The Long-Term Dynamics of Walleye Pollock Stocks in Relation to Oceanographic Changes in the East Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Pollock Statistical Data
2.2. On-Site Pollock Catch Data around the Main Fishing Ground in Korean Waters
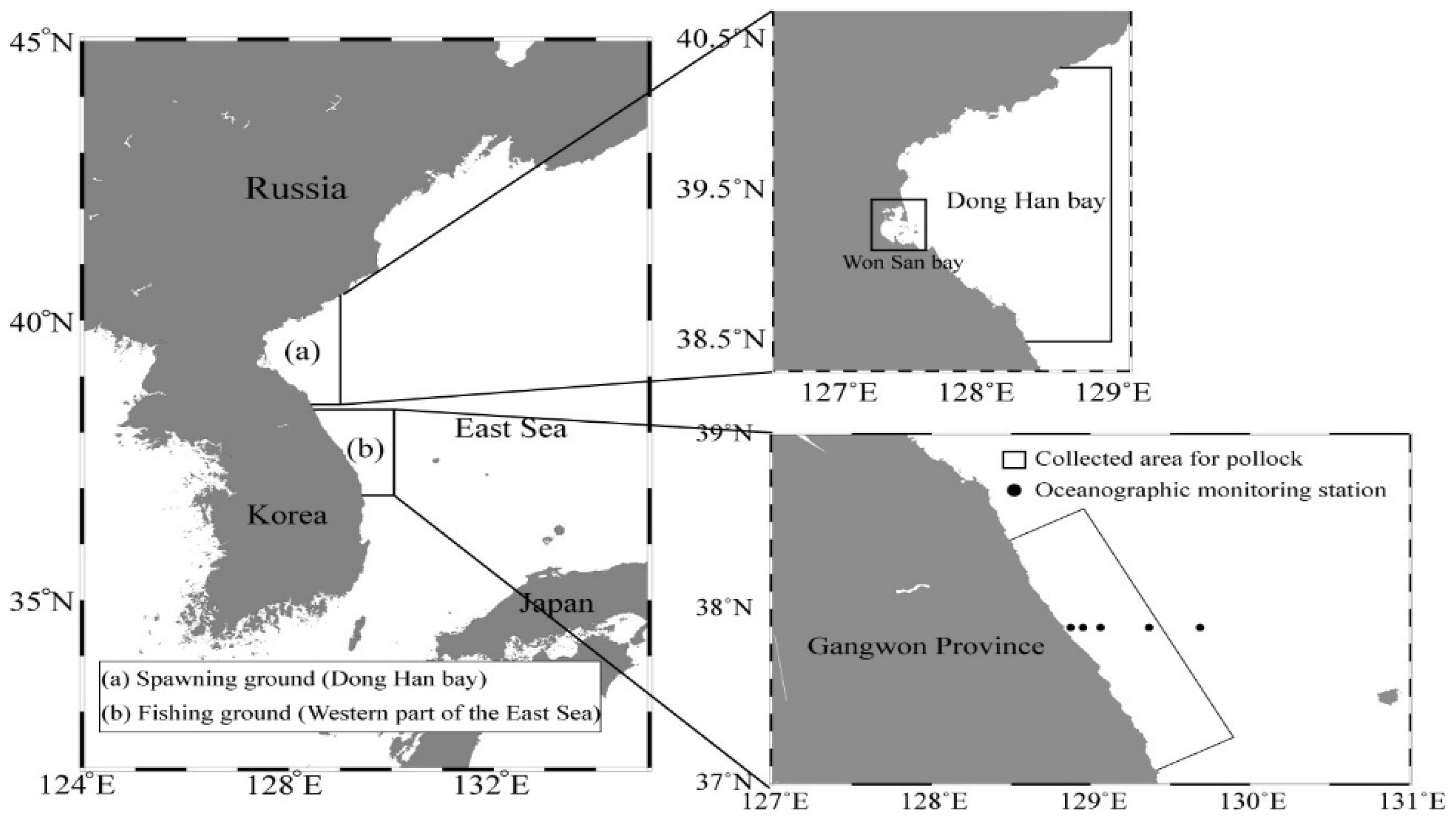
2.3. Long-Term Changes in Oceanic Conditions around Spawning and Fishing Grounds
3. Results
3.1. Long-Term Changes in Catches of Juvenile and Adult Pollock
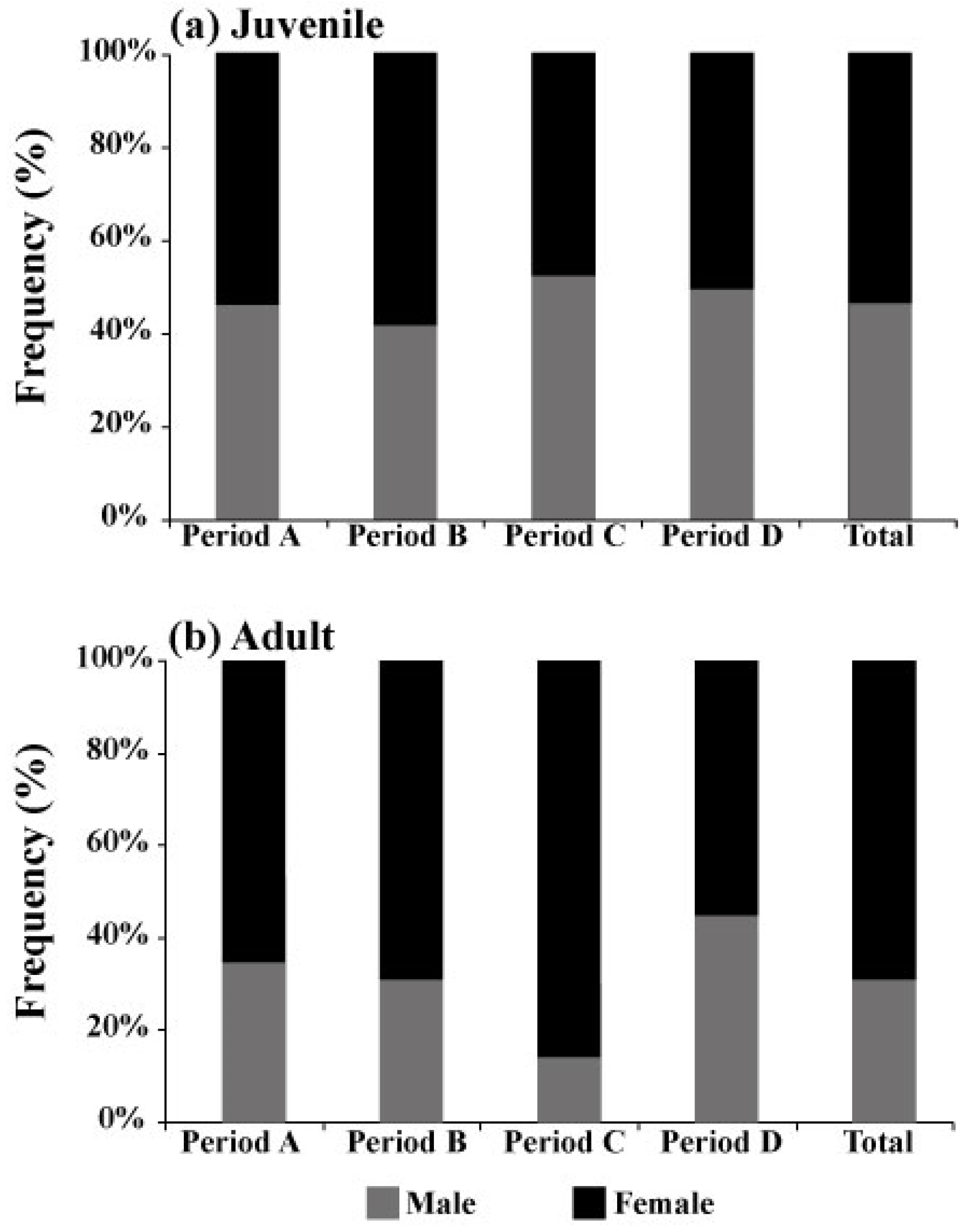
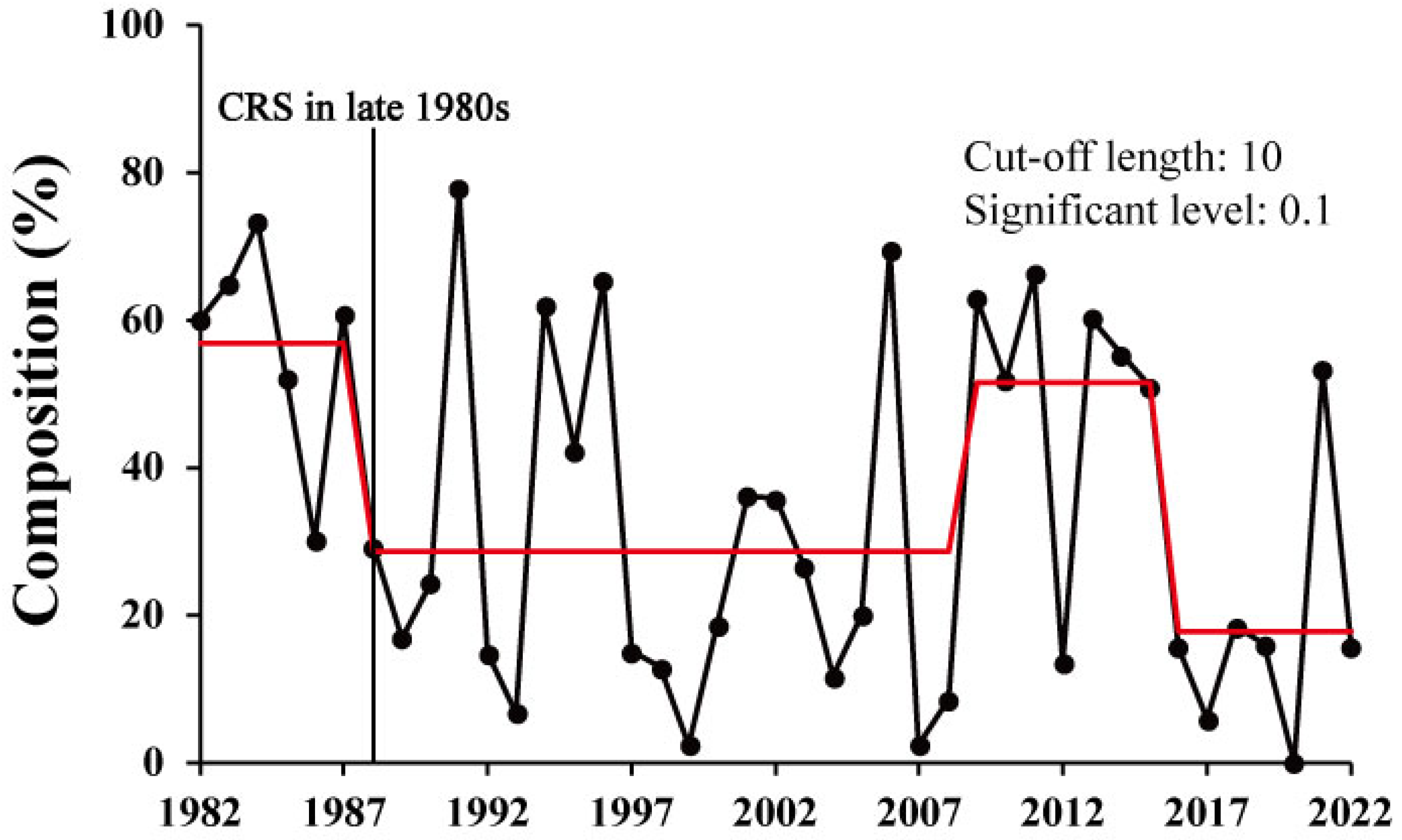
3.2. Long-Term Changes in the Sex Composition of Pollock
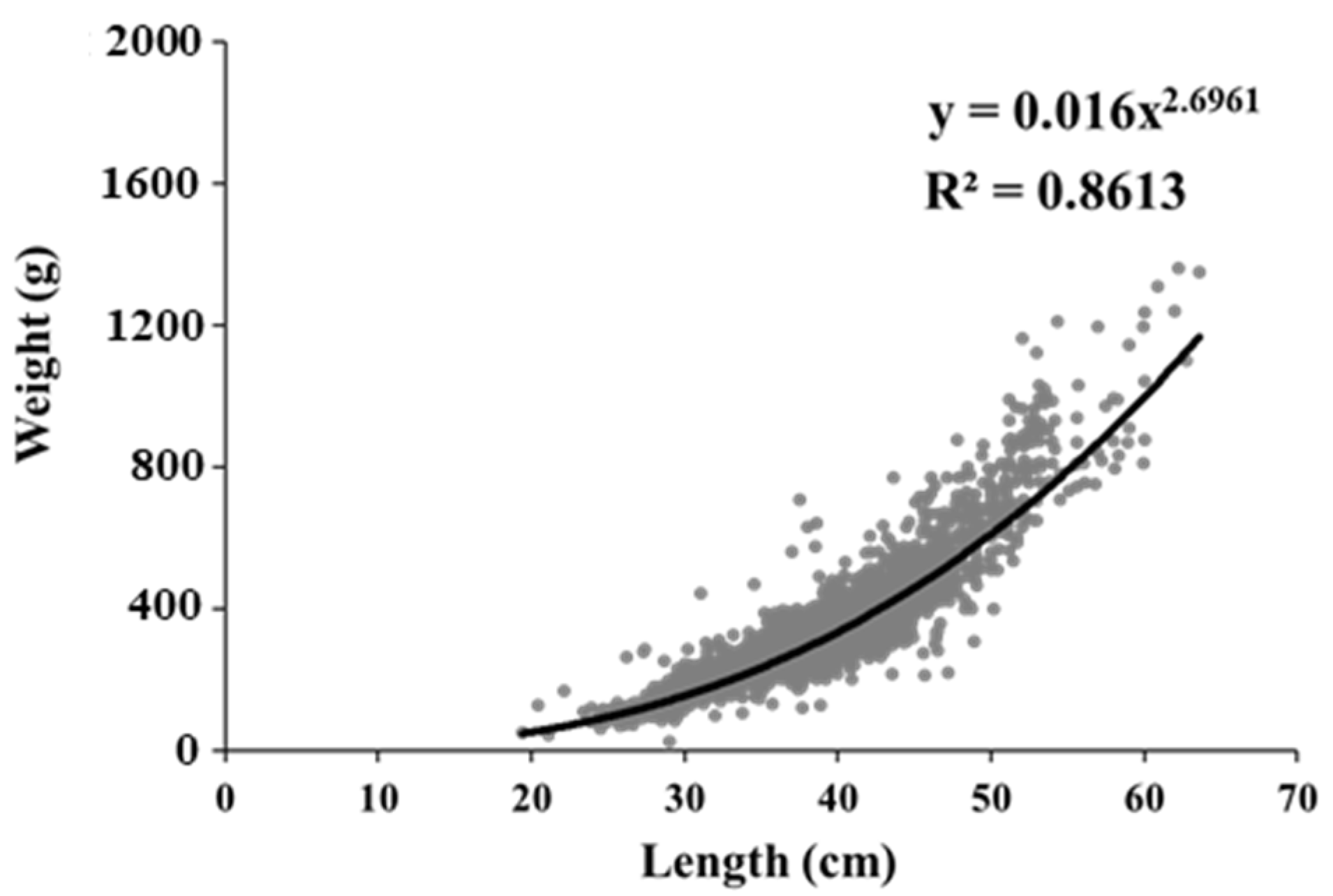
3.3. Long-Term Changes in the Size Spectrum and Condition Factor of Pollock
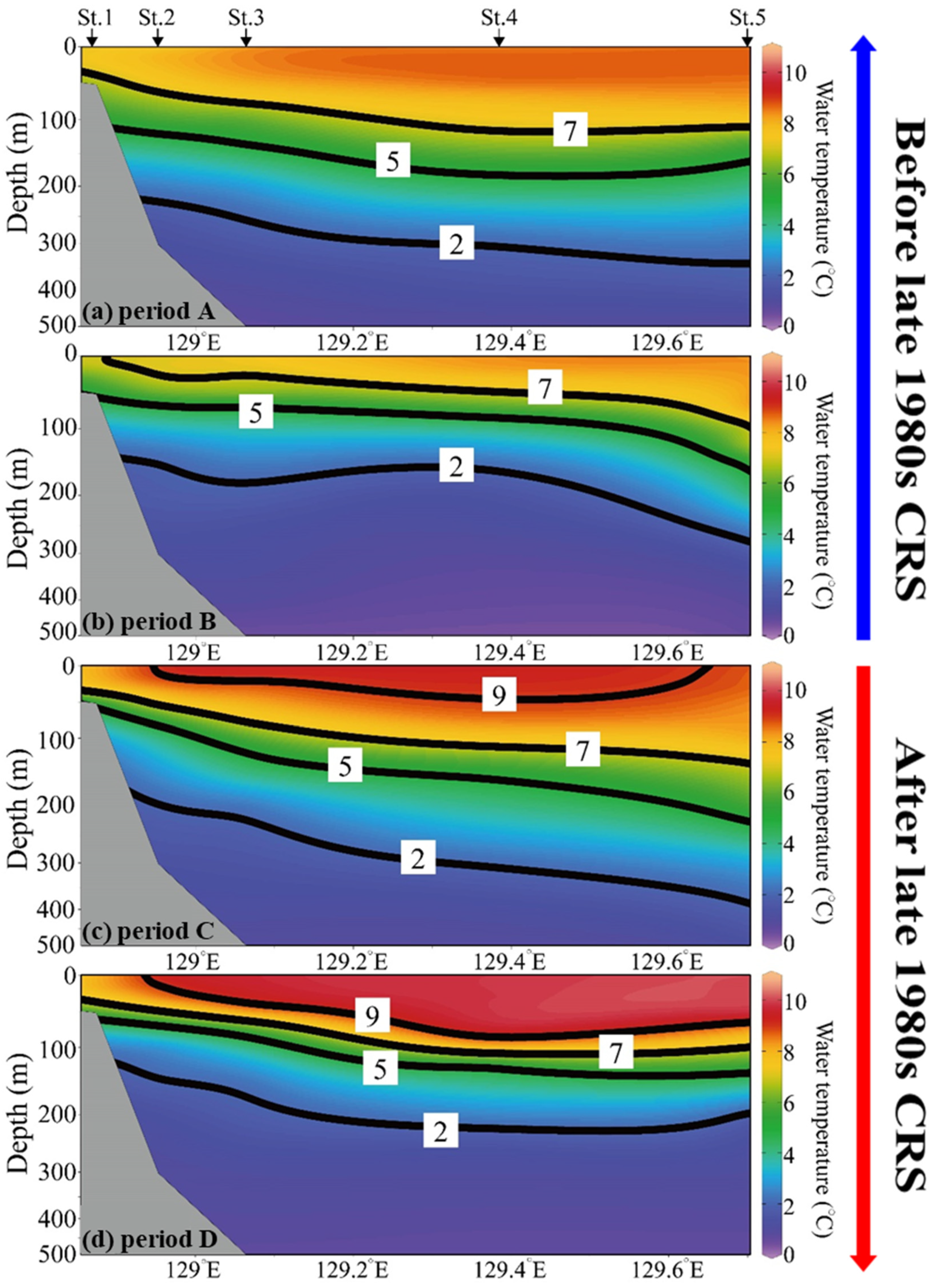
3.4. Water Temperature Structure in the Western Part of the East Sea
3.5. Long-Term Changes in Oceanic Conditions of the Spawning Ground
3.6. Long-Term Changes in the Duration of Suitable Temperature for Spawning (DTS) and Regional Proportion for Suitable Spawning Conditions (RPS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Period | Year | Month | Female | Male | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Length (cm) | Weight (g) | No. | Total Length (cm) | Weight (g) | No. | |||||||
| Mean | STD. | Mean | STD. | Mean | STD. | Mean | STD. | |||||
| A | 1973 | 1 | 44.28 | 8.39 | 483.15 | 261.55 | 26 | 36.33 | 2.52 | 256.63 | 44.42 | 24 |
| 1973 | 2 | 38.69 | 3.91 | 307.41 | 86.93 | 34 | 38.23 | 2.13 | 301.88 | 48.36 | 16 | |
| 1973 | 3 | - | - | - | - | - | - | - | - | - | - | |
| 1974 | 1 | 41.85 | 6.13 | 384.45 | 140.18 | 83 | 39.29 | 5.70 | 326.69 | 119.09 | 35 | |
| 1974 | 2 | 41.13 | 4.36 | 383.61 | 126.33 | 36 | 41.88 | 4.55 | 410.54 | 111.98 | 24 | |
| 1974 | 3 | 38.69 | 4.64 | 300.83 | 134.27 | 36 | 38.26 | 3.18 | 265.14 | 48.44 | 14 | |
| 1975 | 1 | 41.97 | 6.73 | 361.07 | 146.95 | 28 | 38.79 | 6.30 | 305.83 | 164.07 | 12 | |
| 1975 | 2 | 44.51 | 11.05 | 507.89 | 285.16 | 19 | 39.63 | 6.25 | 392.38 | 193.43 | 21 | |
| 1975 | 3 | 40.02 | 4.99 | 361.76 | 161.87 | 33 | 41.53 | 4.12 | 380.71 | 92.02 | 7 | |
| 1976 | 1 | 37.89 | 5.07 | 352.54 | 181.87 | 13 | 37.16 | 6.25 | 300.18 | 177.78 | 44 | |
| 1976 | 2 | - | - | - | - | - | - | - | - | - | - | |
| 1976 | 3 | - | - | - | - | - | - | - | - | - | - | |
| 1977 | 1 | - | - | - | - | - | - | - | - | - | - | |
| 1977 | 2 | 39.77 | 5.21 | 347.06 | 155.11 | 63 | 39.13 | 4.72 | 335.46 | 133.41 | 37 | |
| 1977 | 3 | - | - | - | - | - | - | - | - | - | - | |
| 1978 | 1 | 36.61 | 2.36 | 253.68 | 46.33 | 31 | 36.10 | 2.47 | 251.24 | 43.57 | 29 | |
| 1978 | 2 | 38.03 | 2.04 | 294.22 | 53.38 | 27 | 37.10 | 1.67 | 259.27 | 45.04 | 33 | |
| 1978 | 3 | 35.35 | 2.43 | 232.79 | 54.12 | 29 | 36.88 | 2.79 | 256.35 | 49.21 | 31 | |
| 1979 | 1 | 39.11 | 5.00 | 354.61 | 169.38 | 56 | 35.95 | 3.05 | 255.14 | 70.89 | 64 | |
| 1979 | 2 | 35.73 | 1.97 | 256.41 | 46.73 | 78 | 35.16 | 2.13 | 235.32 | 50.41 | 41 | |
| 1979 | 3 | 34.69 | 3.13 | 225.87 | 56.71 | 81 | 34.29 | 2.73 | 222.79 | 54.47 | 39 | |
| B | 1980 | 1 | 37.93 | 2.20 | 292.30 | 43.29 | 71 | 36.56 | 1.98 | 269.61 | 56.88 | 49 |
| 1980 | 2 | 38.40 | 2.51 | 302.62 | 61.42 | 66 | 37.49 | 2.41 | 268.50 | 49.31 | 54 | |
| 1980 | 3 | - | - | - | - | - | - | - | - | - | - | |
| 1981 | 1 | 38.41 | 2.61 | 291.88 | 69.52 | 69 | 38.37 | 3.05 | 280.12 | 63.74 | 51 | |
| 1981 | 2 | 39.39 | 3.24 | 279.21 | 49.47 | 38 | 38.21 | 2.81 | 252.73 | 46.22 | 22 | |
| 1981 | 3 | - | - | - | - | - | - | - | - | - | - | |
| 1982 | 1 | 37.98 | 3.04 | 266.36 | 62.82 | 67 | 38.48 | 3.06 | 274.12 | 65.83 | 33 | |
| 1982 | 2 | 37.28 | 2.85 | 267.22 | 54.03 | 49 | 37.34 | 2.38 | 258.94 | 53.48 | 51 | |
| 1982 | 3 | - | - | - | - | - | - | - | - | - | - | |
| 1983 | 1 | 38.07 | 2.51 | 277.31 | 52.30 | 75 | 36.84 | 2.34 | 268.88 | 47.27 | 25 | |
| 1983 | 2 | 36.86 | 2.15 | 263.40 | 50.47 | 45 | 36.91 | 2.83 | 265.95 | 53.81 | 65 | |
| 1983 | 3 | - | - | - | - | - | - | - | - | - | - | |
| 1984 | 1 | 37.18 | 3.52 | 264.88 | 62.88 | 67 | 36.38 | 2.36 | 244.85 | 48.95 | 33 | |
| 1984 | 2 | 38.08 | 3.54 | 285.00 | 72.59 | 69 | 36.48 | 3.63 | 259.15 | 53.21 | 20 | |
| 1984 | 3 | - | - | - | - | - | - | - | - | - | - | |
| 1985 | 1 | 37.86 | 3.03 | 265.50 | 52.85 | 88 | 36.45 | 2.34 | 246.45 | 55.80 | 11 | |
| 1985 | 2 | - | - | - | - | - | - | - | - | - | - | |
| 1985 | 3 | - | - | - | - | - | - | - | - | - | - | |
| C | 1991 | 1 | 42.63 | 3.02 | 397.27 | 90.67 | 113 | 41.24 | 2.14 | 381.80 | 67.77 | 15 |
| 1991 | 2 | 38.90 | 6.36 | 302.59 | 120.43 | 63 | 32.07 | 6.01 | 186.23 | 93.83 | 35 | |
| 1991 | 3 | 36.11 | 5.55 | 252.58 | 99.05 | 62 | 35.71 | 5.97 | 246.08 | 108.40 | 37 | |
| 1992 | 1 | 41.31 | 5.65 | 345.13 | 113.75 | 77 | 31.84 | 5.34 | 173.30 | 77.74 | 23 | |
| 1992 | 2 | 39.33 | 6.25 | 334.90 | 134.02 | 60 | 35.23 | 5.84 | 224.37 | 94.06 | 38 | |
| 1992 | 3 | 37.22 | 4.59 | 333.38 | 116.01 | 63 | 30.71 | 4.93 | 217.11 | 114.69 | 37 | |
| 1993 | 1 | - | - | - | - | - | - | - | - | - | - | |
| 1993 | 2 | - | - | - | - | - | - | - | - | - | - | |
| 1993 | 3 | 34.48 | 3.36 | 211.46 | 73.13 | 32 | 33.96 | 3.05 | 195.28 | 37.84 | 39 | |
| 1994 | 1 | - | - | - | - | - | - | - | - | - | - | |
| 1994 | 2 | 41.97 | 4.39 | 386.56 | 117.79 | 39 | 39.07 | 7.09 | 332.44 | 191.22 | 12 | |
| 1994 | 3 | - | - | - | - | - | - | - | - | - | - | |
| 1995 | 1 | 40.49 | 3.25 | 387.25 | 148.51 | 42 | 36.99 | 3.98 | 286.85 | 105.66 | 8 | |
| 1995 | 2 | - | - | - | - | - | - | - | - | - | - | |
| 1995 | 3 | - | - | - | - | - | - | - | - | - | - | |
| D | 2016 | 1 | 45.67 | 6.97 | 550.10 | 271.45 | 30 | 42.35 | 5.91 | 424.96 | 189.48 | 21 |
| 2016 | 2 | 44.08 | 9.13 | 529.36 | 268.57 | 13 | 46.17 | 6.31 | 570.44 | 261.47 | 15 | |
| 2016 | 3 | 44.92 | 7.23 | 517.05 | 290.44 | 19 | 40.29 | 7.57 | 395.71 | 186.78 | 15 | |
| 2017 | 1 | 42.39 | 8.97 | 512.90 | 290.64 | 59 | 41.67 | 7.76 | 457.02 | 223.34 | 38 | |
| 2017 | 2 | 39.14 | 9.71 | 384.13 | 282.10 | 37 | 39.32 | 7.28 | 359.95 | 169.28 | 29 | |
| 2017 | 3 | 38.36 | 10.84 | 420.34 | 340.50 | 41 | 38.29 | 7.18 | 350.49 | 180.54 | 38 | |
| 2018 | 1 | 41.55 | 8.76 | 510.66 | 277.29 | 48 | 39.47 | 7.53 | 408.29 | 194.96 | 35 | |
| 2018 | 2 | 40.97 | 7.42 | 452.71 | 217.74 | 55 | 39.24 | 7.62 | 389.69 | 201.44 | 48 | |
| 2018 | 3 | 32.74 | 6.57 | 254.94 | 180.87 | 139 | 34.27 | 5.32 | 258.99 | 131.95 | 154 | |
References
- Makino, M.; Kim, S.A.; Velikanov, A.; Criddle, K.; Funamoto, T.; Hirota, M.; Sakurai, Y. Introduction: From the Birth to the Table of Walleye Pollock Theragra chalcogramma. Fish. Sci. 2014, 80, 103–107. [Google Scholar] [CrossRef]
- Bailey, K.M.; Quinn, T.J.; Bentzen, R.; Grant, W.S. Population Structure and Dynamics of Walleye Pollock, Theragra chalcogramma. Adv. Mar. Biol. 1999, 37, 179–255. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Napp, J.M.; Kendall, A.W.; Schumacher, J.D. A Synthesis of Biological and Physical Processes Affecting the Feeding Environment of Larval Walleye Pollock (Theragra chalcogramma) in the Eastern Bering Sea. Fish. Oceanogr. 2000, 9, 147–162. [Google Scholar] [CrossRef]
- Mueter, F.J.; Ladd, C.; Palmer, M.C.; Norcross, B.L. Bottom-up and Top-down Controls of Walleye Pollock (Theragra chalcogramma) on the Eastern Bering Sea Shelf. Prog. Oceanogr. 2006, 68, 152–183. [Google Scholar] [CrossRef]
- Noakes, D.J.; Beamish, R.J. Synchrony of Marine Fish Catches and Climate and Ocean Regime Shifts in the North Pacific Ocean. Mar. Coast. Fish. 2009, 1, 155–168. [Google Scholar] [CrossRef]
- Jung, H.K.; Rahman, S.M.; Kang, C.K.; Park, S.Y.; Lee, S.H.; Park, H.J.; Kim, H.W.; Lee, C.I. The Influence of Climate Regime Shifts on the Marine Environment and Ecosystems in the East Asian Marginal Seas and Their Mechanisms. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 143, 110–120. [Google Scholar] [CrossRef]
- Bulatov, O.A. Walleye Pollock: Global Overview. Fish. Sci. 2014, 80, 109–116. [Google Scholar] [CrossRef]
- Seol, K.S.; Lee, C.I.; Jung, H.K. Long Term Changes in Sea Surface Temperature Around Habitat Ground of Walleye Pollock (Gadus chalcogrammus) in the East Sea. J. Korean Soc. Mar. Environ. Saf. 2020, 26, 195–205. [Google Scholar] [CrossRef]
- Park, J.W.; Yoo, H.K.; Jung, H.K.; Park, H.J.; Bae, K.M.; Kang, C.K.; Lee, C.I. Effects of Water Temperature Changes on the Early Life Stages (Egg and Larvae) of Walleye Pollock (Gadus chalcogrammus)—Laboratory Experiments and Field Applications. J. Exp. Mar. Biol. Ecol. 2024, 571, 151980. [Google Scholar] [CrossRef]
- Kim, S.A.; Kang, S.K. The Status and Research Direction for Fishery Resources in the East Sea/Sea of Japan. Korean Soc. Fish. Res 1998, 1, 44–58. [Google Scholar]
- Kim, Y.S.; Han, K.H.; Kang, C.B.; Kim, J.B. Commercial Fishes of the Coastal and Offshore Water in Korea; Hanguel-Publishing: Busan, Republic of Korea, 2004. [Google Scholar]
- Beamish, R.J.; McFarlane, G.A. A Discussion of the Importance of Aging Errors, and an Application to Walleye Pollock: The World’s Largest Fishery. In Recent Developments in Fish Otolith Research; University of South Carolina Press: Columbia, SC, USA, 1995; pp. 545–565. [Google Scholar]
- Lee, C.I.; Han, M.H.; Jung, H.K.; Park, H.J.; Park, J.M. Spawning Season, and Factors Influencing Allometric Growth Pattern and Body Condition of Walleye Pollock Gadus chalcogrammus in the Middle East Sea, Korea. Korean J. Ichthyol. 2019, 31, 141–149. [Google Scholar] [CrossRef]
- Katou, T. Alaska Pollack Trade Relation between Japan and Korea. J. Fish. Bus. Adm. 2002, 33, 109–119. [Google Scholar]
- Kang, S.K.; Kim, S.A. What Caused the Collapse of Walleye Pollock Population in Korean Waters? KMI Int. J. Marit. Aff. Fish. 2015, 7, 43–58. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Kang, Y.K.; Lee, S.T.; Jung, H.K.; Lee, C.I.; Kim, S.; Jeong, K.Y.; Byun, D.S.; Cho, Y.K. Potential Impact of Late 1980s Regime Shift on the Collapse of Walleye Pollock Catch in the Western East/Japan Sea. Front. Mar. Sci. 2022, 9, 802748. [Google Scholar] [CrossRef]
- Bang, M.K.; Kang, S.K.; Kim, S.A.; Jang, C.J. Changes in the Biological Characteristics of Walleye Pollock Related to Demographic Changes in the East Sea during the Late 20th Century. Mar. Coast. Fish. 2018, 10, 91–99. [Google Scholar] [CrossRef]
- Bailey, K.M.; Stabeno, P.J.; Powers, D.A. The Role of Larval Retention and Transport Features in Mortality and Potential Gene Flow of Walleye Pollock. J. Fish Biol. 1997, 51, 135–154. [Google Scholar] [CrossRef]
- Kang, S.K.; Park, J.H.; Kim, S.A. Size-Class Estimation of the Number of Walleye Pollock Theragra chalcogramma Caught in the Southwestern East Sea during the 1970s–1990s. Korean J. Fish. Aquat. Sci. 2013, 46, 445–453. [Google Scholar] [CrossRef][Green Version]
- Nakatani, T.; Maeda, T. Thermal Effect on the Development of Walleye Pollock Eggs and Their Upward Speed to the Surface. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 937–942. [Google Scholar] [CrossRef]
- Bailey, K.M.; Stehr, C.L. Laboratory Studies on the Early Life History of the Walleye Pollock, Theragra chalcogramma (Pallas). J. Exp. Mar. Biol. Ecol. 1986, 99, 233–246. [Google Scholar] [CrossRef]
- Yoo, H.K.; Byun, S.G.; Yamamoto, J.; Sakurai, Y. The Effect of Warmer Water Temperature of Walleye Pollock (Gadus chalcogrammus) Larvae. J. Korean Soc. Mar. Environ. Saf. 2015, 21, 339–346. [Google Scholar] [CrossRef]
- Kim, S.A. Status of Fishery and Science of Bering Sea Walleye Pollock: (II). Ocean Res. 1992, 14, 149–170. [Google Scholar]
- Muigwa, N.M. Vertical Distribution Pattern of Prespawning and Spawning Pollock (Theragra chalcogramma) in Shelikof Strait. In Proceedings of the International Symposium on the Biology and Management of Walleye Pollock, Anchorage, AK, USA, 14–16 November 1989; pp. 403–431. [Google Scholar]
- Park, H.J.; Park, T.H.; Lee, C.I.; Kang, C.K. Ontogenetic Shifts in Diet and Trophic Position of Walleye Pollock, Theragra chalcogramma, in the Western East Sea (Japan Sea) Revealed by Stable Isotope and Stomach Content Analyses. Fish. Res. 2018, 204, 297–304. [Google Scholar] [CrossRef]
- Park, J.M.; Jung, H.K.; Lee, C.I. Factors Influencing Dietary Changes of Walleye Pollock, Gadus chalcogrammus, Inhabiting the East Sea off the Korean Coast. J. Mar. Sci. Eng. 2021, 9, 1154. [Google Scholar] [CrossRef]
- Yamamura, O.; Yabuki, K.; Shida, O.; Watanabe, K.; Honda, S. Spring Cannibalism on 1 Year Walleye Pollock in the Doto Area, Northern Japan: Is It Density Dependent? J. Fish Biol. 2001, 59, 645–656. [Google Scholar] [CrossRef]
- Miyashita, K.; Tetsumura, K.; Honda, S.; Oshima, T.; Kawabe, R.; Sasaki, K. Diel Changes in Vertical Distribution Patterns of Zooplankton and Walleye Pollock (Theragra chalcogramma) off the Pacific Coast of Eastern Hokkaido, Japan, Estimated by the Volume Back Scattering Strength (Sv) Difference Method. Fish. Oceanogr. 2004, 13, 99–110. [Google Scholar] [CrossRef]
- Jung, H.K.; Lee, C.I.; Park, H.J.; Park, J.M. Influences of Oceanographic Features on Spatial and Temporal Distributions of Size Spectrum of Walleye Pollock, Gadus chalcogrammus Inhabiting Middle Eastern Coast of Korea. Korean J. Ichthyol. 2020, 32, 148–159. [Google Scholar] [CrossRef]
- Llopiz, J.; Cowen, R.; Hauff, M.; Ji, R.; Munday, P.; Muhling, B.; Peck, M.; Richardson, D.; Sogard, S.; Sponaugle, S. Early Life History and Fisheries Oceanography: New Questions in a Changing World. Oceanography 2014, 27, 26–41. [Google Scholar] [CrossRef]
- Siddon, E.C.; Kristiansen, T.; Mueter, F.J.; Holsman, K.K.; Heintz, R.A.; Farley, E.V. Spatial Match-Mismatch between Juvenile Fish and Prey Provides a Mechanism for Recruitment Variability across Contrasting Climate Conditions in the Eastern Bering Sea. PLoS ONE 2013, 8, e84526. [Google Scholar] [CrossRef] [PubMed]
- Froese, R.; Pauly, D. Fishbase World Wide Web Electronic Publication; Version 02/2024; ScienceOpen, Inc.: Boston, MA, USA, 2024. [Google Scholar]
- Pauly, D. Some Simple Methods for the Assessment of Tropical Fish Stocks; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1983; p. 52. [Google Scholar]
- NFRDI. Ecology and Fishing Ground of Fisheries Resources in Korean Waters; National Institute of Fisheries Science: Busan, Republic of Korea, 2021. [Google Scholar]
- Tian, Y.; Kidokoro, H.; Watanabe, T.; Iguchi, N. The Late 1980s Regime Shift in the Ecosystem of Tsushima Warm Current in the Japan/East Sea: Evidence from Historical Data and Possible Mechanisms. Prog. Oceanogr. 2008, 77, 127–145. [Google Scholar] [CrossRef]
- Tian, Y.; Ueno, Y.; Suda, M.; Akamine, T. Decadal Variability in the Abundance of Pacific Saury and Its Response to Climatic/Oceanic Regime Shifts in the Northwestern Subtropical Pacific during the Last Half Century. J. Mar. Syst. 2004, 52, 235–257. [Google Scholar] [CrossRef]
- Trenberth, K.E. The Definition of El Niño. Bull. Am. Meteorol. Soc. 1997, 78, 2771–2777. [Google Scholar] [CrossRef]
- Donlon, C.J.; Martin, M.; Stark, J.; Roberts-Jones, J.; Fiedler, E.; Wimmer, W. The Operational Sea Surface Temperature and Sea Ice Analysis (OSTIA) System. Remote Sens. Environ. 2012, 116, 140–158. [Google Scholar] [CrossRef]
- Worley, S.J.; Woodruff, S.D.; Reynolds, R.W.; Lubker, S.J.; Lott, N. ICOADS Release 2.1 Data and Products. Int. J. Climatol. 2005, 25, 823–842. [Google Scholar] [CrossRef]
- Kim, S.A.; Zhang, C.I.; Kim, J.Y.; Oh, J.H.; Kang, S.K.; Lee, J.B. Climate Variability and Its Effects on Major Fisheries in Korea. Ocean Sci. J. 2007, 42, 179–192. [Google Scholar] [CrossRef]
- Kendall, A.W., Jr.; Nakatani, T. Comparisons of Early-Life-History Characteristics of Walleye Pollock Theragra chalcogramma in Shelikof Strait, Gulf of Alaska, and Funka Bay, Hokkaido, Japan. Fish. Bull. 1992, 90, 129–138. [Google Scholar]
- Funamoto, T.; Yamamura, O.; Shida, O.; Itaya, K.; Mori, K.; Hiyama, Y.; Sakurai, Y. Comparison of Factors Affecting Recruitment Variability of Walleye Pollock Theragra chalcogramma in the Pacific Ocean and the Sea of Japan off Northern Japan. Fish. Sci. 2014, 80, 117–126. [Google Scholar] [CrossRef]
- Yoshida, H.; Sakurai, Y. Relationship between Food-Consumption and Growth of Adult Walleye Pollock Theragra chalcogramma in Captivity. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 763–769. [Google Scholar] [CrossRef]
- Smith, R.L.; Paul, A.J.; Paul, J.M. Effect of Food Intake and Temperature on Growth and Conversion Efficiency of Juvenile Walleye Pollock (Theragra chalcogramma (Pallas)): A Laboratory Study. ICES J. Mar. Sci. 1986, 42, 241–253. [Google Scholar] [CrossRef]
- Zhang, C.I.; Yoon, S.C.; Lee, J.B. Effects of the 1988/89 Climatic Regime Shift on the Structure and Function of the Southwestern Japan/East Sea Ecosystem. J. Mar. Syst. 2007, 67, 225–235. [Google Scholar] [CrossRef]
- Lee, C.I.; Jung, Y.W.; Jung, H.K. Response of Spatial and Temporal Variations in the Kuroshio Current to Water Column Structure in the Western Part of the East Sea. J. Mar. Sci. Eng. 2022, 10, 1703. [Google Scholar] [CrossRef]
- Iles, T.D.; Sinclair, M. Atlantic Herring: Stock Discreteness and Abundance. Science 1982, 215, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.M.; Ciannelli, L.; Bond, N.A.; Belgrano, A.; Stenseth, N.C. Recruitment of Walleye Pollock in a Physically and Biologically Complex Ecosystem: A New Perspective. Prog. Oceanogr. 2005, 67, 24–42. [Google Scholar] [CrossRef]
- Hinckley, S.; Hermann, A.J.; Mier, K.L.; Megrey, B.A. Importance of Spawning Location and Timing to Successful Transport to Nursery Areas: A Simulation Study of Gulf of Alaska Walleye Pollock. ICES J. Mar. Sci. 2001, 58, 1042–1052. [Google Scholar] [CrossRef]
- Wilson, M.T.; Brodeur, R.D.; Sarah, H. Distribution and Abundance of Age-O Walleye Pollock, Theragra chalcogramma, in the Western Gulf of Alaska during September 1990. In NOAA Technical Report NMFS 126 Ecology of Juvenile Walleye Pollock, Theragra chalcogramma; Brodeur, R.D., Livingston, P.A., Loughlin, T.R., Hollowed, A.B., Eds.; U.S. Department of Commerce: Seattle, WA, USA, 1996; pp. 11–24. [Google Scholar]
- Honda, S.; Oshima, T.; Nishimura, A.; Hattori, T. Movement of Juvenile Walleye Pollock, Theragra chalcogramma, from a Spawning Ground to a Nursery Ground along the Pacific Coast of Hokkaido, Japan. Fish. Oceanogr. 2004, 13, 84–98. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakagawa, T.; Yokota, T.; Chimura, M.; Yamashita, Y.; Funamoto, T. Effects of Spawning Temperature on the Reproductive Characteristics of Walleye Pollock Gadus chalcogrammus. Fish. Sci. 2019, 85, 901–911. [Google Scholar] [CrossRef]
- McGurk, M.D. Effects of Delayed Feeding and Temperature on the Age of Irreversible Starvation and on the Rates of Growth and Mortality of Pacific Herring Larvae. Mar. Biol. 1984, 84, 13–26. [Google Scholar] [CrossRef]
- Fortier, L.; Sirois, P.; Michaud, J.; Barber, D. Survival of Arctic Cod Larvae (Boreogadus saida) in Relation to Sea Ice and Temperature in the Northeast Water Polynya (Greenland Sea). Can. J. Fish. Aquat. Sci. 2006, 63, 1608–1616. [Google Scholar] [CrossRef]
- Laurel, B.J.; Copeman, L.A.; Spencer, M.; Iseri, P. Comparative Effects of Temperature on Rates of Development and Survival of Eggs and Yolk-Sac Larvae of Arctic Cod (Boreogadus saida) and Walleye Pollock (Gadus chalcogrammus). ICES J. Mar. Sci. 2018, 75, 2403–2412. [Google Scholar] [CrossRef]
- Blood, D.M. Low-Temperature Incubation of Walleye Pollock (Theragra chalcogramma) Eggs from the Southeast Bering Sea Shelf and Shelikof Strait, Gulf of Alaska. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 6095–6108. [Google Scholar] [CrossRef]
- Porter, S.M. Effects of Size and Light on Respiration and Activity of Walleye Pollock (Theragra chalcogramma) Larvae. J. Exp. Mar. Biol. Ecol. 2001, 256, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Hare, S.R.; Mantua, N.J. Empirical Evidence for North Pacific Regime Shifts in 1977 and 1989. Prog. Oceanogr. 2000, 47, 103–145. [Google Scholar] [CrossRef]
- Thompson, D.W.J.; Wallace, J.M. The Arctic Oscillation Signature in the Wintertime Geopotential Height and Temperature Fields. Geophys. Res. Lett. 1998, 25, 1297–1300. [Google Scholar] [CrossRef]
- Jung, Y.; Park, J.-H.; Hirose, N.; Yeh, S.-W.; Kim, K.J.; Ha, H.K. Remote Impacts of 2009 and 2015 El Niño on Oceanic and Biological Processes in a Marginal Sea of the Northwestern Pacific. Sci. Rep. 2022, 12, 741. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, L.; Nürnberg, D.; Chao, W.; Lembke-Jene, L.; Tiedemann, R. ENSO vs Glacial-Interglacial-Induced Changes in the Kuroshio-Oyashio Transition Zone during the Pleistocene. Front. Mar. Sci. 2023, 10, 1074431. [Google Scholar] [CrossRef]
- Ma, C.; Wu, D.; Lin, X.; Yang, J.; Ju, X. On the Mechanism of Seasonal Variation of the Tsushima Warm Current. Cont. Shelf Res. 2012, 48, 1–7. [Google Scholar] [CrossRef]
- Park, S.K.; Ok, Y.S. Bio-Economic Research in Fishery Resource Management: Walleye Pollock. Nongchon-Gyungje 1986, 9, 59–68. [Google Scholar]
- Kim, W.S.; Huh, S.H. Meristic and Morphometric Observations on Nogari and Alaska Pollock. J. Oceanol. Soc. Korea 1978, 13, 26–30. [Google Scholar]
- Solemdal, P. Maternal Effects—A Link between the Past and the Future. J. Sea Res. 1997, 37, 213–227. [Google Scholar] [CrossRef]
- Trippel, E.A. Egg Size and Viability and Seasonal Offspring Production of Young Atlantic Cod. Trans. Am. Fish. Soc. 1998, 127, 339–359. [Google Scholar] [CrossRef]
- Froese, R. Keep It Simple: Three Indicators to Deal with Overfishing. Fish Fish. 2004, 5, 86–91. [Google Scholar] [CrossRef]







| Number of Specimens | ||||
|---|---|---|---|---|
| Period A | Period B | Period C | Period D | |
| Total specimens | 1144 | 1118 | 795 | 834 |
| Female | 673 | 704 | 551 | 441 |
| Male | 471 | 414 | 244 | 393 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.K.; Park, J.W.; Yang, J.H.; Park, J.M.; Han, I.S.; Lee, C.I. The Long-Term Dynamics of Walleye Pollock Stocks in Relation to Oceanographic Changes in the East Sea. Water 2024, 16, 955. https://doi.org/10.3390/w16070955
Jung HK, Park JW, Yang JH, Park JM, Han IS, Lee CI. The Long-Term Dynamics of Walleye Pollock Stocks in Relation to Oceanographic Changes in the East Sea. Water. 2024; 16(7):955. https://doi.org/10.3390/w16070955
Chicago/Turabian StyleJung, Hae Kun, Jong Won Park, Jae Hyeong Yang, Joo Myun Park, In Seong Han, and Chung Il Lee. 2024. "The Long-Term Dynamics of Walleye Pollock Stocks in Relation to Oceanographic Changes in the East Sea" Water 16, no. 7: 955. https://doi.org/10.3390/w16070955
APA StyleJung, H. K., Park, J. W., Yang, J. H., Park, J. M., Han, I. S., & Lee, C. I. (2024). The Long-Term Dynamics of Walleye Pollock Stocks in Relation to Oceanographic Changes in the East Sea. Water, 16(7), 955. https://doi.org/10.3390/w16070955









