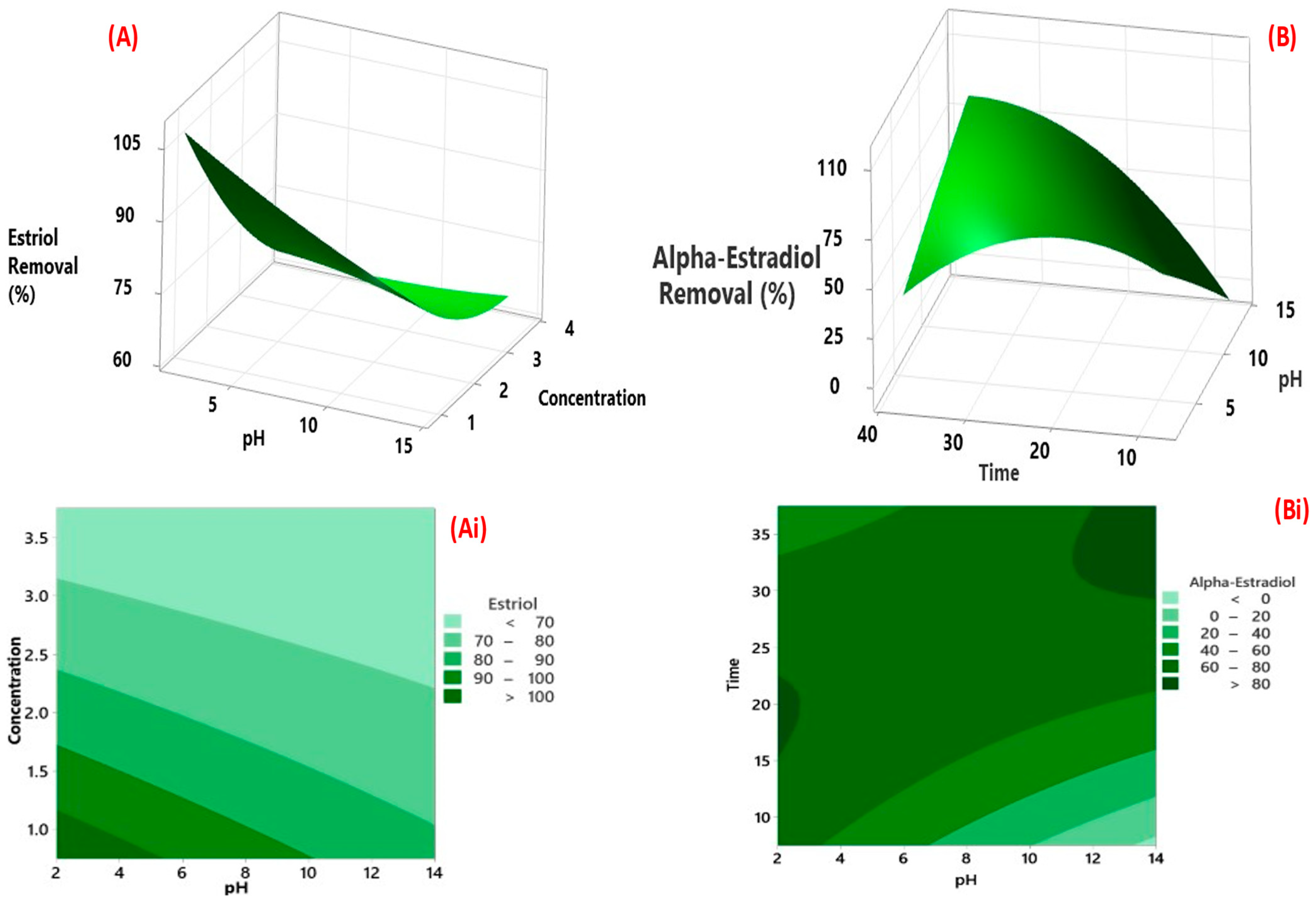
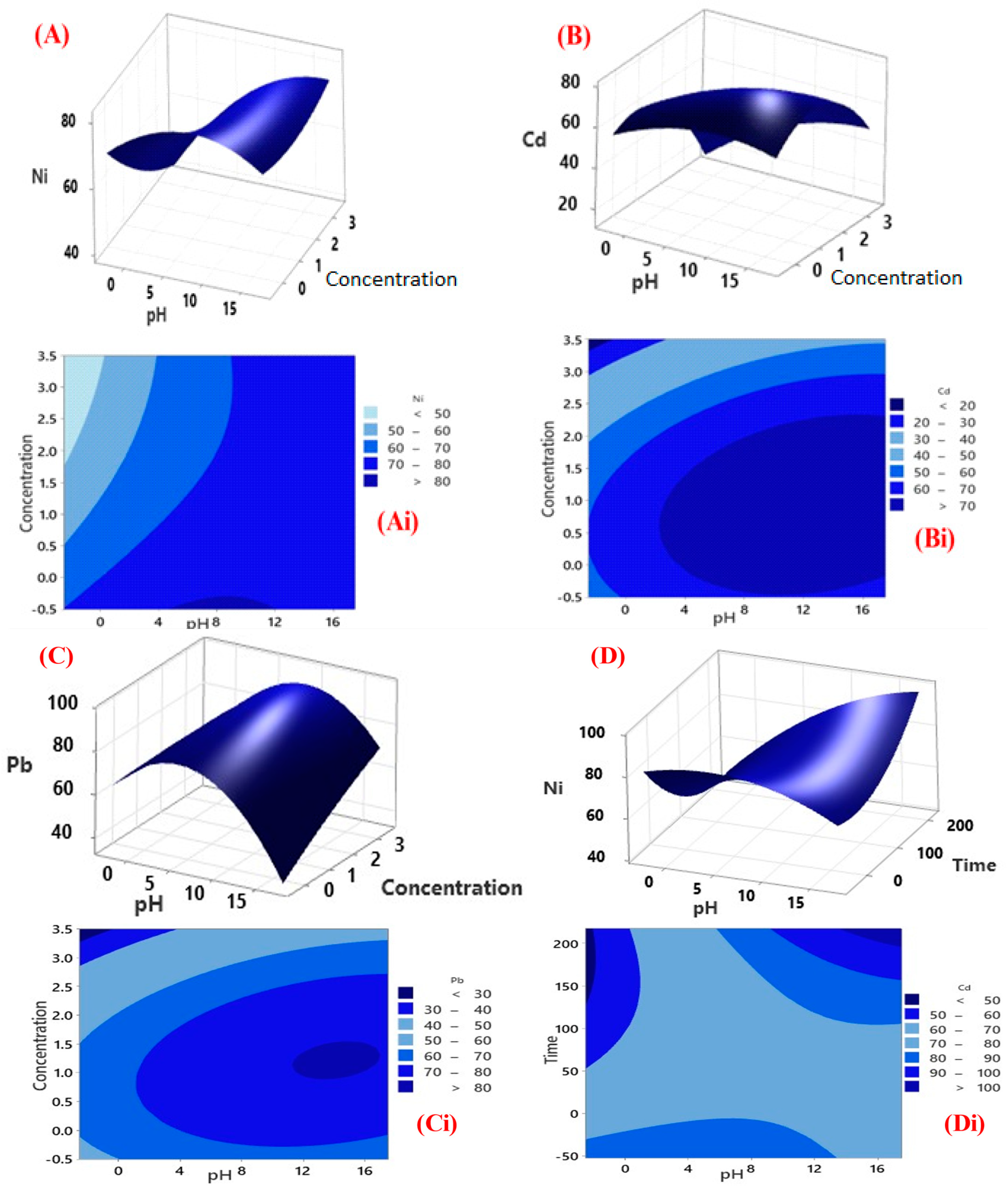
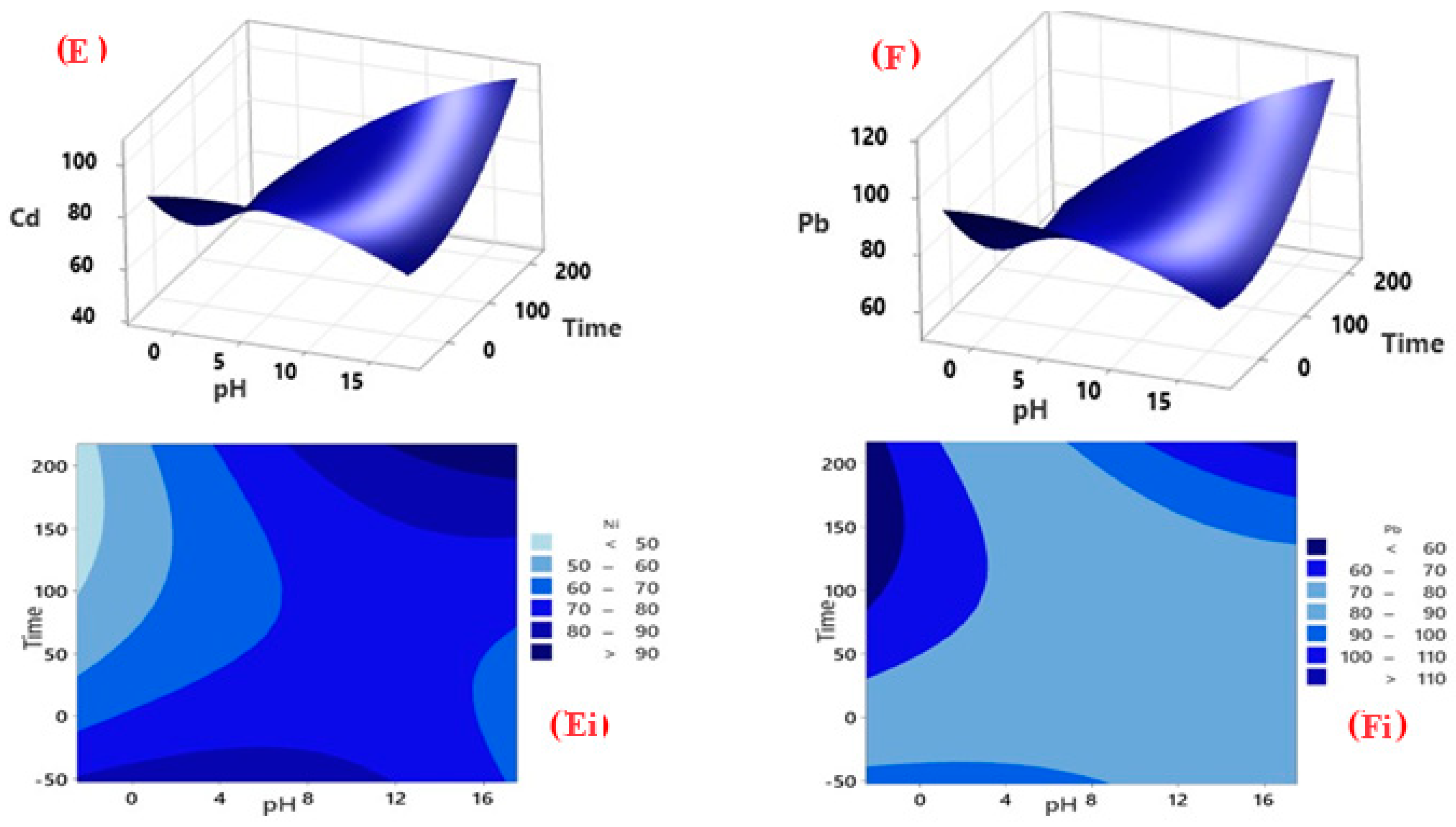
3.3.1. Factorial Design for Optimisation
3.3.2. Validation of the Model and Statistical Analysis
3.3.3. ANOVA Analysis
3.3.4. Ball-Milled Biochar Application in a Real Wastewater Sample
3.3.5. Reusability of the Ball-Milled Biochar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.-Q.; Xing, C.; Cai, Y.-Y.; Yan, X.-T.; Ying, G.-G. How much do human and livestock actually contribute to steroids emission and surface water pollution from past to the future: A global research. Sci. Total Environ. 2021, 772, 145558. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rana, R.; Khanam, R. Determinants of life expectancy in most polluted countries: Exploring the effect of environmental degradation. PLoS ONE 2022, 17, e0262802. [Google Scholar] [CrossRef]
- Chakraborty, P.; Shappell, N.W.; Mukhopadhyay, M.; Onanong, S.; Rex, K.R.; Snow, D. Surveillance of plasticizers, bisphenol A, steroids and caffeine in surface water of River Ganga and Sundarban wetland along the Bay of Bengal: Occurrence, sources, estrogenicity screening and ecotoxicological risk assessment. Water Res. 2021, 19, 116668. [Google Scholar] [CrossRef]
- Azizi-lalabadi, M.; Pirsaheb, M. Investigation of Steroid Hormone Residues in Fish: A Systematic Review. Process Saf. Environ. Prot. 2021, 152, 14–24. [Google Scholar] [CrossRef]
- Zhong, R.; Zou, H.; Gao, J.; Wang, T.; Bu, Q.; Wang, Z.L.; Hu, M.; Wang, Z. A critical review on the distribution and ecological risk assessment of steroid hormones in the environment in China. Sci. Total Environ. 2021, 786, 147452. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, K.; Zhao, Y.; Fent, K. Environmental chemicals affect circadian rhythms: An underexplored effect influencing health and fitness in animals and humans. Environ. Int. 2021, 149, 106159. [Google Scholar] [CrossRef]
- Parrott, J.L.; Blunt, B.R. Life-cycle exposure of fathead minnows (Pimephales promelas) to an ethinylestradiol concentration below 1 ng/L reduces egg fertilization success and demasculinizes males. Environ. Toxicol. 2005, 20, 131–141. [Google Scholar] [CrossRef]
- Chang, M. Dual roles of estrogen metabolism in mammary carcinogenesis. BMB Rep. 2011, 44, 423–434. [Google Scholar] [CrossRef]
- Vasto, S.; Carruba, G.; Candore, G.; Italiano, E.; Di Bona, D.; Caruso, C. Inflammation and prostate cancer. Futur. Oncol. 2008, 4, 637–645. [Google Scholar] [CrossRef]
- Brachet, C.; Heinrichs, C. Central precocious puberty after interpersonal transfer of testosterone gel: Just a coincidence? J. Pediatr. Endocrinol. Metab. 2012, 25, 757–760. [Google Scholar] [CrossRef]
- Lyssimachou, A.; Arukwe, A. Alteration of brain and interrenal StAR protein, P450 scc, and Cyp11β mRNA levels in Atlantic Salmon after nominal waterborne exposure to the synthetic pharmaceutical estrogen ethynylestradiol. J. Toxicol. Environ. Heal. Part. A 2007, 70, 606–613. [Google Scholar] [CrossRef]
- Neczaj, E. Fate of selected emerging contaminants in wastewater treatment systems. Desalin. Water Treat. 2020, 199, 451–463. [Google Scholar] [CrossRef]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Mhuka, V.; Dube, S.; Nindi, M.M. Occurrence of pharmaceutical and personal care products (PPCPs) in wastewater and receiving waters in South Africa using LC-OrbitrapTM MS. Emerg. Contam. 2020, 6, 250–258. [Google Scholar] [CrossRef]
- Naddafi, K.; Mesdaghinia, A.; Abtahi, M.; Hassanvand, M.S.; Beiki, A.; Shaghaghi, G.; Shamsipour, M.; Mohammadi, F.; Saeedi, R. Assessment of burden of disease induced by exposure to heavy metals through drinking water at national and subnational levels in Iran, 2019. Environ. Res. 2022, 204, 112057. [Google Scholar] [CrossRef]
- Gade, M.; Comfort, N.; Re, D.B. Sex-specific neurotoxic effects of heavy metal pollutants: Epidemiological, experimental evidence and candidate mechanisms. Environ. Res. 2021, 201, 111558. [Google Scholar] [CrossRef]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, selective heavy metal removal from water by a metal–organic framework/polydopamine composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Majdoub, M.; Amedlous, A.; Anfar, Z.; Jada, A.; El Alem, N. Engineering of amine-based binding chemistry on functionalized graphene oxide/alginate hybrids for simultaneous and efficient removal of trace heavy metals: Towards drinking water. J. Colloid. Interface Sci. 2021, 589, 511–524. [Google Scholar] [CrossRef]
- Nys, C.; Versieren, L.; Cordery, K.I.; Blust, R.; Smolders, E.; De Schamphelaere, K.A.C. Systematic evaluation of chronic metal-mixture toxicity to three species and implications for risk assessment. Environ. Sci. Technol. 2017, 51, 4615–4623. [Google Scholar] [CrossRef]
- Noutsopoulos, C.; Katsigiannis, A.; Mantziaras, J.; Gioldasi, M.; Katsiri, A. Removal of Anti-Inflammatory Drugs and Endocrine Disrupting Compounds By Granular Activated Carbon. In Proceedings of the International Conference on Environmental Science and Technology, Athens, Greece, 5–7 September 2013. [Google Scholar]
- Peydayesh, M.; Bolisetty, S.; Mohammadi, T.; Mezzenga, R. Assessing the Binding Performance of Amyloid-Carbon Membranes toward Heavy Metal Ions. Langmuir 2019, 35, 4161–4170. [Google Scholar] [CrossRef]
- Lyu, H.; Xia, S.; Tang, J.; Zhang, Y.; Gao, B.; Shen, B. Thiol-modified biochar synthesized by a facile ball-milling method for enhanced sorption of inorganic Hg2+ and organic CH3Hg+. J. Hazard. Mater. 2020, 384, 121357. [Google Scholar] [CrossRef] [PubMed]
- Jean, E.; Villemin, D.; Hlaibi, M.; Lebrun, L. Heavy metal ions extraction using new supported liquid membranes containing ionic liquid as carrier. Sep. Purif. Technol. 2018, 201, 1–9. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Assefi, M.; Maroufi, S.; Sahajwalla, V. Selective isolation of heavy metals from spent electronic waste solution by macroporous ion-exchange resins. J. Hazard. Mater. 2019, 371, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Shariful, M.I.; Sepehr, T.; Mehrali, M.; Ang, B.C.; Amalina, M.A. Adsorption capability of heavy metals by chitosan/poly (ethylene oxide)/activated carbon electrospun nanofibrous membrane. J. Appl. Polym. Sci. 2018, 135, 45851. [Google Scholar] [CrossRef]
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Removal of endocrine disruptors in waters by adsorption, membrane filtration and biodegradation. A review. Environ. Chem. Lett. 2020, 18, 1113–1143. [Google Scholar] [CrossRef]
- Vital, B.; Bartacek, J.; Ortega-Bravo, J.C.; Jeison, D. Treatment of acid mine drainage by forward osmosis: Heavy metal rejection and reverse flux of draw solution constituents. Chem. Eng. J. 2018, 332, 85–91. [Google Scholar] [CrossRef]
- Li, Y.-H.; Ding, J.; Luan, Z.; Di, Z.; Zhu, Y.; Xu, C.; Wu, D.; Wei, B. Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 2003, 41, 2787–2792. [Google Scholar] [CrossRef]
- Wang, X.; Liu, N.; Liu, Y.; Jiang, L.; Zeng, G.; Tan, X.; Liu, S.; Yin, Z.; Tian, S.; Li, J. Adsorption removal of 17β-estradiol from water by rice straw-derived biochar with special attention to pyrolysis temperature and background chemistry. Int. J. Environ. Res. Public Health 2017, 14, 1213. [Google Scholar] [CrossRef]
- Zaib, Q.; Khan, I.A.; Saleh, N.B.; Flora, J.R.V.; Park, Y.G.; Yoon, Y. Removal of bisphenol a and 17β-estradiol by single-walled carbon nanotubes in aqueous solution: Adsorption and molecular modeling. Water. Air. Soil Pollut. 2012, 223, 3281–3293. [Google Scholar] [CrossRef]
- Wan, S.; Wang, S.; Li, Y.; Gao, B. Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Ind. Eng. Chem. 2017, 47, 246–253. [Google Scholar] [CrossRef]
- Das, P.; Nisa, S.; Debnath, A.; Saha, B. Enhanced adsorptive removal of toxic anionic dye by novel magnetic polymeric nanocomposite: Optimization of process parameters. J. Dispers. Sci. Technol. 2022, 43, 880–895. [Google Scholar] [CrossRef]
- Deb, A.; Debnath, A.; Bhowmik, K.; Paul, S.R.; Saha, B. Application of polyaniline impregnated mixed phase Fe2O3, MnFe2O4 and ZrO2 nanocomposite for rapid abatement of binary dyes from aqua matrix: Response surface optimisation. Int. J. Environ. Anal. Chem. 2021, 1–19. [Google Scholar] [CrossRef]
- Das, P.; Debnath, A. Reactive orange 12 dye adsorption onto magnetically separable CaFe2O4 nanoparticles synthesized by simple chemical route: Kinetic, isotherm and neural network modeling. Water Pract. Technol. 2021, 16, 1141–1158. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef]
- Peiris, C.; Nawalage, S.; Wewalwela, J.J.; Gunatilake, S.R.; Vithanage, M. Biochar based sorptive remediation of steroidal estrogen contaminated aqueous systems: A critical review. Environ. Res. 2020, 191, 110183. [Google Scholar] [CrossRef]
- Huang, J.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef]
- Jang, H.M.; Kan, E. Engineered biochar from agricultural waste for removal of tetracycline in water. Bioresour. Technol. 2019, 284, 437–447. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Hassani, A.; Bhatnagar, A. Facile hydrothermal synthesis of novel Fe-Cu layered double hydroxide/biochar nanocomposite with enhanced sonocatalytic activity for degradation of cefazolin sodium. J. Hazard. Mater. 2020, 381, 120742. [Google Scholar] [CrossRef]
- Amusat, S.O.; Kebede, T.G.; Dube, S.; Nindi, M.M. Ball-milling synthesis of biochar and biochar–based nanocomposites and prospects for removal of emerging contaminants: A review. J. Water Process Eng. 2021, 41, 101993. [Google Scholar] [CrossRef]
- Karvelas, M.; Katsoyiannis, A.; Samara, C. Occurrence and fate of heavy metals in the wastewater treatment process. Chemosphere 2003, 53, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Tang, J.; Crittenden, J.C. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chem. Eng. J. 2018, 355, 110–119. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Kebede, T.G.; Dube, S.; Nindi, M.M. Characterisation of water-soluble protein powder and optimisation of process parameters for the removal of sulphonamides from wastewater. Environ. Sci. Pollut. Res. 2019, 26, 21450–21462. [Google Scholar] [CrossRef]
- Mekala, M.; Neerudi, B.; Are, P.R.; Surakasi, R.; Manikandan, G.; Kakara, V.R.; Dhumal, A.A. Water removal from an ethanol-water mixture at azeotropic condition by adsorption technique. Adsorpt. Sci. Technol. 2022, 2022, 10. [Google Scholar] [CrossRef]
- Zhang, D.; He, Q.; Hu, X.; Zhang, K.; Chen, C.; Xue, Y. Enhanced adsorption for the removal of tetracycline hydrochloride (TC) using ball-milled biochar derived from crayfish shell. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 615, 126254. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of pyrolysis temperature on biochar microstructural evolution, physicochemical characteristics, and its influence on biochar/polypropylene composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Lyu, H.; Zhao, Q.; Jiang, L.; Liu, L. Ball-milled biochar for galaxolide removal: Sorption performance and governing mechanisms. Sci. Total Environ. 2019, 659, 1537–1545. [Google Scholar] [CrossRef]
- Kumar, N.S.; Shaikh, H.M.; Asif, M.; Al-Ghurabi, E.H. Engineered biochar from wood apple shell waste for high-efficient removal of toxic phenolic compounds in wastewater. Sci. Rep. 2021, 11, 2586. [Google Scholar] [CrossRef]
- Fang, Z.; Gao, Y.; Bolan, N.; Shaheen, S.M.; Xu, S.; Wu, X.; Xu, X.; Hu, H.; Lin, J.; Zhang, F. Conversion of biological solid waste to graphene-containing biochar for water remediation: A critical review. Chem. Eng. J. 2020, 390, 124611. [Google Scholar] [CrossRef]
- Gupta, N.K.; Prakash, P.; Kalaichelvi, P.; Sheeba, K.N. The effect of temperature and hemicellulose-lignin, cellulose-lignin, and cellulose-hemicellulose on char yield from the slow pyrolysis of rice husk. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 1428–1434. [Google Scholar]
- Sarmah, A.K.; Srinivasan, P.; Smernik, R.J.; Manley-Harris, M.; Antal, M.J.; Downie, A.; Van Zwieten, L. Retention capacity of biochar-amended New Zealand dairy farm soil for an estrogenic steroid hormone and its primary metabolite. Aust. J. Soil. Res. 2010, 48, 648–658. [Google Scholar] [CrossRef]
- Wang, W.; Gong, Q.; Chen, Z.; Wang, W.D.; Huang, Q.; Song, S.; Chen, J.; Wang, X. Adsorption and competition investigation of phenolic compounds on the solid-liquid interface of three-dimensional foam-like graphene oxide. Chem. Eng. J. 2019, 378, 122085. [Google Scholar] [CrossRef]
- Smidt, E.; Meissl, K. The applicability of Fourier transform infrared (FT-IR) spectroscopy in waste management. Waste Manag. 2007, 27, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Tatzber, M.; Stemmer, M.; Spiegel, H.; Katzlberger, C.; Haberhauer, G.; Mentler, A.; Gerzabek, M.H. FTIR-spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na 2CO3 extraction procedures. J. Plant Nutr. Soil Sci. 2007, 170, 522–529. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Huang, J.; Gao, B.; Zhao, L.; Qiu, H.; Cao, X. Sorption of reactive red by biochars ball milled in different atmospheres: Co-effect of surface morphology and functional groups. Chem. Eng. J. 2021, 413, 127468. [Google Scholar] [CrossRef]
- Zhao, S.X.; Ta, N.; Wang, X.D. Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Naderi, H.R.; Norouzi, P.; Ganjali, M.R. Electrochemical study of a novel high performance supercapacitor based on MnO2/nitrogen-doped graphene nanocomposite. Appl. Surf. Sci. 2016, 366, 552–560. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Khan, I.; Kang, S.-M.; Haldorai, Y.; Tripathi, K.M.; Jung, S.; Chen, L.; Kim, T.; Huh, Y.S. A sustainable graphene aerogel capable of the adsorptive elimination of biogenic amines and bacteria from soy sauce and highly efficient cell proliferation. ACS Appl. Mater. Interfaces 2019, 11, 43949–43963. [Google Scholar] [CrossRef]
- Xiang, W.; Wan, Y.; Zhang, X.; Tan, Z.; Xia, T.; Zheng, Y.; Gao, B. Adsorption of tetracycline hydrochloride onto ball-milled biochar: Governing factors and mechanisms. Chemosphere 2020, 255, 127057. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Li, M.; Huang, H.; Xu, H.; Hong, R.; Shen, H. Multifunctional TiO2 nanowires-modified nanoparticles bilayer film for 3D dye-sensitized solar cells. Optoelectron. Adv. Mater. Commun. 2010, 4, 1166–1169. [Google Scholar]
- Fan, Z.; Zhang, Q.; Gao, B.; Li, M.; Liu, C.; Qiu, Y. Removal of hexavalent chromium by biochar supported nZVI composite: Batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention. Chemosphere 2019, 217, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Bokova, S.N.; Obraztsova, E.D.; Grebenyukov, V.V.; Elumeeva, K.V.; Ishchenko, A.V.; Kuznetsov, V.L. Raman diagnostics of multi-wall carbon nanotubes with a small wall number. Phys. Status Solidi Basic Res. 2010, 247, 2827–2830. [Google Scholar] [CrossRef]
- Xing, T.; Li, L.H.; Hou, L.; Hu, X.; Zhou, S.; Peter, R.; Petravic, M.; Chen, Y. Disorder in ball-milled graphite revealed by Raman spectroscopy. Carbon 2013, 57, 515–519. [Google Scholar] [CrossRef]
- El-Khaiary, M.I. Least-squares regression of adsorption equilibrium data: Comparing the options. J. Hazard. Mater. 2008, 158, 73–87. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Ayawei, N.; Angaye, S.S.; Wankasi, D.; Dikio, E.D. Synthesis, Characterization and Application of Mg/Al Layered Double Hydroxide for the Degradation of Congo Red in Aqueous Solution. Open J. Phys. Chem. 2015, 5, 56–70. [Google Scholar] [CrossRef]
- Ayawei, N.; Ekubo, A.T.; Wankasi, D.; Dikio, E.D. Adsorption of congo red by Ni/Al-CO3: Equilibrium, thermodynamic and kinetic studies. Orient. J. Chem. 2015, 31, 1307. [Google Scholar] [CrossRef]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Tong, S.; Chen, N.; Wang, H.; Liu, H.; Tao, C.; Feng, C.; Zhang, B.; Hao, C.; Pu, J.; Zhao, J. Optimization of C/N and current density in a heterotrophic/biofilm-electrode autotrophic denitrification reactor (HAD-BER). Bioresour. Technol. 2014, 171, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, F.; Maleki-Kaklar, M.; Soiltanalinejad, N.; Shabani, F. Central composite design optimization of zinc removal from contaminated soil, using citric acid as biodegradable chelant. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Lima, E.C.; Royer, B.; Vaghetti, J.C.P.; Brasil, J.L.; Simon, N.M.; dos Santos, A.A., Jr.; Pavan, F.A.; Dias, S.L.P.; Benvenutti, E.V.; da Silva, E.A. Adsorption of Cu (II) on Araucaria angustifolia wastes: Determination of the optimal conditions by statistic design of experiments. J. Hazard. Mater. 2007, 140, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Safa, Y.; Bhatti, H.N.; Bhatti, I.A.; Asgher, M. Removal of direct Red-31 and direct Orange-26 by low cost rice husk: Influence of immobilisation and pretreatments. Can. J. Chem. Eng. 2011, 89, 1554–1565. [Google Scholar] [CrossRef]
- El-Sesy, M.E.; Ibrahim, S.S. Application of central composite design approach for optimization nitrate removal from aqueous solution by immobilized Pseudomonas putida. Water Sci. Technol. 2021, 83, 2931–2946. [Google Scholar] [CrossRef]
- Carley, K.M.; Kamneva, N.Y.; Reminga, J. Response Surface Methodology; Carnegie-Mellon University: Pittsburgh, PA, USA, 2004. [Google Scholar]
- Moradi, M.; Fazlzadehdavil, M.; Pirsaheb, M.; Mansouri, Y.; Khosravi, T.; Sharafi, K. Response surface methodology (RSM) and its application for optimization of ammonium ions removal from aqueous solutions by pumice as a natural and low cost adsorbent. Arch. Environ. Prot. 2016, 42, 33–43. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Oturan, N.; Wang, Y.; Chen, L.; Oturan, M.A. Application of response surface methodology to the removal of the antibiotic tetracycline by electrochemical process using carbon-felt cathode and DSA (Ti/RuO2–IrO2) anode. Chemosphere 2012, 87, 614–620. [Google Scholar] [CrossRef]
- Jafari, A.; Mahvi, A.H.; Godini, H.; Rezaee, R.; Hosseini, S.S. Process optimization for fluoride removal from water by Moringa oleifera seed extract. Fluoride 2014, 47, 152–160. [Google Scholar]










| Adsorbent (Pristine and Ball-Milled Biochar) | Surface Area SBET (m2/g) | BJH Pore Volume (cm3/g) | BJH Pore Size (nm) | Grain Size of Pristine and Ball-Milled Biochar (nm) |
|---|---|---|---|---|
| B400 | 53.1 | 0.003 | 4.7 | 112.9 |
| B500 | 220.1 | 0.021 | 3.5 | 27.3 |
| B600 | 278.6 | 0.074 | 5.6 | 21.5 |
| BMB400 | 275.9 | 0.075 | 5.0 | 21.7 |
| BMB500 | 354.6 | 0.097 | 5.6 | 16.9 |
| BMB600 | 311.6 | 0.088 | 3.7 | 19.3 |
| Analyte | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| qmax (mg g−1) | b (L mg−1) | RL | R2 | Kf (L mg−1) | 1/n | R2 | |
| Estriol | 14.53 | 0.2464 | 0.4742 | 0.9840 | 7.70 | 0.4547 | 0.9941 |
| α-Oestradiol | 10.58 | 0.2743 | 0.4475 | 0.9759 | 5.32 | 0.3585 | 0.9843 |
| β-Oestradiol | 12.50 | 0.3060 | 0.4207 | 0.9924 | 7.79 | 0.5014 | 0.9754 |
| Testosterone | 5.73 | 0.6955 | 0.2421 | 0.9238 | 5.51 | 0.5169 | 0.9919 |
| Progesterone | 5.63 | 0.6199 | 0.2639 | 0.9724 | 5.11 | 0.5108 | 0.9998 |
| Bisphenol A | 9.75 | 0.3400 | 0.3953 | 0.9929 | 6.56 | 0.5206 | 0.9546 |
| Nickel | 8.58 | 0.2957 | 0.2483 | 0.9888 | 4.12 | 0.5263 | 0.9768 |
| Cadmium | 4.15 | 0.7615 | 0.2589 | 0.9974 | 5.75 | 0.6289 | 0.7845 |
| Lead | 6.95 | 0.7542 | 0.2568 | 0.9884 | 3.54 | 0.7142 | 0.8692 |
| Factor | Unit | Code | Coded Actual Levels | ||||
|---|---|---|---|---|---|---|---|
| Lowest | Low Level | Mid Level | High Level | Highest | |||
| −α | (−1) | 0 | (+1) | +α | |||
| pH | - | A | 2 | 5 | 8 | 11 | 14 |
| Concentration | ppm | B | 0.75 | 1.5 | 3 | 3.75 | 4.5 |
| Time | min | C | 7.5 | 15 | 22.5 | 30 | 37.5 |
| Temperature | °C | D | 10 | 20 | 30 | 40 | 50 |
| Std Order | Run Order | Pt Type | pH | Conc | Time | Temp | Estriol | Alpha-Oestradiol | Beta-Oestradiol | Testosterone | Progesterone | Bisphenol A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 1 | 1 | 11 | 1.50 | 30.0 | 20 | 90 | 90 | 91 | 95 | 92 | 98 |
| 17 | 2 | −1 | 2 | 2.25 | 22.5 | 30 | 89 | 85 | 90 | 94 | 91 | 96 |
| 16 | 3 | 1 | 11 | 3.00 | 30.0 | 40 | 50 | 52 | 55 | 58 | 54 | 54 |
| 2 | 4 | 1 | 11 | 1.50 | 15.0 | 20 | 47 | 40 | 44 | 38 | 35 | 37 |
| 30 | 5 | 0 | 8 | 2.25 | 22.5 | 30 | 88 | 87 | 90 | 92 | 93 | 90 |
| 31 | 6 | 0 | 8 | 2.25 | 22.5 | 30 | 79 | 77 | 81 | 85 | 80 | 83 |
| 26 | 7 | 0 | 8 | 2.25 | 22.5 | 30 | 81 | 82 | 85 | 83 | 87 | 88 |
| 11 | 8 | 1 | 5 | 3.00 | 15.0 | 40 | 52 | 55 | 56 | 56 | 60 | 51 |
| 22 | 9 | −1 | 8 | 2.25 | 37.5 | 30 | 86 | 85 | 82 | 86 | 79 | 86 |
| 14 | 10 | 1 | 11 | 1.50 | 30.0 | 40 | 80 | 78 | 79 | 72 | 75 | 84 |
| 8 | 11 | 1 | 11 | 3.00 | 30.0 | 20 | 78 | 79 | 81 | 85 | 80 | 81 |
| 24 | 12 | −1 | 8 | 2.25 | 22.5 | 50 | 93 | 95 | 91 | 92 | 89 | 98 |
| 7 | 13 | 1 | 5 | 3.00 | 30.0 | 20 | 70 | 72 | 75 | 78 | 71 | 73 |
| 20 | 14 | −1 | 8 | 3.75 | 22.5 | 30 | 84 | 82 | 87 | 82 | 84 | 87 |
| 18 | 15 | −1 | 14 | 2.25 | 22.5 | 30 | 82 | 77 | 85 | 85 | 85 | 85 |
| 5 | 16 | 1 | 5 | 1.50 | 30.0 | 20 | 81 | 86 | 84 | 79 | 81 | 84 |
| 10 | 17 | 1 | 11 | 1.50 | 15.0 | 40 | 65 | 70 | 69 | 75 | 72 | 69 |
| 27 | 18 | 0 | 8 | 2.25 | 22.5 | 30 | 69 | 75 | 73 | 75 | 73 | 73 |
| 21 | 19 | −1 | 8 | 2.25 | 7.5 | 30 | 26 | 32 | 30 | 33 | 38 | 30 |
| 12 | 20 | 1 | 11 | 3.00 | 15.0 | 40 | 60 | 63 | 61 | 68 | 54 | 61 |
| 19 | 21 | −1 | 8 | 0.75 | 22.5 | 30 | 93 | 94 | 95 | 88 | 84 | 95 |
| 28 | 22 | 0 | 8 | 2.25 | 22.5 | 30 | 65 | 63 | 68 | 70 | 73 | 68 |
| 29 | 23 | 0 | 8 | 2.25 | 22.5 | 30 | 64 | 62 | 66 | 81 | 79 | 66 |
| 15 | 24 | 1 | 5 | 3.00 | 30.0 | 40 | 39 | 42 | 40 | 41 | 45 | 40 |
| 9 | 25 | 1 | 5 | 1.50 | 15.0 | 40 | 80 | 85 | 83 | 83 | 83 | 83 |
| 23 | 26 | −1 | 8 | 2.25 | 22.5 | 10 | 85 | 87 | 80 | 79 | 85 | 89 |
| 1 | 27 | 1 | 5 | 1.50 | 15.0 | 20 | 80 | 82 | 79 | 87 | 83 | 80 |
| 3 | 28 | 1 | 5 | 3.00 | 15.0 | 20 | 65 | 67 | 68 | 70 | 75 | 68 |
| 13 | 29 | 1 | 5 | 1.50 | 30.0 | 40 | 83 | 81 | 87 | 90 | 83 | 86 |
| 4 | 30 | 1 | 11 | 3.00 | 15.0 | 20 | 22 | 20 | 25 | 22 | 20 | 23 |
| 25 | 31 | 0 | 8 | 2.25 | 22.5 | 30 | 75 | 67 | 75 | 80 | 78 | 77 |
| Std Order | Run Order | Pt Type | pH | Concentration | Time | Temperature | Ni | Cd | Pb |
|---|---|---|---|---|---|---|---|---|---|
| 5 | 1 | 1 | 2.5 | 0.5 | 150.0 | 15.0 | 88 | 93 | 94 |
| 6 | 2 | 1 | 12.5 | 0.5 | 150.0 | 15.0 | 89 | 92 | 93 |
| 17 | 3 | −1 | −2.5 | 1.5 | 82.5 | 42.5 | 53 | 56 | 57 |
| 13 | 4 | 1 | 2.5 | 0.5 | 150.0 | 70.0 | 42 | 36 | 38 |
| 22 | 5 | −1 | 7.5 | 1.5 | 217.5 | 42.5 | 89 | 90 | 95 |
| 25 | 6 | 0 | 7.5 | 1.5 | 82.5 | 42.5 | 78 | 83 | 82 |
| 7 | 7 | 1 | 2.5 | 2.5 | 150.0 | 15.0 | 83 | 81 | 89 |
| 3 | 8 | 1 | 2.5 | 2.5 | 15.0 | 15.0 | 55 | 54 | 62 |
| 23 | 9 | −1 | 7.5 | 1.5 | 82.5 | 12.5 | 80 | 84 | 81 |
| 18 | 10 | −1 | 17.5 | 1.5 | 82.5 | 42.5 | 75 | 70 | 77 |
| 19 | 11 | −1 | 7.5 | 0.5 | 82.5 | 42.5 | 80 | 83 | 82 |
| 2 | 12 | 1 | 12.5 | 0.5 | 15.0 | 15.0 | 89 | 90 | 91 |
| 31 | 13 | 0 | 7.5 | 1.5 | 82.5 | 42.5 | 73 | 76 | 73 |
| 8 | 14 | 1 | 12.5 | 2.5 | 150.0 | 15.0 | 85 | 80 | 86 |
| 27 | 15 | 0 | 7.5 | 1.5 | 82.5 | 42.5 | 83 | 83 | 87 |
| 21 | 16 | −1 | 7.5 | 1.5 | 52.5 | 42.5 | 82 | 77 | 83 |
| 24 | 17 | −1 | 7.5 | 1.5 | 82.5 | 97.5 | 65 | 75 | 75 |
| 9 | 18 | 1 | 2.5 | 0.5 | 15.0 | 70.0 | 70 | 72 | 75 |
| 15 | 19 | 1 | 2.5 | 2.5 | 150.0 | 70.0 | 28 | 31 | 40 |
| 10 | 20 | 1 | 12.5 | 0.5 | 15.0 | 70.0 | 60 | 66 | 56 |
| 1 | 21 | 1 | 2.5 | 0.5 | 15.0 | 15.0 | 91 | 86 | 86 |
| 4 | 22 | 1 | 12.5 | 2.5 | 15.0 | 15.0 | 67 | 78 | 75 |
| 16 | 23 | 1 | 12.5 | 2.5 | 150.0 | 70.0 | 62 | 79 | 81 |
| 26 | 24 | 0 | 7.5 | 1.5 | 82.5 | 42.5 | 37 | 39 | 47 |
| 30 | 25 | 0 | 7.5 | 1.5 | 82.5 | 42.5 | 80 | 81 | 85 |
| 12 | 26 | 1 | 12.5 | 2.5 | 15.0 | 70.0 | 78 | 77 | 87 |
| 11 | 27 | 1 | 2.5 | 2.5 | 15.0 | 70.0 | 74 | 85 | 87 |
| 29 | 28 | 0 | 7.5 | 1.5 | 82.5 | 42.5 | 63 | 68 | 77 |
| 28 | 29 | 0 | 7.5 | 1.5 | 82.5 | 42.5 | 82 | 88 | 85 |
| 20 | 30 | −1 | 7.5 | 3.5 | 82.5 | 42.5 | 74 | 20 | 25 |
| 14 | 31 | 1 | 12.5 | 0.5 | 150.0 | 70.0 | 73 | 78 | 79 |
| Analyte Source | Estriol (Values) | α-Oestradiol (Values) | β-Oestradiol (Values) | Testosterone (Values) | Progesterone (Values) | Bisphenol A (Values) | Nickel (Values) | Cadmium (Values) | Lead (Values) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| Model | 12.5 | 0.037 | 13.07 | 0.017 | 13.1 | 0.15 | 12.74 | 0.28 | 13.06 | 0.017 | 12.96 | 0.020 | 14.1 | 0.009 | 14.2 | 0.014 | 13.8 | 0.006 |
| Linear | 4.58 | 0.012 | 5.11 | 0.008 | 5.03 | 0.008 | 4.00 | 0.020 | 4.14 | 0.017 | 4.82 | 0.009 | 5.4 | 0.017 | 6.4 | 0.039 | 5.8 | 0.016 |
| pH | 1.07 | 0.317 | 2.12 | 0.164 | 1.50 | 0.239 | 1.72 | 0.208 | 3.08 | 0.098 | 1.26 | 0.277 | 0.33 | 0.015 | 0.30 | 0.059 | 0.29 | 0.005 |
| Conc | 7.28 | 0.016 | 8.31 | 0.011 | 7.38 | 0.015 | 5.09 | 0.038 | 5.26 | 0.036 | 6.83 | 0.019 | 1.33 | 0.002 | 1.60 | 0.224 | 1.49 | 0.024 |
| Time | 9.97 | 0.006 | 10.00 | 0.006 | 11.24 | 0.004 | 9.13 | 0.008 | 8.20 | 0.011 | 11.38 | 0.004 | 0.50 | 0.489 | 0.60 | 0.449 | 0.52 | 0.048 |
| Temp | 0.01 | 0.910 | 0.01 | 0.927 | 0.01 | 0.938 | 0.5 | 0.828 | 0.00 | 0.963 | 0.00 | 0.978 | 0.00 | 0.982 | 0.07 | 0.788 | 0.02 | 0.081 |
| Square | 2.01 | 0.142 | 1.97 | 0.157 | 2.61 | 0,075 | 1.99 | 0.145 | 2.53 | 0.081 | 2.22 | 0.113 | 0.48 | 0.007 | 0.37 | 0.824 | 0.37 | 0.825 |
| pH*pH | 0.20 | 0.903 | 0.1 | 0.911 | 0.05 | 0.823 | 0.01 | 0.917 | 0.01 | 0.914 | 0.03 | 0.870 | 0.39 | 0.014 | 0.56 | 0.463 | 0.67 | 0.004 |
| Conc*Conc | 0.16 | 0.690 | 0.36 | 0.559 | 0.35 | 0.562 | 0.11 | 0.747 | 0.27 | 0.608 | 0.05 | 0.870 | 0.45 | 0.510 | 0.46 | 0.506 | 0.41 | 0.533 |
| Time*Time | 7.01 | 0.018 | 5.75 | 0.029 | 9.31 | 0.008 | 7.78 | 0.013 | 10.02 | 0.006 | 7.98 | 0.012 | 1.35 | 0.262 | 0.76 | 0.398 | 0.67 | 0.425 |
| Temp*Temp | 0.21 | 0.657 | 0.81 | 0.381 | 0.00 | 0.985 | 0.08 | 0.783 | 0.05 | 0.834 | 0.20 | 0.664 | 0.00 | 0.019 | 0.00 | 0.946 | 0.00 | 0.998 |
| 2-Way Inter | 1.57 | 0.219 | 2.48 | 0.068 | 2.24 | 0.092 | 2.40 | 0.076 | 2.69 | 0.053 | 2.18 | 0.100 | 0.29 | 0.935 | 0.30 | 0.930 | 0.26 | 0.948 |
| pH*Conc | 0.21 | 0.654 | 0.42 | 0.528 | 0.41 | 0.530 | 0.72 | 0.409 | 0.06 | 0.804 | 0.30 | 0.589 | 1.10 | 0.010 | 1.15 | 0.002 | 1.07 | 0.317 |
| pH*Time | 3.60 | 0.076 | 4.68 | 0.046 | 4.34 | 0.054 | 4.31 | 0.054 | 7.46 | 0.015 | 4.70 | 0.046 | 0.22 | 0.016 | 0.14 | 0.716 | 0.12 | 0.731 |
| pH*Temp | 1.11 | 0.307 | 2.19 | 0.158 | 1.50 | 0.238 | 1.93 | 0.184 | 1.69 | 0.213 | 1.62 | 0.221 | 0.23 | 0.035 | 0.24 | 0.629 | 0.20 | 0.065 |
| Conc*Time | 0.18 | 0.679 | 0.12 | 0.737 | 0.24 | 0.633 | 0.02 | 0.901 | 0.11 | 0.746 | 0.43 | 0.522 | 0.00 | 0.979 | 0.00 | 0.945 | 0.01 | 0.040 |
| Conc*Temp | 0.60 | 0.451 | 0.64 | 0.437 | 1.23 | 0.284 | 0.92 | 0.353 | 1.14 | 0.302 | 1.14 | 0.302 | 0.07 | 0.795 | 0.07 | 0.795 | 0.05 | 0.834 |
| Time*Temp | 3.74 | 0.071 | 6.86 | 0.021 | 5.73 | 0.029 | 6.48 | 0.022 | 5.68 | 0.03 | 4.85 | 0.043 | 0.10 | 0.754 | 0.16 | 0.691 | 0.11 | 0.743 |
| Lack of Fit | 3.48 | 0.070 | 6.86 | 0.019 | 5.73 | 0.029 | 6.48 | 0.022 | 5.68 | 0.030 | 4.85 | 0.043 | 0.88 | 0.059 | 1.07 | 0.049 | 0.94 | 0.056 |
| Pure Error | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 16 | 16 | 16 | 16 | 16 | 16 |
| Analyte | Ultrapure Water (% Removal) | Effluent (% Removal) | Influent (% Removal) |
|---|---|---|---|
| Estriol | 93 | 87 | 85 |
| α-Oestradiol | 90 | 86 | 83 |
| β-Oestradiol | 91 | 88 | 86 |
| Testosterone | 92 | 84 | 80 |
| Progesterone | 95 | 89 | 82 |
| Bisphenol A | 94 | 86 | 78 |
| Nickel | 91 | 83 | 75 |
| Cadmium | 90 | 87 | 83 |
| Lead | 93 | 85 | 84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amusat, S.O.; Kebede, T.G.; Nxumalo, E.N.; Dube, S.; Nindi, M.M. Green Synthesis of Surface Modified Biochar for Simultaneous Removal of Steroidal Hormones and Heavy Metals from Wastewater: Optimisation by Central Composite Design. Water 2023, 15, 3703. https://doi.org/10.3390/w15203703
Amusat SO, Kebede TG, Nxumalo EN, Dube S, Nindi MM. Green Synthesis of Surface Modified Biochar for Simultaneous Removal of Steroidal Hormones and Heavy Metals from Wastewater: Optimisation by Central Composite Design. Water. 2023; 15(20):3703. https://doi.org/10.3390/w15203703
Chicago/Turabian StyleAmusat, Sefiu Olaitan, Temesgen Girma Kebede, Edward Ndumiso Nxumalo, Simiso Dube, and Mathew Muzi Nindi. 2023. "Green Synthesis of Surface Modified Biochar for Simultaneous Removal of Steroidal Hormones and Heavy Metals from Wastewater: Optimisation by Central Composite Design" Water 15, no. 20: 3703. https://doi.org/10.3390/w15203703





