Visible Light-Driven Photocatalytic Degradation of Tetracycline Using p-n Heterostructured Cr2O3/ZrO2 Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Cr2O3 Nano-Cubes
2.2. Preparation of Cr2O3-ZrO2 Nanocomposite
2.3. Instrumentations
2.4. Photocatalytic Methodology
3. Results and Discussion
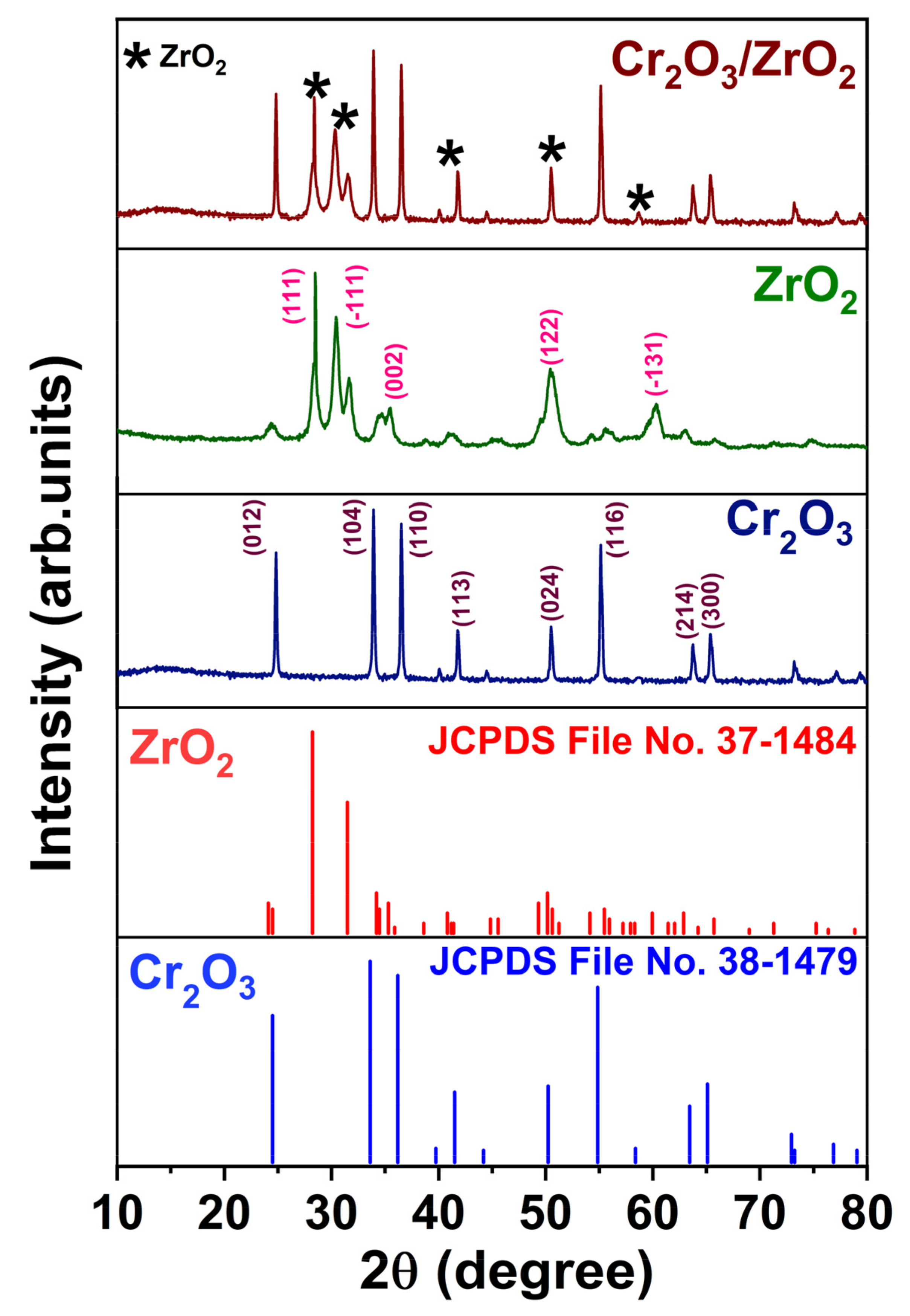
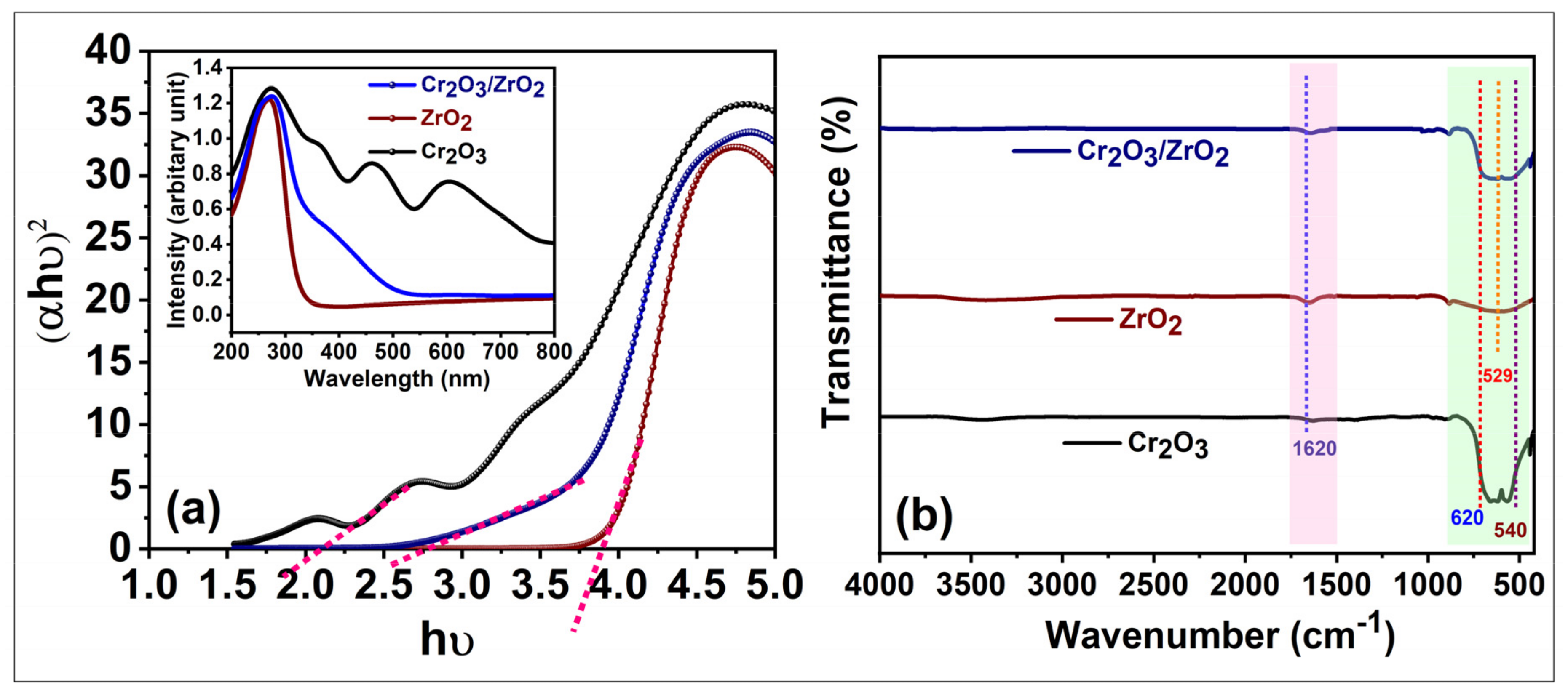
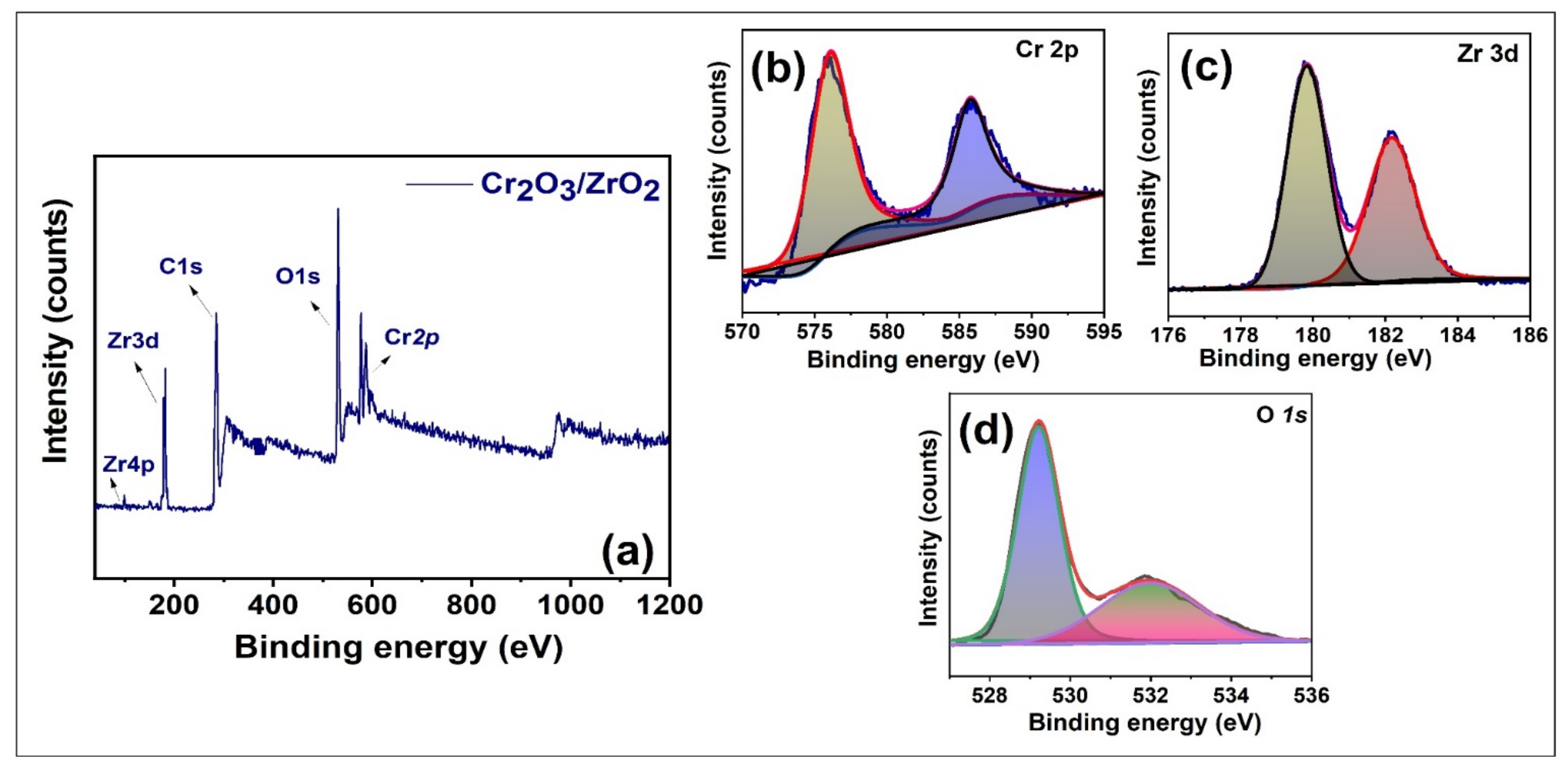
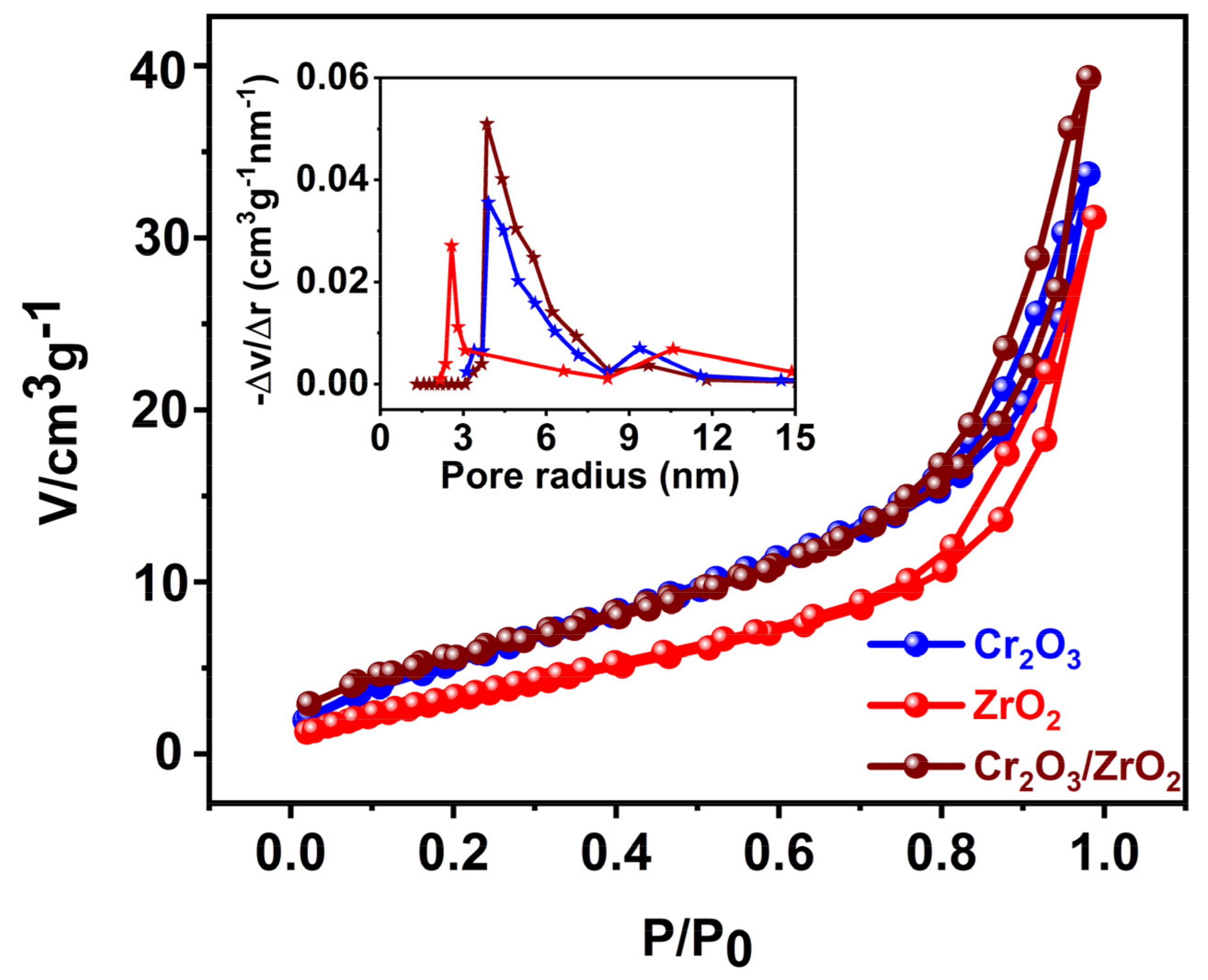
3.1. Characterization
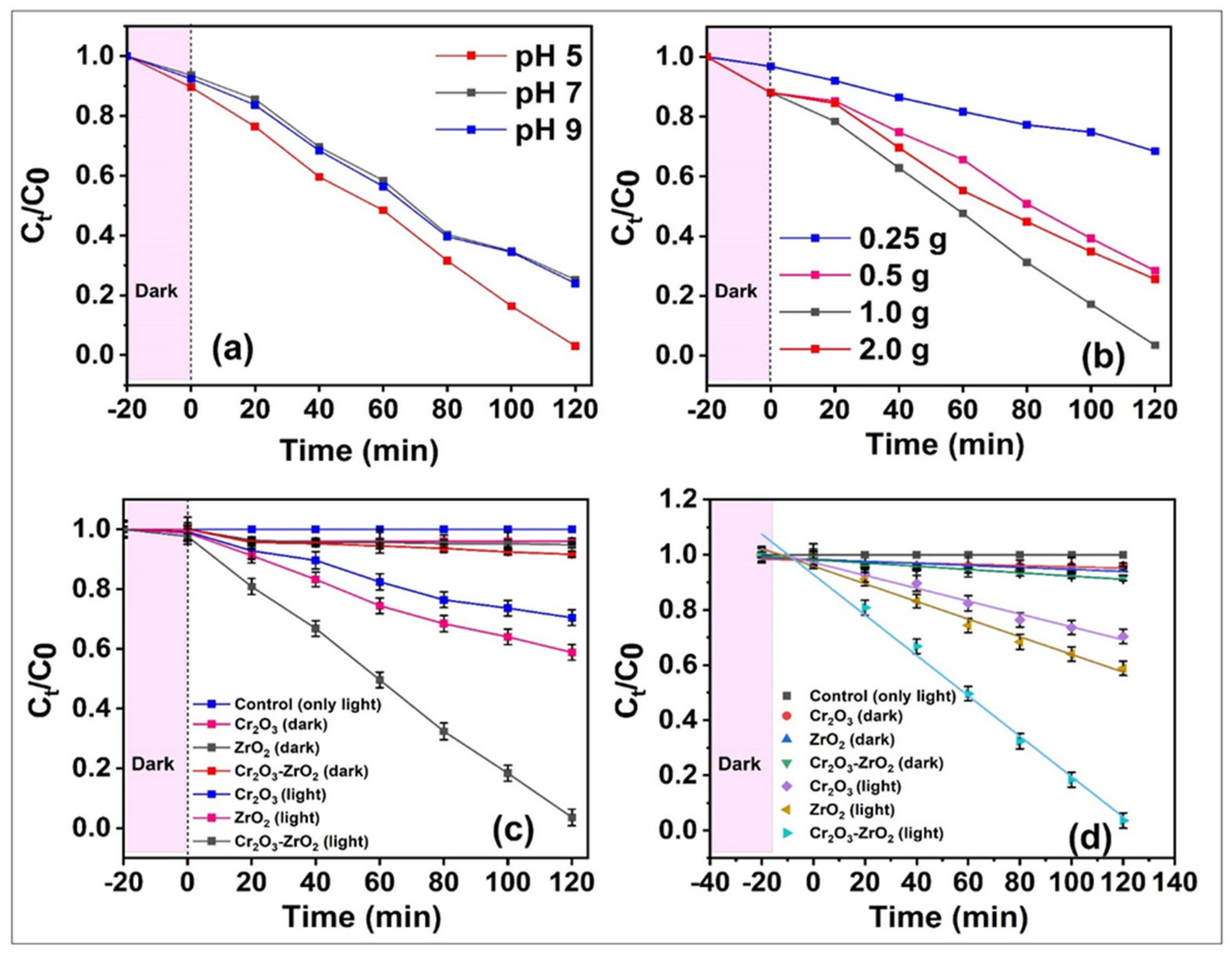
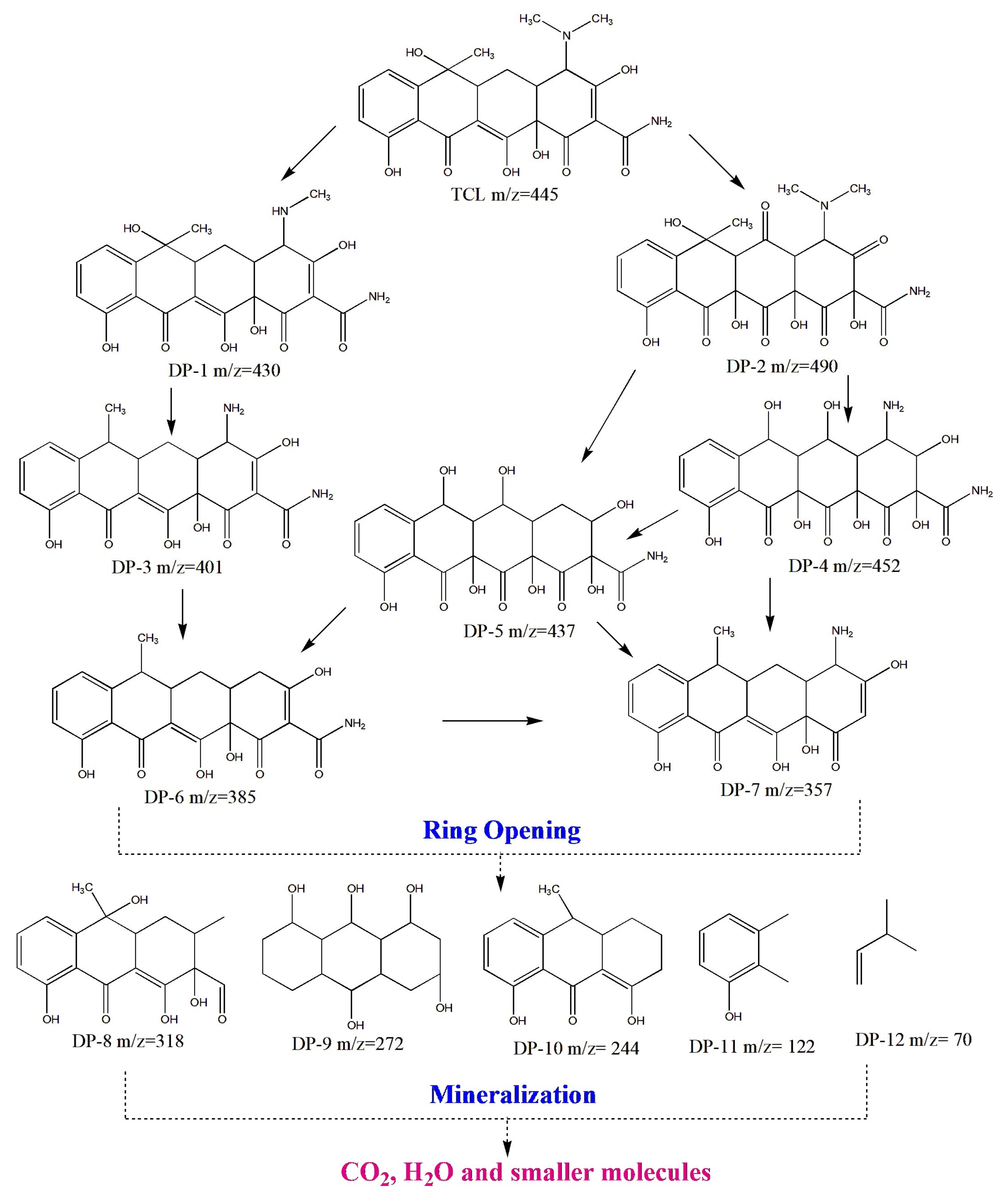
3.2. Catalytic Degradation of TCL and Pathway of the Cr2O3-ZrO2 Composite
3.3. Photocatalysis Degradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, Y.; Xu, D.; Zhang, J.; Wang, Q.; Pang, S.; Zhang, G.; Campos, L.C.; Lv, L.; Liu, X.; Gao, W.; et al. Enhanced antibiotic wastewater degradation by intimately coupled B-Bi3O4Cl photocatalysis and biodegradation reactor: Elucidating degradation principle systematically. J. Hazard. Mater. 2023, 445, 130364. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hu, X.; Li, M.; Fang, J.; Leng, C.; Zhu, X.; Xu, W.; Qin, J.; Yao, L.; Liu, Z.; et al. Constructing mesoporous Zr-doped SiO2 onto efficient Z-scheme TiO2/g-C3N4 heterojunction for antibiotic degradation via adsorption-photocatalysis and mechanism insight. Environ. Res. 2022, 214, 114189. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Zhu, M.; Mei, Q.; Tang, X.; Wu, Y.; Yue, S.; Tang, Y.; Wang, Q. Constructing aloe-emodin/FeOOH organic-inorganic heterojunction for synergetic photocatalysis-Fenton eliminating antibiotic pollutants. J. Environ. Chem. Eng. 2023, 11, 109775. [Google Scholar] [CrossRef]
- Sharma, M.; Mandal, M.K.; Pandey, S.; Kumar, R.; Dubey, K.K. Visible-Light-Driven Photocatalytic Degradation of Tetracycline Using Heterostructured Cu2O-TiO2 Nanotubes, Kinetics, and Toxicity Evaluation of Degraded Products on Cell Lines. ACS Omega 2022, 7, 33572–33586. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hu, Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Li, C.; Tian, Q.; Zhang, Y.; Li, Y.; Yang, X.; Zheng, H.; Chen, L.; Li, F. Sequential combination of photocatalysis and microalgae technology for promoting the degradation and detoxification of typical antibiotics. Water Res. 2022, 210, 117985. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Zhang, J.; Zhang, C.; Li, N.; Zhao, T.; Chen, F.; Hu, L.; Zhang, S.; Wang, Z. Vertically Aligned ZnO@ZnS Nanorod Chip with Improved Photocatalytic Activity for Antibiotics Degradation. ACS Appl. Nano Mater. 2018, 1, 793–799. [Google Scholar] [CrossRef]
- Naraginti, S.; Yu, Y.Y.; Fang, Z.; Yong, Y.C. Novel tetrahedral Ag3PO4@N-rGO for photocatalytic detoxification of sulfamethoxazole: Process optimization, transformation pathways and biotoxicity assessment. Chem. Eng. J. 2019, 375, 122035. [Google Scholar] [CrossRef]
- Tan, R.; Wang, Y.; Jin, Z.; Zhang, P.; Luo, H.; Liu, D.; Mamba, B.B.; Kuvarega, A.T.; Gui, J. Preparation of carbon-coated brookite@anatase TiO2 heterophase junction nanocables with enhanced photocatalytic performance. Photochem. Photobiol. Sci. 2020, 19, 966–975. [Google Scholar] [CrossRef]
- Sompalli, N.K.; Mohanty, A.; Mohan, A.M.; Deivasigamani, P. Heterojunction Cr2O3-Ag2O nanocomposite decorated porous polymer monoliths a new class of visible light fast responsive heterogeneous photocatalysts for pollutant clean-up. J. Environ. Chem. Eng. 2021, 9, 104846. [Google Scholar] [CrossRef]
- Sompalli, N.K.; Das, A.; De, S.S.; Mohan, A.M.; Deivasigamani, P. Mesoporous monolith designs of mixed phased titania codoped Sm3+/Er3+ composites: A super responsive visible light photocatalysts for organic pollutant clean-up. Appl. Surf. Sci. 2020, 504, 144350. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Wen, Y.; Feng, M.; Zhang, P.; Zhou, H.-C.; Sharma, V.K.; Ma, X. Metal Organic Frameworks (MOFs) as Photocatalysts for the Degradation of Agricultural Pollutants in Water. ACS ES&T Eng. 2021, 1, 804–826. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Wang, Y.; Tang, N.; He, R. Semiconductor Photocatalysis for Water Purification; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Elaheh, K.; Naimeh, S. The Application of Photocatalytic Materials for Efficient Air Purification; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Olatunji, O.S. Photocatalytic degradation of tetracycline in aqueous systems under visible light irridiation using needle-like SnO2 nanoparticles anchored on exfoliated g-C3N4. Environ. Sci. Eur. 2022, 34, 5. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, X.; Han, W.; Han, Y.; Guo, M.; Peng, Z.; Fan, Z.; Shi, Y.; Wan, S. Tetracycline Removal by Hercynite-Biochar from the Co-Pyrolysis of Red Mud-Steel Slag-Sludge. Nanomaterials 2022, 12, 2595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Hong, P.; Shi, X.; Li, Y.; Yao, K.; Zhang, W.; Wang, C.; He, J.; Zhang, K.; Kong, L. Efficient degradation of tetracycline by a novel nanoconfinement structure Cu2O/Cu@MXene composite. J. Hazard. Mater. 2023, 448, 130995. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Liu, G.; Lang, D.N.; Chen, S.F.; Yang, C.; Wu, R.L.; Wang, W.; Wang, J.D. Fast photodegradation of antibiotics and dyes by an anionic surfactant-aided CdS/ZnO nanodispersion. New J. Chem. 2022, 46, 11303–11314. [Google Scholar] [CrossRef]
- He, Y.; Wang, D.; Li, X.; Fu, Q.; Yin, L.; Yang, Q.; Chen, H. Photocatalytic degradation of tetracycline by metal-organic frameworks modified with Bi2WO6 nanosheet under direct sunlight. Chemosphere 2021, 284, 131386. [Google Scholar] [CrossRef]
- He, X.; Kai, T.; Ding, P. Heterojunction Photocatalysts for Degradation of the Tetracycline Antibiotic: A Review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef]
- Singh, K.K.; Senapati, K.K.; Borgohain, C.; Sarma, K.C. Newly developed Fe3O4–Cr2O3 magnetic nanocomposite for photocatalytic decomposition of 4-chlorophenol in water. J. Environ. Sci. 2017, 52, 333–340. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Abou-Gamra, Z.M.; Salem, A.M. Photocatalytic degradation of methylene blue dye over novel spherical mesoporous Cr2O3/TiO2 nanoparticles prepared by sol-gel using octadecylamine template. J. Environ. Chem. Eng. 2017, 5, 4251–4261. [Google Scholar] [CrossRef]
- Mohanapandian, K.; Krishnan, A. Synthesis, structural, morphological and optical properties of Cu2+ doped Cr2O3 nanoparticles. Int. J. Adv. Eng. Technol. E 2016, 7, 273–279. Available online: http://www.technicaljournalsonline.com/ijeat/VOL%20VII/IJAET%20VOL%20VII%20ISSUE%20II%20APRIL%20JUNE%202016/20167250.pdf (accessed on 16 October 2023).
- Rani, V.; Sharma, A.; Kumar, A.; Singh, P.; Thakur, S.; Singh, A.; Van Le, Q.; Nguyen, V.H.; Raizada, P. ZrO2-Based Photocatalysts for Wastewater Treatment: From Novel Modification Strategies to Mechanistic Insights. Catalysts 2022, 12, 1418. [Google Scholar] [CrossRef]
- Agorku, E.S.; Kuvarega, A.T.; Mamba, B.B.; Pandey, A.C.; Mishra, A.K. Enhanced visible-light photocatalytic activity of multi-elements-doped ZrO2 for degradation of indigo carmine. J. Rare Earths 2015, 33, 498–506. [Google Scholar] [CrossRef]
- Kadam, A.; Dhabbe, R.; Gophane, A.; Sathe, T.; Garadkar, K. Template free synthesis of ZnO/Ag2O nanocomposites as a highly efficient visible active photocatalyst for detoxification of methyl orange. J. Photochem. Photobiol. B Biol. 2016, 154, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Aldeen, E.M.S.; Jalil, A.A.; Mim, R.S.; Alhebshi, A.; Hassan, N.S.; Saravanan, R. Altered zirconium dioxide based photocatalyst for enhancement of organic pollutants degradation: A review. Chemosphere 2022, 304, 135349. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.N.; Yamini, Y.; Asiabi, H. Multiwall carbon nanotube-zirconium oxide nanocomposite hollow fiber solid phase microextraction for determination of polyaromatic hydrocarbons in water, coffee and tea samples. J. Chromatogr. A 2018, 1554, 8–15. [Google Scholar] [CrossRef]
- Ivanova, T.; Gesheva, K.; Cziraki, A.; Szekeres, A.; Vlaikova, E. Structural transformations and their relation to the optoelectronic properties of chromium oxide thin films. J. Phys. Conf. Ser. 2008, 113, 012030. [Google Scholar] [CrossRef]
- Ocaa, M. Nanosized Cr2O3 hydrate spherical particles prepared by the urea method. J. Eur. Ceram. Soc. 2001, 21, 931–939. [Google Scholar] [CrossRef]
- Jagadeesan, D.; Sompalli, N.K.; Mohan, A.M.; Rao, C.V.S.B.; Nagarajan, S.; Deivasigamani, P. ZrO2–Ag2O nanocomposites encrusted porous polymer monoliths as high-performance visible light photocatalysts for the fast degradation of pharmaceutical pollutants. Photochem. Photobiol. Sci. 2022, 21, 1273–1286. [Google Scholar] [CrossRef]
- Kadari, A.; Schemme, T.; Kadri, D.; Wollschläger, J. XPS and morphological properties of Cr2O3 thin films grown by thermal evaporation method. Results Phys. 2017, 7, 3124–3129. [Google Scholar] [CrossRef]
- Cao, Z.; Zuo, C. Cr2O3/carbon nanosheet composite with enhanced performance for lithium ion batteries. RSC Adv. 2017, 7, 40243–40248. [Google Scholar] [CrossRef]
- Liu, J.; Liao, M.; Imura, M.; Tanaka, A.; Iwai, H.; Koide, Y. Low on-resistance diamond field effect transistor with high-k ZrO2 as dielectric. Sci. Rep. 2014, 4, 2–6. [Google Scholar] [CrossRef]
- Luo, J.; Luo, X.; Hu, C.; Crittenden, J.C.; Qu, J. Zirconia (ZrO2) Embedded in Carbon Nanowires via Electrospinning for Efficient Arsenic Removal from Water Combined with DFT Studies. ACS Appl. Mater. Interfaces 2016, 8, 18912–18921. [Google Scholar] [CrossRef] [PubMed]
- Divakaran, K.; Baishnisha, A.; Balakumar, V.; Perumal, K.N.; Meenakshi, C.; Kannan, R.S. Photocatalytic degradation of tetracycline under visible light using TiO2@ sulfur doped carbon nitride nanocomposite synthesized via in-situ method. J. Environ. Chem. Eng. 2021, 9, 105560. [Google Scholar] [CrossRef]
- McGrady, J.; Yamashita, S.; Kano, S.; Yang, H.; Abe, H. Charge transfer across the Cr2O3, Fe2O3, and ZrO2 oxide/water interface: A pulse radiolysis study. Radiat. Phys. Chem. 2021, 180, 109240. [Google Scholar] [CrossRef]
- Safari, G.; Hoseini, M.; Seyedsalehi, M.; Kamani, H.; Jaafari, J.; Mahvi, A. Photocatalytic degradation of tetracycline using nanosized titanium dioxide in aqueous solution. Int. J. Environ. Sci. Technol. 2015, 12, 603–616. [Google Scholar] [CrossRef]
- Linh, N.X.D.; Hanh, N.T.; Cuong, L.M.; Huong, N.T.; Ha, N.T.T.; Trinh, T.D.; Van Noi, N.; Cam, N.T.D.; Pham, T.D. Facile Fabrication of α-Fe2O3/g-C3N4 Z-Scheme Heterojunction for Novel Degradation of Residual Tetracycline. Top. Catal. 2023, 66, 139–148. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Gao, X.; Ma, J.; Chen, Z.; Kang, B.; Liu, J.; Li, H.; Feng, Z.; Huang, J. Novel BP/BiOBr S-scheme nano-heterojunction for enhanced visible-light photocatalytic tetracycline removal and oxygen evolution activity. J. Hazard. Mater. 2020, 387, 121690. [Google Scholar] [CrossRef]
- Chen, L.; Xu, B.; Jin, M.; Chen, L.; Yi, G.; Xing, B.; Zhang, Y.; Wu, Y.; Li, Z. Excellent photocatalysis of Bi2WO6 structured with oxygen vacancies in degradation of tetracycline. J. Mol. Struct. 2023, 1278, 134911. [Google Scholar] [CrossRef]
- Pan, T.; Chen, D.; Xu, W.; Fang, J.; Wu, S.; Liu, Z.; Wu, K.; Fang, Z. Anionic polyacrylamide-assisted construction of thin 2D-2D WO3/g-C3N4 Step-scheme heterojunction for enhanced tetracycline degradation under visible light irradiation. J. Hazard. Mater. 2020, 393, 122366. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Chen, P.; Zheng, H.; Shi, J.; Shu, H.; Liu, Y. Photocatalytic degradation of tetracycline by using a regenerable (Bi)BiOBr/rGO composite. J. Clean. Prod. 2022, 339, 130771. [Google Scholar] [CrossRef]
- Phuong, D.M.; Duong, T.A.; Huong, N.T.; Khoa, N.V.; Hanh, N.T.; Phuong, N.M.; Pham, T.D.; Trang, H.T.; Van Noi, N. Enhancement of visible light photocatalytic removal of residual tetracycline by Ni doped WO3 nano structures. Inorg. Chem. Commun. 2023, 157, 111329. [Google Scholar] [CrossRef]
- Fu, Q.; Meng, Y.; Yao, Y.; Shen, H.; Xie, B.; Ni, Z.; Xia, S. Construction of facet orientation-supported Z-scheme heterojunction of BiVO4 (110)-Fe2O3 and its photocatalytic degradation of tetracycline. J. Environ. Chem. Eng. 2023, 11, 111060. [Google Scholar] [CrossRef]
- Siddhardhan, E.V.; Surender, S.; Arumanayagam, T. Degradation of tetracycline drug in aquatic environment by visible light active CuS/CdS photocatalyst. Inorg. Chem. Commun. 2023, 147, 110244. [Google Scholar] [CrossRef]




| S. No. | Photocatalyst | Light Source | Degradation Time | Degradation (%) | Catalyst Amount (mg) | Drug Conc. (mg/L) | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | α-Fe2O3/g-C3N4 NCs | Visible (32 mW/cm2, W) | 180 min | 95.0 | 50 | 10 | [40] |
| 2. | Black Phosphorus/BiOBr NCs | Visible (300 W/cm2, Xe) | 90 min | 79.0 | 100 | 50 | [41] |
| 3. | BiWO6 | Visible (300 W/cm2, Xe) | 180 min | 79.7 | 30 | 20 | [42] |
| 4. | WO3/g-C3N4 | Visible (300 W/cm2, Xe) | 60 min | 78.0 | 50 | 80 | [43] |
| 5. | (Bi)BiOBr/rGO NCs | Visible (300 W/cm2, Xe) | 140 min | 98.0 | 50 | 20 | [44] |
| 6. | Ni-WO3 | LED (35W) | 105 min | 76.0 | 50 | 10 | [45] |
| 7. | BiVO4/Fe2O3 | Visible (300 W/cm2, Xe) | 60 min | 91.5 | 30 | 15 | [46] |
| 8. | CuS/CdS NCs | Solar Light | 50 min | 90.0 | 50 | 20 | [47] |
| 9. | Cr2O3/ZrO2 | Visible (300 W/cm2, Xe) | 120 min | 97.1 | 100 | 50 | Present Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Naraginti, S.; Chen, P.; Li, J.; Yang, X.; Li, B. Visible Light-Driven Photocatalytic Degradation of Tetracycline Using p-n Heterostructured Cr2O3/ZrO2 Nanocomposite. Water 2023, 15, 3702. https://doi.org/10.3390/w15203702
Wei X, Naraginti S, Chen P, Li J, Yang X, Li B. Visible Light-Driven Photocatalytic Degradation of Tetracycline Using p-n Heterostructured Cr2O3/ZrO2 Nanocomposite. Water. 2023; 15(20):3702. https://doi.org/10.3390/w15203702
Chicago/Turabian StyleWei, Xueyu, Saraschandra Naraginti, Pengli Chen, Jiyuan Li, Xiaofan Yang, and Buwei Li. 2023. "Visible Light-Driven Photocatalytic Degradation of Tetracycline Using p-n Heterostructured Cr2O3/ZrO2 Nanocomposite" Water 15, no. 20: 3702. https://doi.org/10.3390/w15203702
APA StyleWei, X., Naraginti, S., Chen, P., Li, J., Yang, X., & Li, B. (2023). Visible Light-Driven Photocatalytic Degradation of Tetracycline Using p-n Heterostructured Cr2O3/ZrO2 Nanocomposite. Water, 15(20), 3702. https://doi.org/10.3390/w15203702





