Improvement of the Carbocatalytic Degradation of Pharmaceuticals in Water by the Use of Ultrasound Waves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Methods
2.2.1. Carbonaceous Material Preparation
2.2.2. Reaction Systems
2.3. Analyses
2.3.1. Chromatographic Analyses
2.3.2. Electron Paramagnetic Resonance Spectroscopy (EPR)
2.3.3. SW-Mn Characterization
2.3.4. Theoretical Calculations
3. Results
3.1. Brief Characterization of the SW-Mn Material
3.2. Effect of Pollutant
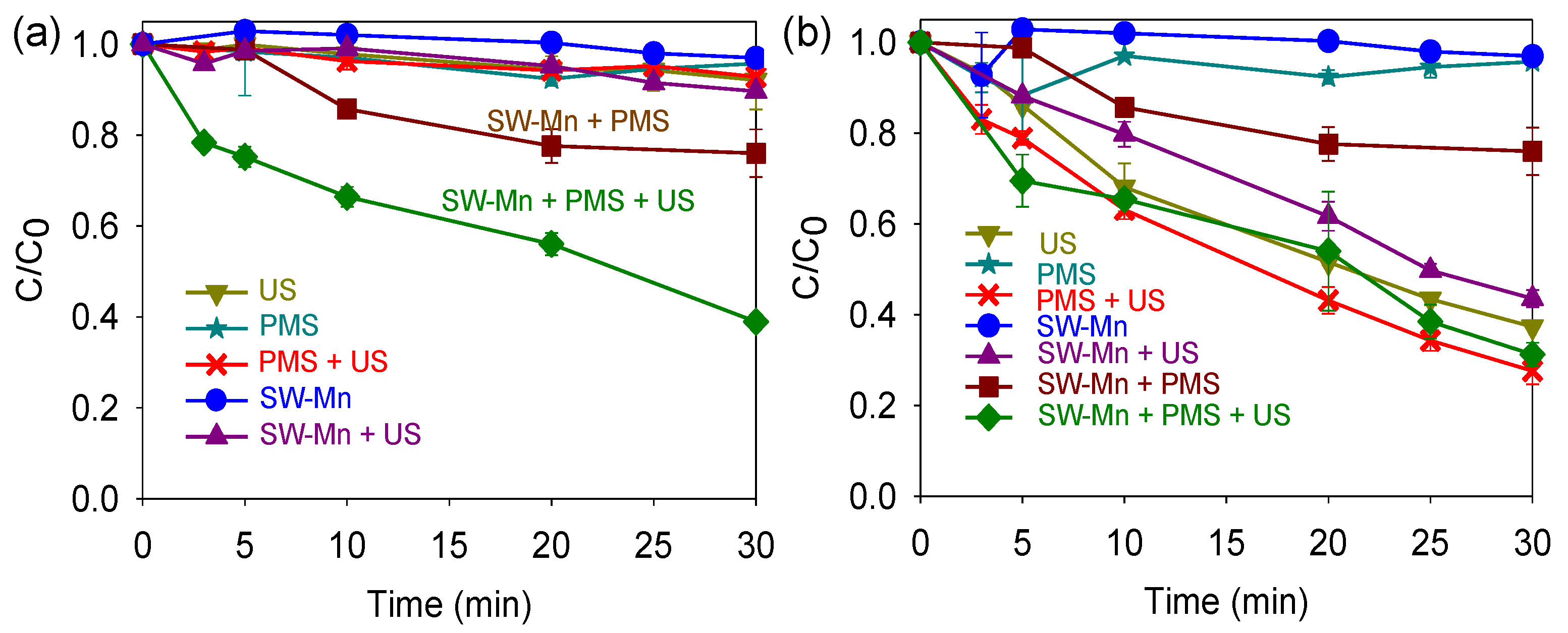
3.3. Sonocarbocatalysis Process
3.3.1. Effect of Ultrasound Frequency
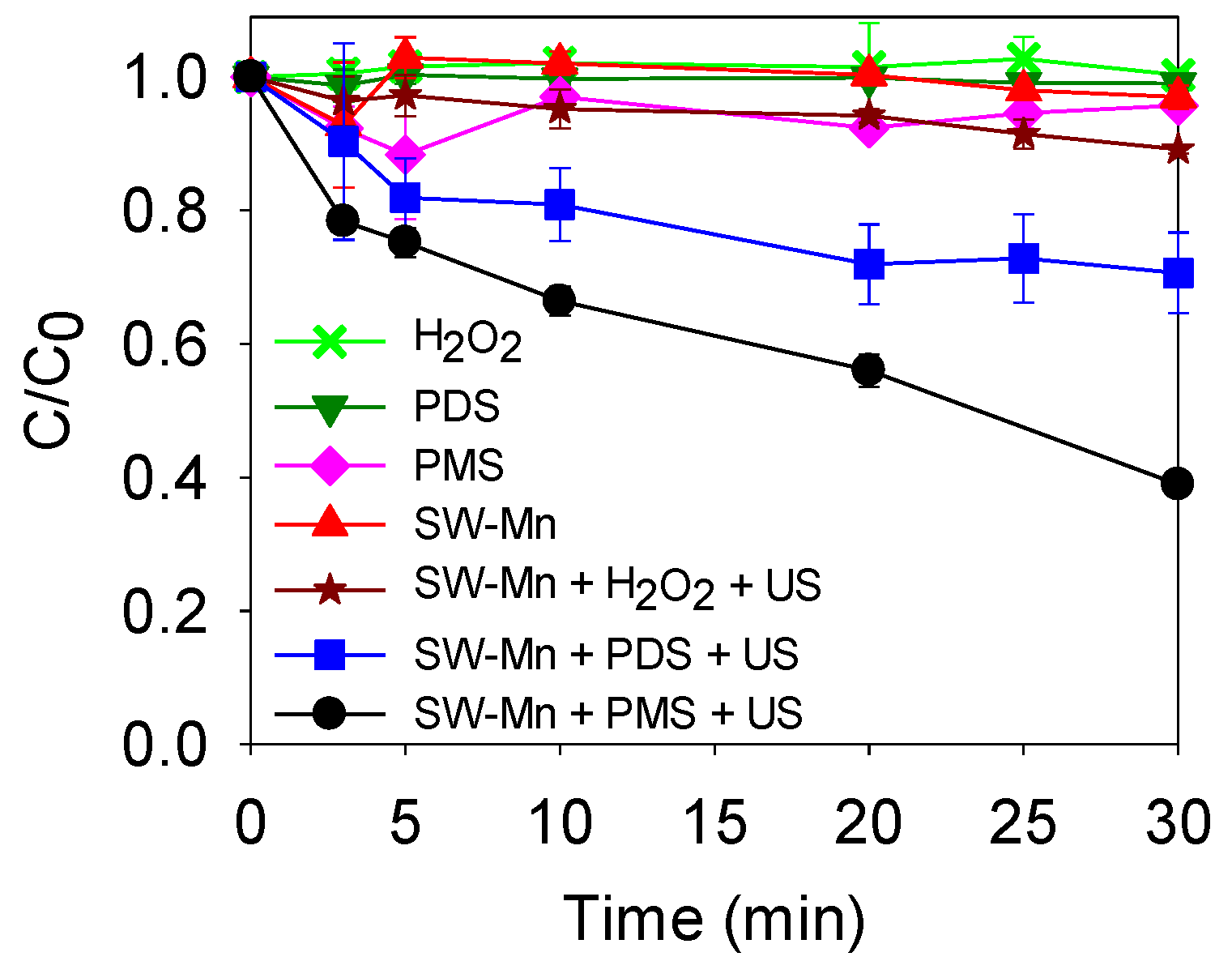
3.3.2. Effect of the Peroxide Type (H2O2, PDS, and PMS)
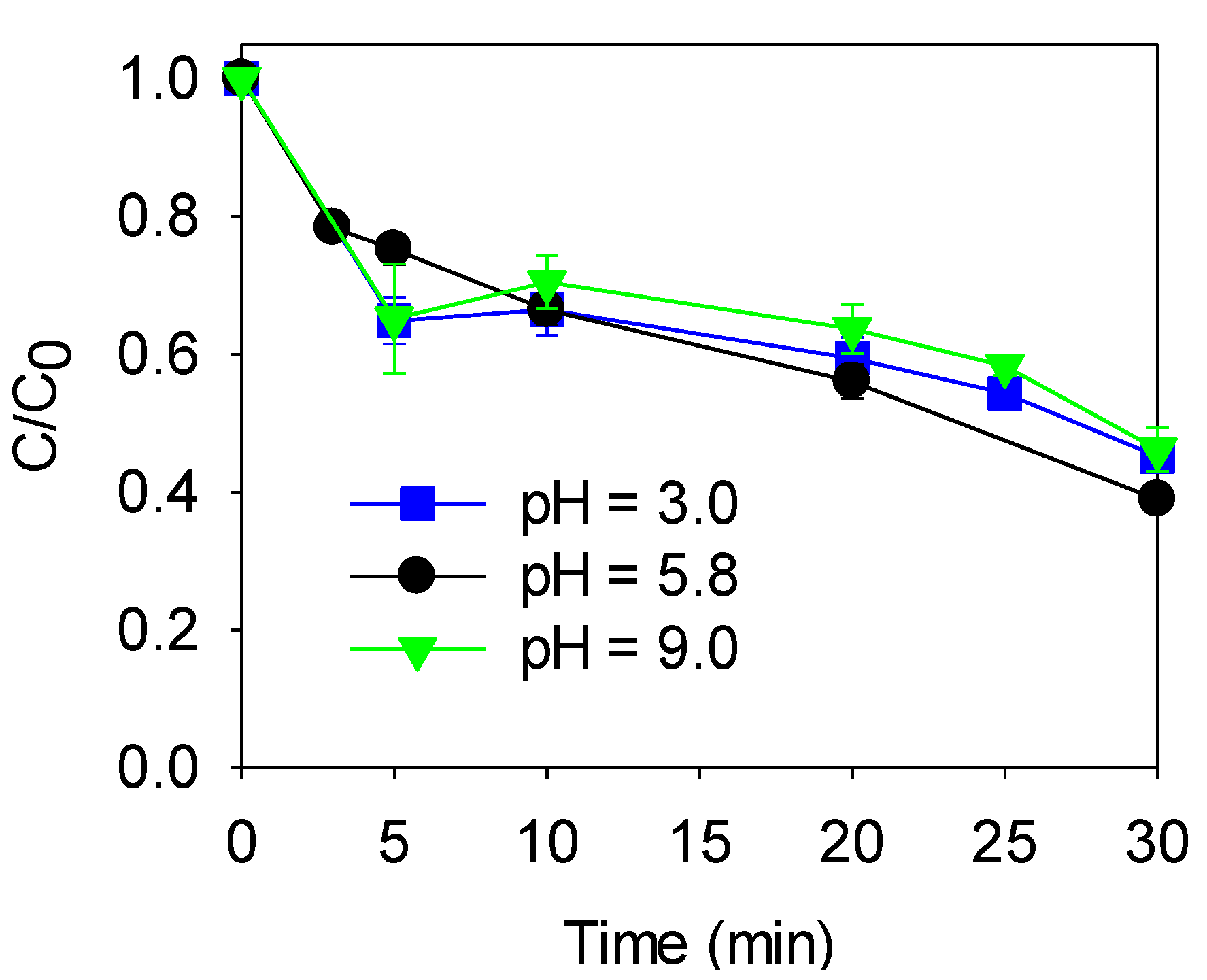
3.3.3. Effect of the pH
3.4. Identification of ROS and Reaction Mechanism during the Carbocatalytic and Sonocarbocatalytic Treatments
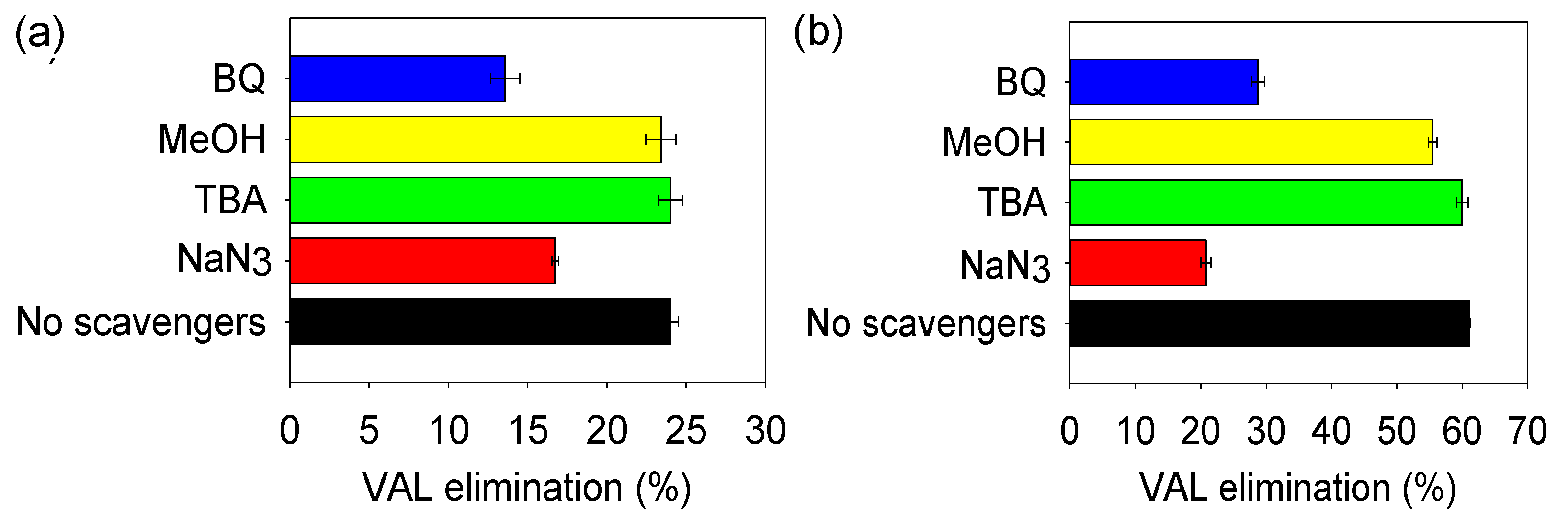
3.4.1. Role of the Reactive Oxygen Species (ROS) in the Processes
3.4.2. Role of Manganese in the Processes
3.5. Reuse of the Carbocatalyst and Effect of Water Matrix upon Sonocarbocatalysis
4. Discussion
4.1. Adsorption and Carbocatalytic Processes
4.2. Sonocarbocatalysis Process
4.3. Identification of ROS and Reaction Mechanism during the Carbocatalytic and Sonocarbocatalytic Treatment
4.3.1. Role of the Reactive Oxygen Species in the Processes
4.3.2. Role of Manganese in the Processes
4.3.3. Understanding the Interaction between SW-Mn and PMS
4.4. Applications: Reuse and Effect of Water Matrix upon Sonocarbocatalysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dhawande, A.; Moon, S.; Kale, V.; Pethe, A.M.; Raut, N.A. Chapter 1—Pharmaceutical waste: An emerging threat to the ecosystem. In 360-Degree Waste Management; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2, pp. 3–37. [Google Scholar]
- Glassman, P.M.; Muzykantov, V.R. Pharmacokinetic and pharmacodynamic properties of drug delivery systems. J. Pharmacol. Exp. Ther. 2019, 370, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Lakkaboyana, S.K.; Sharma, N.; Chakraborty, P.; Umesh, M.; Pasrija, R.; Thomas, J.; Kalebar, V.U.; Jayaraj, I.; Awasthi, M.K.; et al. A critical assessment of technical advances in pharmaceutical removal from wastewater—A critical review. Case Stud. Chem. Environ. Eng. 2023, 8, 100363. [Google Scholar] [CrossRef]
- Insani, W.N.; Qonita, N.A.; Jannah, S.S.; Nuraliyah, N.M.; Supadmi, W.; Gatera, V.A.; Alfian, S.D.; Abdulah, R. Improper disposal practice of unused and expired pharmaceutical products in Indonesian households. Heliyon 2020, 6, 6–10. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Tan, Y.H.; Mazari, S.A.; Karri, R.R.; Abdullah, E.C. Emerging pollutants and their removal using visible-light responsive photocatalysis—A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 106643. [Google Scholar] [CrossRef]
- González-González, R.B.; Sharma, P.; Singh, S.P.; Américo-Pinheiro, J.H.P.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Persistence, environmental hazards, and mitigation of pharmaceutically active residual contaminants from water matrices. Sci. Total Environ. 2022, 821, 153329. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.F.; Moreira, W.M.; de Araújo, T.P.; Bergamasco, R.; Ostroski, I.C.; de Barros, M.A.S.D. Non-conventional processes applied for the removal of pharmaceutics compounds in waters: A review. Process Saf. Environ. Prot. 2022, 167, 527–542. [Google Scholar] [CrossRef]
- Bayer, A.; Asner, R.; Schüssler, W.; Kopf, W.; Weiß, K.; Sengl, M.; Letzel, M. Behavior of sartans (antihypertensive drugs) in wastewater treatment plants, their occurrence and risk for the aquatic environment. Environ. Sci. Pollut. Res. 2014, 21, 10830–10839. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Meena, R.A.A.; Palanisami, T.; Ashokkumar, V.; Palvannan, T.; Gu, F.L. Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota—A review. Sci. Total Environ. 2020, 698, 134057. [Google Scholar] [CrossRef]
- Botero-Coy, A.M.; Martínez-Pachón, D.; Boix, C.; Rincón, J.R.; Castillo, N.; Arias-Marín, L.P.; Manrique-Losada, L.; Torres-Palma, R.A.; Moncayo-Lasso, A.; Hernández, F. An investigation into the occurrence and removal of pharmaceuticals in colombian wastewater. Sci. Total Environ. 2018, 62, 842–853. [Google Scholar] [CrossRef]
- Jasim, N.A. The design for wastewater treatment plant (WWTP) with GPS X modelling. Cogent Eng. 2020, 7, 1723782. [Google Scholar] [CrossRef]
- Infrastructures, H. Design and construction of wastewater treatment plant (WWTP). Available online: https://www.sice.com/sites/Sice/files/2016-10/ENV_EDAR_Construction_ENG_(9).pdf (accessed on 18 October 2023).
- Englande, A.J.; Krenkel, P.A. Waste Water Treatment and Water Reclamation. In Encyclopedia of Physical Science and Technology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 639–670. [Google Scholar]
- Bwapwa, J.; Jaiyeola, A. Emerging Contaminants in Drinking Water and Wastewater, Effects on Environment and Remediation. Int. J. Appl. Eng. Res. 2019, 14, 539–546. [Google Scholar]
- Arrubla, J.P.; Cubillos, J.A.; Ramírez, C.A.; Arredondo, J.A.; Arias, C.A.; Paredes, D. Pharmaceutical and personal care products in domestic wastewater and their removal in anaerobic treatment systems: Septic tank—Up flow anaerobic filter|Presencia de productos farmacéuticos y de cuidado personal en aguas residuales domésticas y su elim. Ing. E Investig. 2016, 36, 70–78. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Martínez-Mena, Y.L.; Arboleda-Echavarría, J.; Hoyos-Ayala, D.A.; Echavarría-Isaza, A.; Torres-Palma, R.A. Zeolite 4A activates peroxymonosulfate toward the production of singlet oxygen for the selective degradation of organic pollutants. Chem. Eng. Res. Des. 2023, 193, 121–131. [Google Scholar] [CrossRef]
- Ayanda, S. Water Treatment Technologies: Principles, Applications, Successes and Limitations of Bioremediation, Membrane Bioreactor and the Advanced Oxidation Processes; OMICS International: Hyderabad, India, 2017; ISBN 9781632780584. [Google Scholar]
- Yu, J.; Zhu, Z.; Zhang, H.; Shen, X.; Qiu, Y.; Yin, D.; Wang, S. Persistent free radicals on N-doped hydrochar for degradation of endocrine disrupting compounds. Chem. Eng. J. 2020, 398, 125538. [Google Scholar] [CrossRef]
- Gasim, M.F.; Veksha, A.; Lisak, G.; Low, S.C.; Hamidon, T.S.; Hussin, M.H.; Oh, W. Da Importance of carbon structure for nitrogen and sulfur co-doping to promote superior ciprofloxacin removal via peroxymonosulfate activation. J. Colloid Interface Sci. 2023, 634, 586–600. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Qu, S.; Yuan, Y.; Yang, X.; Xu, H.; Mohamed, A.K.; Zhang, J.; Zhao, C.; Liu, L.; Wang, B.; Wang, X.; et al. Carbon defects in biochar facilitated nitrogen doping: The significant role of pyridinic nitrogen in peroxymonosulfate activation and ciprofloxacin degradation. Chem. Eng. J. 2022, 441, 135864. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 132387. [Google Scholar] [CrossRef]
- Oh, W.D.; Lim, T.T. Design and application of heterogeneous catalysts as peroxydisulfate activator for organics removal: An overview. Chem. Eng. J. 2019, 358, 110–133. [Google Scholar] [CrossRef]
- Liu, H.; Ye, M.; Ren, Z.; Lichtfouse, E.; Chen, Z. Towards synergistic combination of biochar/ultrasonic persulfate enhancing removal of natural humic acids from water. J. Environ. Chem. Eng. 2022, 10, 107809. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Li, J.; Qiu, J.; Cai, C.; Zhang, H. Degradation of Acid Orange 7 by persulfate activated with zero valent iron in the presence of ultrasonic irradiation. Sep. Purif. Technol. 2014, 122, 41–46. [Google Scholar] [CrossRef]
- Diao, Z.H.; Dong, F.X.; Yan, L.; Chen, Z.L.; Qian, W.; Kong, L.J.; Zhang, Z.W.; Zhang, T.; Tao, X.Q.; Du, J.J.; et al. Synergistic oxidation of Bisphenol A in a heterogeneous ultrasound-enhanced sludge biochar catalyst/persulfate process: Reactivity and mechanism. J. Hazard. Mater. 2020, 384, 121385. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, H.; Zhong, X.; Hou, L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe-Co/SBA-15 catalyst for the degradation of Orange II in water. J. Hazard. Mater. 2015, 283, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Xiang, L.; Royer, S.; Valange, S.; Barrault, J.; Zhang, H. Degradation of C.I. Acid Orange 7 by heterogeneous Fenton oxidation in combination with ultrasonic irradiation. J. Chem. Technol. Biotechnol. 2011, 86, 970–977. [Google Scholar] [CrossRef]
- Liu, F.; Yi, P.; Wang, X.; Gao, H.; Zhang, H. Degradation of Acid Orange 7 by an ultrasound/ZnO-GAC/persulfate process. Sep. Purif. Technol. 2018, 194, 181–187. [Google Scholar] [CrossRef]
- Cherifi, Y.; Addad, A.; Vezin, H.; Barras, A.; Ouddane, B.; Chaouchi, A.; Szunerits, S.; Boukherroub, R. PMS activation using reduced graphene oxide under sonication: Efficient metal-free catalytic system for the degradation of rhodamine B, bisphenol A, and tetracycline. Ultrason. Sonochem. 2019, 52, 164–175. [Google Scholar] [CrossRef]
- Sun, X.; Xu, D.; Dai, P.; Liu, X.; Tan, F.; Guo, Q. Efficient degradation of methyl orange in water via both radical and non-radical pathways using Fe-Co bimetal-doped MCM-41 as peroxymonosulfate activator. Chem. Eng. J. 2020, 402, 125881. [Google Scholar] [CrossRef]
- Grisales-Cifuentes, C.M.; Serna-Galvis, E.A.; Acelas, N.; Porras, J.; Flórez, E.; Torres-Palma, R.A. Biochar from palm fiber wastes as an activator of different oxidants for the elimination of pharmaceuticals from diverse classes in aqueous samples. J. Environ. Manage. 2022, 323, 116148. [Google Scholar] [CrossRef]
- Ademollo, N.; Spataro, F.; Rauseo, J.; Pescatore, T.; Fattorini, N.; Valsecchi, S.; Polesello, S.; Patrolecco, L. Occurrence, distribution and pollution pattern of legacy and emerging organic pollutants in surface water of the Kongsfjorden (Svalbard, Norway): Environmental contamination, seasonal trend and climate change. Mar. Pollut. Bull. 2021, 163, 111900. [Google Scholar] [CrossRef]
- Zou, Y.; Li, W.; Yang, L.; Xiao, F.; An, G.; Wang, Y.; Wang, D. Activation of peroxymonosulfate by sp2-hybridized microalgae-derived carbon for ciprofloxacin degradation: Importance of pyrolysis temperature. Chem. Eng. J. 2019, 370, 1286–1297. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.D.; Lim, T.T. Graphene-and CNTs-based carbocatalysts in persulfates activation: Material design and catalytic mechanisms. Chem. Eng. J. 2018, 354, 941–976. [Google Scholar] [CrossRef]
- Torres-Palma, R.A.; Nieto, J.I.; Combet, E.; Pétrier, C.; Pulgarin, C. An innovative ultrasound, Fe2+ and TiO2 photoassisted process for bisphenol a mineralization. Water Res. 2010, 44, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ansari, K. Chemically treated Lawsonia inermis seeds powder (CTLISP): An eco-friendly adsorbent for the removal of brilliant green dye from aqueous solution. Groundw. Sustain. Dev. 2020, 11, 100417. [Google Scholar] [CrossRef]
- Shankar, U.; Gogoi, R.; Sethi, S.K.; Verma, A. Forcefields for Atomistic-Scale Simulations: Materials and Applications; Springer: Berlin/Heidelberg, Germany, 2022; Volume 99, pp. 299–313. ISBN 9789811930928. [Google Scholar]
- Wang, H.; Lu, Z.; Qian, D.; Li, Y.; Zhang, W. Single-crystal α-MnO2 nanorods: Synthesis and electrochemical properties. Nanotechnology 2007, 18, 2–7. [Google Scholar] [CrossRef]
- Racik, K.M.; Guruprasad, K.; Mahendiran, M.; Madhavan, J.; Maiyalagan, T.; Raj, M.V.A. Enhanced Electrochemical Performance of MnO2/NiO Nanocomposite for Supercapacitor Electrode with Excellent Cycling Stability. J. Mater. Sci. Mater. Electron. 2019, 30, 5222–5232. [Google Scholar] [CrossRef]
- Tang, N.; Tian, X.; Yang, C.; Pi, Z.; Han, Q. Facile Synthesis of α-MnO2 Nanorods for High-Performance Alkaline Batteries. J. Phys. Chem. Solids 2010, 71, 258–262. [Google Scholar] [CrossRef]
- Motozuka, S.; Tagaya, M.; Hayashi, K.; Kameyama, T.; Oguri, H.; Xu, Z. Mechanochemical Surface Modification of Carbon Fibers Using a Simple Rubbing Method. J. Compos. Mater. 2017, 51, 3577–3584. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Dang, B.; Xiong, Y.; Yao, Q.; Wang, C.; Sun, Q.; Jin, C. Bio-Inspired Nacre-like Nanolignocellulose-Poly (Vinyl Alcohol)-TiO2 Composite with Superior Mechanical and Photocatalytic Properties. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Shircliff, R.A.; Stradins, P.; Moutinho, H.; Fennell, J.; Ghirardi, M.L.; Cowley, S.W.; Branz, H.M.; Martin, I.T. Angle-Resolved XPS Analysis and Characterization of Monolayer and Multilayer Silane Films for DNA Coupling to Silica. Langmuir 2013, 29, 4057–4067. [Google Scholar] [CrossRef]
- Guateque-Londoño, J.F.; Serna-Galvis, E.A.; Ávila-Torres, Y.; Torres-Palma, R.A. Degradation of Losartan in Fresh Urine by Sonochemical and Photochemical Advanced Oxidation Processes. Water (Switzerland) 2020, 12. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Q.; Zhang, C.; Wang, R.; Deng, R.; Luo, H.; Li, T.; Li, J.; Chen, S.; Liu, C. Mn Doped Magnetic Biochar as Persulfate Activator for the Degradation of Tetracycline. Chem. Eng. J. 2020, 391, 123532. [Google Scholar] [CrossRef]
- Ren, W.; Nie, G.; Zhou, P.; Zhang, H.; Duan, X.; Wang, S. The Intrinsic Nature of Persulfate Activation and N-Doping in Carbocatalysis. Environ. Sci. Technol. 2020, 54, 6438–6447. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Ngo, H.H.; Guo, W.; Li, Z.; Wang, X.; Zhang, J.; Long, T. A New Spent Coffee Grounds Based Biochar-Persulfate Catalytic System for Enhancement of Urea Removal in Reclaimed Water for Ultrapure Water Production. Chemosphere 2022, 288, 132459. [Google Scholar] [CrossRef] [PubMed]
- Forouzesh, M.; Ebadi, A.; Aghaeinejad-Meybodi, A. Degradation of Metronidazole Antibiotic in Aqueous Medium Using Activated Carbon as a Persulfate Activator. Sep. Purif. Technol. 2019, 210, 145–151. [Google Scholar] [CrossRef]
- Ji, R.; Chen, J.; Liu, T.; Zhou, X.; Zhang, Y. Critical Review of Perovskites-Based Advanced Oxidation Processes for Wastewater Treatment: Operational Parameters, Reaction Mechanisms, and Prospects. Chinese Chem. Lett. 2022, 33, 643–652. [Google Scholar] [CrossRef]
- Guateque-Londoño, J.F.; Serna-Galvis, E.A.; Silva-Agredo, J.; Ávila-Torres, Y.; Torres-Palma, R.A. Dataset on the Degradation of Losartan by TiO2-Photocatalysis and UVC/Persulfate Processes. Data Br. 2020, 31, 105692. [Google Scholar] [CrossRef]
- Chen, X.-L.; Li, H.; Lai, L.; Zhang, Y.; Chen, Y.; Li, X.; Liu, B.; Wang, H. Peroxymonosulfate Activation Using MnFe2O4 Modified Biochar for Organic Pollutants Degradation: Performance and Mechanisms. Sep. Purif. Technol. 2023, 308, 122886. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of Persulfate (PS) and Peroxymonosulfate (PMS) and Application for the Degradation of Emerging Contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Mojović, M.; Vuletić, M.; Bačić, G.G. Detection of Oxygen-Centered Radicals Using EPR Spin-Trap DEPMPO: The Effect of Oxygen. Ann. N. Y. Acad. Sci. 2005, 1048, 471–475. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, C.H. Oxidative Transformation of Fluoroquinolone Antibacterial Agents and Structurally Related Amines by Manganese Oxide. Environ. Sci. Technol. 2005, 39, 4474–4483. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Z.; Yang, K.; Yu, Z.; Zuo, Y.; Cheng, L.; Wang, Y.; Sun, H.; Yu, G.; Zhang, C.; et al. Periodate Activated by Different Crystalline Phases MnO2 for Profound Oxidation Tetracycline Hydrochloride: Oxygen Vacancy-Dominated Active Pivots and Mechanism. Sep. Purif. Technol. 2022, 301, 122022. [Google Scholar] [CrossRef]
- Gan, L.; Fang, X.; Xu, L.; Wang, L.; Wu, Y.; Dai, B.; He, W.; Shi, J. Boosted Activity of δ-MnO2 by Kenaf Derived Carbon Fiber for High-Efficient Oxidative Degradation of Bisphenol A in Water. Mater. Des. 2021, 203, 1–11. [Google Scholar] [CrossRef]
- Audi, A.A.; Sherwood, P.M.A. Valence-Band x-Ray Photoelectron Spectroscopic Studies of Manganese and Its Oxides Interpreted by Cluster and Band Structure Calculations. Surf. Interface Anal. 2002, 33, 274–282. [Google Scholar] [CrossRef]
- Rosso, J.J.; Hochella, M.F. Natural Iron and Manganese Oxide Samples by XPS. Surf. Sci. Spectra 1996, 4, 253–265. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of Catalyst Deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Effect of Inorganic Anions on the Performance of Advanced Oxidation Processes for Degradation of Organic Contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Hagan, G.B.; Minkah, R.; Yiran, G.A.B.; Dankyi, E. Assessing Groundwater Quality in Peri-Urban Accra, Ghana: Implications for Drinking and Irrigation Purposes. Groundw. Sustain. Dev. 2022, 17, 100761. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Rosseto, M.; Nazari, M.T.; Ostwald, B.E.P.; Alessandretti, I.; Manera, C.; Piccin, J.S.; Dettmer, A. Adsorption of Diclofenac Sodium by Composite Beads Prepared from Tannery Wastes-Derived Gelatin and Carbon Nanotubes. J. Environ. Chem. Eng. 2021, 9, 105030. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Bairamis, F.; Konstantinou, I.; Mantzavinos, D.; Frontistis, Z. Degradation of Antihypertensive Drug Valsartan in Water Matrices by Heat and Heat/Ultrasound Activated Persulfate: Kinetics, Synergy Effect and Transformation Products. Chem. Eng. J. Adv. 2020, 4, 100062. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. A Novel Strategy of Successive Non-Radical and Radical Process for Enhancing the Utilization Efficiency of Persulfate. Chemosphere 2020, 245, 125555. [Google Scholar] [CrossRef]
- Wang, G.; Hambly, A.C.; Zhao, D.; Wang, G.; Tang, K.; Andersen, H.R. Peroxymonosulfate Activation by Suspended Biogenic Manganese Oxides for Polishing Micropollutants in Wastewater Effluent. Sep. Purif. Technol. 2023, 306, 122501. [Google Scholar] [CrossRef]
- Zhao, W.; Duan, Z.; Zheng, Z.; Li, B. Efficient Diclofenac Removal by Superoxide Radical and Singlet Oxygen Generated in Surface Mn(II)/(III)/(IV) Cycle Dominated Peroxymonosulfate Activation System: Mechanism and Product Toxicity. Chem. Eng. J. 2022, 433, 133742. [Google Scholar] [CrossRef]
- Camargo-Perea, A.L.; Serna-Galvis, E.A.; Lee, J.; Torres-Palma, R.A. Understanding the Effects of Mineral Water Matrix on Degradation of Several Pharmaceuticals by Ultrasound: Influence of Chemical Structure and Concentration of the Pollutants. Ultrason. Sonochem. 2021, 73, 105500. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Son, Y. Ultrasonics Sonochemistry Effects of Gas Saturation and Sparging on Sonochemical Oxidation Activity under Different Liquid Level and Volume Conditions in 300-KHz Sonoreactors: Zeroth- and First-Order Reaction Comparison Using KI Dosimetry and BPA Degrada. Ultrason. Sonochem. 2023, 98, 106521. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Karami, N.; Zinatizadeh, A.A.; Falahi, F.; Aghamohammadi, N.; Almasi, A. Using High Frequency and Low-Intensity Ultrasound to Enhance Activated Sludge Characteristics. Ultrason. Sonochem. 2019, 54, 274–280. [Google Scholar] [CrossRef]
- McKenzie, T.G.; Karimi, F.; Ashokkumar, M.; Qiao, G.G. Ultrasound and Sonochemistry for Radical Polymerization: Sound Synthesis. Chem.-A Eur. J. 2019, 25, 5372–5388. [Google Scholar] [CrossRef]
- Ziylan-Yavaş, A.; Ince, N.H. Enhanced Photo-Degradation of Paracetamol on n-Platinum-Loaded TiO2: The Effect of Ultrasound and [Rad]OH/Hole Scavengers. Chemosphere 2016, 162, 324–332. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Sun, Y.; Gong, H.; Guo, M.; Zhang, X.; Meng, L.; Gan, L. Mechanistic Study on the Combination of Ultrasound and Peroxymonosulfate for the Decomposition of Endocrine Disrupting Compounds. Ultrason. Sonochem. 2020, 60, 104749. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Zhou, X.; Wu, Q.; Zheng, H.; Chang, J.; Ren, N. Enhanced Peroxymonosulfate Activation for Sulfamethazine Degradation by Ultrasound Irradiation: Performances and Mechanisms. Chem. Eng. J. 2018, 335, 145–153. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Chen, Z.; Zeng, Q.; Hu, C. Insights into the Difference in Metal-Free Activation of Peroxymonosulfate and Peroxydisulfate. Chem. Eng. J. 2020, 394, 123936. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, T.; Li, R.; Cao, X.; Kan, Y.; Wei, B.; Sun, X. Efficient Degradation of Ibuprofen by Co/Fe@CNFs Catalyst in the Presence of Peroxymonosulfate and Persulfate: Characterization, Performance, and Mechanism Comparison. J. Taiwan Inst. Chem. Eng. 2022, 131, 104161. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Liu, P.; Bai, X.; Du, X.; Jin, P.; Yang, L.; Jin, X.; Shi, X.; Wang, Y. Peroxymonosulfate Activation by Nitrogen-Doped Biochar from Sawdust for the Efficient Degradation of Organic Pollutants. Chem. Eng. J. 2020, 387, 124065. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Xu, H.; Wen, Q. Efficient Heterogeneous Activation of Peroxymonosulfate by Modified CuFe2O4 for Degradation of Tetrabromobisphenol A. Chem. Eng. J. 2020, 389. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Li, L.Y.; Mohamed, B.A.; Ramim, S.S. Adsorption of P-Benzoquinone at Low Concentrations from Aqueous Media Using Biosolid-Based Activated Carbon. J. Environ. Manag. 2022, 316, 115263. [Google Scholar] [CrossRef]
- Li, G.; Zhu, W.; Zhu, L.; Chai, X. Effect of Pyrolytic Temperature on the Adsorptive Removal of P-Benzoquinone, Tetracycline, and Polyvinyl Alcohol by the Biochars from Sugarcane Bagasse. Korean J. Chem. Eng. 2016, 33, 2215–2221. [Google Scholar] [CrossRef]
- Guoting, L.; Yanmin, F.; Xiaoqi, C.; Erming, L.; Qingtong, W.; Hao, Z. Efficient Adsorptive Removal of P-Benzoquinone by Lanthanum Modified Activated Carbon Fibre. J. Environ. Eng. 2016, 10, 1638–1644. [Google Scholar]
- Lee, J.; Von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Yang, S.; Xu, S.; Tong, J.; Ding, D.; Wang, G.; Chen, R.; Jin, P.; Wang, X.C. Overlooked Role of Nitrogen Dopant in Carbon Catalysts for Peroxymonosulfate Activation: Intrinsic Defects or Extrinsic Defects? Appl. Catal. B Environ. 2021, 295, 120291. [Google Scholar] [CrossRef]
- Tan, J.; Xu, C.; Zhang, X.; Huang, Y. MOFs-Derived Defect Carbon Encapsulated Magnetic Metallic Co Nanoparticles Capable of Efficiently Activating PMS to Rapidly Degrade Dyes. Sep. Purif. Technol. 2022, 289, 120812. [Google Scholar] [CrossRef]
- Zhao, Y.; An, H.; Dong, G.; Feng, J.; Wei, T.; Ren, Y.; Ma, J. Oxygen Vacancies Induced Heterogeneous Catalysis of Peroxymonosulfate by Ni-Doped AgFeO2 Materials: Evolution of Reactive Oxygen Species and Mechanism. Chem. Eng. J. 2020, 388, 124371. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A Review of the Innovations in Metal- and Carbon-Based Catalysts Explored for Heterogeneous Peroxymonosulfate (PMS) Activation, with Focus on Radical vs. Non-Radical Degradation Pathways of Organic Contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, S.; Zhong, H.; Yan, M.; Yang, X. Activation of Persulfates by Carbonaceous Materials: A Review. Chem. Eng. J. 2021, 418, 129297. [Google Scholar] [CrossRef]
- Fang, Z.; Qi, J.; Xu, Y.; Liu, Y.; Qi, T.; Xing, L.; Dai, Q.; Wang, L. Promoted Generation of Singlet Oxygen by Hollow-Shell CoS/g-C3N4 Catalyst for Sulfonamides Degradation. Chem. Eng. J. 2022, 441, 136051. [Google Scholar] [CrossRef]
- Wu, S.; Yang, C.; Lin, Y.; Cheng, J.J. Efficient Degradation of Tetracycline by Singlet Oxygen-Dominated Peroxymonosulfate Activation with Magnetic Nitrogen-Doped Porous Carbon. J. Environ. Sci. (China) 2022, 115, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, X.; Guo, Q.; Wang, X.; Zhang, L.; Zhu, W.; Luo, Y. Activation of Peroxymonosulfate by the CoFe/ZSM-5 for Efficient Sulfamethoxazole Degradation. J. Environ. Chem. Eng. 2022, 10, 107012. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Chen, D.; Dai, G.; Wei, D.; Shu, Y. Activation of Persulfate with Biochar for Degradation of Bisphenol A in Soil. Chem. Eng. J. 2020, 381, 122637. [Google Scholar] [CrossRef]
- Guo, J.; Wen, X.; Yang, J.; Fan, T. Removal of Benzo(a)Pyrene in Polluted Aqueous Solution and Soil Using Persulfate Activated by Corn Straw Biochar. J. Environ. Manage. 2020, 272, 111058. [Google Scholar] [CrossRef]
- Lara-Pérez, C.; Leyva, E.; Zermeño, B.; Osorio, I.; Montalvo, C.; Moctezuma, E. Photocatalytic degradation of diclofenac sodium salt: Adsorption and reaction kinetic studies. Environ. Earth Sci. 2020, 79, 1–13. [Google Scholar] [CrossRef]
- Siddiqui, N.; Husain, A.; Chaudhry, L.; Alam, S.S.; Mitra, M.; Bhasin, P.S. Pharmacological and pharmaceutical profile of valsartan: A review. J. Appl. Pharm. Sci. 2011, 1, 12–19. [Google Scholar]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Nackiewicz, J.; Gąsowska-Bajger, B.; Kołodziej, Ł.; Poliwoda, A.; Pogoda-Mieszczak, K.; Skonieczna, M. Comparison of the degradation mechanisms of diclofenac in the presence of iron octacarboxyphthalocyanine and myeloperoxidase. Spectrochim. Acta–Part A Mol. Biomol. Spectrosc. 2023, 287. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, M.S.; Azha, S.F.; Ismail, S. A review of diclofenac occurrences, toxicology, and potential adsorption of clay-based materials with surfactant modifier. J. Environ. Chem. Eng. 2022, 10, 107541. [Google Scholar] [CrossRef]
- Vasantha Raman, N.; Gsell, A.S.; Voulgarellis, T.; van den Brink, N.W.; de Senerpont Domis, L.N. Moving beyond standard toxicological metrics: The effect of diclofenac on planktonic host-parasite interactions. Aquat. Toxicol. 2023, 254, 106370. [Google Scholar] [CrossRef] [PubMed]


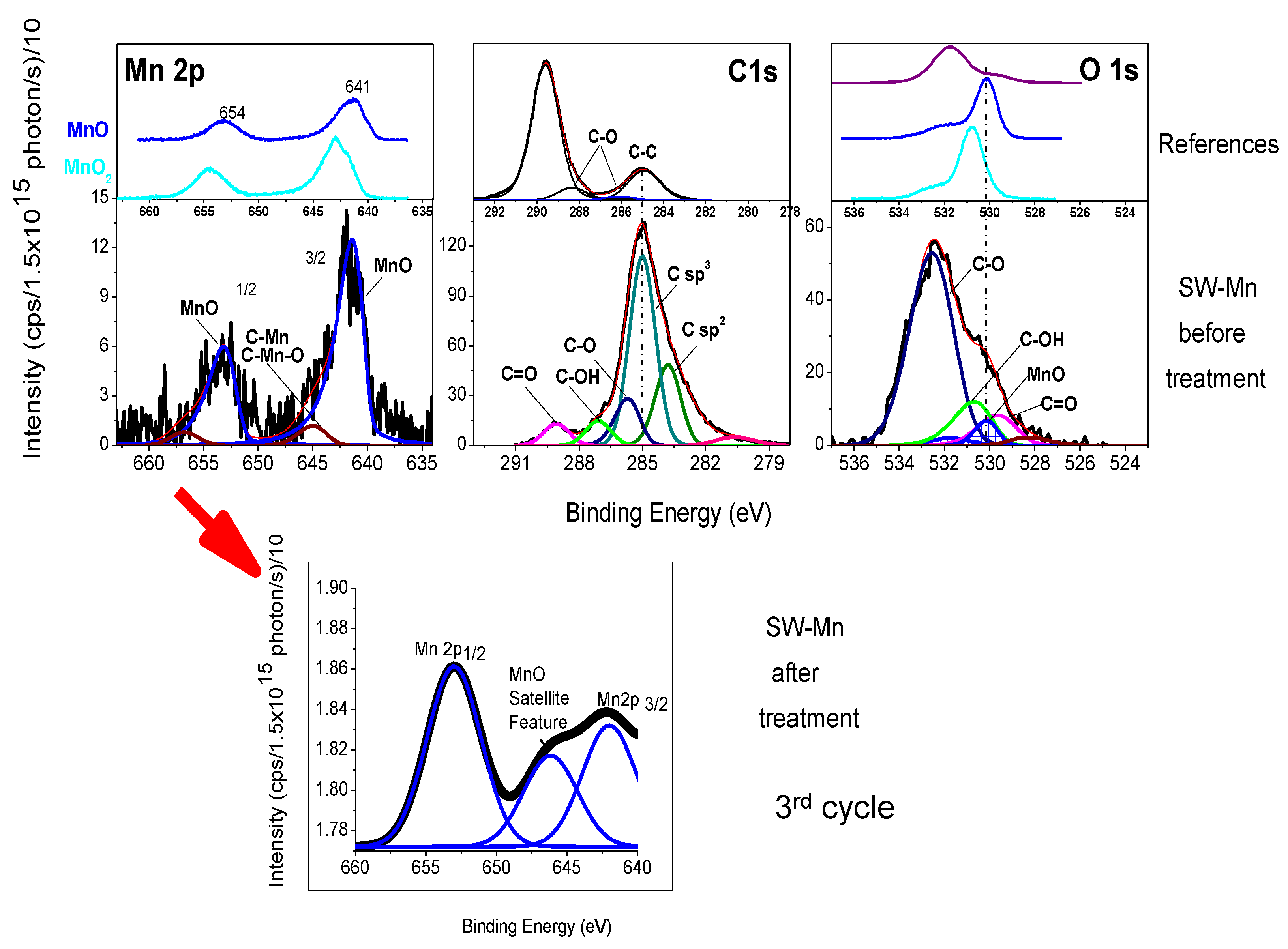



| Mn 2p3/2 | C 1s | O 1s | Si 2p | |||||||||||||
| Binding Energy (eV) | ||||||||||||||||
| Sample (Chem %) * | MnO | MnO2 | Mn-C-O Mn-C | CH-CH Sp2 | CH-CH Sp3 | C-O | C-OH | C=O | C-Mn-O C-Mn | O-C | O=C | OH-C | MnO sat | MnO2 sat | C-Mn-O C-Mn | Si-O |
| C-Mn | 641.40 | --- | 645.00 | 283.79 | 285.00 | 285.70 | 287.10 | 289.08 | 282.69 280.63 | 532.55 | 529.65 | 530.68 | 530.13 531.74 | --- | 528.30 --- | 102.70 |
| % | (91.5) | --- | (8.5) | (19.3) | (46.2) | (11.1) | (5.8) | (5.2) | (9.5) (3.0) | (67.8) | (9.3) | (13.6) | (6.9) | --- | (2.5) | (100) |
| SW-Mn before | 641.40 | 642.80 | 645.00 | 283.79 | 285.00 | 285.64 | 287.10 | 289.08 | 282.69 280.20 | 532.66 | 529.65 | 530.55 | 530.13 531.74 | 530.78 532.48 | 527.60 525.60 | 102.70 |
| % | (91.5) | (0) | (8.5) | (8.1) | (40.1) | (29.4) | (4.1) | (3.0) | (8.8) (6.4) | (47.2) | (18.4) | (11.4) | (14.4) | 0 | (7.9) (0.6) | (100) |
| SW-Mn after | 641.40 | 642.80 | 645.00 | 283.79 | 285.00 | 285.70 | 287.10 | 289.08 | 282.69 --- | 532.45 | 529.65 | 530.50 | 530.13 531.74 | 530.13 531.74 | 528.30 --- | 102.70 |
| % | (96.5) | (0) | (3.5) | (10.7) | (53.1) | (21.0) | (5.1) | (3.5) | (6.6) | (52.7) | (14.0) | (15.8) | (10.7) | (0) | (4.7) | (100) |
| MnO MnO2 | 641.40 | 542.80 | 530.13 531.74 | 530.78 532.48 | ||||||||||||
| Sample | Mn | C | O | Si | ||||||||||||
| Elemental composition (%) ** | ||||||||||||||||
| C-Mn | 4.3 | 76.1 | 18.7 | 1.0 | ||||||||||||
| SW-Mn before% | 9.8 | 62.7 | 25.7 | 1.8 | ||||||||||||
| SW-Mn after% | 8.4 | 64.7 | 24.6 | 2.3 | ||||||||||||
| Parameters | Average Value |
|---|---|
| pH | 7.5 |
| Conductivity (µS cm–1) | 770 |
| TOC (mg L–1) | 27.6 |
| COD (mg L–1) | 54.4 |
| Chlorides (mg L–1) | 68.5 |
| Alkalinity (mg L–1) | 84.7 |
| Process | SBET (m2 g−1) | VµP (cm3 g−1) | VMP (cm3 g−1) | VTP (cm3 g−1) | DAP (mm) |
|---|---|---|---|---|---|
| DCF | |||||
| Before | 103.7 ± 5.6 | 0.075 | 0.007 | 0.082 | 3.51 |
| Adsorption | 83.9 ± 4.3 | 0.064 | 0.01 | 0.074 | 3.53 |
| Carbocatalysis | 53.2 ± 8.2 | 0.045 | 0.007 | 0.052 | 3.91 |
| VAL | |||||
| Before | 103.7 ± 5.6 | 0.075 | 0.007 | 0.082 | 3.51 |
| Adsorption | 96.8 ± 9.4 | 0.075 | 0.007 | 0.078 | 3.22 |
| Carbocatalysis | 68.9 ± 6.6 | 0.064 | 0.005 | 0.070 | 3.75 |
| Sonocarbocatalysis | 55.5 ± 8.3 | 0.060 | 0.004 | 0.064 | 3.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quimbaya-Ñañez, C.; Serna-Galvis, E.A.; Silva-Agredo, J.; García-Rubio, I.; Torres-Palma, R.A.; Ávila-Torres, Y.P. Improvement of the Carbocatalytic Degradation of Pharmaceuticals in Water by the Use of Ultrasound Waves. Water 2023, 15, 3679. https://doi.org/10.3390/w15203679
Quimbaya-Ñañez C, Serna-Galvis EA, Silva-Agredo J, García-Rubio I, Torres-Palma RA, Ávila-Torres YP. Improvement of the Carbocatalytic Degradation of Pharmaceuticals in Water by the Use of Ultrasound Waves. Water. 2023; 15(20):3679. https://doi.org/10.3390/w15203679
Chicago/Turabian StyleQuimbaya-Ñañez, Carolina, Efraím A. Serna-Galvis, Javier Silva-Agredo, Inés García-Rubio, Ricardo A. Torres-Palma, and Yenny P. Ávila-Torres. 2023. "Improvement of the Carbocatalytic Degradation of Pharmaceuticals in Water by the Use of Ultrasound Waves" Water 15, no. 20: 3679. https://doi.org/10.3390/w15203679





