Sorption of 2,4-Dichlorophenoxyacetic Acid from Agricultural Leachate Using Termite Mound Soil: Optimization Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent Preparation
2.2. Adsorbent Characterization
2.3. Sorption Experiment
2.3.1. Effect of Contact Time
2.3.2. Effect of pH
2.3.3. Effect of TMS Dose
2.3.4. Effect of 2,4-D Concentration
2.4. Adsorption Kinetics
2.5. Adsorption Isotherm
2.6. Response Surface Modeling and Experimental Design
3. Results and Discussion
3.1. Adsorbent Characterization
3.1.1. Chemical Composition
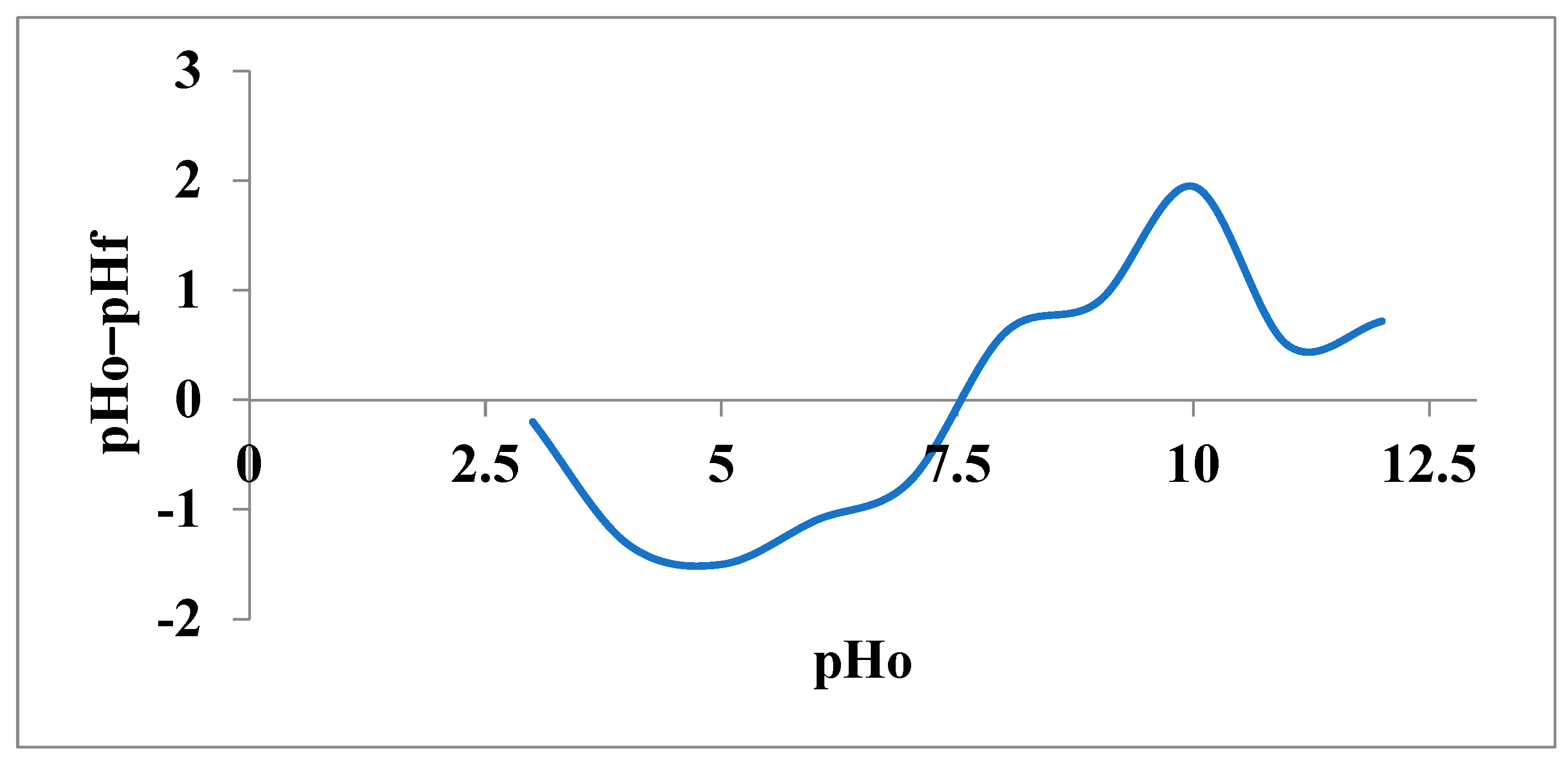
3.1.2. Point of Zero Charge (pHpzc)
3.1.3. Specific Surface Area
3.1.4. SEM Examination
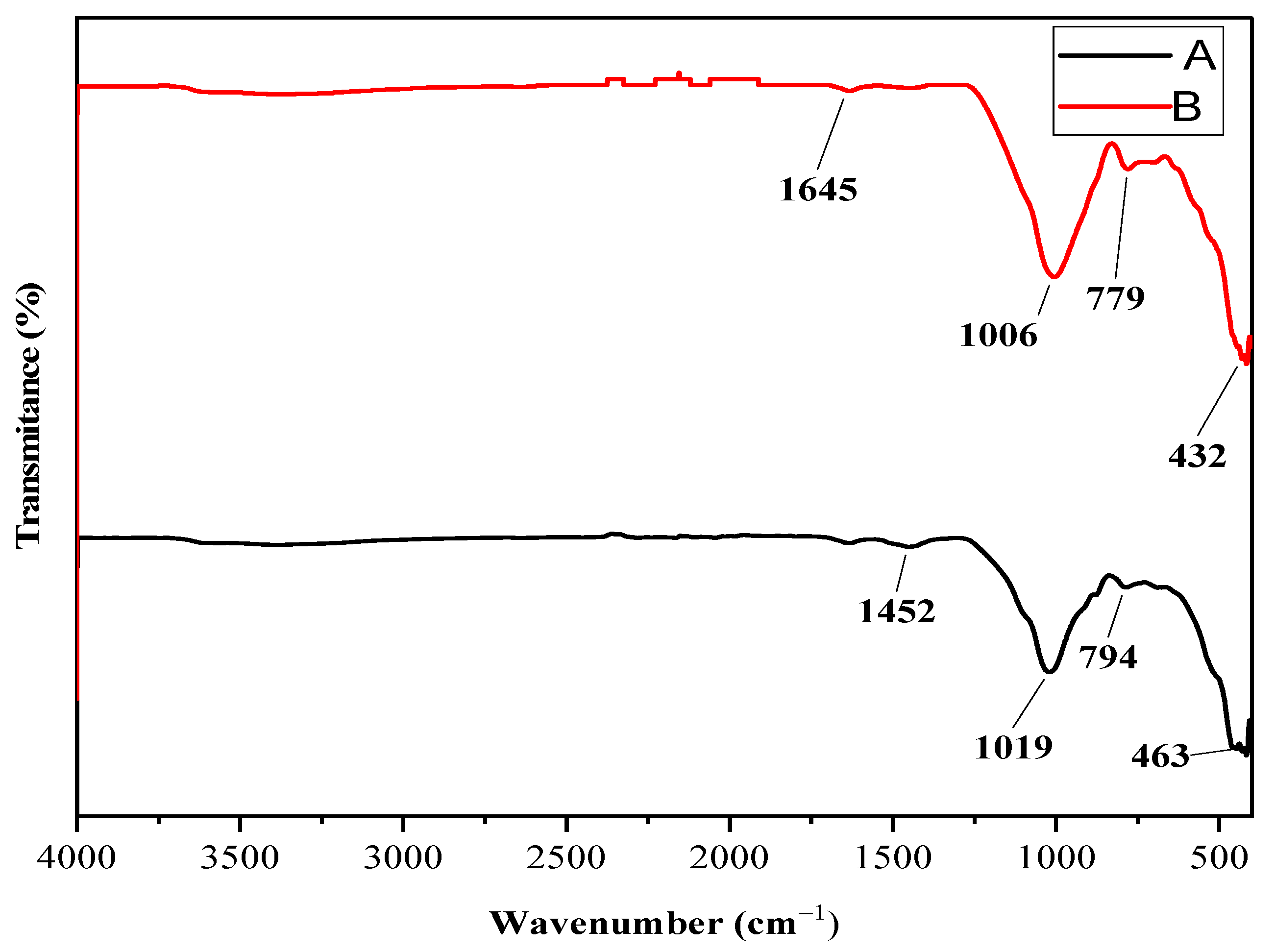
3.1.5. FTIR Analysis
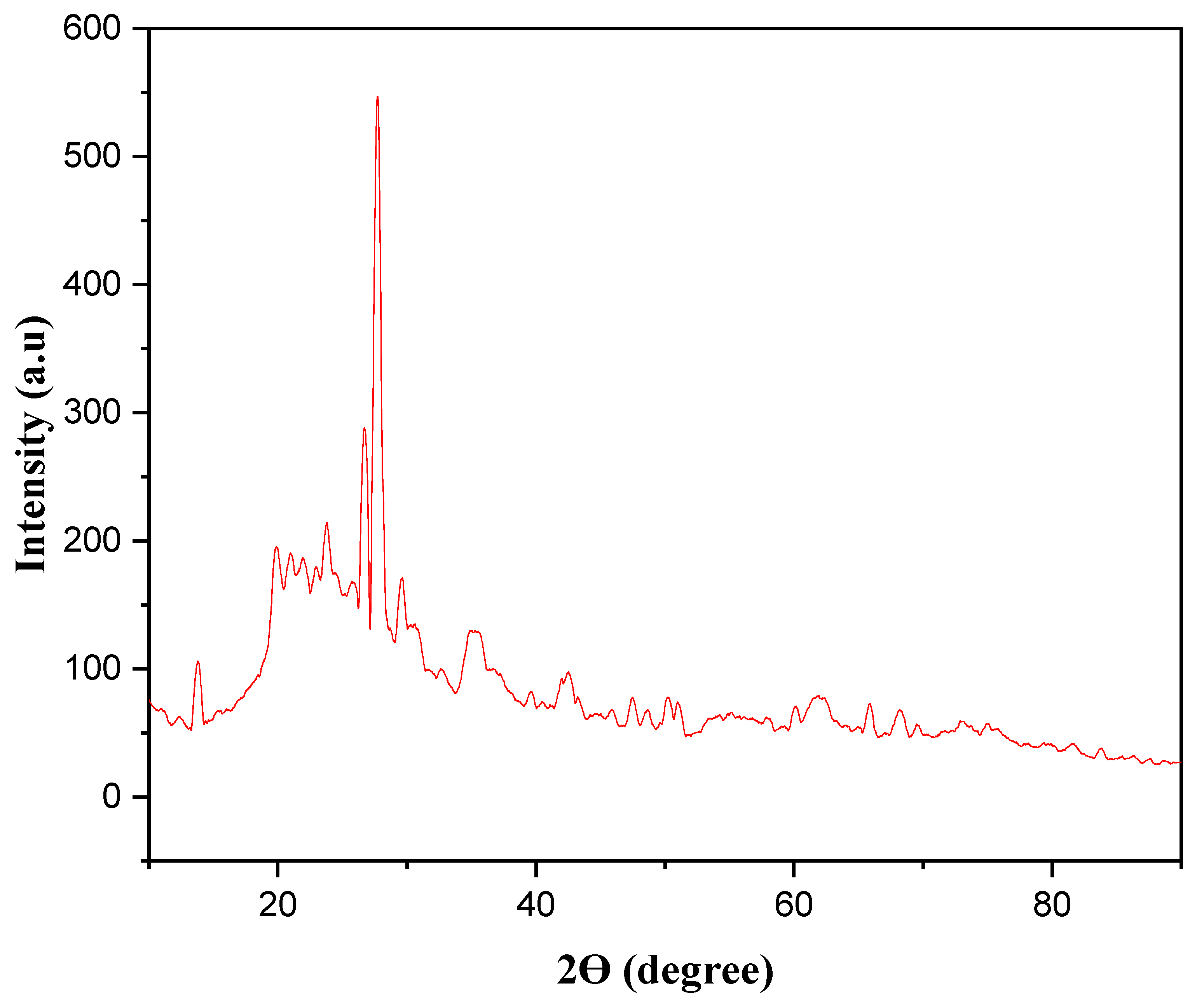
3.1.6. XRD Analysis
3.2. 2,4-D Sorption
3.2.1. Effect of pH
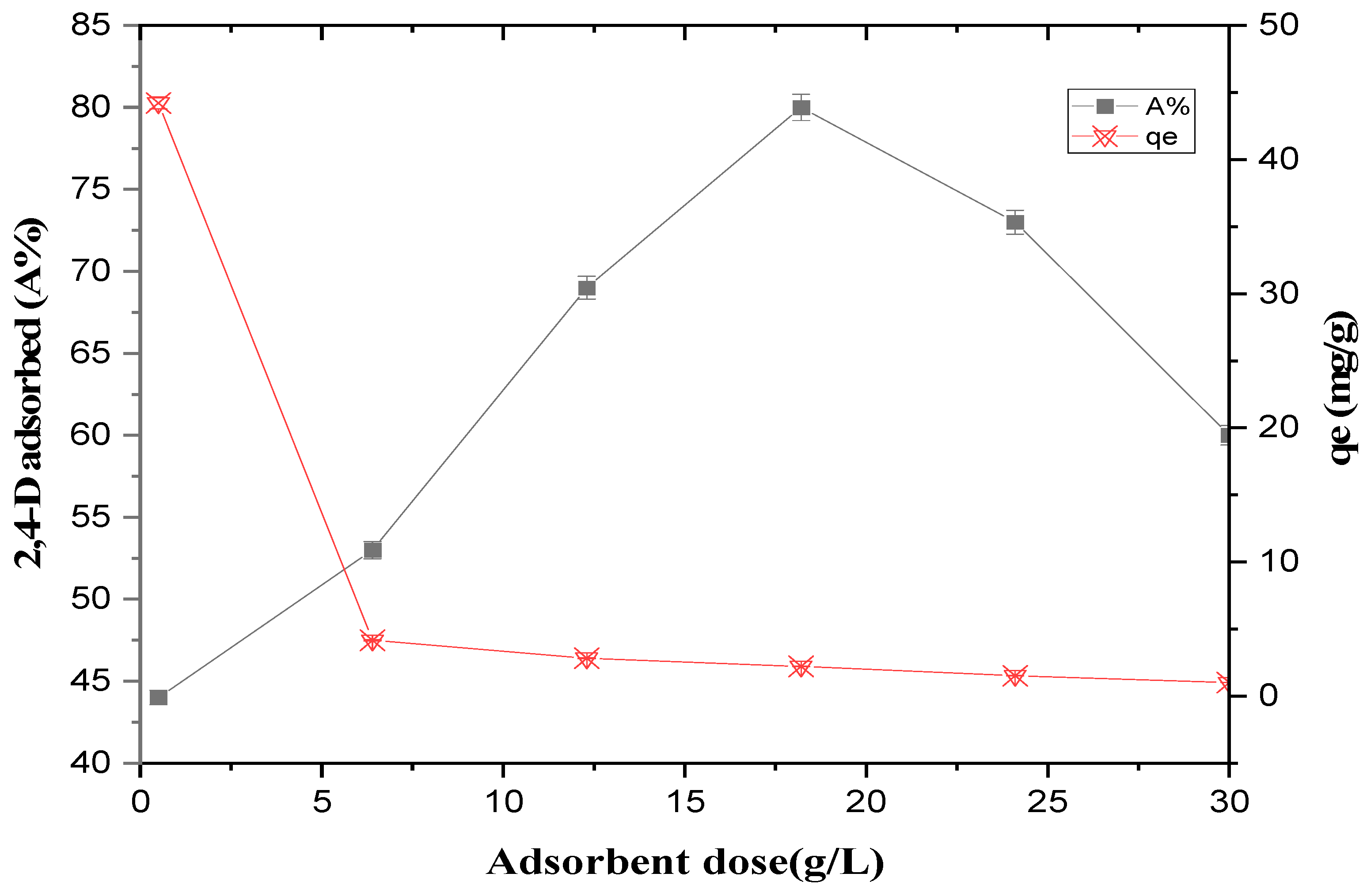
3.2.2. Effect of TMS Dose
3.2.3. Effect of 2,4-D Initial Concentration
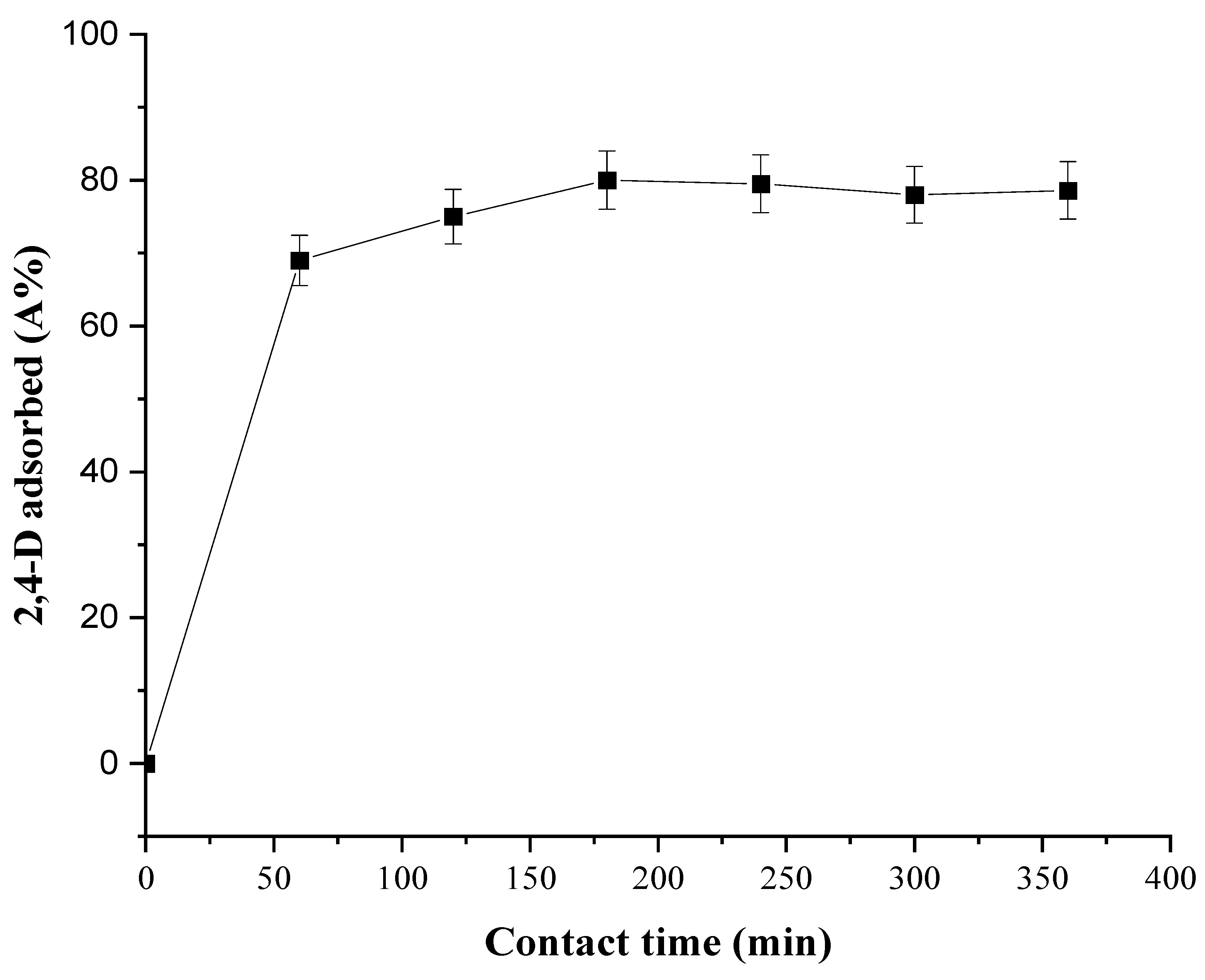
3.2.4. Effect of Contact Time
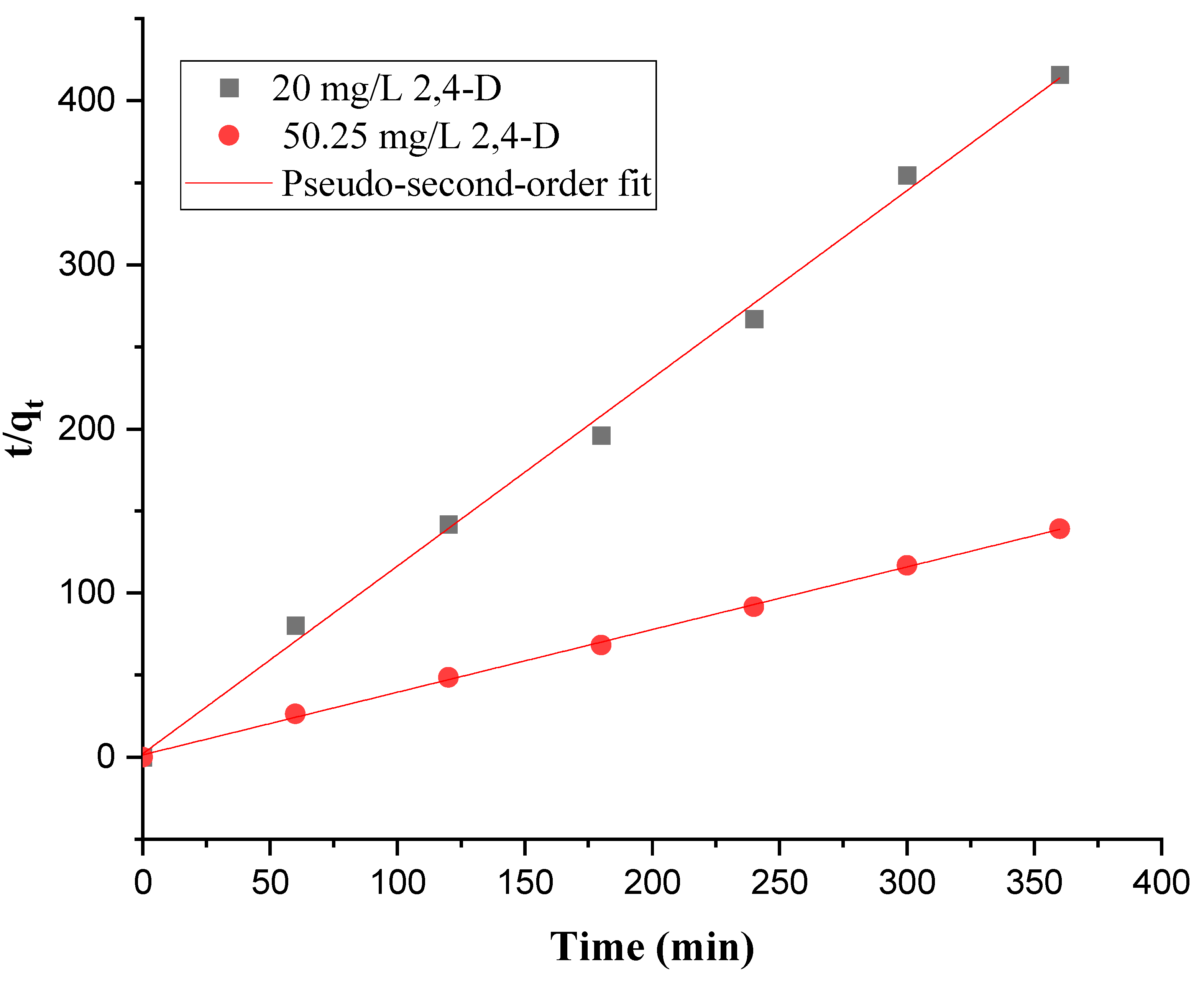
3.2.5. Adsorption Kinetics
3.2.6. Adsorption Isotherm
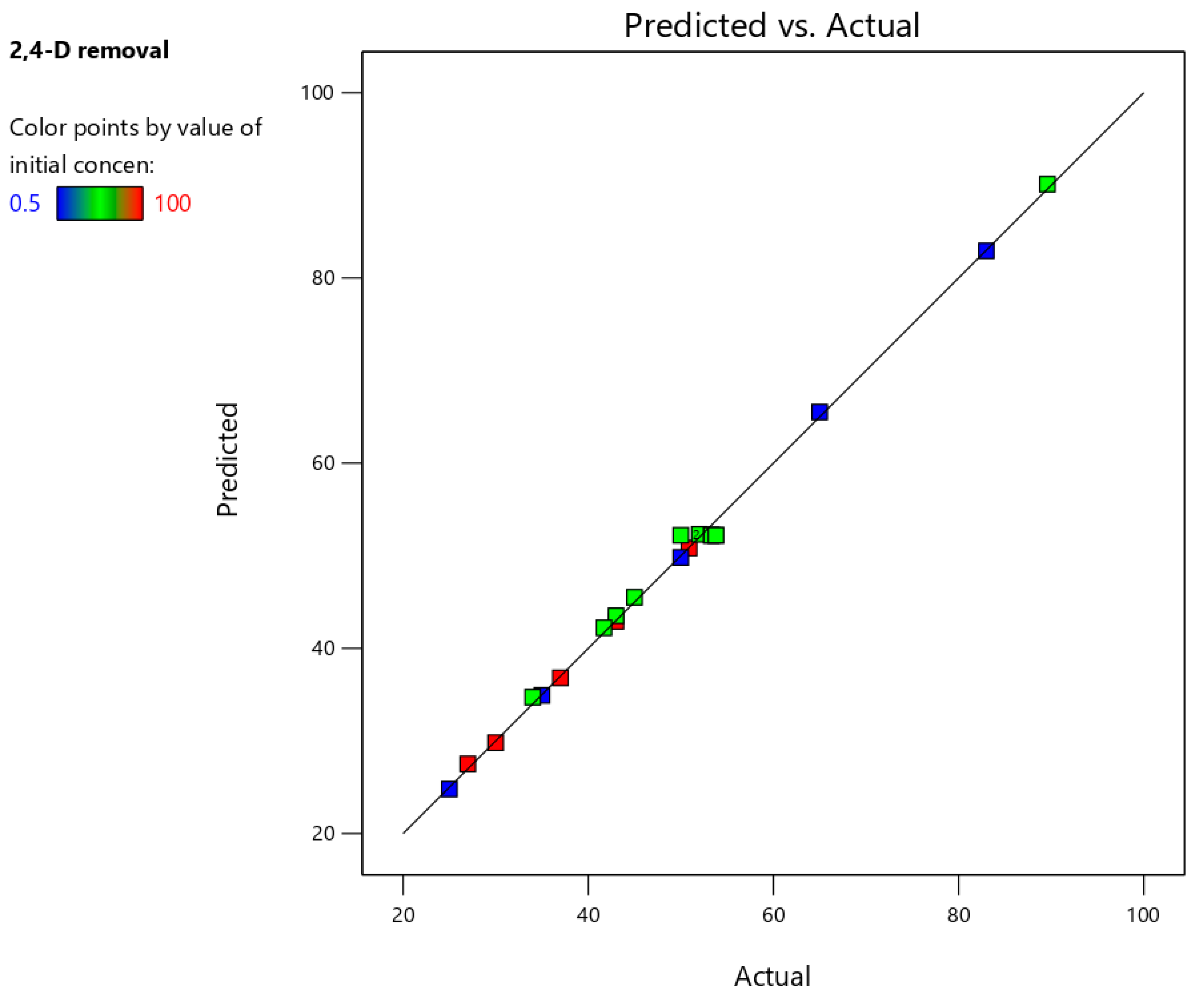
3.3. Central Composite Design (CCD)
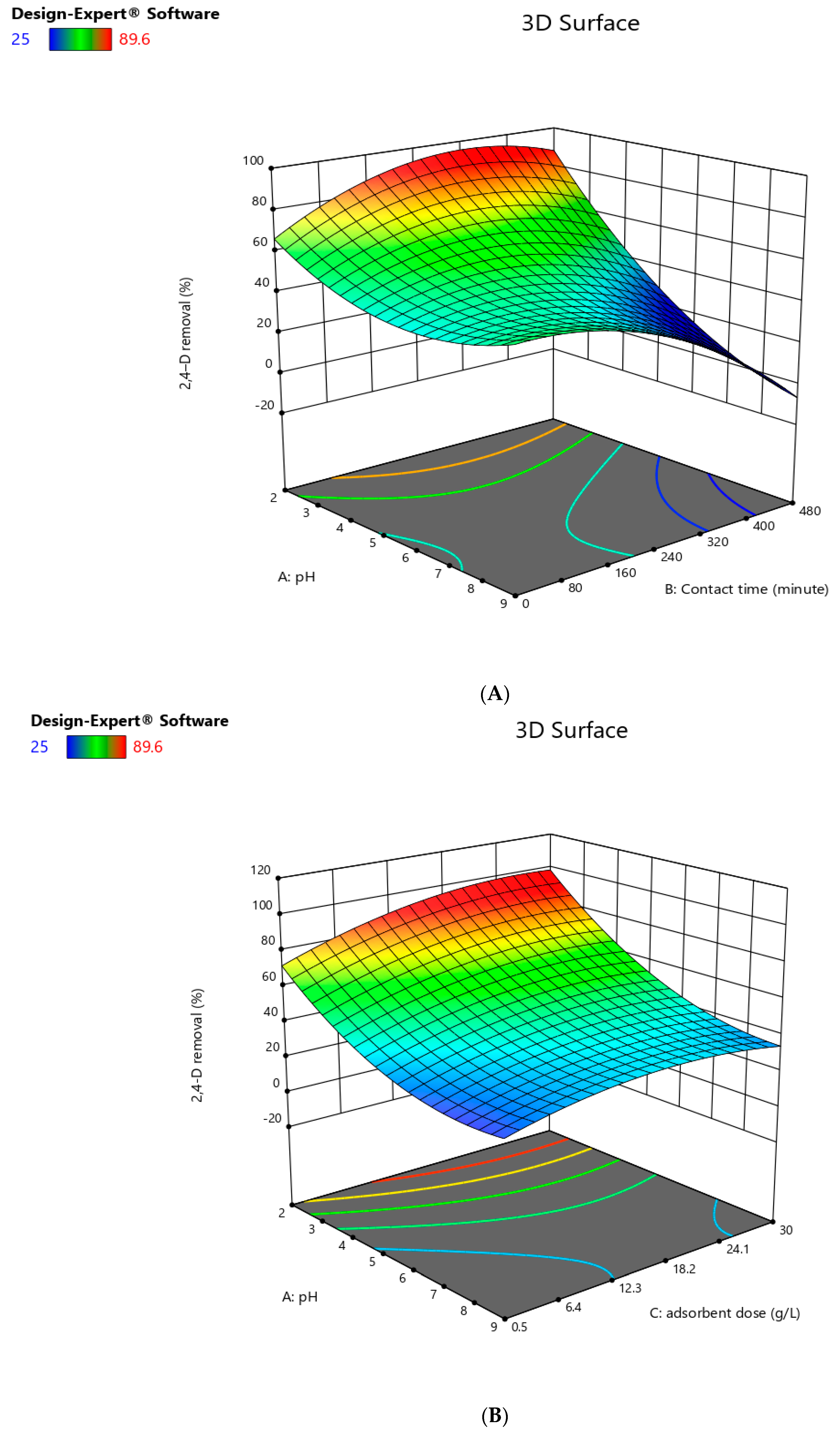
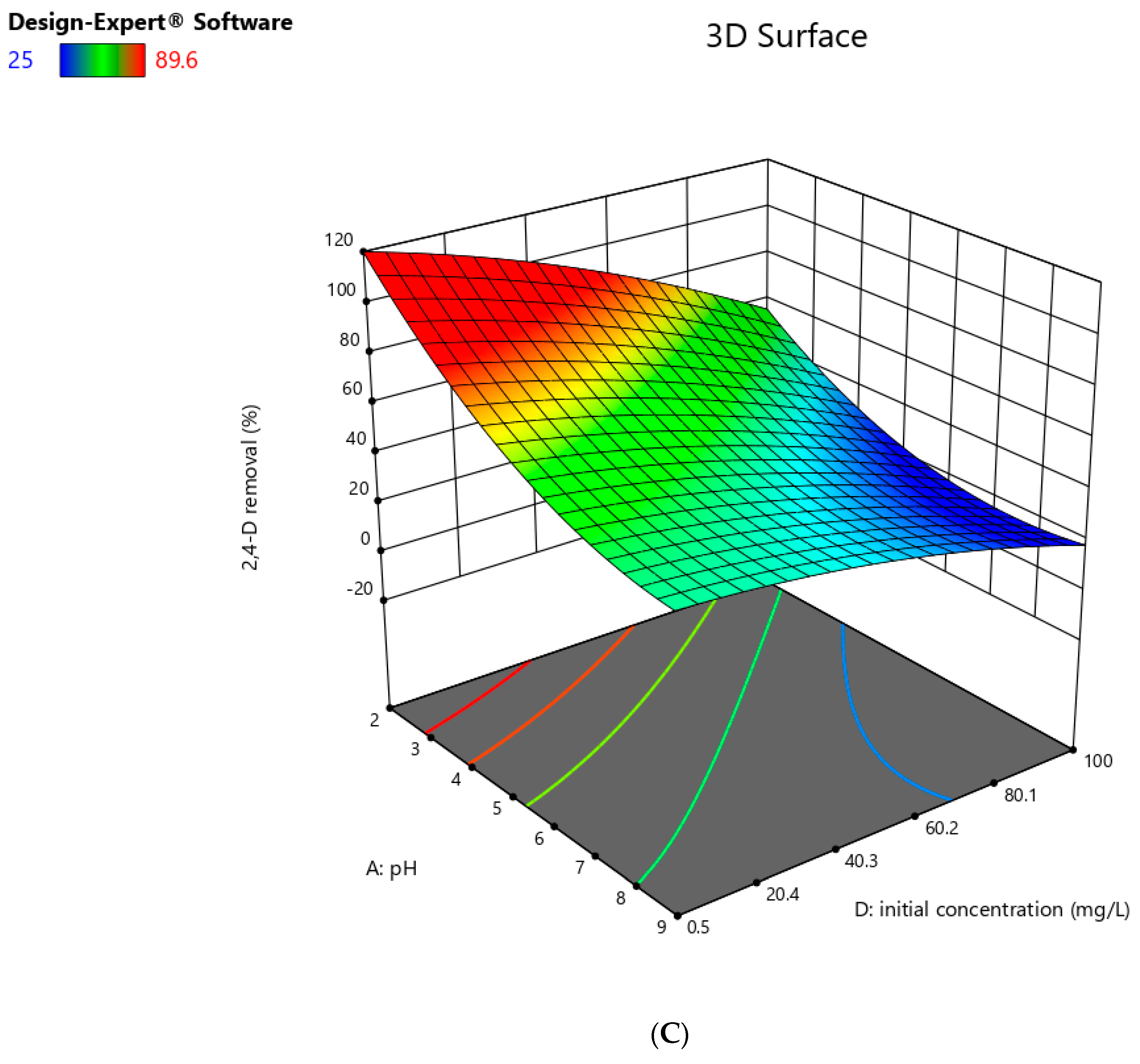
Interaction between the Relevant Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rani, K.; Dhania, G. Bioremediation and biodegradation of pesticide from contaminated soil and water—A noval approach. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 23–33. [Google Scholar]
- Islam, F.; Wang, J.; Farooq, M.A.; Khan, M.S.; Xu, L.; Zhu, J.; Zhao, M.; Muños, S.; Li, Q.X.; Zhou, W. Potential impact of the herbicide 2,4-dichlorophenoxyacetic acid on human and ecosystems. Environ. Int. 2018, 111, 332–351. [Google Scholar] [CrossRef] [PubMed]
- Goscianska, J.; Olejnik, A. Removal of 2,4-D herbicide from aqueous solution by aminosilane-grafted mesoporous carbons. Adsorption 2019, 25, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Souza, F.; Saéz, C.; Llanos, J.; Lanza, M.R.; Cañizares, P.; Rodrigo, M.A. Solar-powered electrokinetic remediation for the treatment of soil polluted with the herbicide 2,4-D. Electrochim. Acta 2016, 190, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, Y.A.; Abdul-Hameed, H.M.; Razak, Z.A. Biodegradation of 2,4-dichlorophenoxyacetic acid contaminated soil in a roller slurry bioreactor. CLEAN-Soil Air Water 2015, 43, 1241–1247. [Google Scholar] [CrossRef]
- Bazrafshan, E.; KORD, M.F.; Faridi, H.; Farzadkia, M.; Sargazi, S.; Sohrabi, A. Removal of 2,4-dichlorophenoxyacetic acid (2,4-D) from aqueous environments using single-walled carbon nanotubes. Health Scope 2013, 2, 39–46. [Google Scholar] [CrossRef]
- Bartczak, P.; Żółtowska, S.; Norman, M.; Klapiszewski, Ł.; Zdarta, J.; Komosa, A.; Kitowski, I.; Ciesielczyk, F.; Jesionowski, T. Saw-sedge Cladium mariscus as a functional low-cost adsorbent for effective removal of 2,4-dichlorophenoxyacetic acid from aqueous systems. Adsorption 2016, 22, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, N.S.; Kharkar, R.A.; Mandavgane, S.A. 2,4-Dichlorophenoxyacetic acid adsorption on adsorbent prepared from groundnut shell: Effect of preparation conditions on equilibrium adsorption capacity. Arab. J. Chem. 2019, 12, 4541–4549. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, N.S.; Mandavgane, S.A. Fundamentals of 2,4 dichlorophenoxyacetic acid removal from aqueous solutions. Sep. Purif. Rev. 2018, 47, 337–354. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Yang, Z.; Yang, Q.; Li, B.; Bai, Z. Heterogeneous fenton-like catalytic degradation of 2,4-dichlorophenoxyacetic acid in water with FeS. Chem. Eng. J. 2015, 273, 481–489. [Google Scholar] [CrossRef]
- Guleri, S.; Singh, K.; Kaushik, R.; Dhankar, R.; Tiwari, A. Phycoremediation: A novel and synergistic approach in wastewater remediation. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 98–106. [Google Scholar] [CrossRef]
- Zhou, T.; Fang, L.; Wang, X.; Han, M.; Zhang, S.; Han, R. Adsorption of the herbicide 2,4-dichlorophenoxyacetic acid by Fe-crosslinked chitosan complex in batch mode. Desalin Water Treat. 2017, 70, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Deokar, S.K.; Mandavgane, S.A.; Kulkarni, B.D. Adsorptive removal of 2,4-dichlorophenoxyacetic acid from aqueous solution using bagasse fly ash as adsorbent in batch and packed-bed techniques. Clean Technol. Environ. Policy 2016, 18, 1971–1983. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Lee, S.-M. Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv. Colloid Interface Sci. 2013, 201, 68–93. [Google Scholar] [CrossRef]
- Tilahun, A.; Kebede, F.; Yamoah, C.; Erens, H.; Mujinya, B.; Verdoodt, A.; Van Ranst, E. Quantifying the masses of Macrotermes subhyalinus mounds and evaluating their use as a soil amendment. Agric. Ecosyst. Environ. 2012, 157, 54–59. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Maheshwari, D.K.; Dheeman, S.; Bajpai, V.K. Termitarium-inhabiting Bacillus spp. enhanced plant growth and bioactive component in turmeric (Curcuma longa L.). Curr. Microbiol. 2017, 74, 184–192. [Google Scholar] [CrossRef]
- Enagbonma, B.J.; Babalola, O.O. Environmental sustainability: A review of termite mound soil material and its bacteria. Sustainability 2019, 11, 3847. [Google Scholar] [CrossRef] [Green Version]
- Amiri, M.J.; Bahrami, M.; Beigzadeh, B.; Gil, A. A response surface methodology for optimization of 2,4-dichlorophenoxyacetic acid removal from synthetic and drainage water: A comparative study. Environ. Sci. Pollut. Res. 2018, 25, 34277–34293. [Google Scholar] [CrossRef]
- Moradi, M.; Fazlzadehdavil, M.; Pirsaheb, M.; Mansouri, Y.; Khosravi, T.; Sharafi, K. Response surface methodology (RSM) and its application for optimization of ammonium ions removal from aqueous solutions by pumice as a natural and low cost adsorbent. Arch. Environ. Prot. 2016, 42, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Appel, C.; Ma, L. Concentration, pH, and surface charge effects on cadmium and lead sorption in three tropical soils. J. Environ. Qual. 2002, 31, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, T.; Saddique, M.T.; Naeem, A.; Westerhoff, P.; Mustafa, S.; Alum, A. Comparison of different methods for the point of zero charge determination of NiO. Ind. Eng. Chem. Res. 2011, 50, 10017–10023. [Google Scholar] [CrossRef]
- Tebeje, A.; Worku, Z.; Nkambule, T.; Fito, J. Adsorption of chemical oxygen demand from textile industrial wastewater through locally prepared bentonite adsorbent. Int. J. Environ. Sci. Technol. 2022, 19, 1893–1906. [Google Scholar] [CrossRef]
- Bedada, D.; Angassa, K.; Tiruneh, A.; Kloos, H.; Fito, J. Chromium removal from tannery wastewater through activated carbon produced from Parthenium hysterophorus weed. Energy Ecol. Environ. 2020, 5, 184–195. [Google Scholar] [CrossRef]
- OECD. 106: Adsorption–Desorption Using a Batch Equilibrium Method; OECD: Paris, France, 2000. [Google Scholar] [CrossRef]
- Fufa, F.; Alemayehu, E.; Lennartz, B. Sorptive removal of arsenate using termite mound. J. Environ. Manag. 2014, 132, 188–196. [Google Scholar] [CrossRef]
- Mohamed, H.S.; Soliman, N.; Abdelrheem, D.A.; Ramadan, A.A.; Elghandour, A.H.; Ahmed, S.A. Adsorption of Cd2+ and Cr3+ ions from aqueous solutions by using residue of Padina gymnospora waste as promising low-cost adsorbent. Heliyon 2019, 5, e01287. [Google Scholar] [CrossRef] [Green Version]
- Vyas, B.; Kumar Singh, A.; Singh Cameotra, S. Sorption behaviour of maneb in the agriculture soils and its correlation with soil properties. Int. J. Eng. Sci. 2015, 1, 1–8. [Google Scholar]
- Taktak, F.; İlbay, Z.; Şahin, S. Evaluation of 2,4-D removal via activated carbon from pomegranate husk/polymer composite hydrogel: Optimization of process parameters through face centered composite design. Korean J. Chem. Eng. 2015, 32, 1879–1888. [Google Scholar] [CrossRef]
- Huang, L.; Kong, J.; Wang, W.; Zhang, C.; Niu, S.; Gao, B. Study on Fe (III) and Mn (II) modified activated carbons derived from Zizania latifolia to removal basic fuchsin. Desalination 2012, 286, 268–276. [Google Scholar] [CrossRef]
- Du Changwen, M.F.; Yuzhen, L.; Jianmin, Z. 6 Soil Fertility Assessed by Infrared Spectroscopy. Soil-Specif. Farming: Precis. Agric. 2015, 22, 155. [Google Scholar]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of cellulosic fibers by FTIR spectroscopy for their further implementation to building materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Kajjumba, G.W.; Yıldırım, E.; Aydın, S.; Emik, S.; Ağun, T.; Osra, F.; Wasswa, J. A facile polymerisation of magnetic coal to enhanced phosphate removal from solution. J. Environ. Manag. 2019, 247, 356–362. [Google Scholar] [CrossRef]
- Gülen, J.; Arslan, S. Adsorption of 2,4-D on carbonized chest nut shell. Phys. Rev. Res. Int. 2013, 3, 531–540. [Google Scholar]
- Gupta, V.K.; Ali, I.; Saini, V.K. Adsorption of 2,4-D and carbofuran pesticides using fertilizer and steel industry wastes. J. Colloid Interface Sci. 2006, 299, 556–563. [Google Scholar] [CrossRef]
- Njoku, V.; Hameed, B. Preparation and characterization of activated carbon from corncob by chemical activation with H3PO4 for 2,4-dichlorophenoxyacetic acid adsorption. Chem. Eng. J. 2011, 173, 391–399. [Google Scholar] [CrossRef]
- Salman, J.; Njoku, V.; Hameed, B. Batch and fixed-bed adsorption of 2,4-dichlorophenoxyacetic acid onto oil palm frond activated carbon. Chem. Eng. J. 2011, 174, 33–40. [Google Scholar] [CrossRef]
- Hameed, B.; Salman, J.; Ahmad, A. Adsorption isotherm and kinetic modeling of 2,4-D pesticide on activated carbon derived from date stones. J. Hazard. Mater. 2009, 163, 121–126. [Google Scholar] [CrossRef]
- Aksu, Z.; Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, C.; Huang, X.; Li, B.; Niu, J.; Shen, Z. Role of uniform pore structure and high positive charges in the arsenate adsorption performance of Al13-modified montmorillonite. J. Hazard. Mater. 2012, 203, 317–325. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A. A comparative study of dye removal using fly ash treated by different methods. Chemosphere 2005, 60, 1401–1407. [Google Scholar] [CrossRef]
- Solaimany Nazar, A.R.; Jokar Baloochi, S.; Farhadian, M.; Goshadrou, A. 2,4-dichlorophenoxyacetic acid adsorption from contaminated water through activated carbon reclaimed with zero-valent iron and titanium dioxide. Sci. Iran. 2018, 25, 1395–1411. [Google Scholar]
- Deokar, S.K.; Mandavgane, S.A. Rice husk ash for fast removal of 2,4-dichlorophenoxyacetic acid from aqueous solution. Adsorpt. Sci. Technol. 2015, 33, 429–440. [Google Scholar] [CrossRef]
- Trivedi, N.S.; Mandavgane, S.A.; Kulkarni, B.D. Mustard plant ash: A source of micronutrient and an adsorbent for removal of 2,4-dichlorophenoxyacetic acid. Environ. Sci. Pollut. Res. 2016, 23, 20087–20099. [Google Scholar] [CrossRef] [PubMed]
- de Souza, F.M.; Dos Santos, O.A.A.; Vieira, M.G.A. Adsorption of herbicide 2,4-D from aqueous solution using organo-modified bentonite clay. Environ. Sci. Pollut. Res. 2019, 26, 18329–18342. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.; Nasseri, S.; Karamimanesh, M. Removal of 2,4-Dichlorophenolyxacetic acid (2,4-D) herbicide in the aqueous phase using modified granular activated carbon. J. Environ. Health Sci. Eng. 2014, 12, 28. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Shahmoradi, A.; Ghasemi, J. Pesticides removal using conventional and low-cost adsorbents: A review. CLEAN-Soil Air Water 2011, 39, 1105–1119. [Google Scholar] [CrossRef]
- Ani, J.; Okoro, U.; Aneke, L.; Onukwuli, O.; Obi, I.; Akpomie, K.; Ofomatah, A. Application of response surface methodology for optimization of dissolved solids adsorption by activated coal. Appl. Water Sci. 2019, 9, 60. [Google Scholar] [CrossRef]





| Name (Factor) | Units | Low (−) | Middle (0) | High (+) | −α | +α |
|---|---|---|---|---|---|---|
| pH (A) | 2 | 5.5 | 9 | 1.5 | 12.5 | |
| Contact time (B) | Minute | 1 | 180.5 | 360 | 121.4 | 482.4 |
| Adsorbent dose (C) | g/L | 0.5 | 15.25 | 30 | 9.6 | 40.1 |
| Initial 2,4-D concentration (D) | mg/L | 0.5 | 50.25 | 100 | 33.4 | 133.9 |
| Content (%) | Value |
|---|---|
| SiO2 | 60.36 |
| Al2O3 | 10.25 |
| Fe2O3 | 6.18 |
| TiO2 | 0.31 |
| MgO | 1.06 |
| Na2O | 1.68 |
| K2O | 2.74 |
| CaO | 2.34 |
| MnO | 0.12 |
| P2O5 | 0.33 |
| SO3 | <0.01 |
| LOI | 8.38 |
| Total C | 2.3 |
| Total S | ND |
| Total N | 0.13 |
| Pb | ND |
| Ni | ND |
| Cd | ND |
| Model | Parameters | 2,4-D Concentration (mg/L) | |
|---|---|---|---|
| 20 | 50.25 | ||
| Pseudo-first -order | qe,exp (mg/g) | 0.92 | 2.64 |
| K1 (min−1) | −1.71 | −2.3 | |
| qe,cal (mg/g) | 0.92 | 0.36 | |
| R2 | 0.53 | 0.35 | |
| Pseudo-second-order | K2 g/(mg.min) | 0.63 | 0.103 |
| qe,cal (mg/g) | 0.87 | 2.62 | |
| R2 | 0.99 | 0.99 | |
| Intraparticle diffusion | Kp mg/(g.min0.5) | 0.043 | 0.128 |
| C (mg/g) | 0.214 | 0.631 | |
| R2 | 0.73 | 0.75 | |
| Isotherm/Models | Freundlich Constants | Langmuir Constants | |||||
|---|---|---|---|---|---|---|---|
| Variables | 1/n | K | R2 | qmax (mg/g) | KL (L/mg) | RL | R2 |
| Values | −0.23 | 3.47 | 0.8164 | 22.78 | 0.06 | 0.25 | 0.9687 |
| Adsorbent | Adsorption Capacity (mg/g) | References |
|---|---|---|
| Rice husk ash | 1.4 | [35] |
| Activated carbon from corncob | 95.26 | [13] |
| Mustard plant ash | 0.76 | [44] |
| Bentonite clay | 136.14 | [45] |
| Granular activated carbon | 0.688 | [46] |
| Cladium mariscus | 65.58 | [7] |
| Bagasse fly ash | 5.63 | [37] |
| Termite mound soil | 22.78 | This study |
| Run Order | pH | Contact Time (Min) | Adsorbent Dose (g/L) | Initial 2,4-D Concentration (mg/L) | 2,4-D Removal (%) |
|---|---|---|---|---|---|
| 1 | 2 | 360 | 30 | 100 | 50.9 |
| 2 | 2 | 1 | 0.5 | 0.5 | 50 |
| 3 | 5.5 | 180.5 | 15.25 | 33.4 | 65 |
| 4 | 9 | 1 | 0.5 | 100 | 37 |
| 5 | 5.5 | 180.5 | 15.25 | 50.25 | 53.3 |
| 6 | 5.5 | 180.5 | 15.25 | 50.25 | 53.8 |
| 7 | 5.5 | 121.4 | 15.25 | 50.25 | 43 |
| 8 | 2 | 360 | 0.5 | 100 | 30 |
| 9 | 9 | 360 | 30 | 0.5 | 35 |
| 10 | 5.5 | 180.5 | 40.1 | 50.25 | 52 |
| 11 | 12.5 | 180.5 | 15.25 | 50.25 | 45 |
| 12 | 2 | 180.5 | 15.25 | 50.25 | 89.6 |
| 13 | 5.5 | 180.5 | 15.25 | 133.9 | 27 |
| 14 | 5.5 | 180.5 | 15.25 | 50.25 | 50 |
| 15 | 5.5 | 180.5 | 9.6 | 50.25 | 50 |
| 16 | 5.5 | 482.4 | 15.25 | 50.25 | 34 |
| 17 | 1.5 | 1 | 30 | 0.5 | 83 |
| 18 | 5.5 | 180.5 | 15.25 | 50.25 | 50.1 |
| 19 | 9 | 1 | 30 | 100 | 43 |
| 20 | 5.5 | 180.5 | 15.25 | 50.25 | 50 |
| 21 | 9 | 360 | 0.5 | 0.5 | 25 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 5261.85 | 14 | 375.85 | 152.76 | <0.0001 | Significant |
| A-pH | 1307.51 | 1 | 1307.51 | 531.41 | <0.0001 | |
| B-contact time | 69.63 | 1 | 69.63 | 28.30 | 0.0018 | |
| C-adsorbent dose | 628.45 | 1 | 628.45 | 255.42 | <0.0001 | |
| D-initial concentration | 957.11 | 1 | 957.11 | 389.00 | <0.0001 | |
| AB | 359.40 | 1 | 359.40 | 146.07 | < 0.0001 | |
| AC | 179.55 | 1 | 179.55 | 72.98 | 0.0001 | |
| AD | 111.89 | 1 | 111.89 | 45.48 | 0.0005 | |
| BC | 8.20 | 1 | 8.20 | 3.33 | 0.1177 | |
| BD | 273.01 | 1 | 273.01 | 110.96 | <0.0001 | |
| CD | 32.40 | 1 | 32.40 | 13.17 | 0.0110 | |
| A2 | 622.12 | 1 | 622.12 | 252.85 | <0.0001 | |
| B2 | 222.66 | 1 | 222.66 | 90.50 | <0.0001 | |
| C2 | 192.74 | 1 | 192.74 | 78.34 | 0.0001 | |
| D2 | 82.63 | 1 | 82.63 | 33.58 | 0.0012 | |
| Residual | 14.76 | 6 | 2.46 | |||
| Lack of Fit | 4.42 | 2 | 2.21 | 0.8551 | 0.4907 | not significant |
| Pure Error | 10.34 | 4 | 2.59 | |||
| Cor Total Std. Dev. | 5276.61 1.57 | R2 | 0.9972 | |||
| Mean | 48.35 | Adjusted R2 | 0.9907 | |||
| C.V.% | 3.24 | Predicted R2 | 0.8836 | |||
| Adeq Precision | 49.2631 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debebe, Y.; Alemayehu, E.; Worku, Z.; Bae, W.; Lennartz, B. Sorption of 2,4-Dichlorophenoxyacetic Acid from Agricultural Leachate Using Termite Mound Soil: Optimization Using Response Surface Methodology. Water 2023, 15, 327. https://doi.org/10.3390/w15020327
Debebe Y, Alemayehu E, Worku Z, Bae W, Lennartz B. Sorption of 2,4-Dichlorophenoxyacetic Acid from Agricultural Leachate Using Termite Mound Soil: Optimization Using Response Surface Methodology. Water. 2023; 15(2):327. https://doi.org/10.3390/w15020327
Chicago/Turabian StyleDebebe, Yalemtsehay, Esayas Alemayehu, Zemene Worku, Wookeun Bae, and Bernd Lennartz. 2023. "Sorption of 2,4-Dichlorophenoxyacetic Acid from Agricultural Leachate Using Termite Mound Soil: Optimization Using Response Surface Methodology" Water 15, no. 2: 327. https://doi.org/10.3390/w15020327







