Seasonal Changes in Upper Thermal Tolerances of Freshwater Thai Fishes
Abstract
:1. Introduction
2. Materials and Methods
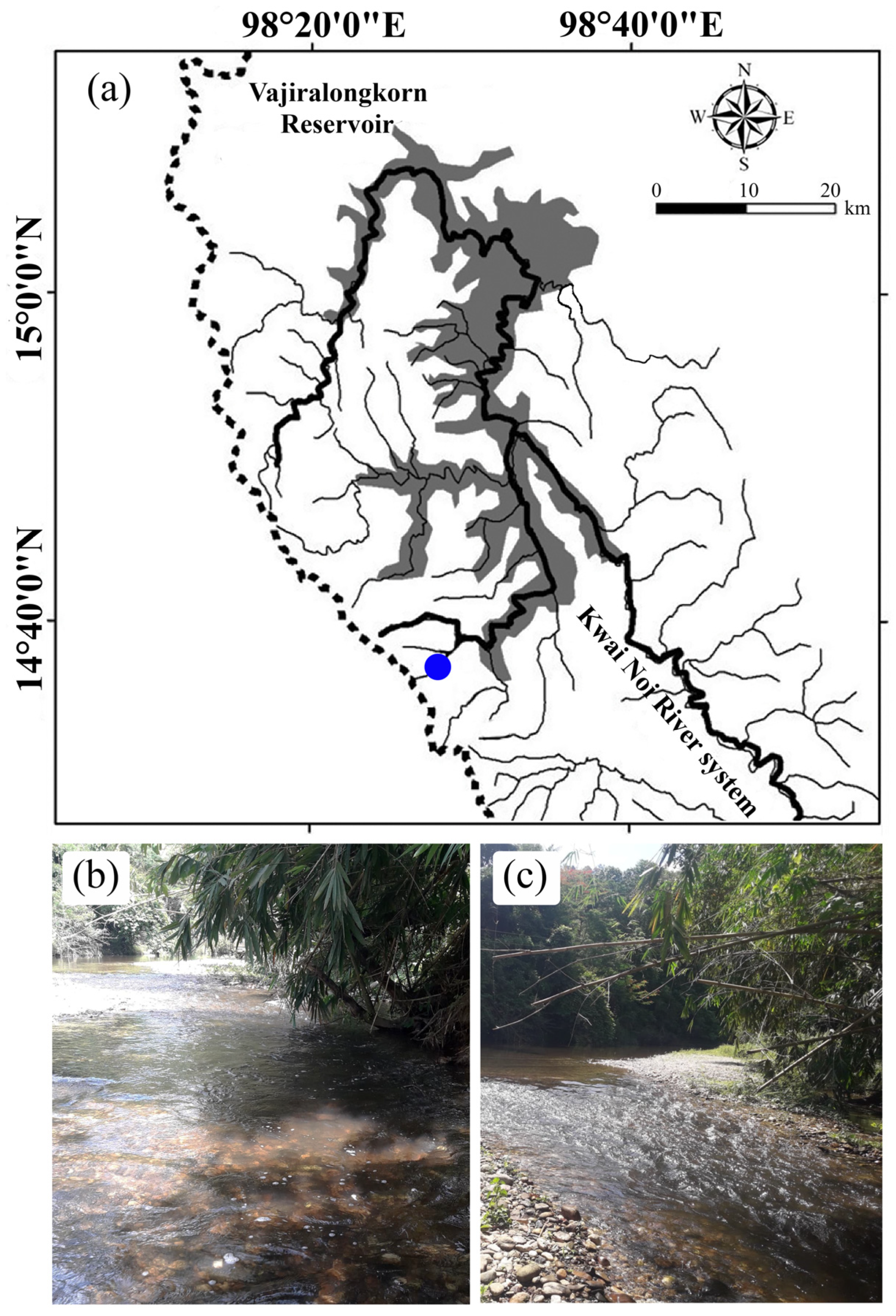
2.1. Study Area
2.2. Fish Samples
2.3. Fish Rearing Condition
2.4. CTmax Measurement
2.5. Statistical Analysis
3. Results
3.1. Environmental Data
3.2. CTmax of the Fish in Wet Season and Dry Season
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Viron, O.; Dehant, V.; Goosse, H.; Crucifix, M. Effect of global warming on the length-of-day. Geophys. Res. Lett. 2002, 29, 1146. [Google Scholar] [CrossRef]
- Landerer, F.W.; Jungclaus, J.H.; Marotzke, J. Ocean bottom pressure changes lead to a decreasing length-of-day in a warming climate. Geophys. Res. Lett. 2007, 34, L06307. [Google Scholar] [CrossRef] [Green Version]
- Scott, S.G.; Pankhurst, N.W. Interannual variation in the reproductive cycle of the New Zealand snapper Pagrus auratus (Bloch & Schneider) (Sparidae). J. Fish Biol. 1992, 41, 685–696. [Google Scholar]
- Shimizu, A. Effect of photoperiod and temperature on gonadal activity and plasma steroid levels in a reared strain of the mummichog (Fundulus heteroclitus) during different phases of its annual reproductive cycle. Gen. Comp. Endocrinol. 2003, 131, 310–324. [Google Scholar] [CrossRef]
- Moyle, P.B.; Cech, J.J. Fishes: An Introduction to Ichthyology, 4th ed.; Prentice Hall: Uppersaddle River, NJ, USA, 2000; p. 621. [Google Scholar]
- Pankhurst, N.W.; Munday, P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Beitinger, T.L.; Bennett, W.A.; McCauley, R.W. Temperature tolerance of North American freshwater fishes exposed to dynamic changes in temperature. Environ. Biol. Fishes 2000, 58, 237–275. [Google Scholar] [CrossRef]
- Brett, J.R. Some lethal Temperature Relations of Algonquin Park Fishes; University of Toronto studied biological series, No.52. Publications of the Ontario fisheries research laboratory, No.63; University of Toronto Press: Toronto, ON, Canada, 1944; pp. 1–49. [Google Scholar]
- Golovanov, V.K.; Ruchin, A.B. Critical thermal maximum of Amur Sleeper Perccottus glenii in different season of the year. J. Ichthyol. 2011, 51, 788–793. [Google Scholar] [CrossRef]
- Alexandre, C.M.; Ferreira, M.T.; Almeida, P.R. Life history of a cyprinid species in non-regulated and regulated rivers from permanent and temporary Mediterranean basins. Ecohydrology 2015, 8, 1137–1153. [Google Scholar] [CrossRef]
- Cereja, R. Critical thermal maxima in aquatic ectotherms. Ecol. Indic. 2020, 119, 106856. [Google Scholar] [CrossRef]
- Terpin, K.M.; Spotila, J.R.; Koons, R.R. Effect of photoperiod on the temperature tolerance of the blacknose dace. Rhinichthys atratulus. Comp. Biochem. Physiol. 1976, 53A, 241–244. [Google Scholar] [CrossRef]
- Guderley, H.; Leroy, P.H.; Gagné, A. Thermal acclimation, growth, and burst swimming of threespine stickleback: Enzymatic correlates and influence of photoperiod. Physiol. Biochem. Zool. 2001, 74, 66–74. [Google Scholar] [CrossRef]
- Healy, T.M.; Schulte, P.M. Factors affecting plasticity in whole-organism thermal tolerance in common killifish (Fundulus heteroclitus). J. Comp. Physiol.B 2012, 182, 49–62. [Google Scholar] [CrossRef]
- Huey, R.B.; Buckley, L.B. Designing a seasonal acclimation study presents challenges and opportunities. Integr. Org. Biol. 2022, 4, obac016. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, D.I.; Sparks, R.W.; De Oca, R.M.; Skolik, R.; Menze, M.A.; Martinez, E. Seasonal changes in mitochondrial bioenergetics and physiological performance of the bluegill sunfish, Lepomis macrochirus, from a shallow, Midwest river. J. Therm. Biol. 2022, 104, 103186. [Google Scholar] [CrossRef]
- Kitano, H. Systems biology: A brief overview. Sciences 2002, 295, 1662–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, C.M.; McDonald, D.G. Global Warming: Implications for Freshwater and Marine Fish; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Payne, N.L.; Smith, J.A.; van der Meulen, D.E.; Taylor, M.D.; Watanabe, Y.Y.; Takahashi, A.; Marzullo, T.A.; Gray, C.A.; Cadiou, G.; Suthers, I.M. Temperature dependence of fish performance in the wild: Links with species biogeography and physiological thermal tolerance. Funct. Ecol. 2016, 30, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Turko, A.J.; Nolan, C.B.; Balshine, S.; Scott, G.R.; Pitcher, T.E. Thermal tolerance depends on season, age and body condition in imperilled redside dace Clinostomus elongatus. Conserv. Physiol. 2020, 8, coaa062. [Google Scholar] [CrossRef]
- Li, D.; Dorber, M.; Barbarossa, V.; Verones, F. Global characterization factors for quantifying the impacts of increasing water temperature on freshwater fish. Ecol. Indic. 2022, 142, 109201. [Google Scholar] [CrossRef]
- Becker, C.D.; Genoway, R.G. Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environ. Biol. Fishes 1979, 4, 245–256. [Google Scholar] [CrossRef]
- Calosi, P.; Bilton, D.T.; Spicer, J.I. Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol. Lett. 2008, 4, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Tongnunui, S.; Beamish, F.W.H. Critical thermal maximum, temperature acclimation and climate effects on Thai freshwater fishes. EA. 2017, 10, 109–117. [Google Scholar]
- Beamish, F.W.H.; Sa-ardrit, P.; Tongnunui, S. Habitat characteristics of the Cyprinidae in small rivers in Central Thailand. Environ. Biol. Fishes 2006, 76, 237–253. [Google Scholar] [CrossRef]
- Hayden, B.; Tongnunui, S.; Beamish, F.W.H.; Nithirojpakdee, P.; Cunjak, R.A. Variation in stable-isotope ratios between fin and muscle tissues can alter assessment of resource use in tropical river fishes. J. Fish Biol. 2017, 91, 574–586. [Google Scholar] [CrossRef]
- Hayden, B.; Tongnunui, S.; Beamish, F.W.H.; Nithirojpakdee, P.; Soto, D.X.; Cunjak, R.A. Functional and trophic diversity of tropical headwater stream communities inferred from carbon, nitrogen and hydrogen stable isotope ratios. Food Webs 2021, 26, e00181. [Google Scholar] [CrossRef]
- Tongnunui, S.; Sooksawat, T.; Kohkaew, R.; Teampanpong, J.; Wattanakornsiri, A. Accumulation of microplastics in the freshwater shrimp, Macrobrachium lanchesteri, from Khwae Noi Watershed in Western Thailand. EnvironmentAsia 2022, 15, 25–37. [Google Scholar]
- Erdfelder, E.; Faul, F.; Buchner, A. GPOWER: A general power analysis program. BRMIC 1996, 28, 1–11. [Google Scholar] [CrossRef]
- Hoar, W.S. Seasonal variation in the resistance of goldfish to temperature. Trans. R. Soc. Can. 1955, 49, 25–34. [Google Scholar]
- Hoar, W.S. Photoperiodism and thermal resistance of goldfish. Nature 1956, 178, 364–365. [Google Scholar] [CrossRef]
- Hoar, W.S.; Robertson, G.B. Temperature resistance of goldfish maintained under controlled photoperiods. Can. J. Zool. 1959, 37, 419–428. [Google Scholar] [CrossRef]
- Sharma, N.K.; Akhtar, M.S.; Pandey, N.; Singh, R.; Singh, A.K. Seasonal variation in thermal tolerance, oxygen consumption, antioxidative enzymes and non-specific immune indices of Indian hill trout, Barilius bendelisis (Hamilton, 1870) from central Himalaya, India. J. Therm. Biol. 2015, 52, 166–176. [Google Scholar] [CrossRef]
- Hines, C.W.; Fang, Y.; Chan, V.K.; Stiller, K.T.; Brauner, C.J.; Richards, J.G. The effect of salinity and photoperiod on thermal tolerance of Atlantic and coho salmon reared from smolt to adult in recirculating aquaculture systems. Comp. Biochem. Physiol., Mol. Amp; Integr. Physiol. 2019, 230, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tyler, A.V. Some lethal temperature relations of two minnows of the genus Chrosomus. Can. J. Zool. 1966, 44, 349–364. [Google Scholar] [CrossRef]
- Morgan, R.; Sundin, J.; Finnøen, M.H.; Dresler, G.; Vendrell, M.M.; Dey, A.; Sarkar, K.; Jutfelt, F. Are model organisms representative for climate change research? Testing thermal tolerance in wild and laboratory zebrafish populations. Conserv. Physiol. 2019, 7, coz036. [Google Scholar] [CrossRef]
- Haworth, M.R.; Bestgen, K.R. Flow and water temperature affect reproduction and recruitment of a Great Plains cyprinid. Can. J. Fish. Aquat. Sci. 2017, 74, 853–863. [Google Scholar] [CrossRef]
- Lowe-McConnell, R.H. Ecological Studies in Tropical Fish Communities; Cambridge University Press: Cambridge, UK, 1987; p. 382. [Google Scholar]
- Martin-Smith, K.; Laird, L. Reproductive patterns in some Cypriniformes from Borneo. In Proceedings of the 5th Indo Pacific Fish Conference, Paris, Society of French Ichthyology, Noumea, French, 3–8 November 1997. [Google Scholar]
- Muchilisin, Z.; Musman, M.; Azizah, M.S. Spawning season of Rasbora tawarensis (Pisves: Cyprinidae) in Lake Laut Tawar, Aceh Province, Indonesia. Reprod. Biol. Endocrinol. 2010, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winemiller, K.O. Patterns of life history among South American fishes in seasonal environments. Oecologia 1989, 81, 225–241. [Google Scholar] [CrossRef]
- Thapanand-Chaidee, T. Fecundity relationship, maturity size and spawning season of shark catfish Helicophagus waandersii Bleeker, 1858 in the Mun River, Thailand. J. Fish. Environ. 2008, 32, 17–29. [Google Scholar]
- Valbo-Jørgensen, J.; Poulsen, A.F. Using local knowledge as a research tool in the study of river fish biology: Experiences from the Mekong. Environ. Dev. Sustain. 2000, 2, 253–376. [Google Scholar] [CrossRef]
- Petitgas, P.; Rijnsdorp, A.D.; Dickey-Collas, M.; Engelhard, G.H.; Peck, M.A.; Pinnegar, J.K.; Drinkwater, K.; Huret, M.; Nash, R.D. Impacts of climate change on the complex life cycles of fish. Fish. Oceanogr. 2013, 22, 121–139. [Google Scholar] [CrossRef]
- Beamish, F.W.H. Migration and spawning energetics of the anadromous sea lamprey, Petromyzon marinus. Environ. Biol. Fish 1979, 4, 3–7. [Google Scholar]
- Boulton, A.J.; Boyero, L.; Covich, A.P.; Dobson, M.; Lake, S.; Pearson, R. Are tropical streams ecologically different from temperate streams? In Tropical Stream Ecology; Dudgeon, D., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 257–284. [Google Scholar]
- Hontela, A.; Stacey, N. Cyprinidae. In Reproductive Seasonality in Teleosts; Munro, A.D., Scott, A.P., Lam, T.J., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 53–78. [Google Scholar]
- Shultz, A.D.; Zuckerman, Z.C.; Suski, C.D. Thermal tolerance of nearshore fishes across seasons: Implications for coastal fish communities in a changing climate. Mar. Biol. 2016, 163, 1–10. [Google Scholar] [CrossRef]
- Suwanpakdee, S.; Sriyasak, P.; Pimolrat, P. Risk of climate variability on tilapia cage culture in songkhram river in northeastern Thailand. AJSTR 2021, 24, 76–83. [Google Scholar] [CrossRef]
- Phanprasit, W.; Rittaprom, K.; Dokkem, S.; Meeyai, A.C.; Boonyayothin, V.; Jaakkola, J.J.; Näyhä, S. Climate warming and occupational heat and hot environment standards in Thailand. Saf. Health Work 2021, 12, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, M.; Takata, K.; Hanasaki, N.; Archevarahuprok, B.; Champathong, A.; Ikoma, E.; Jaikaeo, C.; Kaewrueng, S.; Kanae, S.; Kazama, S.; et al. A review of climate-change impact and adaptation studies for the water sector in Thailand. Environ. Res. Lett. 2021, 16, 023004. [Google Scholar] [CrossRef]
- Liu, S.; Xie, Z.; Liu, B.; Wang, Y.; Gao, J.; Zeng, Y.; Xie, J.; Xie, Z.; Jia, B.; Qin, P.; et al. Global river water warming due to climate change and anthropogenic heat emission. Glob. Planet. Chang. 2020, 193, 103289. [Google Scholar] [CrossRef]
- Kail, J.; Palt, M.; Lorenz, A.; Hering, D. Woody buffer effects on water temperature: The role of spatial configuration and daily temperature fluctuations. Hydrol. Process. 2021, 35, e14008. [Google Scholar] [CrossRef]
- Coats, W.A.; Jackson, C.R. Riparian canopy openings on mountain streams: Landscape controls upon temperature increases within openings and cooling downstream. Hydrol. Process. 2020, 34, 1966–1980. [Google Scholar] [CrossRef]
- Loicq, P.; Moatar, F.; Jullian, Y.; Dugdale, S.J.; Hannah, D.M. Improving representation of riparian vegetation shading in a regional stream temperature model using LiDAR data. Sci. Total Environ. 2018, 624, 480–490. [Google Scholar] [CrossRef]
- Hopkin, R.S.; Qari, S.; Bowler, K.; Hyde, D.; Cuculescu, M. Seasonal thermal tolerance in marine Crustacea. J. Exp. Mar. Biol. Ecol. 2006, 331, 74–81. [Google Scholar] [CrossRef]
- Magozzi, S.; Calosi, P. Integrating metabolic performance, thermal tolerance, and plasticity enables for more accurate predictions on species vulnerability to acute and chronic effects of global warming. Glob. Chang. Biol. 2015, 21, 181–194. [Google Scholar] [CrossRef]
- Barley, J.M.; Cheng, B.S.; Sasaki, M.; Gignoux-Wolfsohn, S.; Hays, C.G.; Putnam, A.B.; Sheth, S.; Villeneuve, A.R.; Kelly, M. Limited plasticity in thermally tolerant ectotherm populations: Evidence for a trade-off. Proc. R. Soc. B Biol. Sci. B 2021, 288, 20210765. [Google Scholar] [CrossRef] [PubMed]
- Rivera, H.E.; Aichelman, H.E.; Fifer, J.E.; Kriefall, N.G.; Wuitchik, D.M.; Wuitchik, S.J.; Davies, S.W. A framework for understanding gene expression plasticity and its influence on stress tolerance. Mol. Ecol. 2021, 30, 1381–1397. [Google Scholar] [CrossRef] [PubMed]






| Physicochemical Parameters | Wet Season (May and August) | Dry Season (November and February) | ||
|---|---|---|---|---|
| Year 2017 | Year 2022 | Year 2017 | Year 2022 | |
| Dissolved oxygen (DO, mgO2/L) | 8.1–8.5 | 8.7–9.1 | 8.7–9.1 | 8.6–9.1 |
| 8.3 ± 0.1 | 8.9 ± 0.1 | 8.8 ± 0.1 | 8.8 ± 2.0 | |
| Alkalinity (mgCaCO3/L) | 34–36 | 34–38 | 34–36 | 32–36 |
| 34.7 ± 0.8 | 35.7–1.5 | 35.0 ± 0.7 | 34.1 ± 1.6 | |
| Silica (mgSiO2/L) | 34–35 | 34–37 | 35–38 | 35–38 |
| 37.7 ± 0.4 | 35.5 ± 1.2 | 36.3 ± 1.3 | 36.5 ± 1.2 | |
| Ammonia (mgNH3N/L) | 0.01–0.02 | 0.01–0.02 | 0.01–0.02 | 0.01–0.2 |
| 0.012 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.04 ± 0.1 | |
| Total iron (mgFe/L) | 0.01–0.02 | 0.01–0.2 | 0.01–0.02 | 0.01–0.02 |
| 0.014 ± 0.00 | 0.02 ± 0.05 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| pH | 7.4–7.8 | 6.5–7.8 | 7.4–8.1 | 7.4–8.2 |
| 7.5 ± 0.1 | 7.4 ± 0.4 | 7.7 ± 0.3 | 7.8 ± 0.3 | |
| Canopy (%) | 45% | 40% | 30% | 30% |
| 45.0 ± 0.0 | 40.0 ± 0.0 | 30.0 ± 0.0 | 30.0 ± 0.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tongnunui, S.; Sooksawat, T.; Chotwiwatthanakun, C.; Supiwong, W.; Wattanakornsiri, A.; Beamish, F.W.H. Seasonal Changes in Upper Thermal Tolerances of Freshwater Thai Fishes. Water 2023, 15, 350. https://doi.org/10.3390/w15020350
Tongnunui S, Sooksawat T, Chotwiwatthanakun C, Supiwong W, Wattanakornsiri A, Beamish FWH. Seasonal Changes in Upper Thermal Tolerances of Freshwater Thai Fishes. Water. 2023; 15(2):350. https://doi.org/10.3390/w15020350
Chicago/Turabian StyleTongnunui, Sampan, Treerat Sooksawat, Charoonroj Chotwiwatthanakun, Weerayuth Supiwong, Amnuay Wattanakornsiri, and F. W. H. Beamish. 2023. "Seasonal Changes in Upper Thermal Tolerances of Freshwater Thai Fishes" Water 15, no. 2: 350. https://doi.org/10.3390/w15020350






