Chemical Characterization and Genesis of Thermal Reservoir Water in the Southern Part of the Jizhong Depression
Abstract
:1. Introduction
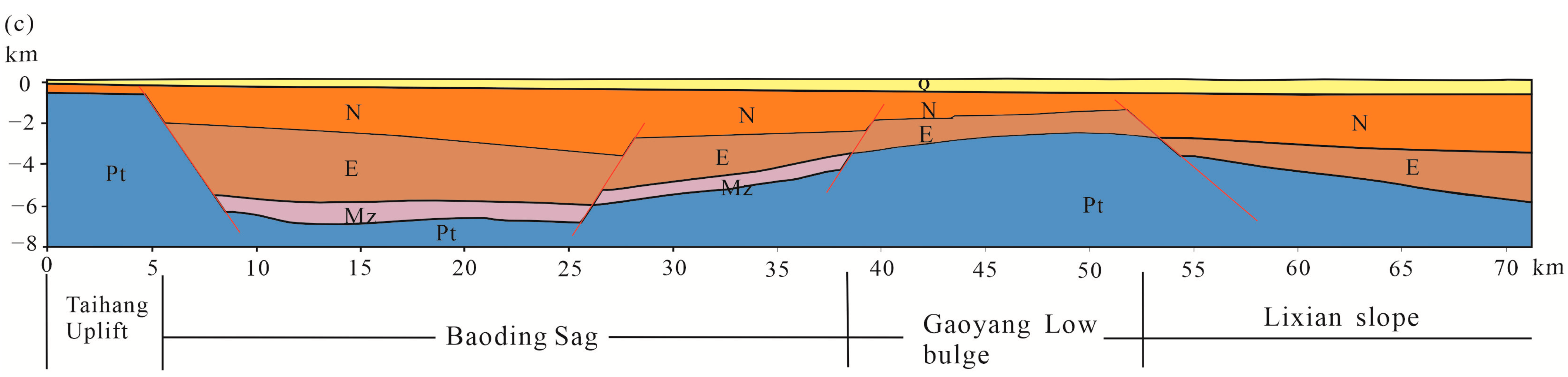
2. Regional Geological Setting
3. Materials and Methods
3.1. Hydrochemical Sample
3.2. Geothermometer
3.2.1. Cationic Geothermal Temperature Scale
3.2.2. Silicon Dioxide Temperature Scale
3.3. Multi-Mineral Equilibrium Graphical Method
3.4. Water–Rock Balance Analysis
4. Results
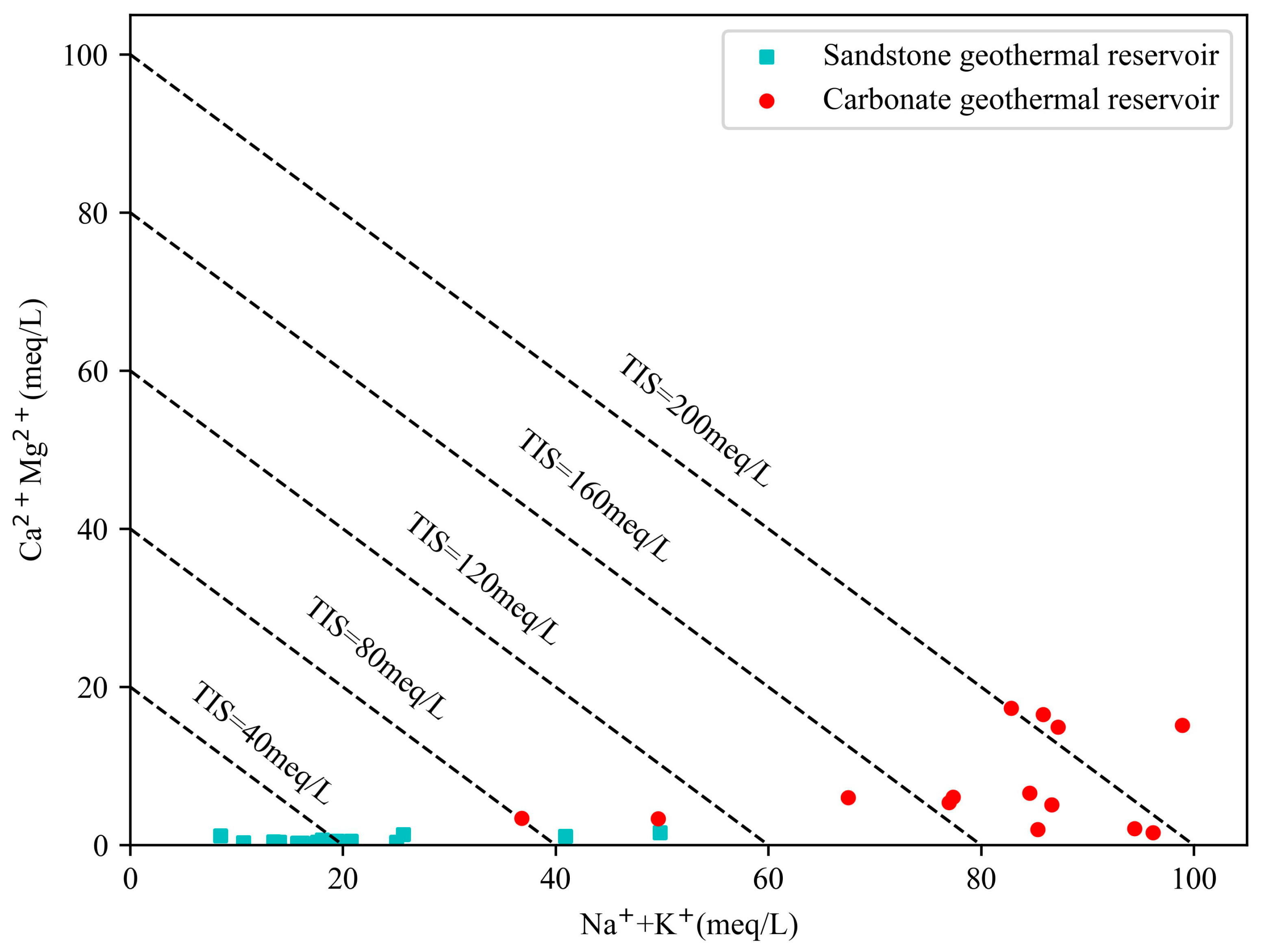
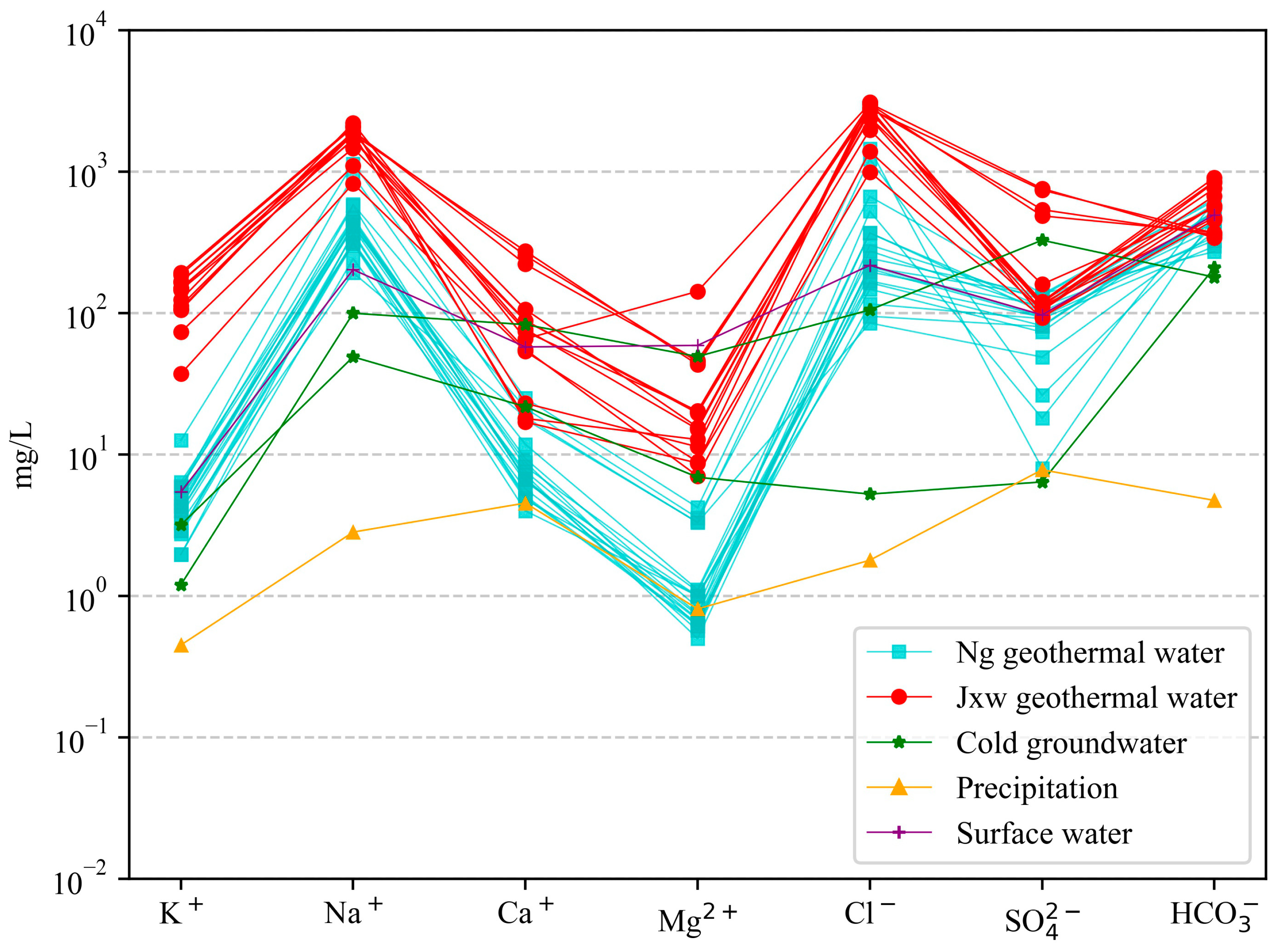
4.1. Hydrochemical Characteristics
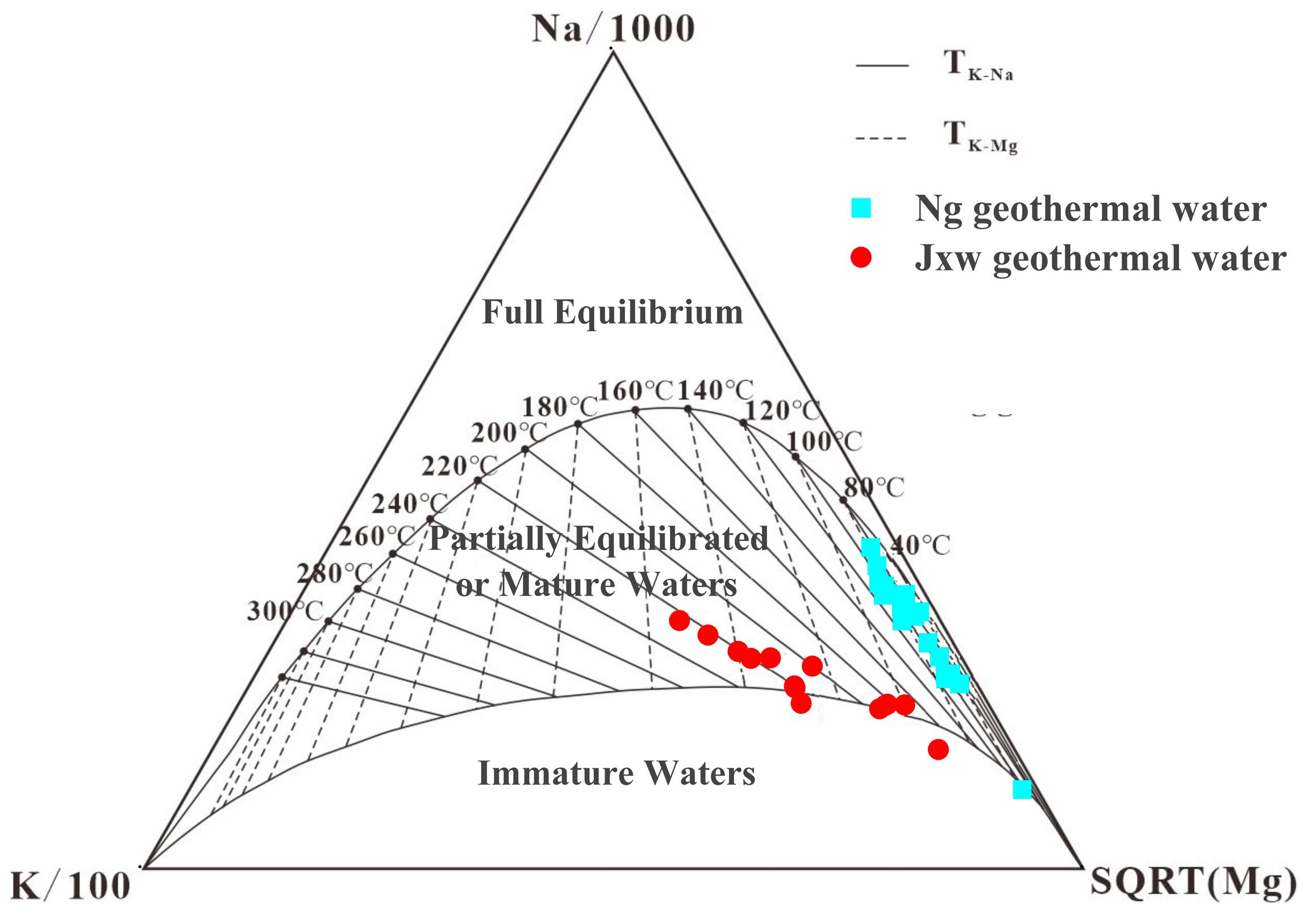
4.2. Estimation of Deep Thermal Storage Temperature
4.3. Water Rock Balance Results
5. Discussion
5.1. Analysis of Hydrochemical Evolution
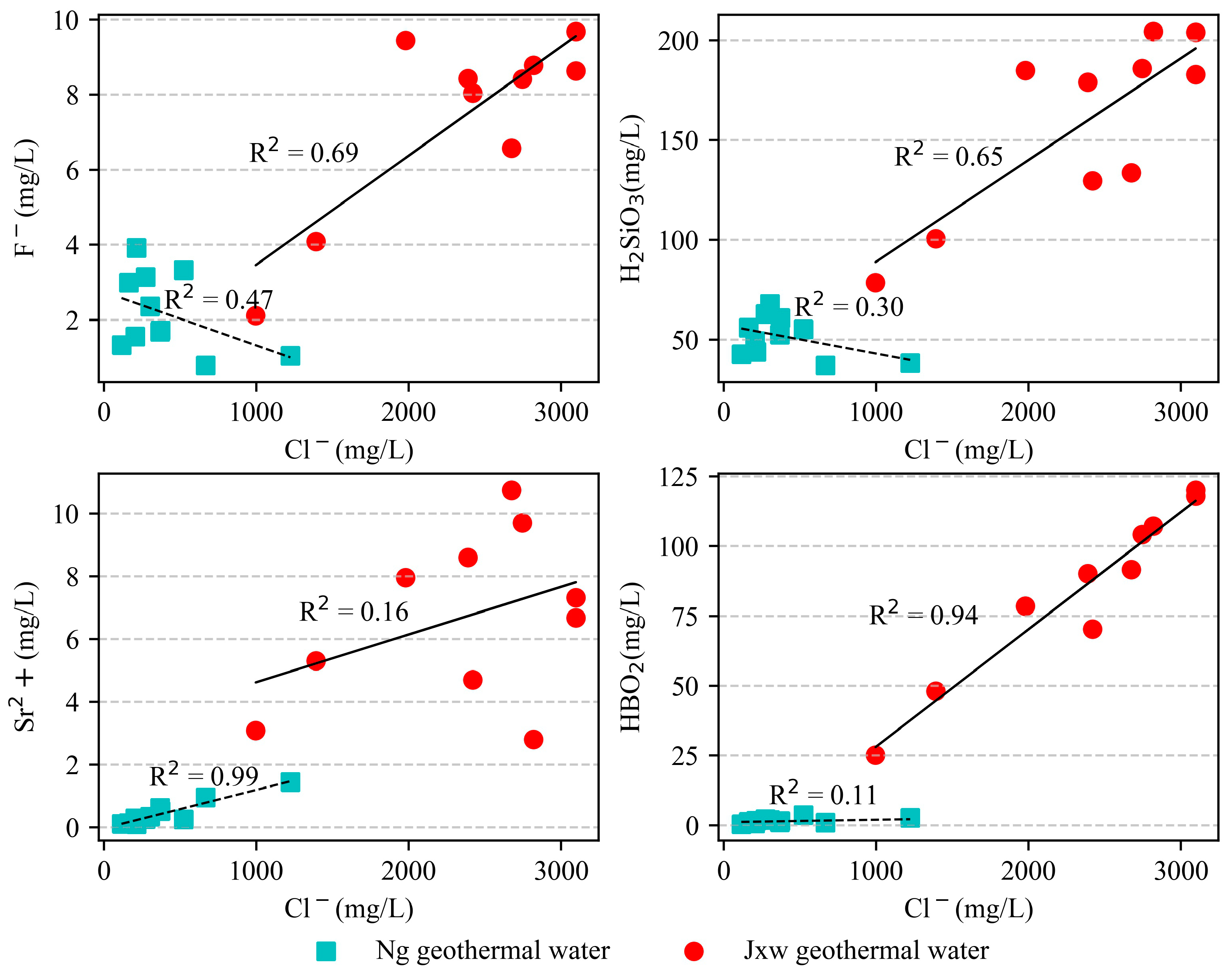
5.1.1. Correlation Analysis of Hydrochemical Components
5.1.2. Characteristic Coefficients of Geothermal Water
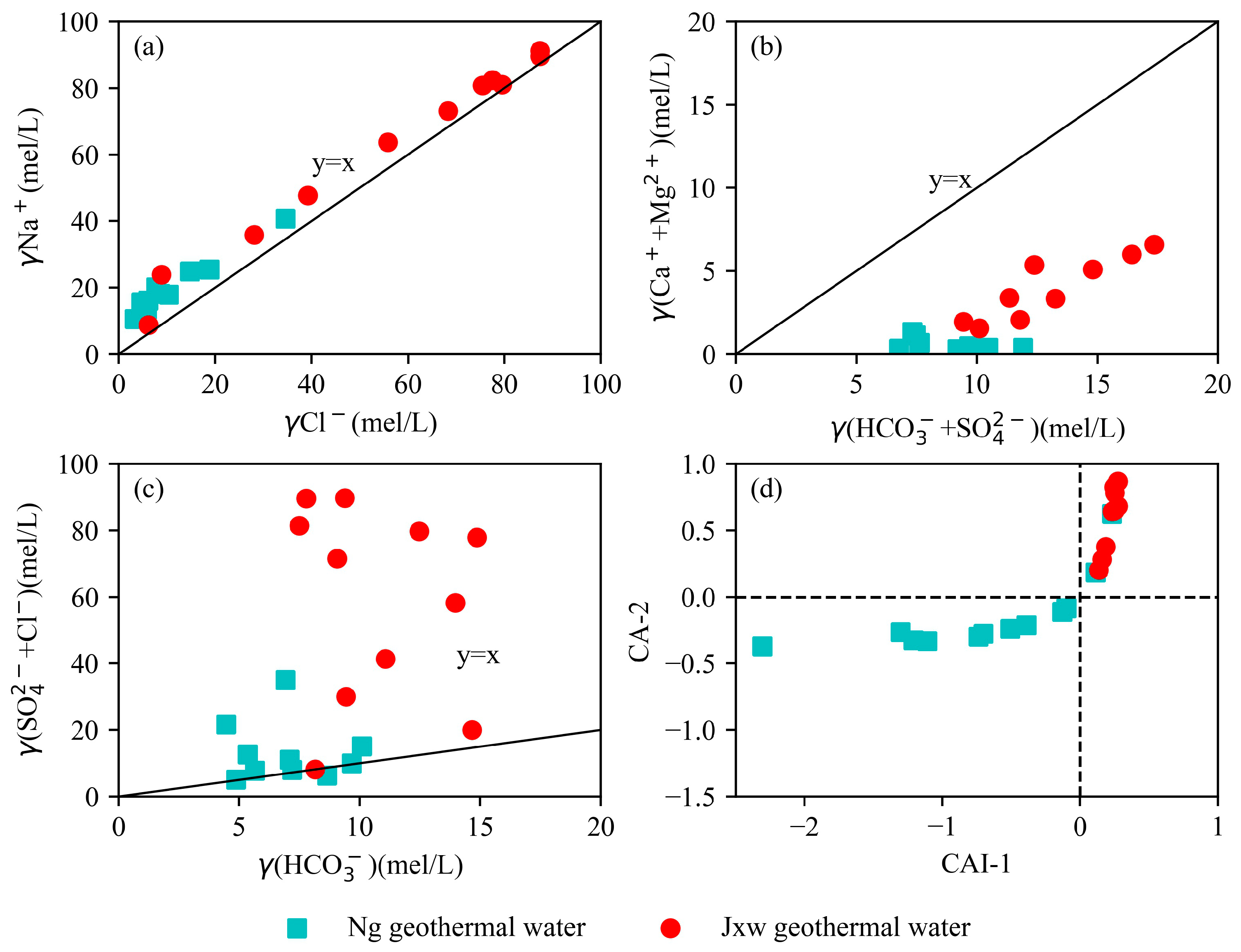
5.1.3. Main Ion Sources of Geothermal Water
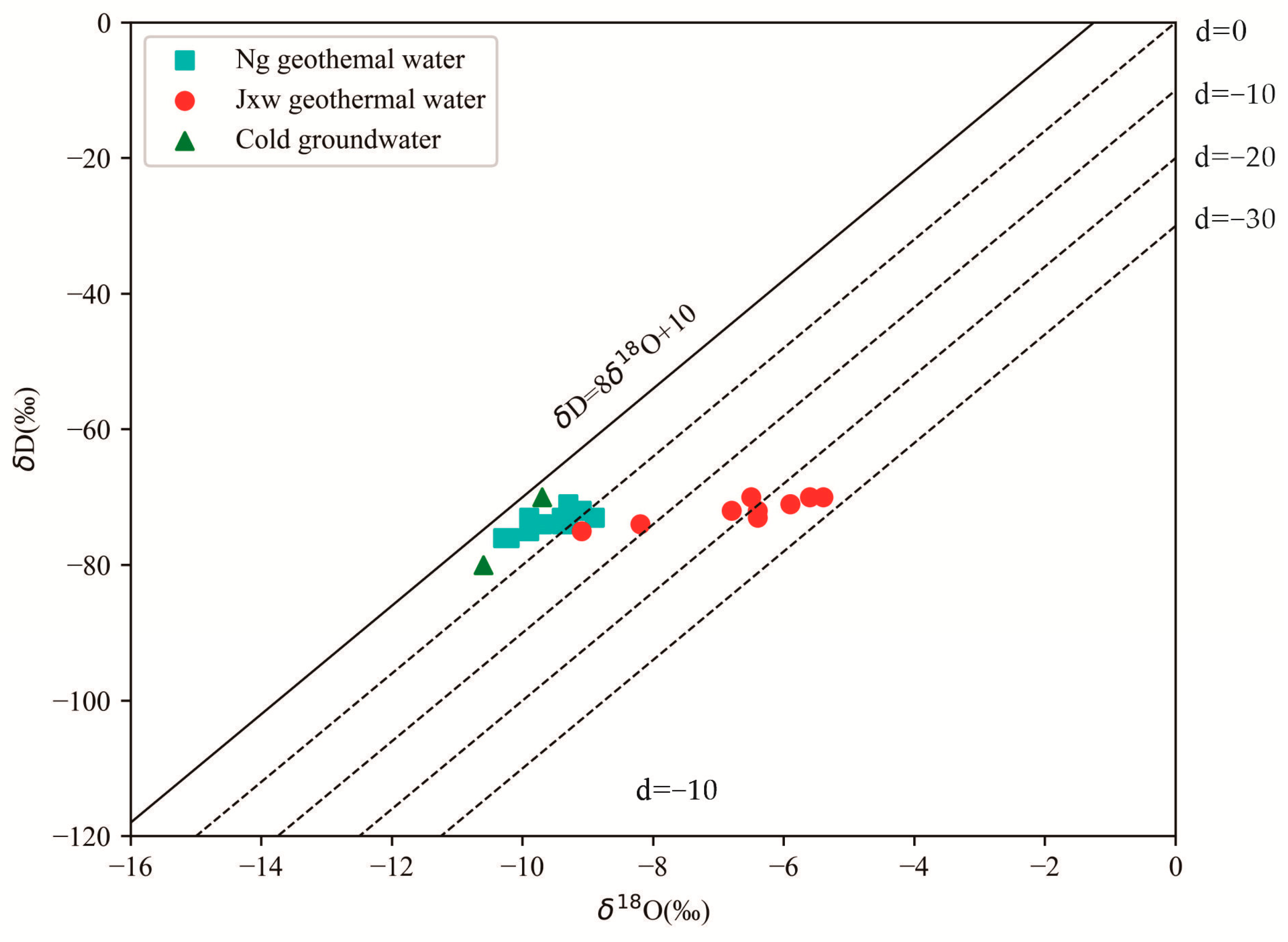
5.1.4. Source of Geothermal Water Supply
5.1.5. Elevation of Geothermal Water Supply
5.2. Deep Thermal Cycling Process
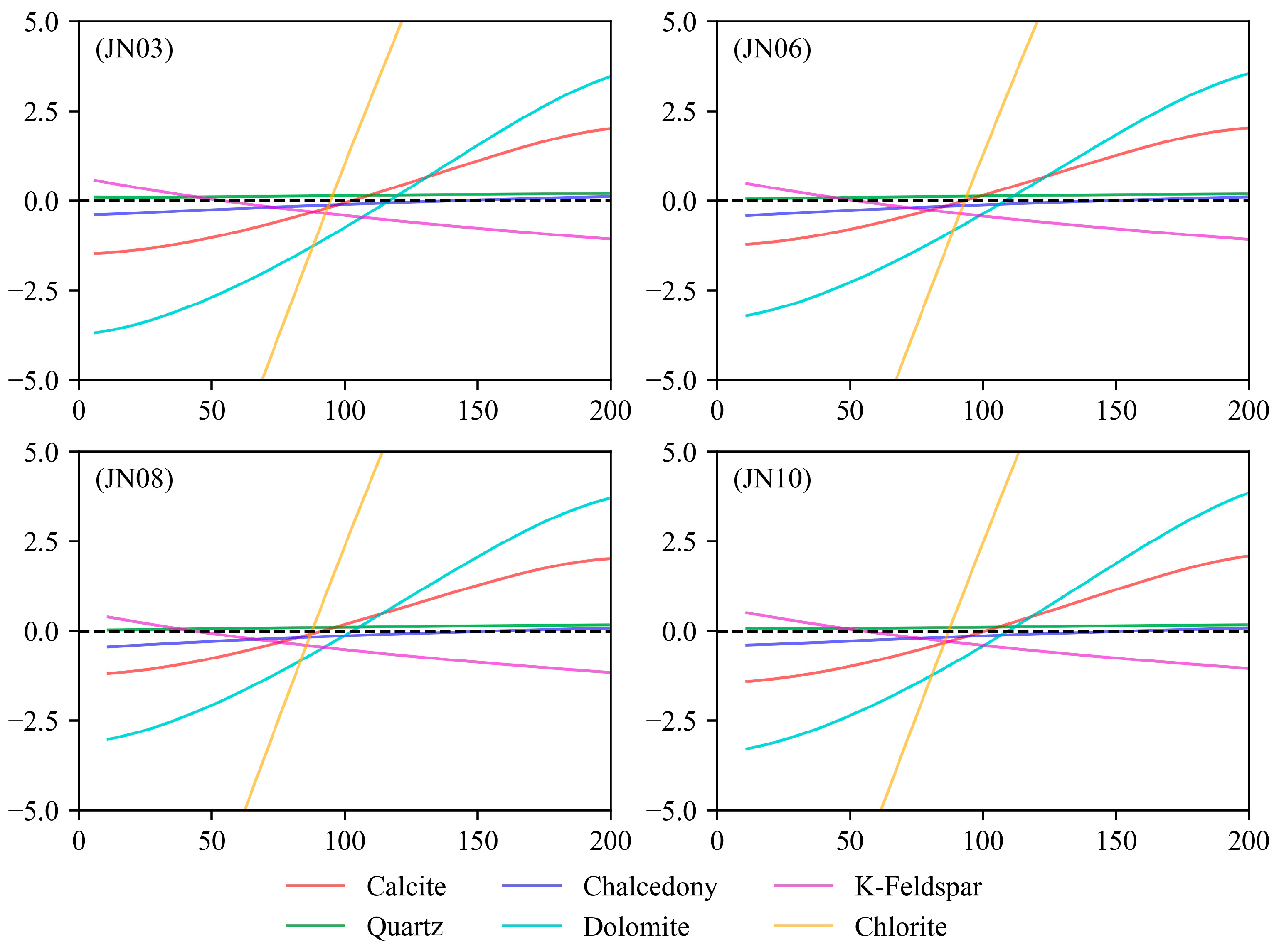
5.2.1. Deep Thermal Storage Water–Rock Equilibrium State
5.2.2. Thermal Storage Temperature
5.2.3. Thermal Cycle Depth
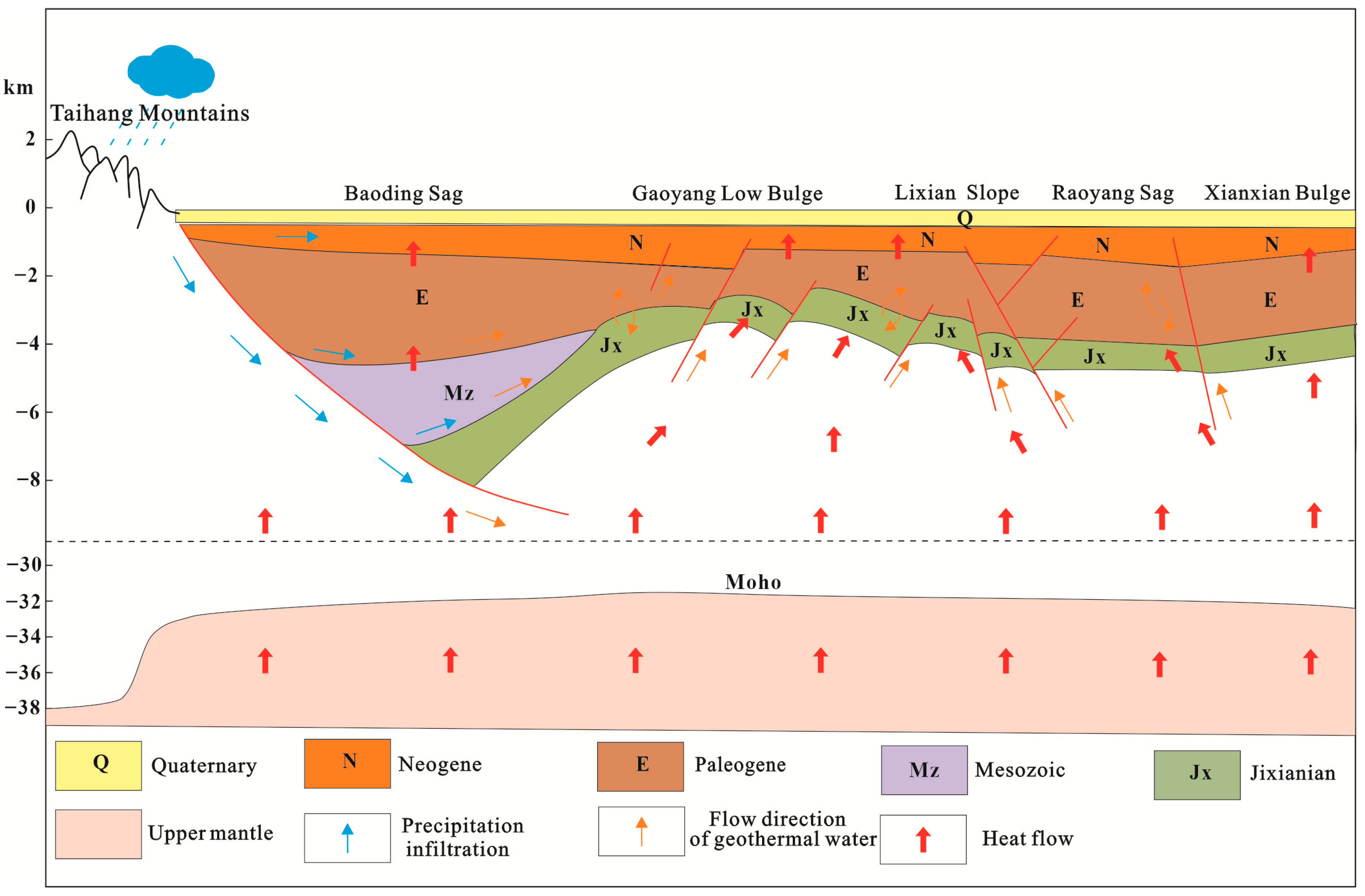
5.3. Conceptual Circulation of the Thermal Waters
- (1)
- Heat source: The destruction of the North China Craton causes thinning of the lithosphere and uplift of the Moho surface at the bottom of the depression, which is more conducive to the conduction of deep heat.
- (2)
- Thermal reservoirs: The research area contains multiple layers of high permeability thermal reservoirs in the Neogene Guantao Formation (Ng), while in the hidden bedrock uplift area, there are underlying thermal reservoirs in the Middle Neoproterozoic Jixian Wumishan Formation (Jxw), which contain abundant geothermal resources.
- (3)
- Channel: The structural pattern of the alternating concavity and convexity in the research area has formed large and deep faults, and the widely developed fractures in the deep carbonate thermal reservoirs provide good channels for the migration of groundwater and thermal conduction.
- (4)
- Cover layer: The thermal reservoir in the southern part of the Jizhong Depression is covered by a Neogene-Paleogene sandstone layer, which has a low thermal conductivity. The upper part is covered by the Quaternary system, and the lithology is mainly composed of clay and sand layers, which have a poor thermal conductivity. Therefore, the thermal insulation effect is significant. The research area is covered with a double insulation layer, which makes the heat of the thermal reservoir less likely to dissipate and provides a good insulation effect for the heat in the thermal reservoir.
6. Conclusions
- (1)
- According to the geochemical and isotopic analyses of geothermal water samples from the study area, the hydrochemical types of the geothermal fluids in the sandstone thermal storage are mainly the Cl·HCO3−Na type, while the geothermal fluids in the carbonate rock thermal storage are mainly the Cl-Na type and Cl·HCO3−Na type. The content of the main ions (Na+, K+, and Cl−) in the water samples from the Jxw thermal storage is greater than that in the Ng thermal storage. The reason for this is that the Jxw thermal storage has a longer water cycle path, it experiences more complete leaching and filtration, and a large amount of soluble substances in the surrounding rock enter the hot water. According to the ion ratios, the components of the geothermal water are mainly controlled by dissolution and cation adsorption of the carbonate rocks. According to the calculated characteristic coefficients, the geological environment where the Jxw thermal storage is located is more enclosed than that of the Ng thermal storage, with a longer flow path, slower water circulation, and higher salinity.
- (2)
- By analyzing the hydrogen and oxygen isotope compositions of the thermal storage water in the study area, it was found that the main source of the Ng thermal storage water and shallow groundwater is atmospheric precipitation. The Jxw thermal storage water undergoes a more rightward oxygen drift compared to the Ng thermal storage water. This is due to the higher elevation of the deep Jxw thermal storage, which is recharged laterally from the distant high mountains, while the Ng thermal storage is recharged in a small amount in the lower altitude piedmont plain. The elevation of the geothermal water supply area in the research area is 763–1063 m, which is consistent with the elevation of the Taihang Mountains in the western part of the Jizhong Depression. It is speculated that the geothermal water is mainly supplied by precipitation in the mountainous areas of the western Taihang Mountains.
- (3)
- The measured temperature of the Ng thermal storage in the research area is 69.2–88 °C, and the measured temperature of the Jxw thermal storage is 82–100 °C. The Na-K-Ca temperature scale and the multi-mineral equilibrium method have relatively small errors and are suitable for the southern region of the Jizhong Depression, with average errors of 21.44 °C and 32.64 °C, respectively. The depth of the Jxw thermal storage cycle in the research area is 3033–5187 m, and the depth of the Ng thermal storage cycle is 1360–2862 m. The geothermal water sample is in partial equilibrium or is mixed water due to the entry of cold water into the thermal reservoir, and the water–rock interaction does not reach equilibrium after mixing with the hot water.
- (4)
- The geothermal genesis model for the study area is as follows. The precipitation in the Taihang Mountains infiltrates via faults and is supplied laterally from northwest to southeast. During the long-term runoff process, the groundwater undergoes water–rock reactions and changes in hydrochemical type and is continuously heated by the deep heat flow and radioactive heat from the rocks to form geothermal water. Some of the geothermal water forms thermal convection locally, while the rest is transferred upwards along the fault channel. Heat accumulation occurs in the structural bulge area, resulting in the formation of a convection–conduction-type geothermal system.
- (5)
- The bedrock thermal storage in the study area is widely distributed and has a great resource potential. Karst fissures are relatively well developed, but there are problems such as strong sealing of the geothermal water and long supply cycles. It is difficult to meet large-scale development needs by relying on natural supply. Therefore, the characteristics of the reservoir fissure development should be utilized, and artificial recharge should be conducted to achieve sustainable utilization of the thermal resources in this area.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fagundo-Castillo, J.R.; Carrillo-Rivera, J.J.; Antigüedad-Auzmendi, I.; González-Hernández, P.; Peláez-Díaz, R.; Hernández-Díaz, R.; Cáceres-Govea, D.; Hernández-Santana, J.R.; Suárez-Muñoz, M.; Melián-Rodríguez, C.; et al. Chemical and Geological Control of Spring Water in Eastern Guaniguanico Mountain Range, Pinar Del Rìo, Cuba. Environ. Geol. 2008, 55, 247–267. [Google Scholar] [CrossRef]
- Mongillo, M.A.; Axelsson, G. Preface to Geothermics Special Issue on Sustainable Geothermal Utilization. Geothermics 2010, 39, 279–282. [Google Scholar] [CrossRef]
- Axelsson, G.; Gunnlaugsson, E.; Jonasson, T.; Olafasson, M. Low-Temperature Geothermal Utilization in Iceland-Decades of Experience. Geothermics 2010, 39, 329–338. [Google Scholar] [CrossRef]
- Gude, V.G. Geothermal Source Potential for Water Desalination—Current Status and Future Perspective. Renew. Sustain. Energy Rev. 2016, 57, 1038–1065. [Google Scholar] [CrossRef]
- Najafi, G.; Ghobadian, B. Geothermal Resources in Iran: The Sustainable Future. Renew. Sustain. Energy Rev. 2011, 15, 3946–3951. [Google Scholar] [CrossRef]
- Wang, G.L.; Lin, W.J. Main Hydro- Geothermal Systems and Their Genetic Models in China. Acta Geol. Sin. 2020, 94, 1923–1937. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.L.; Liu, F.; Xing, L.X.; Li, M. Characteristics of Geothermal Resources in Sedimentary Basins. Geol. China 2019, 46, 255–268. [Google Scholar]
- Prada, S.; Cruz, J.V.; Figueira, C. Using Stable Isotopes to Characterize Groundwater Recharge Sources in the Volcanic Island of Madeira, Portugal. J. Hydrol. 2016, 536, 409–425. [Google Scholar] [CrossRef]
- Négrel, P.; Petelet-Giraud, E.; Millot, R. Tracing Water Cycle in Regulated Basin Using Stable δ18O–δ2H Isotopes: The Ebro River Basin (Spain). Chem. Geol. 2016, 422, 71–81. [Google Scholar] [CrossRef]
- Belhai, M.; Fujimitsu, Y.; Barragán, R.M.; Iwanaga, T.; Haouchine, A. Insights into Geochemical Characteristics of the Geothermal Fluids of Northwestern Algeria: An Updated Conceptual Model. Geothermics 2022, 99, 102304. [Google Scholar] [CrossRef]
- Ellis, A.J. Quantitative Interpretation of Chemical Characteristics of Hydrothermal Systems. Geothermics 1970, 2, 516–527. [Google Scholar] [CrossRef]
- Truesdell, A.H.; Nathenson, M.; Rye, R.O. The Effects of Subsurface Boiling and Dilution on the Isotopic Compositions of Yellowstone Thermal Waters. Geophys. Res. Part B Solid Earth 1977, 82, 3694–3704. [Google Scholar] [CrossRef]
- Armannsson, H. The Fluid Geochemistry of Icelandic High Temperature Geothermal Areas. Appl. Geochem. 2016, 66, 4–64. [Google Scholar] [CrossRef]
- Procesi, M.; Marini, L.; Cinti, D.; Sciarra, A.; Basile, P.; Mazzoni, T.; Zarlenga, F. Preliminary Fluid Geochemical Survey in Tete Province and Prospective Development of Geothermics in Mozambique. Geotherm. Energy 2022, 10, 2. [Google Scholar] [CrossRef]
- Pang, J.N.; Zhong, H.; Lv, M.; Tian, J.; Kong, Y.L. Geochemical and Isotopic Characteristics of Fluids in the Niutuozhen Geothermal Field, North China. Environ. Earth Sci. 2018, 77, 12. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Zhang, W.; Ma, F.; Zhu, X.; Zhang, H.X.; Wang, G.L. Geothermal Fluid Chemistry Characteristics of Rongcheng Geothermal Field in Xiong’an New Area. Acta Geol. Sin. 2020, 94, 1991–2001. [Google Scholar]
- Xing, Y.F.; Wang, H.Q.; Li, j.; Teng, Y.G.; Zhang, B.J.; Li, y.y.; Wang, G.L. Analysis of the Chemical Field Characteristics and Influencing Factors of Geothermal Water in Xiong’an New Area. Geol. China 2022, 49, 1711–1722. [Google Scholar]
- Zhang, H.X.; Wang, G.L.; Zhang, W.; Ma, F.; Zhu, X.; Yue, G.F. Characteristics of the Rongcheng Bulge Geothermal Field and the Evolution of Geothermal Fluids, Xiong’an New Area, China. Water 2022, 14, 2468. [Google Scholar] [CrossRef]
- Deng, J.Z. Chemical Characteristics and Genesis of Thermal Water Storage in Carbonate Rocks in the Northern Part of the Jizhong Depression; East China University of Science and Technology: Shanghai, China, 2022. [Google Scholar]
- Zhao, Z.R.; Zhang, W.; Wang, G.L.; Xing, L.X.; Zhang, H.X.; Zhao, J.Y. Hydrogeochemical Characteristics of Gaoyang Geothermal Field in the Jizhong Depression and Their Constraints on Geothermal Genesis. Geol. China 2023, 11, 1167. [Google Scholar]
- Liang, S.J. The Geological Structural Characteristics and Oil and Gas Occurrence of the Late Cenozoic in the Jizhong Depression; Northwest University: Kirkland, WA, USA, 2001. [Google Scholar]
- Zuo, Y.H.; Qiu, N.S.; Chang, J.; Hao, Q.Q.; Li, Z.X.; Li, J.W.; Li, W.Z.; Xie, C.H. Thermal Structure of Mesozoic and Cenozoic Lithosphere in Bohai Bay Basin. Acta Geol. Sin. 2013, 87, 145–153. [Google Scholar]
- Wu, Z.P.; Hou, X.B.; Li, W. Discussion on Mesozoic Basin Pattern and Evolution Process in Eastern North China. Tecton Miner. 2007, 4, 385–399. [Google Scholar]
- Zhang, W.C.; Yang, D.X.; Chen, Y.J.; Qian, Z.; Zhang, C.W.; Liu, H.F. Characteristics of Paleogene Sedimentary structures and distribution of oil and gas in Jizhong Depression. Acta Geol. Sin. 2008, 82, 1103–1112. [Google Scholar]
- Chang, J.; Qiu, N.; Zhao, X.; Xu, w.; Xu, Q.; Jin, F.; Han, C.; Ma, X.; Dong, X.; Liang, X. Present-Day Geothermal Regime of the Jizhong Depression in Bohai Bay Basin, East China. Chin. Geophys 2016, 59, 14. [Google Scholar]
- Song, J.J.; Wang, G.L.; Xing, L.X.; Lu, C.; Qian, J.Z. Influence of Rock Thermal Conductivity Correction on the Calculated Value of Terrestrial Heat Flow—A Case Study of Jizhong Depression, Bohai Bay Basin. Geol. Rev. 2023, 69, 1349–1364. [Google Scholar]
- GB 8538-2016; National Food Safety Standard Methods for Examination of Drinking Natural Mineral Water. Standards Press of China: Beiing, China, 2017. (In Chinese)
- GB/T 5750-2006; Standard Examination Methods for Drinking Water. Standards Press of China: Beiing, China, 2007. (In Chinese)
- DZ/T 0064-1993; Analysis Method of Groundwater Uality. Standards Press of China: Beiing, China, 1994. (In Chinese)
- Giggenbach, W.F. Geothermal Solute Equilibria. Derivation of Na-K-Mg-Ca Geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar] [CrossRef]
- Fournier, R.O. A Revised Equation for the Na/K Geothermometer. Trans. Geotherm. Resour. Counc. 1979, 3, 221–224. [Google Scholar]
- Fournier, R.O.; Truesdell, A.H. An Empirical Na-K-Ca Geothermometer for Natural Waters. Geochim. Cosmochim. Acta 1973, 37, 1255–1275. [Google Scholar] [CrossRef]
- Chiodini, G.; Frondini, F. Theoretical Geothermometers and PCOzindicators for Aqueous Solutions Coming from Hydrothermal Systems of Medium-Low Temperature Hosted in Carbonate-Evaporite Rocks. Application to the Thermal Springs of the Etruscan Swell, Italy. Appl. Geochem. 1995, 10, 337–346. [Google Scholar] [CrossRef]
- Li, Y.M.; Pang, Z.H.; Luo, Q.; Chen, K. Applicability Analysis of Boiling Correction Method for SiO2 Ground Thermometer in Plateau Areas. Geol. Rev. 2021, 67, 1050–1056. [Google Scholar]
- Fournier, R.O. Chemical Geothermometers and Mixing Models for Geothermal Systems. Geothermics 1977, 5, 41–50. [Google Scholar] [CrossRef]
- Lin, W.J.; Chen, X.Y.; Gan, H.N.; Yue, G.F. Geothermal Geological Characteristics and Exploration Direction of Dry Hot Rocks in the Xiamen Bay Zhangzhou Basin along the Southeast Coast. Acta Geol. Sin. 2020, 94, 2066–2077. [Google Scholar]
- Chen, M.X. Review and Prospect on Geothermal Studies in China. Acta Geophys. Sin. 1994, 37, 19. [Google Scholar]
- Wang, D.C. Fundamentals of Hydrogeology; Geological Publishing Co.: Beijing, China, 1986. [Google Scholar]
- Apollaro, C.; Vespasiano, G.; De Rosa, R.; Marini, L. Use of Mean Residence Time and Flowrate of Thermal Waters to Evaluate the Volume of Reservoir Water Contributing to the Natural Discharge and the Related Geothermal Reservoir Volume. Geothermics 2015, 58, 62–74. [Google Scholar] [CrossRef]
- Yuan, J.F. Transport of Boron in the Aquatic Environment of the Yangbajing Geothermal Field, Tibet; China University of Geosciences: Beijing, China, 2010. [Google Scholar]
- Du, Y.Q.; Zhu, G.Y.; Ruan, Z.; Cao, Y.H.; Shen, Z.H.; Chang, Q.H.; Chen, G.P.; Yu, B.S. Hydrochemical Characteristics, Genesis and Simulation of Mineral Dissolution-Precipitation in Cambrian Strata of the Tabei Rise, Tarim Basin. Oil Gas Geol. 2022, 43, 11. [Google Scholar]
- Sun, H.Y.; Wei, X.F.; Gan, F.W.; Wang, H.; Jia, F.C.; He, Z.X.; Li, D.J.; Li, J.; Zhang, J. Genetic Types and Formation Mechanisms of Strontium Rich Groundwater in the Middle and Upper Reaches of the Luanhe River Basin. Acta Geosci. Sin. 2020, 41, 65–79. [Google Scholar]
- Li, J.; Zhou, S.Z.; Zhao, Y.; Zhao, R.K.; Dang, Z.W.; Pan, M.Q.; Zhu, D.N.; Zhou, C.S. Analysis of the Main Ion Characteristics and Genesis of Groundwater in Huixian Karst Wetland. Environ. Sci. 2021, 42, 1750–1760. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Yin, Z.Y.; Luo, Q.K.; Wu, J.F.; Xu, S.H.; Wu, J.C. Identification of the Long-Term Variations of Groundwater and Their Governing Factors Based on Hydrochemical and Isotopic Data in a River Basin. J. Hydrol. 2021, 592, 125604. [Google Scholar] [CrossRef]
- Craig, H. The Isotopic Geochemistry of Water and Carbon in Geothermal Area. In Nuclear Geology in Geothermal Areas, Spoleto; Tongiori, E., Ed.; Consiglio Nazionale delle Ricerche: Roma, Italy, 1963; pp. 17–53. [Google Scholar]
- Liu, K.; Liu, Y.C.; Sun, Y.; Liu, J.R.; Wang, S.F.; Liu, Z.M. Characteristics of Deuterium Excess Parameters of Geothermal Water in Beijing. Geol. China 2015, 42, 2029–2035. [Google Scholar] [CrossRef]
- Zhang, B.J.; Wang, S.Q.; Kang, F.G.; Wu, Y.Q.; Li, Y.Y.; Gao, J.; Yuan, W.Z.; Xing, Y.F. Heat Accumulation Mechanism of the Gaoyang Carbonatite Geothermal Field, Hebei Province, North China. Front. Earth Sci. 2022, 10, 858814. [Google Scholar] [CrossRef]
- Liu, J.R.; Song, X.F.; Yue, G.F.; Sun, X.M.; Liu, X.; Wang, S.Q. Characteristics of δ18O in Precipitation over Eastern Monsoon China and the Water Vapor Sources. Chin. Sci. Bull. 2009, 54, 3521–3531. [Google Scholar]
- Chen, Z.Y. Study on Groundwater Resources Evolution from Paleoenvironmental Information of Groundwater System in North China Plain; Jilin University: Changchun, China, 2001. [Google Scholar]
- Zhang, Z.S.; Sun, Z.X.; Li, M.G.; Wang, S.J. Application of Fixed Aluminum Log (Q/K) Diagram in Hot Springs. Earth Sci. 2004, 3, 352–356. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.L.; Zhao, J.Y.; Liu, F. Geochemical Characteristics of Medium-High Temperature Geothermal Fluids in West Sichuan and Their Geological Implications. Geoscience 2021, 35, 188–198. [Google Scholar]




| Sample NO. | Stratigraphic | T (°C) | Ph | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | TDS | Sr2+ | δDVSMOW | δ18OVSMOW | F− | H2SiO3 | HBO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JN01 | Ng | 69.2 | 7.95 | 4.6 | 445.1 | 9.37 | 0.83 | 369.8 | 102 | 396.9 | 1183 | 0.536 | −74 | −9.7 | 1.72 | 60.89 | 1.62 |
| JN02 | Ng | 82 | 8.16 | 5.03 | 416.9 | 8.83 | 0.61 | 303.4 | 124.2 | 432.4 | 1135 | 0.346 | −74 | −9.3 | 2.36 | 68.02 | 1.92 |
| JN03 | Ng | 67 | 8.04 | 4 | 412 | 11.81 | 1.11 | 368 | 110.4 | 325.8 | 1115 | 0.636 | −75 | −9.9 | 1.69 | 52.62 | 1.12 |
| JN04 | Ng | 46.1 | 8.16 | 2.74 | 308.3 | 7.61 | 0.75 | 206.1 | 94.28 | 344.8 | 834.6 | 0.3 | −76 | −10.3 | 1.56 | 49.46 | 0.64 |
| JN05 | Ng | 44.8 | 8.58 | 1.97 | 242.9 | 5.71 | 0.68 | 116 | 91.4 | 296.8 | 661.1 | 0.116 | −76 | −10.2 | 1.33 | 42.93 | 0.46 |
| JN06 | Ng | 62 | 7.94 | 4.29 | 463.6 | 6.7 | 0.57 | 275 | 108.1 | 589.4 | 1209 | 0.28 | −74 | −9.4 | 3.14 | 62.92 | 2.3 |
| JN07 | Ng | 73.4 | 8.19 | 2.9 | 359.6 | 4.97 | 0.62 | 164.1 | 81.49 | 527.2 | 925.7 | 0.148 | −73 | −9.4 | 2.99 | 56.11 | 1.26 |
| JN08 | Ng | 68 | 8.12 | 4.29 | 573.2 | 6.59 | 0.73 | 524.1 | 18.06 | 615.5 | 1487 | 0.259 | −73 | −8.9 | 3.33 | 55.28 | 3.72 |
| JN09 | Ng | 56.1 | 8.41 | 1.96 | 370.5 | 5.11 | 0.64 | 214.3 | 96.45 | 438.3 | 967.7 | 0.114 | −72 | −9.1 | 3.92 | 43.99 | 1.76 |
| JN10 | Ng | 48.2 | 8.14 | 5.83 | 586.5 | 21.23 | 3.56 | 667.7 | 137.1 | 271.9 | 1592 | 0.965 | −73 | −9.9 | 0.79 | 37.29 | 0.92 |
| JN11 | Ng | 52.9 | 8.17 | 5.89 | 937.5 | 17.69 | 3.32 | 1224 | 26.24 | 421.8 | 2465 | 1.46 | −71 | −9.3 | 1.04 | 38.5 | 2.77 |
| JN12 | Ng | 72 | 8.7 | 4 | 310.2 | 4 | 1 | 95 | 80.3 | 510.1 | 1063.1 | 43.54 | / | / | / | / | / |
| JN13 | Ng | 79 | 7.94 | 3.3 | 192.9 | 18.4 | 3.3 | 85.1 | 48.9 | 373.4 | 753.6 | 34.11 | / | / | / | / | / |
| JN14 | Ng | 80 | 8.4 | 2.9 | 316.9 | 4.8 | 1 | 147.5 | 73.5 | 524.7 | 1114 | 38.96 | / | / | / | / | / |
| JN15 | Ng | 80 | 8.36 | 3.7 | 319.7 | 6.4 | 1 | 168.8 | 93.7 | 478.4 | 1125.5 | 57.46 | / | / | / | / | / |
| JN16 | Ng | 75 | 8.81 | 4.9 | 401 | 7.2 | 0.5 | 195.7 | 113.1 | 578.4 | 1350 | 33.29 | / | / | / | / | / |
| JN17 | Ng | 76 | 8.45 | 5.98 | 441.7 | 6.74 | 0.69 | 211.8 | 132.8 | 622.2 | 1498 | 79.43 | / | / | / | / | / |
| JN18 | Ng | 88 | 7.98 | 6.39 | 473.4 | 8.22 | 1.08 | 244.2 | 135.6 | 641.7 | 1585 | 85.54 | / | / | / | / | / |
| JN19 | Ng | 76.4 | 7.78 | 12.61 | 1138 | 25.08 | 4.22 | 1453 | 8 | 482.1 | 3193 | 59.8 | / | / | / | / | / |
| JJ01 | Jxw | 82 | 7.46 | 149.3 | 1691 | 88.56 | 20.08 | 2387 | 104.9 | 838.2 | 5122 | 8.611 | −72 | −6.4 | 8.43 | 179.1 | 90.26 |
| JJ02 | Jxw | 81.6 | 7.53 | 167 | 1894 | 77.05 | 15.09 | 2746 | 112.3 | 761.2 | 5677 | 9.714 | −71 | −5.9 | 8.42 | 186 | 104.2 |
| JJ03 | Jxw | 66.8 | 7.03 | 147.9 | 1857 | 106.3 | 15.6 | 2675 | 120.6 | 906.3 | 5618 | 10.74 | −73 | −6.4 | 6.58 | 133.8 | 91.72 |
| JJ04 | Jxw | 71 | 7.34 | 37.19 | 823.9 | 53.59 | 8.86 | 996.2 | 92.81 | 574.6 | 2398 | 3.091 | −75 | −9.1 | 2.11 | 78.78 | 25.18 |
| JJ05 | Jxw | 65.6 | 7.05 | 73.45 | 1098 | 55.31 | 7.04 | 1391 | 104.5 | 675.3 | 3212 | 5.309 | −74 | −8.2 | 4.09 | 100.6 | 48.17 |
| JJ06 | Jxw | 100 | 8.38 | 186.5 | 2062 | 23.03 | 11.34 | 3097 | 116.1 | 571.6 | 6461 | 6.689 | −71 | −5.9 | 8.63 | 204.1 | 118.1 |
| JJ07 | Jxw | 84.1 | 7.08 | 145.6 | 1466 | 88.15 | 19.6 | 1978 | 118.3 | 852.5 | 4882 | 7.97 | −72 | −6.8 | 9.45 | 185 | 78.48 |
| JJ08 | Jxw | 87.6 | 8.4 | 193.4 | 2097 | 16.99 | 8.7 | 3096 | 111.7 | 473.9 | 6109 | 7.327 | −70 | −5.4 | 9.68 | 182.9 | 120 |
| JJ09 | Jxw | 88.2 | 8.46 | 165.6 | 1865 | 18 | 12.76 | 2818 | 94.16 | 456.1 | 5563 | 2.811 | −70 | −5.6 | 8.78 | 204.4 | 107.2 |
| JJ10 | Jxw | 100 | 7.65 | 150.1 | 1682 | 74.08 | 20.36 | 2421 | 159.7 | 552.2 | 4968 | 4.705 | −70 | −6.5 | 8.04 | 129.7 | 70.36 |
| JJ11 | Jxw | 83 | 7.79 | 124.4 | 2201.1 | 65.7 | 142.5 | 3034.8 | 760.7 | 341.7 | 6742.6 | 81.82 | / | / | / | / | / |
| JJ12 | Jxw | 81 | 7.01 | 107.1 | 1911 | 255.4 | 45.22 | 2777 | 741.8 | 367.3 | 6297 | 62.53 | / | / | / | / | / |
| JJ13 | Jxw | 75.7 | 6.9 | 112.8 | 1838 | 274.3 | 43.13 | 3000.7 | 487 | 371.2 | 6227.4 | 63.96 | / | / | / | / | / |
| JJ14 | Jxw | 89.1 | 7.46 | 105.1 | 1944 | 221.9 | 46.39 | 2827 | 538.7 | 356.9 | 6147 | 64.61 | / | / | / | / | / |
| JQ01 | Q | 14.9 | 7.83 | 1.2 | 99.82 | 82.89 | 49.34 | 105.4 | 327.7 | 179.5 | 889.7 | 0.897 | −70 | −9.7 | 0.43 | <0.20 | 22.97 |
| JQ02 | Q | 21.5 | 8.09 | 3.18 | 49.05 | 21.81 | 6.91 | 5.25 | 6.4 | 209.4 | 326.7 | 0.297 | −80 | −10.6 | 0.37 | <0.20 | 21.47 |
| JD01 | / | 19 | 5.86 | 0.45 | 2.83 | 4.54 | 0.81 | 1.79 | 7.8 | 4.74 | 35.62 | 0.016 | −55 | −8.5 | 0.15 | <1.00 | <0.20 |
| JB01 | / | 15.2 | 8.03 | 5.44 | 202.9 | 57.65 | 59.1 | 218.1 | 97.05 | 497 | 1167 | 0.647 | −34 | −2.7 | 0.81 | 12.89 | 0.96 |
| Simple No. | Measuring Temperature | Na-K | Na-K-Ca | Ca-Mg | K-Mg | Quartz | Chalcedony | SI Method |
|---|---|---|---|---|---|---|---|---|
| JN01 | 69.2 | 82.31 | 78.42 | 435.31 | 86.02 | 138.30 | 111.72 | 97 |
| JN02 | 82 | 89.43 | 84.45 | 457.66 | 89.70 | 149.67 | 124.40 | 96 |
| JN03 | 67 | 79.52 | 71.62 | 430.79 | 75.46 | 142.80 | 116.72 | 92 |
| JN04 | 46.1 | 75.64 | 67.40 | 428.60 | 69.72 | 133.93 | 106.88 | 83 |
| JN05 | 44.8 | 71.71 | 61.10 | 415.00 | 63.10 | 132.23 | 105.00 | 92 |
| JN06 | 62 | 77.40 | 81.32 | 438.53 | 91.81 | 141.59 | 115.38 | 81 |
| JN07 | 73.4 | 71.47 | 70.91 | 409.49 | 82.33 | 126.07 | 98.22 | 89 |
| JN08 | 68 | 68.33 | 78.29 | 416.01 | 87.54 | 59.65 | 27.44 | 81 |
| JN09 | 56.1 | 54.48 | 61.66 | 409.05 | 69.47 | 135.19 | 108.27 | 83 |
| JN10 | 48.2 | 80.57 | 66.86 | 386.22 | 78.81 | 155.61 | 131.09 | 82 |
| JN11 | 52.9 | 61.20 | 67.89 | 376.93 | 88.48 | 73.97 | 42.35 | 71 |
| JN12 | 72 | 92.58 | 72.84 | 364.20 | 96.79 | 125.29 | 97.37 | 99 |
| JN13 | 79 | 106.59 | 55.18 | 386.59 | 53.23 | 100.78 | 70.77 | 78 |
| JN14 | 80 | 76.91 | 65.42 | 375.15 | 81.70 | 120.69 | 92.33 | 85 |
| JN15 | 80 | 87.49 | 71.02 | 393.93 | 83.52 | 133.59 | 106.50 | 96 |
| JN16 | 75 | 90.03 | 86.30 | 457.45 | 93.10 | 144.19 | 118.27 | 78 |
| JN17 | 76 | 94.92 | 87.28 | 423.54 | 103.10 | 153.68 | 128.91 | 89 |
| JN18 | 88 | 94.78 | 83.29 | 404.23 | 101.51 | 154.94 | 130.33 | 98 |
| JN19 | 76.4 | 85.48 | 83.25 | 383.02 | 101.74 | 32.20 | 125.31 | 90 |
| JJ01 | 82 | 215.20 | 131.26 | 362.50 | 193.24 | 139.88 | 113.48 | 117 |
| JJ02 | 81.6 | 215.08 | 139.65 | 370.81 | 197.42 | 143.78 | 117.82 | 125 |
| JJ03 | 66.8 | 206.58 | 135.06 | 389.33 | 187.58 | 147.93 | 122.46 | 110 |
| JJ04 | 71 | 164.08 | 101.96 | 385.29 | 129.18 | 133.07 | 105.93 | 121 |
| JJ05 | 65.6 | 192.67 | 125.38 | 401.85 | 164.22 | 139.67 | 113.24 | 121 |
| JJ06 | 100 | 217.26 | 148.32 | 321.82 | 212.80 | 145.71 | 119.97 | 149 |
| JJ07 | 84.1 | 225.42 | 130.85 | 364.16 | 189.28 | 146.80 | 121.19 | 115 |
| JJ08 | 87.6 | 218.94 | 154.32 | 319.99 | 217.57 | 143.47 | 117.47 | 153 |
| JJ09 | 88.2 | 215.68 | 142.19 | 306.22 | 212.13 | 133.86 | 106.80 | 150 |
| JJ10 | 100 | 216.11 | 131.21 | 351.67 | 196.20 | 165.13 | 141.86 | 140 |
| JJ11 | 83 | 179.97 | 97.00 | 265.41 | 176.87 | 293.81 | 297.40 | 122 |
| JJ12 | 81 | 179.36 | 108.81 | 376.73 | 142.28 | 291.14 | 293.97 | 148 |
| JJ13 | 75.7 | 186.09 | 110.99 | 384.45 | 141.88 | 249.93 | 242.21 | 148 |
| JJ14 | 89.1 | 176.74 | 107.90 | 366.57 | 145.98 | 259.26 | 253.74 | 150 |
| T (°C) | pH | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | TDS | |
|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | 1 | |||||||||
| pH | −0.097 | 1 | ||||||||
| K+ | 0.285 | −0.431 | 1 | |||||||
| Na+ | −0.055 | −0.433 | 0.848 ** | 1 | ||||||
| Ca2+ | −0.101 | −0.570 * | 0.686 ** | 0.678 ** | 1 | |||||
| Mg2+ | −0.097 | −0.459 * | 0.618 ** | 0.645 ** | 0.951 ** | 1 | ||||
| Cl− | −0.162 | −0.462 * | 0.788 ** | 0.976 ** | 0.769 ** | 0.740 ** | 1 | |||
| SO42− | 0.026 | 0.246 | −0.273 | −0.494 * | −0.359 | −0.473 * | −0.555 * | 1 | ||
| HCO3− | 0.611 ** | 0.116 | 0.209 | 0.094 | −0.401 | −0.353 | −0.101 | −0.034 | 1 | |
| TDS | 0.099 | −0.365 | 0.911 ** | 0.973 ** | 0.674 ** | 0.662 ** | 0.931 ** | −0.460 * | 0.196 | 1 |
| T (°C) | 1 | |||||||||
| pH | 0.661 * | 1 | ||||||||
| K+ | 0.606 * | 0.619 * | 1 | |||||||
| Na+ | 0.513 | 0.444 | 0.717 ** | 1 | ||||||
| Ca2+ | −0.128 | −0.648 * | −0.349 | 0.144 | 1 | |||||
| Mg2+ | 0.044 | −0.048 | −0.137 | 0.441 | 0.259 | 1 | ||||
| Cl− | 0.509 | 0.427 | 0.713 ** | 0.982 ** | 0.215 | 0.357 | 1 | |||
| SO42− | 0.017 | −0.253 | −0.275 | 0.428 | 0.679 ** | 0.823 ** | 0.397 | 1 | ||
| HCO3− | −0.305 | −0.265 | 0.18 | −0.348 | −0.363 | −0.512 | −0.382 | −0.699 ** | 1 | |
| TDS | 0.481 | 0.308 | 0.638 * | 0.976 ** | 0.309 | 0.474 | 0.974 ** | 0.527 | −0.384 | 1 |
| Sample No. | Stratigraphic | γNa+/γCl− | 100 × γSO42−/γCl− | γCl−/(γHCO3− + CO32−) |
|---|---|---|---|---|
| JN01 | Ng | 1.86 | 20.33 | 1.60 |
| JN02 | 2.12 | 30.26 | 1.21 | |
| JN03 | 1.73 | 22.16 | 1.94 | |
| JN04 | 2.31 | 33.73 | 1.03 | |
| JN05 | 3.23 | 58.10 | 0.67 | |
| JN06 | 2.60 | 28.99 | 0.80 | |
| JN07 | 3.38 | 36.72 | 0.54 | |
| JN08 | 1.69 | 2.57 | 1.46 | |
| JN09 | 2.66 | 33.22 | 0.84 | |
| JN10 | 1.35 | 15.14 | 4.22 | |
| JN11 | 1.18 | 1.59 | 5.00 | |
| JJ01 | Jxw | 1.09 | 3.24 | 4.90 |
| JJ02 | 1.06 | 3.02 | 6.21 | |
| JJ03 | 1.07 | 3.33 | 5.08 | |
| JJ04 | 1.28 | 6.87 | 2.98 | |
| JJ05 | 1.22 | 5.56 | 3.54 | |
| JJ06 | 1.03 | 2.77 | 9.32 | |
| JJ07 | 1.14 | 4.41 | 3.99 | |
| JJ08 | 1.04 | 2.67 | 11.24 | |
| JJ09 | 1.02 | 2.47 | 10.63 | |
| JJ10 | 1.07 | 4.88 | 7.54 | |
| JQ01 | Q | 2.72 | 127.10 | 0.60 |
| JQ02 | 1.44 | 32.85 | 0.75 |
| Sample No. | Supply Elevation (m) | Sample No. | Supply Elevation (m) |
|---|---|---|---|
| JN01 | 963 | JJ01 | 863 |
| JN02 | 963 | JJ02 | 813 |
| JN03 | 1013 | JJ03 | 913 |
| JN04 | 1063 | JJ04 | 1013 |
| JN05 | 1063 | JJ05 | 963 |
| JN06 | 963 | JJ06 | 813 |
| JN07 | 913 | JJ07 | 863 |
| JN08 | 913 | JJ08 | 763 |
| JN09 | 863 | JJ09 | 763 |
| JN10 | 913 | JJ10 | 763 |
| JN11 | 813 |
| No. | Temperature Scale Type | Error Range (°C) | Average Error (°C) |
|---|---|---|---|
| 1 | Na-k | ±0.33–141.32 | 57.52 |
| 2 | Na-K-Ca | ±0.84–68.26 | 21.44 |
| 3 | K-Mg | ±1.7–129.97 | 50.06 |
| 4 | Ca-Mg | ±181.42–382.50 | 312.32 |
| 5 | Quartz | ±8.35–210.81 | 73.82 |
| 6 | Chalcedony | ±8.23–214.4 | 53.73 |
| 7 | SI Method | ±1.00–72.3 | 32.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xing, L.; Lin, W.; Zhang, W.; Zhao, Z.; Zhao, J.; Zhai, T. Chemical Characterization and Genesis of Thermal Reservoir Water in the Southern Part of the Jizhong Depression. Water 2023, 15, 3532. https://doi.org/10.3390/w15203532
Wang L, Xing L, Lin W, Zhang W, Zhao Z, Zhao J, Zhai T. Chemical Characterization and Genesis of Thermal Reservoir Water in the Southern Part of the Jizhong Depression. Water. 2023; 15(20):3532. https://doi.org/10.3390/w15203532
Chicago/Turabian StyleWang, Lijun, Linxiao Xing, Wenjing Lin, Wei Zhang, Zirui Zhao, Jiayi Zhao, and Tianlun Zhai. 2023. "Chemical Characterization and Genesis of Thermal Reservoir Water in the Southern Part of the Jizhong Depression" Water 15, no. 20: 3532. https://doi.org/10.3390/w15203532
APA StyleWang, L., Xing, L., Lin, W., Zhang, W., Zhao, Z., Zhao, J., & Zhai, T. (2023). Chemical Characterization and Genesis of Thermal Reservoir Water in the Southern Part of the Jizhong Depression. Water, 15(20), 3532. https://doi.org/10.3390/w15203532







