Frog Crabs (Ranina ranina) in South Penghu Marine National Park, Taiwan: A Case Study of Population Dynamics and Recreational Fishing Sustainable Development
Abstract
:1. Introduction
2. Materials and Methods
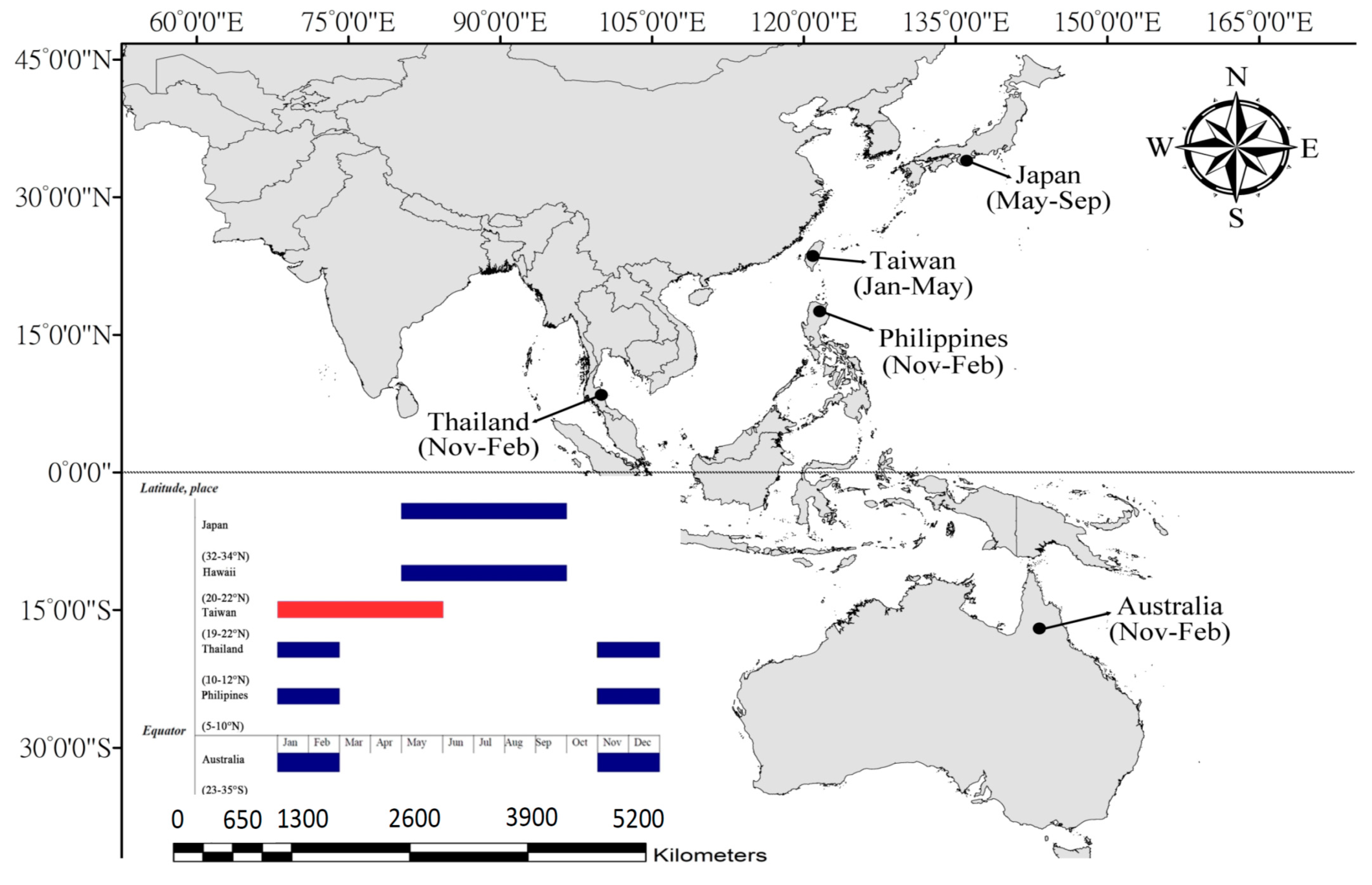
2.1. Study Area
2.2. Biological Sampling
2.3. Biological Characteristics
2.4. Reproduction Analysis
3. Results and Discussion
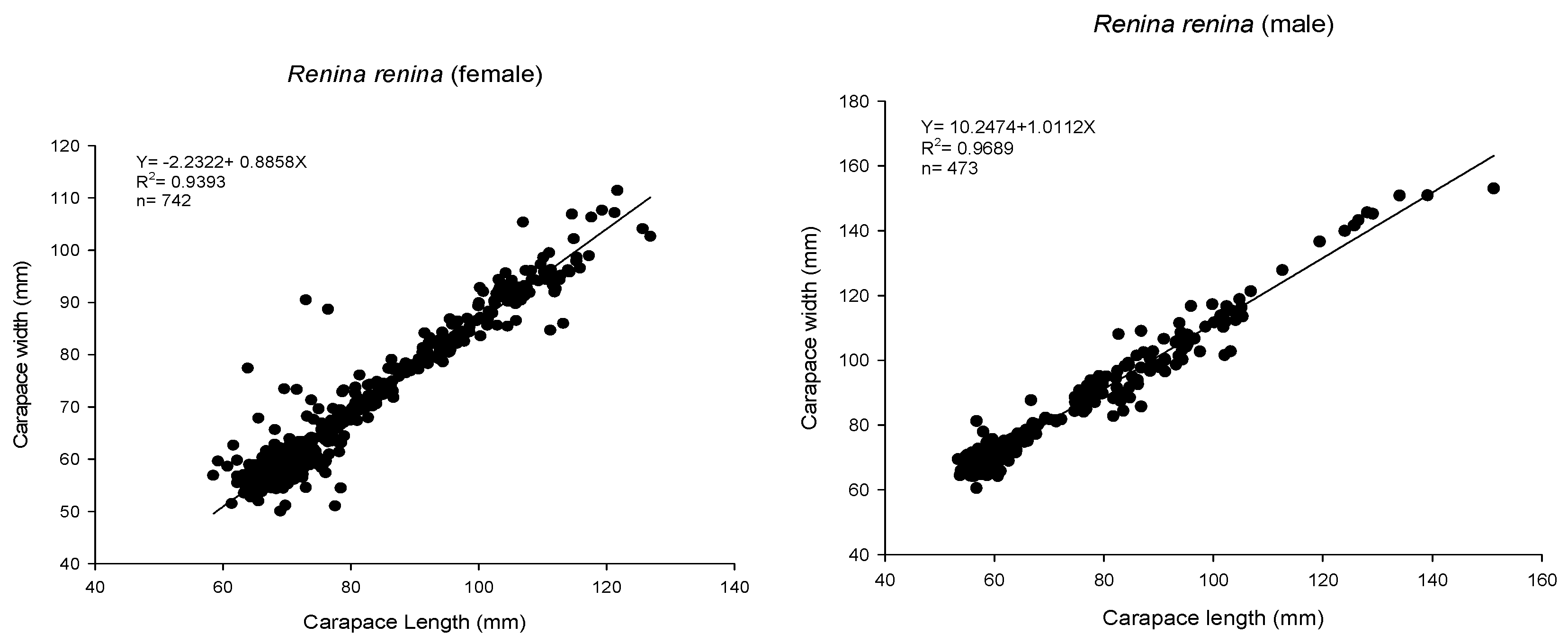
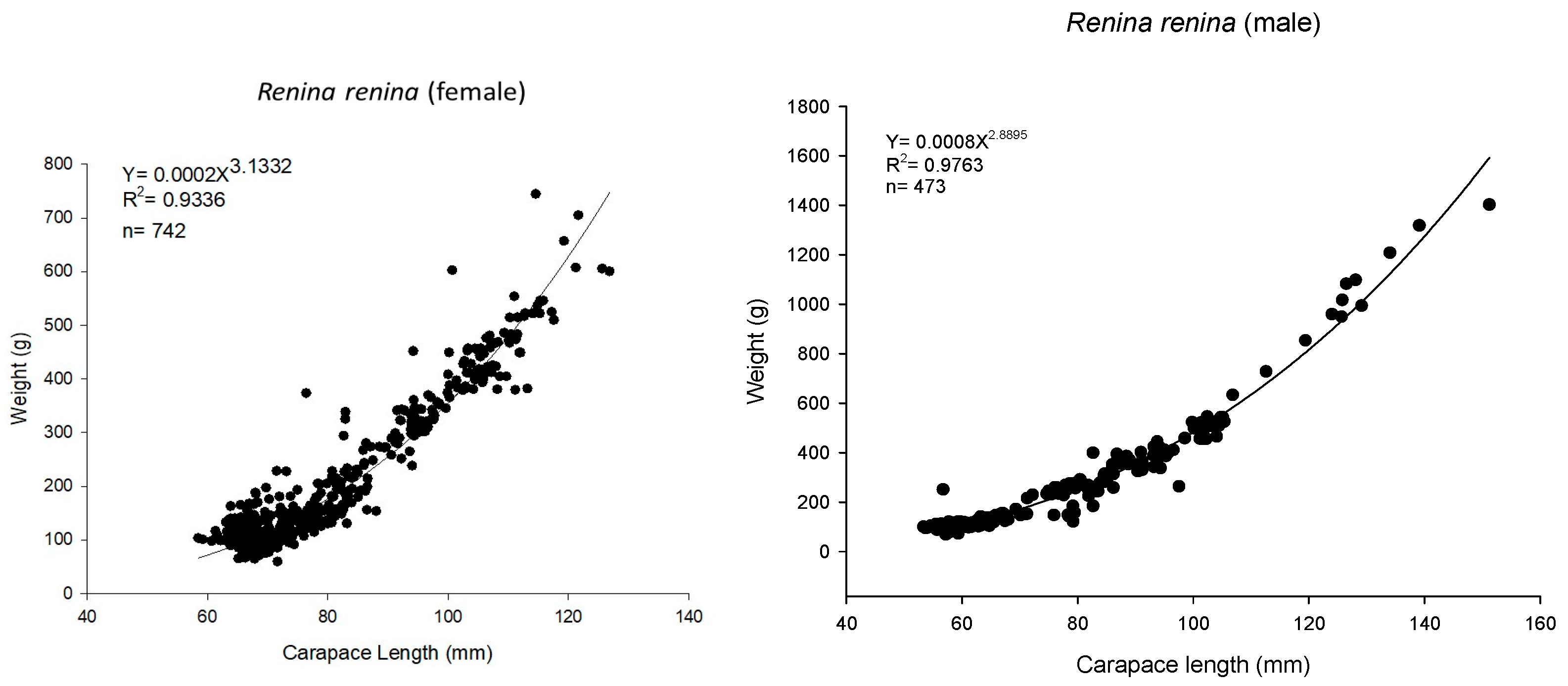
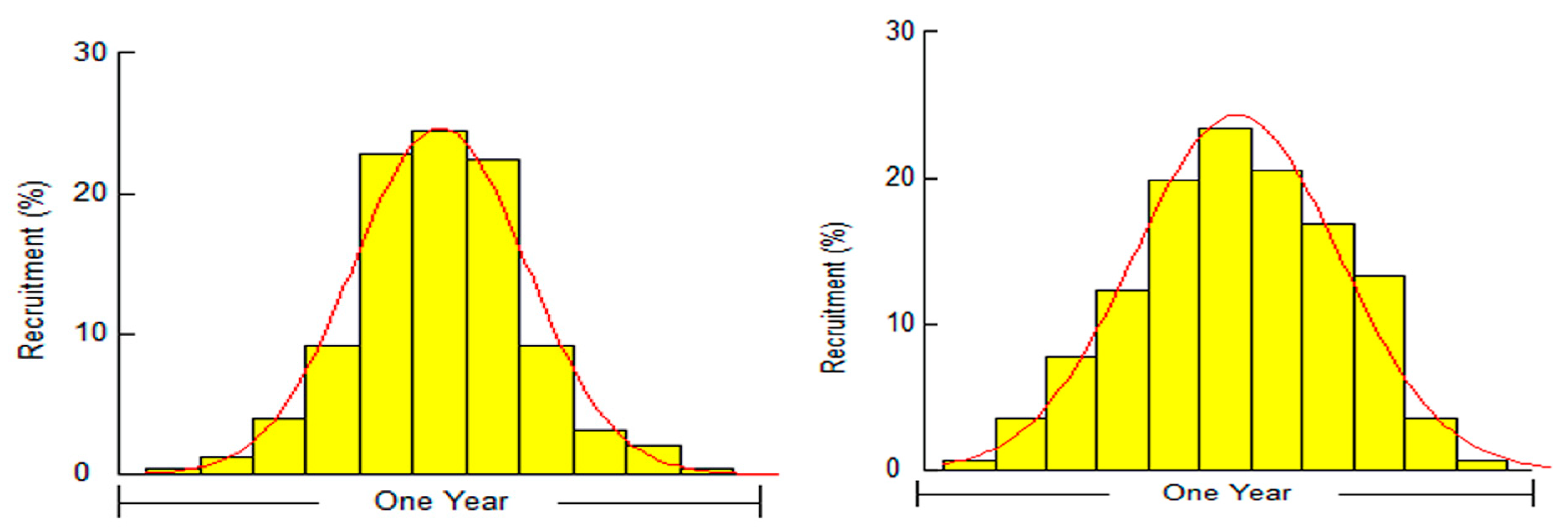
3.1. Biological Survey and Population Structure
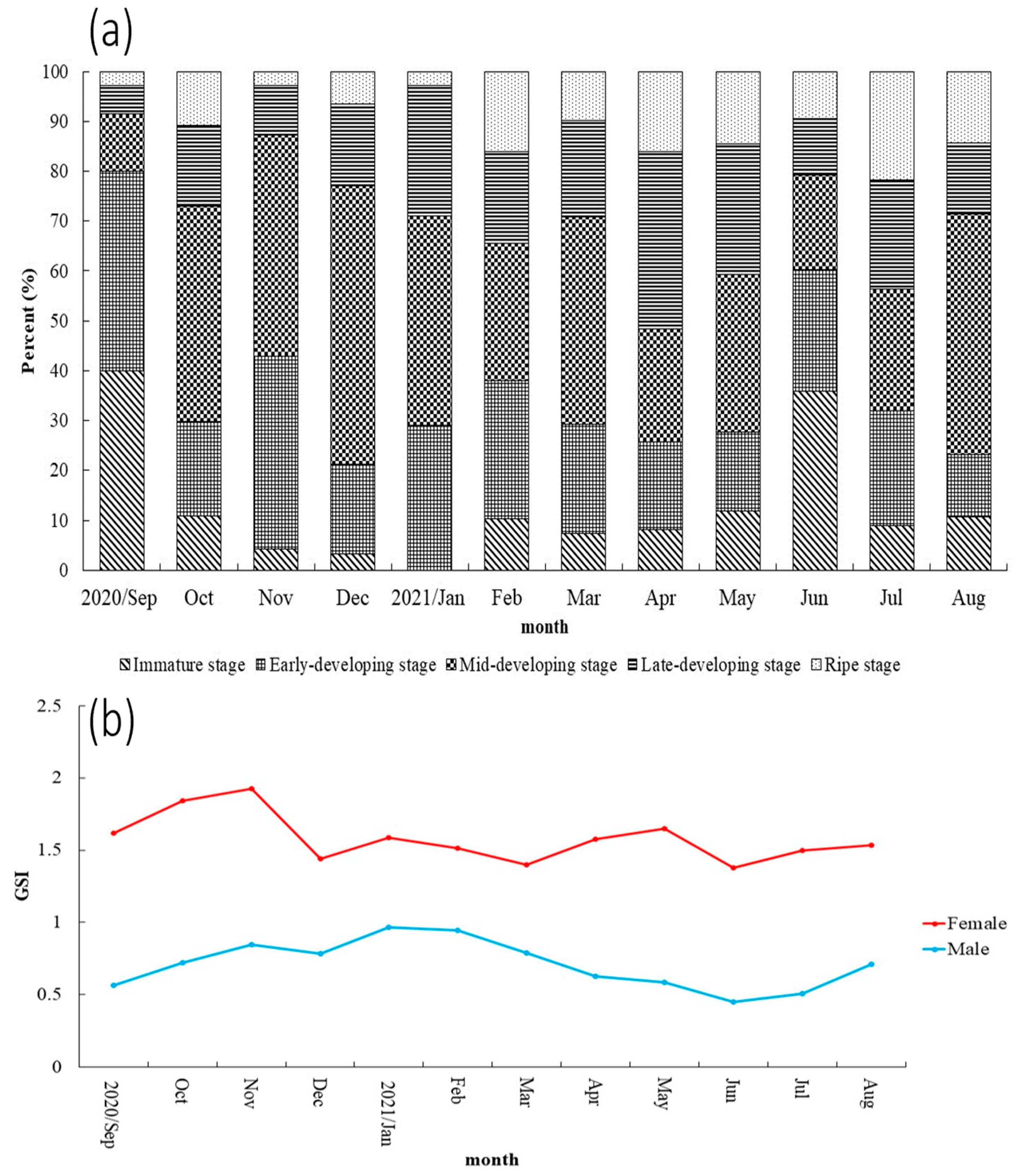
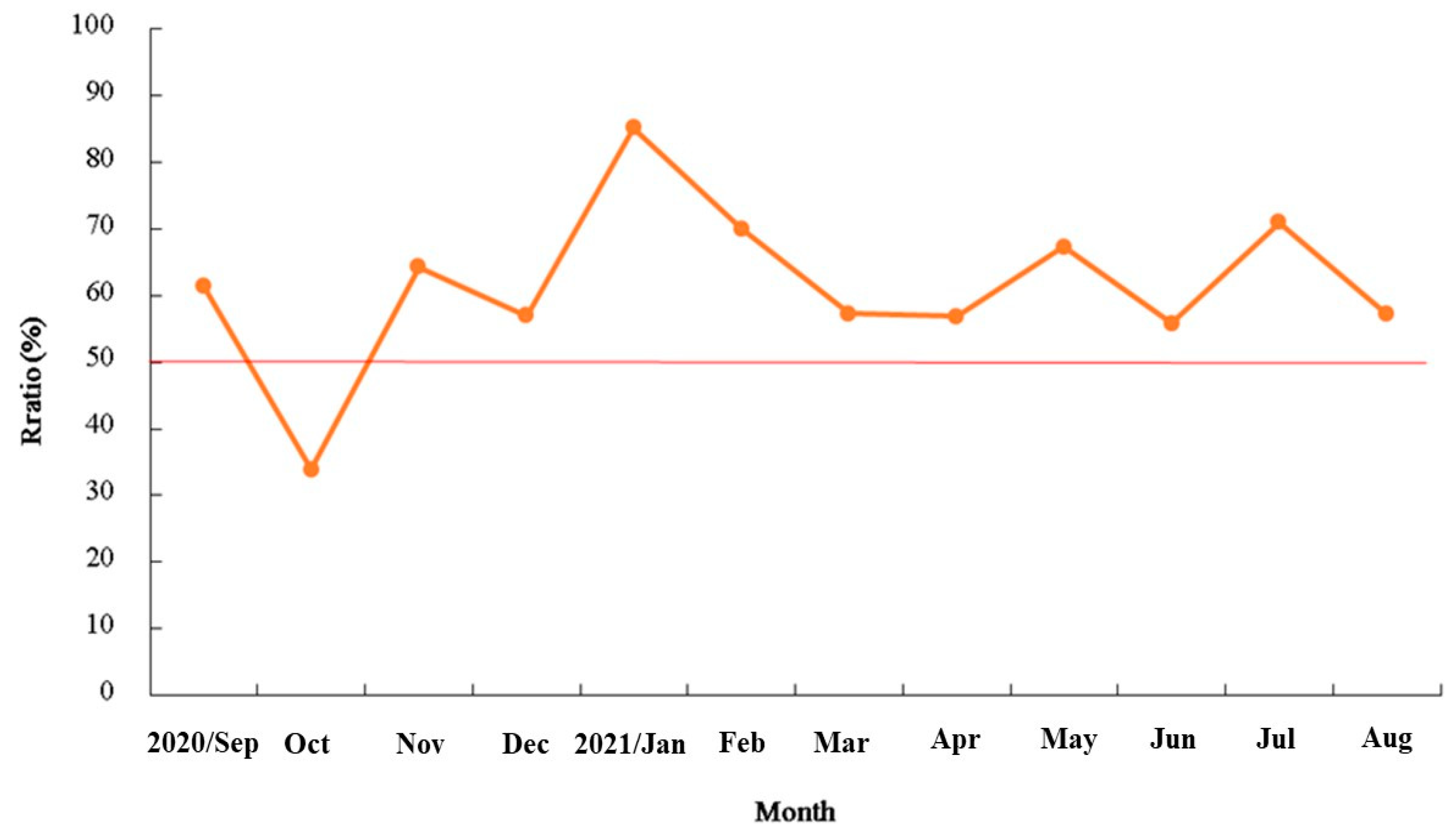
3.2. Reproduction Biology
3.3. Frog Crabs and the Impact of Recreational Fishing: A Policy Relationship
- (i)
- Monitoring and Research: detailed analysis is vital to understand the frog crab’s population dynamics, habitat needs, and potential threats, ensuring that recreational fishing adopts a sustainable approach.
- (ii)
- Community Engagement: engaging local communities in conservation activities will make them proactive partners in safeguarding the frog crab and broader marine ecosystems.
- (iii)
- Integrative Approach: adopting a holistic strategy that includes other species ensures that the focus on frog crabs do not overshadow other biodiversity concerns.
- (iv)
- Global Collaboration: partnering with international organizations and neighboring countries to share best practices and resources ensures a unified effort in marine conservation.
- (v)
- Policy Review and Update: regularly revisiting and updating policies related to the frog crab, in light of new scientific findings and societal needs, guarantees the strategies remain effective.
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, P.H. Crabs: Common Seafood of Taiwan, 2nd ed.; Taiwan Fisheries Department: Taipei, Taiwan, 1996; pp. 8–48. (In Chinese) [Google Scholar]
- Ng, P.K. Lamoha, a replacement name for Hypsophrys Wood-Mason & Alcock, 1891 (Brachyura, Homolidae), a junior synonym of Hypsophrys Agassiz, 1859 (Pisces, Teleostei, Cichlidae). Crustaceana 1998, 71, 121–125. [Google Scholar]
- Kennelly, S.J.; Scandol, J.P. Using a fishery-independent survey to assess the status of a spanner crab Ranina ranina fishery: Univariate analyses and biomass modelling. Crustaceana 2002, 75, 13–40. [Google Scholar]
- Thomas, L.R.; DiNardo, G.T.; Lee, H.H.; Piner, K.R.; Kahng, S.E. Factors influencing the distribution of kona crabs Ranina ranina (Brachyura: Raninidae) catch rates in the main Hawaiian Islands. J. Crustac. Biol. 2013, 33, 633–640. [Google Scholar] [CrossRef]
- Spencer, D.M.; Brown, I.W.; Lee, S.Y.; Lemckert, C.J. Physical oceanographic processes affecting catchability of spanner crab (Ranina ranina)—A review. Fish. Res. 2017, 186, 248–257. [Google Scholar] [CrossRef]
- Spencer, D.M.; Brown, I.W.; Doubell, M.J.; Brown, C.J.; Redondo Rodriguez, A.; Lee, S.Y.; Zhang, H.; Lemckert, C.J. Bottom boundary layer cooling and wind-driven upwelling enhance the catchability of spanner crab (Ranina ranina) in South-East Queensland, Australia. Fish. Oceanogr. 2019, 28, 317–326. [Google Scholar] [CrossRef]
- Marine Conservation Institute. Tracking Promises. Available online: https://www.mpatlas.org/promises/ (accessed on 15 May 2023).
- Garcia-Rubies, A.; Cebrian, E.; Schembri, P.J.; Evans, J.; Macpherson, E. Ecological Effects and Benefits of Mediterranean Marine Protected Areas: Management Implications, 1st ed.; John Wiley & Sons: Chichester, UK, 2017; pp. 24–63. [Google Scholar]
- Pryor, S.H.; Schultz, A.L.; Malcolm, H.A.; Smith, S.D.A. Partial protection disallowing trawling has conservation benefits in a subtropical marine park. Ocean. Coast. Manag. 2020, 183, 105027. [Google Scholar] [CrossRef]
- MacDonald, P.D.; Green, P.E.J. User’s Guide to Program MIX: An Interactive Program for Fitting Mixtures of Distribution; Ichtthus Data Systems: Hamilton, ON, Canada, 1985; pp. 4–68. [Google Scholar]
- Fournier, D.A.; Sibert, J.R.; Majkowski, J.; Hampton, J. MULTIFAN a Iikelihoodbase method for estimating growth parameter and composition from multiple length frequency data sets illustrated using data for southern bluefin tuna (Thunnus maccoyii). Can. J. Fish. Aquat. Sci. 1990, 47, 301–317. [Google Scholar] [CrossRef]
- Pauly, D.; Ingles, J.; Neal, R. Application to Shrimp Stocks of Objective Methods for the Estimation of Growth, Mortality and Recruitment Related Parameters from Length Frequency Data, ELEFAN I and II. In Penaeid Shrimps: Their Biology and Management, 1st ed.; Fishing News Books: Farnham, UK, 1984; pp. 21–59. [Google Scholar]
- Parrack, M.L. Aspects of brown shrimps, Penaeus aztecus, growth in the northern Gulf of Mexico. Fish. Bull. 1979, 76, 827–837. [Google Scholar]
- Garcia, S.; Le Reste, L. Life Cycles, Dynamics, Exploitation and Management of Coastal Penaeid Shrimp Stocks; FAO Technical Papers; Food and Agriculture Organization: Rome, Italy, 1981; Volume 203, pp. 1–215. [Google Scholar]
- Fre’chette, J.; Parsons, D.G. Report of shrimp aging workshop held at Ste. Foy, Quebec, in May and at Dartmouth, Nova Scotia, in November 1981. NAFO Sci. Counc. Stud. 1983, 6, 79–100. [Google Scholar]
- Garcia, S. Tropical Penaeid Prawns. In Fish Population Dynamics: The Implications for Management, 1st ed.; John Wiley and Sons: Hoboken, NJ, USA, 1988; pp. 45–65. [Google Scholar]
- Baelde, P. Growth, mortality and yield-per-recruit of deep-water royal red prawns (Haliporoides sibogae) off eastern Australia, using the length-based MULTIFAN method. Mar. Biol. 1994, 118, 617–625. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Xu, B.; Xue, Y.; Ren, Y. Selecting optimal bin size to account for growth variability in Electronic LEngth Frequency ANalysis (ELEFAN). Fish. Res. 2020, 225, 105474. [Google Scholar] [CrossRef]
- Fisch, N.; Camp, E.; Shertzer, K.; Ahrens, R. Assessing likelihoods for fitting composition data within stock assessments, with emphasis on different degrees of process and observation error. Fish. Res. 2021, 243, 106069. [Google Scholar] [CrossRef]
- Enin, U.I.; Lowenberg, U.; Kunzel, T. Population dynamics of the estuarine prawn (Nematopalaemon hastatus Aurivillius 1898) off the southeast coast of Nigeria. Fish. Res. 1996, 26, 17–35. [Google Scholar] [CrossRef]
- Etim, L.; Sankare, Y. Growth and Mortality, Recruitment and Yield of the Fresh-Water Shrimp, Macrobrachium vollenhovenii, Herklots 1851 (Crustacea, Palaemonidae) in the Fache Reservoir; Cote d’Ivoire Publishers Inc.: Yamoussoukro, Côte d’Ivoire, 1998; pp. 21–64. [Google Scholar]
- Oh, C.W.; Hartnoll, G.; Nash, R.D.M. Population dynamics of the common shrimp, Crangon crangon (L.), in Port Erin Bay, Isle of Man, Irish Sea. ICES J. Mar. Sci. 1999, 56, 718–733. [Google Scholar] [CrossRef]
- Ng, P.K.L. The Indo-Pacific Pilumnidae XIII. On the identity of Pilumnus dofleini Balss, 1933, with description of a new species (Crustacea: Decapoda: Brachyura) from Taiwan and the South China Sea. Zool. Res. 2000, 39, 301–306. [Google Scholar]
- Pauly, D.; Gaschutz, G. A Simple Method for Fitting Oscillating Length Growth Data, with a Program for Pocket Calculator; International Council for the Exploration of the Sea, Council Meeting G24, Demersal Fish Committee, 1979; pp. 15–34. Available online: https://www.seaaroundus.org/doc/Researcher+Publications/dpauly/PDF/1979/Other/OscillatingLengthData.pdf (accessed on 22 August 2023).
- Oh, C.W. Population biology of the swimming crab, Portunus trituberculatus (Miers, 1876) (Decapoda, Brachyura) on the western coast of Korea, Yellow sea. Crustaceana 2011, 84, 1251–1267. [Google Scholar]
- de Lestang, G.; Norman, H.S.; Potter, I.C. Reproductive biology of the blue swimmer crab (Portunus pelagicus, Decapoda: Portunidae) in five bodies of water on the west coast of Australia. Fish. Bull. 2003, 101, 745–757. [Google Scholar]
- Gayanilo, F.C.; Sparre, P.; Pauly, D. The FAO-ICLARM Stock Assessment Tools (FiSAT) User’s Guide. FAO Computerized Information Series (Fisheries), 1st ed.; FAO: Rome, Italy, 1995; pp. 7–20. [Google Scholar]
- Somers, I.F. On a seasonally oscillating growth function. Fishbyte 1988, 6, 13–14. [Google Scholar]
- Kirkwood, J.M.; Brown, I.W.; Gaddes, S.W.; Hoyle, S. Juvenile length-at-age data reveal that spanner crabs (Ranina ranina) grow slowly. Mar. Biol. 2005, 147, 331–339. [Google Scholar] [CrossRef]
- Krajangdara, T.; Watanabe, S. Growth and reproduction of the red frog crab, Ranina ranina (Linnaeus, 1758), in the Andaman Sea off Thailand. Fish. Sci. 2005, 71, 20–28. [Google Scholar] [CrossRef]
- Campbell, A. Application of a Yield and Egg-per-Recruit Model to the Lobster Fishery in the Bay of Fundy. N. Am. J. Fish. Manag. 1985, 5, 91–104. [Google Scholar] [CrossRef]
- Hoenig, J.M.; Csirke, J.; Sanders, M.J.; Abella, A.; Andreoli, M.G.; Levi, D.; Ragonese, S.; AI-Shoushani, M.; EI-Musa, M.M. Data Acquisition for Length-Based Stock Assessment: Report of Working Group I. In Length-Based Methods in Fisheries Research; Pauly, D., Morgan, R., Eds.; ICLARM Conference Proceedings, 13; ICLARM Publishers Inc.: Manila, Philippines, 1987; pp. 9–45. [Google Scholar]
- Yu, H.; Chen, T.Y. The Illustrated Penaeoid Prawns of Taiwan; Southem Material Center Publishers Inc.: Taipei, Taiwan, 1986. [Google Scholar]
- Wolf, M. A proposed method for standardization of the selection of class interval for length-frequency analysis. Fishbyte 1989, 7, 5. [Google Scholar]
- Tzeng, T.D.; Yeh, S.Y. Estimates of biological parameters of sword prawn (Parapenaeopsis hardwickii) in the adjacent waters off Taichung Harbor. J. Fish. Soc. Taiwan 2000, 27, 241–251. [Google Scholar]
- Ahmadi, I.S.; Sho, S. Trapping survey for the red frog crabs (Ranina ranina) using the baited tangle nets and pots in offshore Tanegashima, Japan. Int. J. Innov. Stud. Aquat. Biol. Fish. 2015, 1, 30–37. [Google Scholar]
- Baylon, J.C.; Tito, O.D. Reproductive biology of the red frog crab, Ranina ranina (Linnaeus, 1758) (Crustacea: Decapoda: Raninidae) from southwestern Mindanao, Philippines. Asian Fish. Sci. 2012, 25, 113–123. [Google Scholar] [CrossRef]
- Fielding, A.; Haley, S.R. Sex ratio, size at reproductive maturity, and reproduction of the Hawaiian kona crab, Ranina ranina (Linnaeus) (Brachyura, Gymnopleura: Raninidae). Pac. Sci. 1976, 30, 131–145. [Google Scholar]
- Minagawa, M.; Chiu, J.R.; Kudo, M.; Ito, F.; Takashima, F. Female reproductive biology and oocyte development of the red frog crab, Ranina ranina, off Hachijojima, Izu Islands, Japan. Mar. Biol. 1993, 115, 613–623. [Google Scholar] [CrossRef]
- Skinner, D.G.; Hill, B.J. Catch rate and emergence of male and female spanner crabs (Ranina ranina) in Australia. Mar. Biol. 1986, 91, 461–465. [Google Scholar] [CrossRef]
- Kennelly, S.J.; Watkins, D. Fecundity and reproductive period, and their relationship to catch rates of spanner crabs, Ranina ranina, off the east coast of Australia. J. Crust. Biol. 1994, 14, 146–150. [Google Scholar] [CrossRef]
- Kailola, P.J.; Williams, M.J.; Stewart, P.C.; Reichelt, R.E.; McNee, A.; Grieve, C. Australian Fisheries Resources; Bureau of Resource Sciences, Commonwealth of Australia Publishers Inc.: Canberra, Australia, 1993; pp. 4–28. [Google Scholar]
- Batoy, C.B.; Sarmago, J.F.; Pilapil, B.C. Breeding season, sexual maturity and fecundity of the blue crab, Portunus pelagicus (L.) in selected coastal waters in Leyte and vicinity, Philippines. Ann. Trop. Res. 1987, 9, 157–177. [Google Scholar]
- Lai, H.L.; Gunderson, D.R. Effects of ageing errors on estimates of growth, mortality and yield per recruit for walleye pollock (Theragra chalcogramma). Fish. Res. 1987, 5, 287–302. [Google Scholar]
- Lai, Y.M.; Yang, F.P.; Pao, C.C. Human papillomavirus deoxyribonucleic acid and ribonucleic acid in seminal plasma and sperm cells. Fertil. Steril. 1996, 65, 1026–1030. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, C.-H. Frog Crabs (Ranina ranina) in South Penghu Marine National Park, Taiwan: A Case Study of Population Dynamics and Recreational Fishing Sustainable Development. Water 2023, 15, 3689. https://doi.org/10.3390/w15203689
Shih C-H. Frog Crabs (Ranina ranina) in South Penghu Marine National Park, Taiwan: A Case Study of Population Dynamics and Recreational Fishing Sustainable Development. Water. 2023; 15(20):3689. https://doi.org/10.3390/w15203689
Chicago/Turabian StyleShih, Chun-Han. 2023. "Frog Crabs (Ranina ranina) in South Penghu Marine National Park, Taiwan: A Case Study of Population Dynamics and Recreational Fishing Sustainable Development" Water 15, no. 20: 3689. https://doi.org/10.3390/w15203689




