Environmentally Realistic Waterborne Atrazine Exposure Affects Behavior in Poecilia latipinna
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects, Housing, and Atrazine Preparation
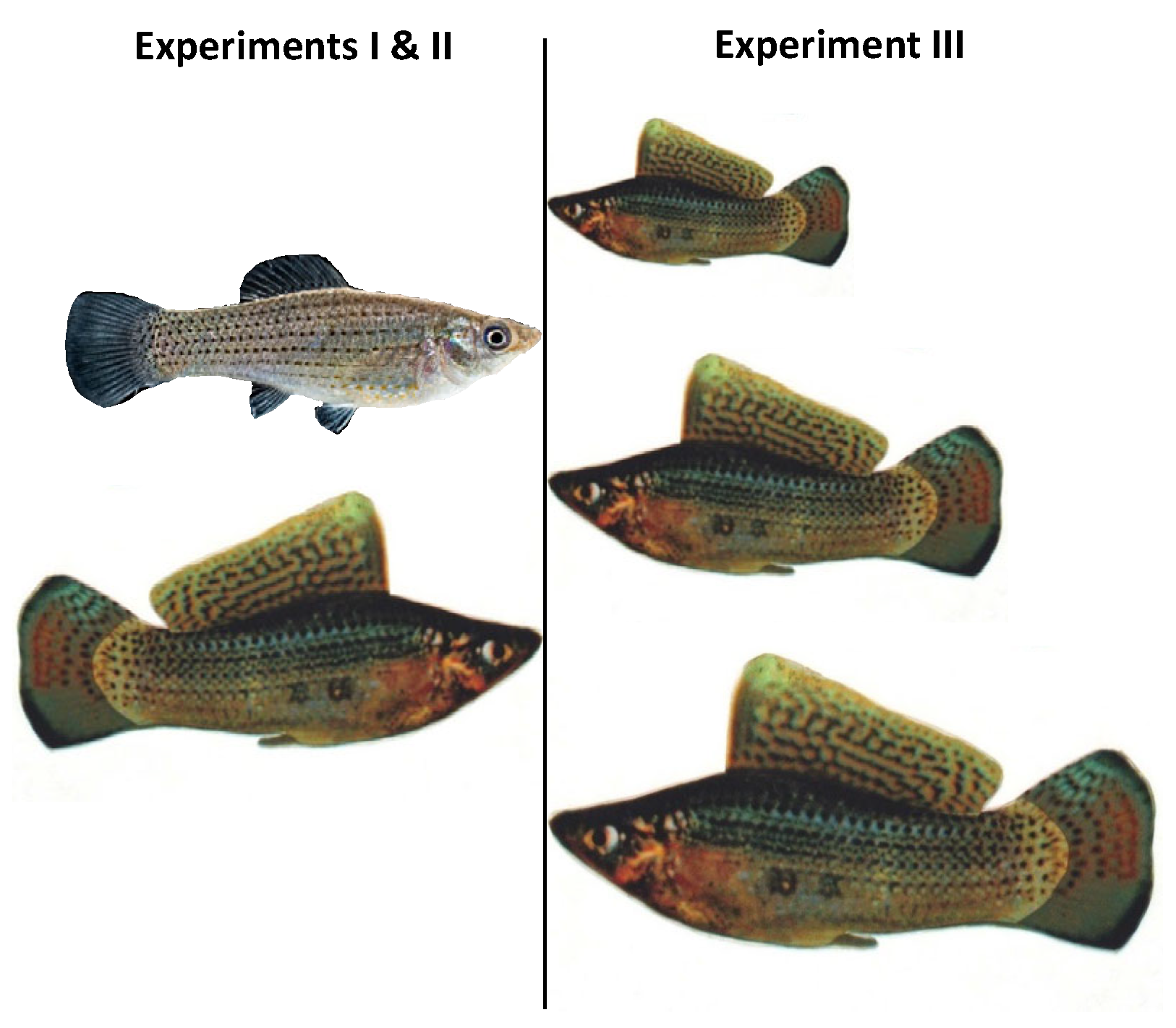
2.2. Dummy Construction
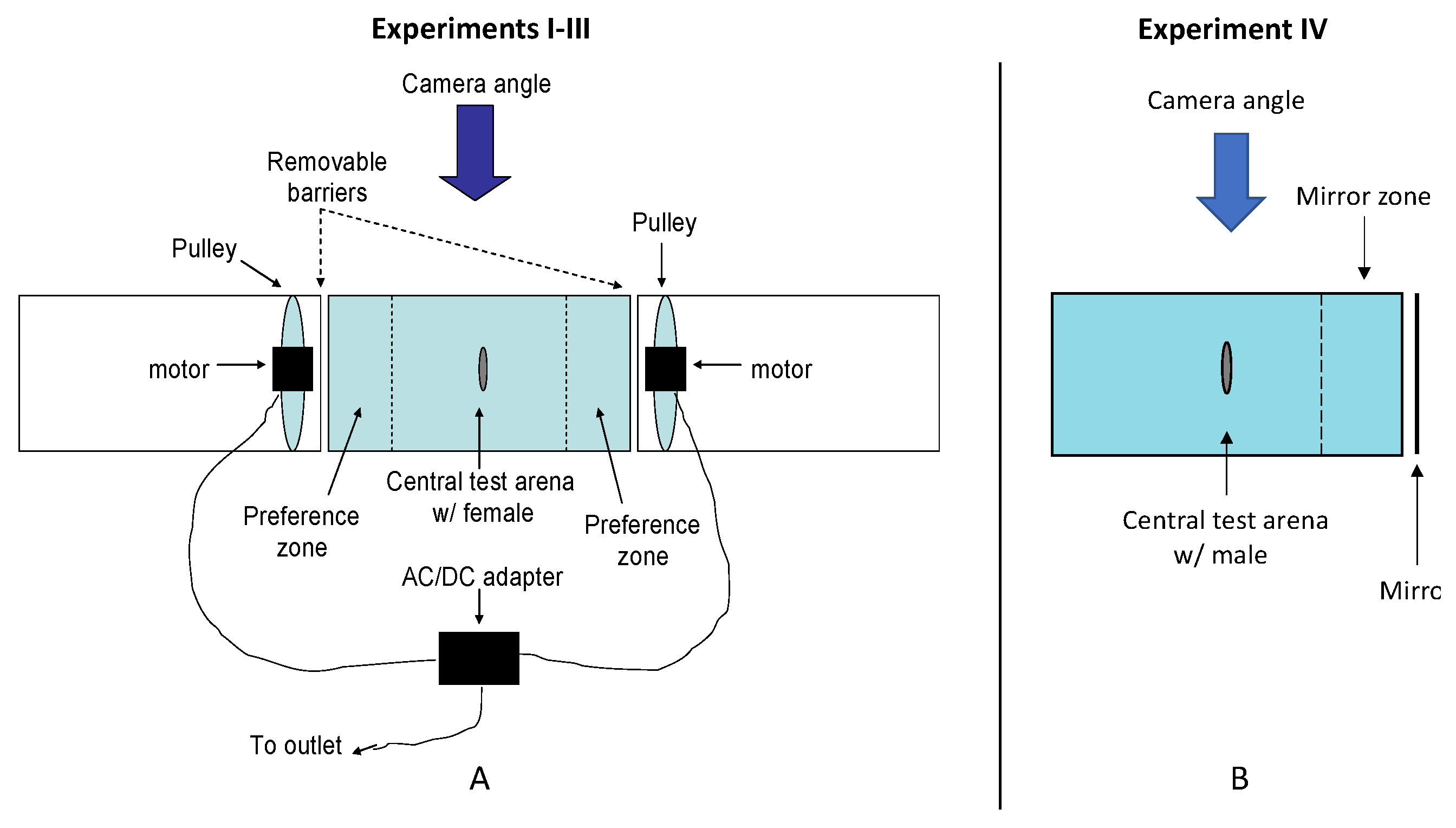
2.3. Testing Apparatus and Procedures for Experiments I–III
2.4. Procedure for Experiment IV
2.5. Novel Arena Protocol
2.6. Behavioral Measures and Statistical Analyses
3. Results
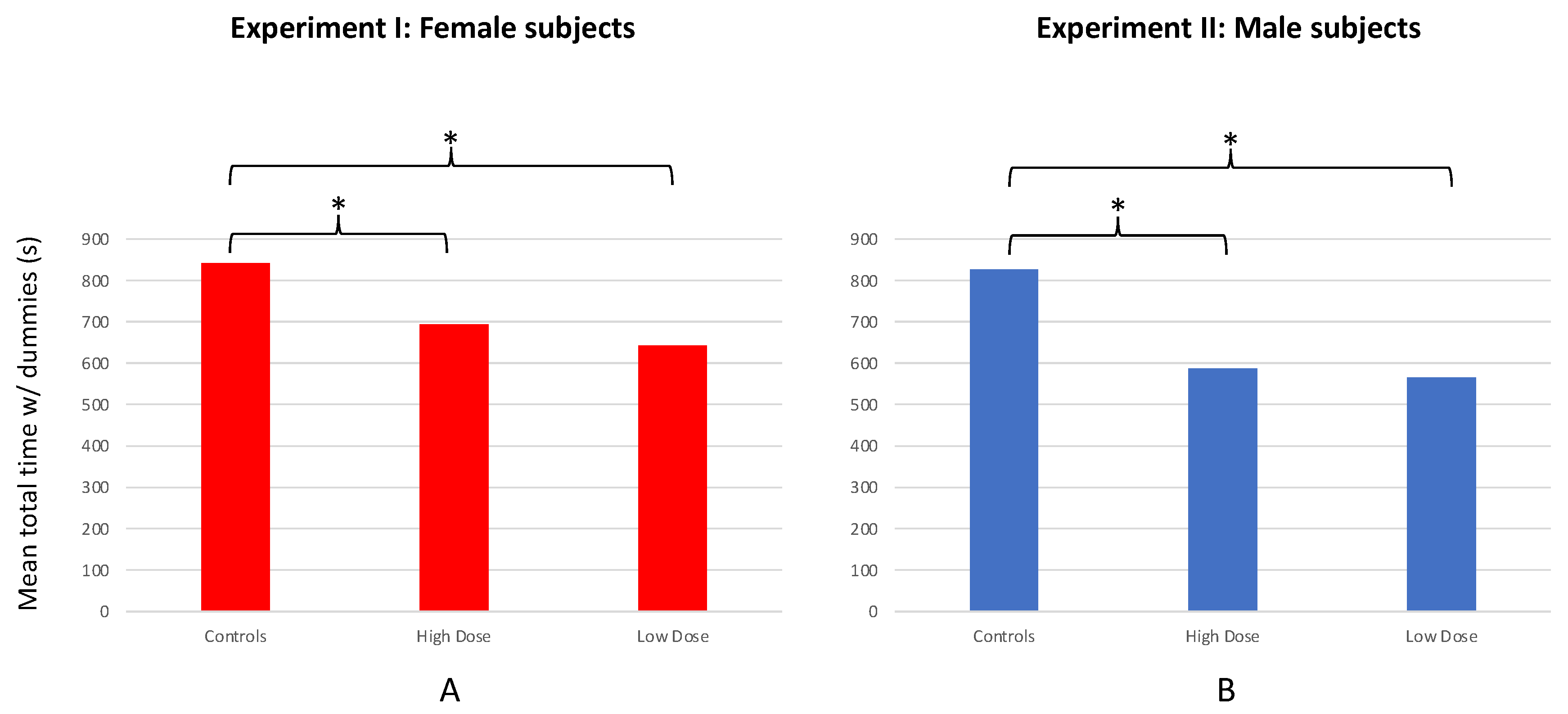
3.1. Experiment I: Comparison of Preferences for Male vs. Female Stimuli in AZT-Exposed vs. Unexposed Females
3.2. Experiment II: Comparison of Preferences for Male vs. Female Stimuli in AZT-Exposed vs. Unexposed Males
3.3. Experiment III: Comparison of Preferences for Male Body Size in AZT-Exposed vs. Unexposed Females
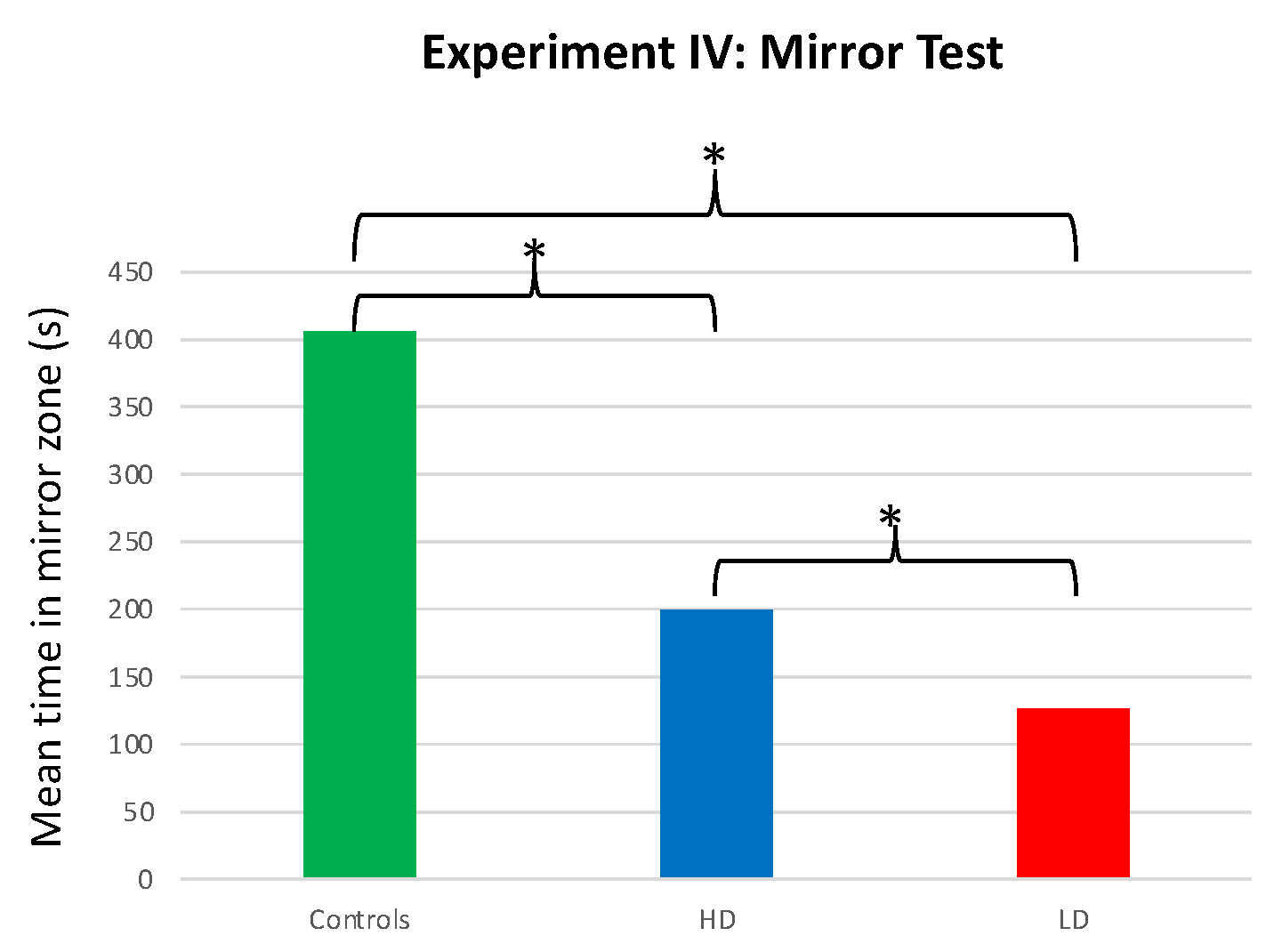
3.4. Experiment IV: Comparison of Time Spent in the Mirror Zone for AZT-Exposed vs. Unexposed Males
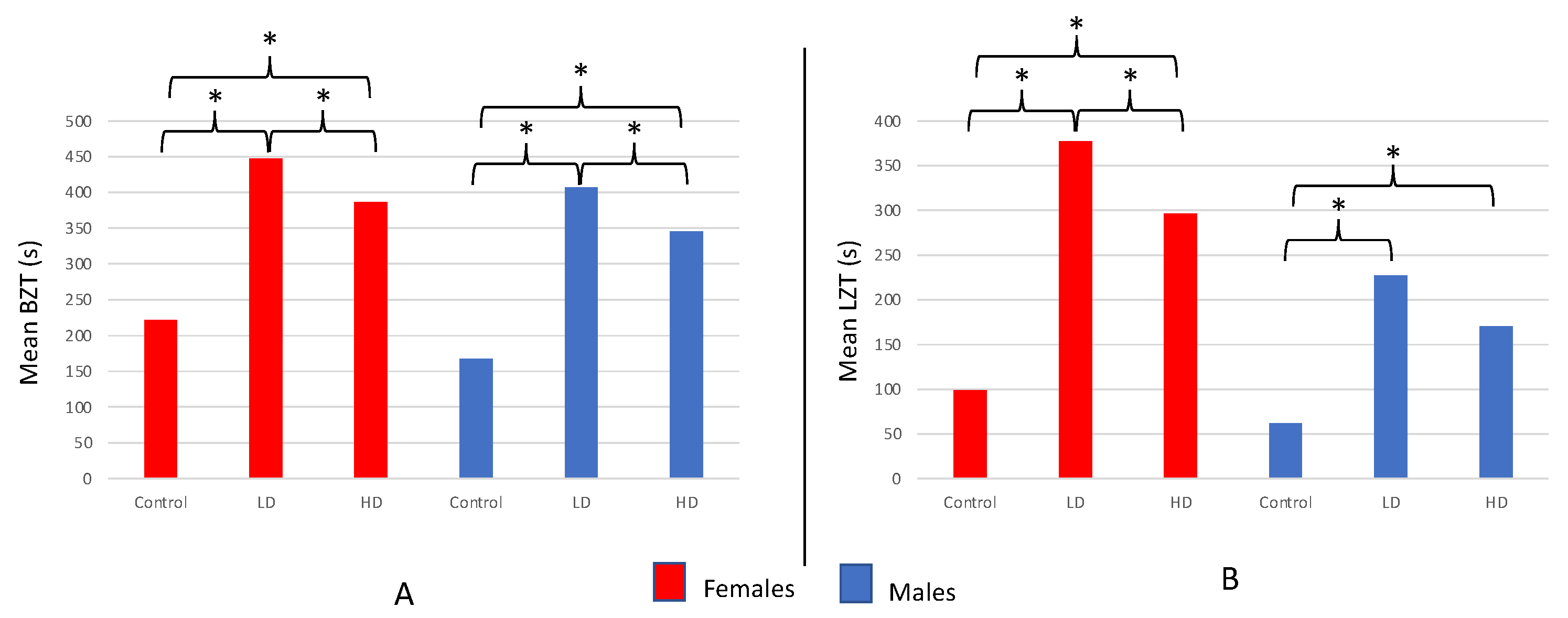
3.5. Comparison of Novel Arena Test Results for AZT-Exposed vs. Unexposed Fish from All Four Experiments
4. Discussion
4.1. Effects on Female Behavior
4.2. Effects on Male Behavior
4.3. Novel Area Test
4.4. Behavior As An Indicator of Environmental Health
4.5. Possible Mechanisms Underlying Atrazine’s Effects on Behavior
4.6. Justification of Methods
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markman, S.; Guschina, I.A.; Barnsley, S.; Buchanan, K.L.; Pascoe, D.; Müller, C.T. Endocrine disrupting chemicals accumulate in earthworms exposed to sewage effluent. Chemosphere 2007, 70, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, K. Environmentally realistic exposure to the herbicide atrazine alters some sexually selected traits in male guppies. PLoS ONE 2012, 7, e30611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filby, A.L.; Paull, G.C.; Hickmore, T.F.; Tyler, C.R. Unravelling the neurophysiological basis of aggression in a fish model. BMC Genom. 2010, 11, 498. [Google Scholar] [CrossRef] [Green Version]
- Thornqvist, P.-O.; Hoglund, E.; Winberg, S. Natural selection constrain personality and brain gene expression differences in Atlantic salmon (Salmo salar). J. Exp. Biol. 2015, 218, 1077–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitfield, C.W.; Cziko, A.-M.; Robinson, G.E. Gene expression profiles in the brain predict behavior in individual honey bees. Science 2003, 302, 296–299. [Google Scholar] [CrossRef]
- Wiese, A.-S.; Needham, E.K.; Noer, C.L.; Balsby, T.J.S.; Dabelsteen, T.; Pakkenberg, B. The number of neurons in specific amygdala regions is associated with boldness in mink: A study in animal personality. Brain Struct. Funct. 2018, 223, 1989–1998. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Hotchkiss, A.K.; Rider, C.V.; Blystone, C.R.; Wilson, V.S.; Hartig, P.C.; Ankley, G.T.; Foster, P.M.; Gray, C.L.; Gray, L.E. Fifteen years after “Wingspread”—Environmental endocrine disrupters and human and wildlife health: Where we are today and where we need to go. Toxicol. Sci. 2008, 105, 235–259. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Baccarelli, A.; Pesatori, A.C.; Bertazzi, P.A. Occupational and environmental agents as endocrine disruptors: Experimental and human evidence. J. Endocrinol. Investig. 2000, 23, 771–781. [Google Scholar] [CrossRef]
- Frye, C.A.; Bo, E.; Calamandreis, G.; Calza, L.; Dessi-Fulgheri, F.; Fernández, M.; Fusani, L.; Kah, O.; Kajta, M.; Le Page, Y.; et al. Endocrine disrupters: A review of some sources, effects, andmechanisms of actions on behavior and neuroendocrine systems. J. Neuroendocrinol. 2012, 24, 144–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLachlan, J. Environmental signaling: What embryos and evolution teach us about endocrine disrupting chemicals. Endocr. Rev. 2001, 22, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Clotfelter, E.D.; Bell, A.M.; Levering, K.R. The role of animal behavior in the study of endocrine-disrupting chemicals. Anim. Behav. 2004, 68, 665–676. [Google Scholar] [CrossRef]
- Little, E.E. Behavioral measures of environmental stressors in fish. In Biological Indicators of Aquatic Ecosystem Stress; Adams, S.M., Ed.; American Fisheries Society: Bethesda, MD, USA, 2002; pp. 431–472. [Google Scholar]
- Perreault, H.A.N.; Semsar, K.; Godwin, J. Fluoxetine treatment decreases territorial aggression in a coral reef fish. Physiol. Behav. 2003, 70, 719–724. [Google Scholar] [CrossRef]
- Lynn, S.E.; Egar, J.M.; Walker, B.G.; Sperry, T.S.; Ramenofsky, M. Fish on Prozac: A simple, noninvasive physiology laboratory investigating the mechanisms of aggressive behavior in Betta splendens. Adv. Physiol. Educ. 2007, 31, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Schultz, M.M.; Painter, M.M.; Bartell, S.E.; Logue, A.; Furlong, E.T.; Werner, S.L.; Schoenfuss, H.L. Selective uptake and biological consequences of environmentally relevant antidepressant pharmaceutical exposures on male fathead minnows. Aquat. Toxicol. 2011, 104, 38–47. [Google Scholar] [CrossRef]
- Tyler, C.R.; Jobling, S.; Sumpter, J.P. Endocrine disruption in wildlife: A critical review of the evidence. Crit. Rev. Toxicol. 1998, 28, 319–361. [Google Scholar] [CrossRef]
- Shenoy, K. Prenatal exposure to low doses of atrazine affects mating behaviors in male guppies. Horm. Behav. 2014, 66, 439–448. [Google Scholar] [CrossRef]
- Soffker, M.; Tyler, C. Endocrine disrupting chemicals and sexual behaviors in fish—A critical review on effects and possible consequences. Crit. Rev. Toxicol. 2012, 42, 8. [Google Scholar] [CrossRef]
- Bertram, M.G.; Saaristo, M.; Ecker, T.E.; Baumgartner, J.B.; Wong, B. An androgenic endocrine disruptor alters male mating behavior in the guppy (Poecilia reticulata). Behav. Ecol. 2018, 29, 1255–1263. [Google Scholar] [CrossRef]
- Bell, A.M. Effects of an endocrine disrupter on courtship and aggressive behaviour of male three-spined stickleback, Gasterosteus aculeatus. Anim. Behav. 2001, 62, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Clotfelter, E.D.; Rodriguez, A.C. Behavioral changes in fish exposed to phytoestrogens. Environ. Pollut. 2006, 144, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Martinović, D.; Hogarth, W.T.; Jones, R.E.; Sorensen, P.W. Environmental estrogens suppress hormones, behavior and reproductive fitness in male fathead minnows. Environ. Toxicol. Chem. 2007, 26, 271–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisp, T.M.; Clegg, E.D.; Cooper, R.L.; Wood, W.P.; Anderson, D.G.; Baetcke, K.P.; Hoffman, J.L.; Morrow, M.S.; Rodier, D.J.; Schaeffer, J.E.; et al. Environmental endocrine disruption: An effects assessment and analysis. Environ. Health Perspect. 1998, 106, 11–56. [Google Scholar] [PubMed]
- Dzieweczynski, T.L.; Hebert, O.L. The effects of short-term exposure to an endocrine disrupter on behavioral consistency in male juvenile and adult Siamese fighting fish. Arch. Environ. Contam. Toxicol. 2013, 64, 316–326. [Google Scholar] [CrossRef]
- Shenoy, K.; Crowley, P.H. Endocrine disruption of male mating signals: Ecological and evolutionary implications. Funct. Ecol. 2011, 25, 433–448. [Google Scholar] [CrossRef]
- Volkova, K.; Caspillo, N.R.; Porseryd, T.; Hallgren, S.; Dinnétz, P.; Porsch-Hällström, I. Developmental exposure of zebrafish (Danio rerio) to 17α-ethinylestradiol affects non-reproductive behavior and fertility as adults, and increases anxiety in unexposed progeny. Horm. Behav. 2015, 73, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Dzieweczynski, T.L. Short-term exposure to an endocrine disruptor affects behavioural consistency in male threespine stickleback. Aquat. Toxicol. 2011, 105, 681–687. [Google Scholar] [CrossRef]
- Dzieweczynski, T.L.; Campbell, B.A.; Marks, J.M.; Logan, B. Acute exposure to 17α-ethinylestradiol alters boldness behavioral syndrome in female Siamese fighting fish. Horm. Behav. 2014, 66, 577–584. [Google Scholar] [CrossRef]
- Hebert, O.L.; Lavin, L.E.; Marks, J.M.; Dzieweczynski, T.L. The effects of 17α-ethinyloestradiol on boldness and its relationship to decision making in male Siamese fighting fish. Anim. Behav. 2014, 87, 203–212. [Google Scholar] [CrossRef]
- Lagesson, A.; Saaristo, M.; Brodin, T.; Fick, J.; Klaminder, J.; Martin, J.; Wong, B.B.M. Fish on steroids: Temperature-dependent effects of 17β-trenbolone on predator escape, boldness, and exploratory behaviors. Environ. Pollut. 2019, 245, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Porseryd, T.; Kellner, M.; Caspillo, N.R.; Volkova, K.; Elabbas, L.; Ullah, S.; Olsén, H.; Dinnétz, P.; Hällström, I.P. Combinatory effects of low concentrations of 17α-etinylestradiol and citalopram on non-reproductive behavior in adult zebrafish (Danio rerio). Aquat. Toxicol. 2017, 193, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lamb, S.; Chia, J.; Johnson, S. Paternal exposure to a common herbicide alters the behavior and serotonergic system of zebrafish offspring. PLoS ONE 2020, 15, e0228357. [Google Scholar] [CrossRef]
- Barr, D.B.; Panuwet, P.; Nguyen, J.V.; Udunka, S.; Needham, L.L. Assessing exposure to atrazine and its metabolites using biomonitoring. Environ. Health Perspect. 2007, 115, 1474–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, K.R.; Baker, D.B.; Richards, R.P.; Dixon, K.R.; Klaine, S.J.; La Point, T.W.; Kendall, R.J.; Weisskopf, C.P.; Giddings, J.M.; Giesy, J.P.; et al. Ecological risk assessment of atrazine in North American surface waters. Environ. Toxicol. Chem. 1996, 15, 31–76. [Google Scholar] [CrossRef]
- Wirbisky, S.E.; Weber, G.J.; Sepúlveda, M.S.; Xiao, C.; Cannon, J.R.; Freeman, J.L. Developmental origins of neurotransmitter and transcriptome alterations in adult female zebrafish exposed to atrazine during embryogenesis. Toxicology 2015, 333, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Wirbisky, S.E.; Weber, G.J.; Sepúlveda, M.S.; Lin, T.-L.; Jannasch, A.S.; Freeman, J.L. An embryonic atrazine exposure results in reproductive dysfunction in adult zebrafish and morphological alterations in their offspring. Sci. Rep. 2016, 6, 21337. [Google Scholar] [CrossRef] [Green Version]
- Hayes, T.B.; Collins, A.; Lee, M.; Mendoza, M.; Noriega, N.; Stuart, A.A.; Vonk, A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl. Acad. Sci. USA 2002, 99, 5476–5480. [Google Scholar] [CrossRef] [Green Version]
- Hayes, T.B.; Khoury, V.; Narayan, A.; Nazir, M.; Park, A.; Brown, T.; Adame, L.; Chan, E.; Buchholz, D.; Stueve, T.; et al. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc. Natl. Acad. Sci. USA 2010, 107, 4612–4617. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Dodd, C.A.; Filipov, N.M. Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicol. Teratol. 2013, 39, 26–35. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Reidenbach, L.S.; Thanki, D.H.; Winchester, A.E.; Qualizza, B.A.; Ryan, G.A.; Egan, K.E.; Hedrick, V.E.; Sobreira, T.J.; Peterson, S.M.; et al. Embryonic atrazine exposure elicits proteomic, behavioral, and brain abnormalities with developmental time specific gene expression signatures. J. Proteom. 2018, 186, 71–82. [Google Scholar] [CrossRef]
- Graymore, M.; Stagnitti, F.; Allinson, G. Impacts of atrazine in aquatic ecosystems. Environ. Int. 2001, 26, 483–495. [Google Scholar] [CrossRef]
- Grube, A.; Donaldson, D.; Kiely, T.; Wu, L. Pesticides Industry Sales and Usage: 2006 and 2007 Market Estimates; Environmentla Protection Agency (EPA): Washington, DC, USA, 2011; 733-R-11-001. [Google Scholar]
- Richter, C.A.; Papoulias, D.M.; Whyte, J.J.; Tillitt, D.E. Evaluation of potential mechanisms of atrazine-induced reproductive impairment in fathead minnow (Pimephales promelas) and Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 2016, 35, 2230–2238. [Google Scholar] [CrossRef]
- Foradori, C.D.; Hinds, L.R.; Hanneman, W.H.; Legare, M.E.; Clay, C.M.; Handa, R.J. Atrazine inhibits pulsatile luteinizing hormone release without altering pituitary sensitivity to a gonadotropin-releasing hormone receptor agonist in female Wistar rats. Biol. Reprod. 2009, 81, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foradori, C.D.; Zimmerman, A.D.; Hinds, L.R.; Zuloaga, K.L.; Breckenridge, C.B.; Handa, R.J. Atrazine inhibits pulsatile gonadotropin-releasing hormone (GnRH) release without altering GnRH messenger RNA or protein levels in the female rat. Biol. Reprod. 2013, 88, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.L.; Laws, S.C.; Das, P.C.; Narotsky, M.G.; Goldman, J.M.; Lee Tyrey, E.; Stoker, T.E. Atrazine and reproductive function: Mode and mechanism of action studies. Birth Defects Res. B Dev. Reprod Toxicol. 2007, 80, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Dodd, C.A.; Xiao, S.; Krishna, S.; Ye, X.; Filipov, N.M. Gestational and lactational exposure to atrazine via the drinking water causes specific behavioral deficits and selectively alters monoaminergic systems in C57BL/6 mouse dams, juvenile and adult offspring. Toxicol. Sci. 2014, 141, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.T.; Boerma, J.; Lansbergen, G.W.; van den Berg, M. Induction and inhibition of aromatase (CYP19) activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol. Appl. Pharmacol. 2002, 182, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.; Farombi, E.; Kashyap, M.; Pant, A. Atrazine induces transcriptional changes in marker genes associated with steroidogenesis in primary cultures of rat Leydig cells. Toxicol. In Vitro 2011, 25, 1588–1595. [Google Scholar] [CrossRef]
- Kucka, M.; Pogrmic-Majkic, K.; Fa, S.; Stojilkovic, S.S.; Kovacevic, R. Atrazine acts as an endocrine disrupter by inhibiting cAMP-specific phosphodiesterase-4. Toxicol. Appl. Pharmacol. 2012, 265, 19–26. [Google Scholar] [CrossRef]
- Hayes, T.B. Welcome to the revolution: Integrative biology and assessing the impact of endocrine disruptors on environmental and public health. Integr. Comp. Biol. 2005, 45, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yanase, T.; Morinaga, H.; Gondo, S.; Okabe, T.; Nomura, M.; Komatsu, T.; Morohashi, K.I.; Hayes, T.B.; Takayanagi, R.; et al. Atrazine-induced aromatase expression is SF-1 dependent: Implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ. Health Perspect. 2007, 115, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Schmidel, A.J.; Assmann, K.L.; Werlang, C.C.; Bertoncello, K.T.; Francescon, F.; Rambo, C.L.; Beltrame, G.M.; Calegari, D.; Batista, C.B.; Blaser, R.E.; et al. Subchronic atrazine exposure changes defensive behaviour profile and disrupts brain acetylcholinesterase activity of zebrafish. Neurotoxicol. Teratol. 2014, 44, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.N.; Moore, P.A. Express yourself: Individuals with bold personalities exhibit increased behavioral sensitivity to dynamic herbicide exposure. Ecotoxicol. Environ. Saf. 2019, 179, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Bi, H.; Ma, K.; Li, B. Developmental exposure to atrazine impairs spatial memory and downregulates the hippocampal D1 dopamine receptor and cAMP-dependent signaling pathway in rats. Int. J. Mol. Sci. 2018, 19, 2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardullas, U.; Giordano, M.; Rodríguez, V.M. Chronic atrazine exposure causes disruption of the spontaneous locomotor activity and alters the striatal dopaminergic system of the male Sprague–Dawley rat. Neurotoxicol. Teratol. 2011, 33, 263–272. [Google Scholar] [CrossRef]
- MacLaren, R.D.; Rowland, W.J.; Morgan, N. Female preferences for sailfin and body size in the sailfin molly, Poecilia latipinna. Ethology 2004, 110, 363–379. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Chauhan, A.; Datta, S.; Wani, A.B.; Singh, N.; Singh, J. Toxicity, degradation and analysis of the herbicide atrazine. Environ. Chem. Lett. 2018, 16, 211–237. [Google Scholar] [CrossRef]
- Rohr, J.R.; McCoy, K.A. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environ. Health Perspect. 2010, 118, 20–32. [Google Scholar] [CrossRef] [Green Version]
- MacLaren, R.D.; Daniska, D. Female preferences for dorsal fin and body size in Xiphophorus helleri: Further investigation of the LPA bias in Poeciliid fishes. Behaviour 2008, 145, 897–913. [Google Scholar] [CrossRef]
- MacLaren, R.D.; Fontaine, A. Female bias for male lateral projection area in Poecilia reticulata. Environ. Biol. Fishes 2012, 93, 105–119. [Google Scholar] [CrossRef]
- MacLaren, R.D.; Fontaine, A. Incongruence between the sexes in preferences for body and dorsal fin size in Xiphophorus variatus. Behav. Proc. 2013, 92, 99–106. [Google Scholar] [CrossRef]
- MacLaren, R.D.; He, R.; Gagnon, J. Bias for enlarged male body and dorsal fins in female Xiphophorus variatus. Behav. Proc. 2011, 87, 197–202. [Google Scholar] [CrossRef]
- MacLaren, R.D.; Rowland, W.J. Female preference for male lateral projection area in the shortfin molly Poecilia mexicana; evidence for a preexisting bias. Ethology 2006, 112, 678–690. [Google Scholar] [CrossRef]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maximino, C.; de Oliveira, D.L.; Rosemberg, D.B.; Batista, E.D.J.O.; Herculano, A.M.; Oliveira, K.R.M.; Benzecry, R.; Blaser, R. A comparison of the light/dark and novel tank tests in zebrafish. Behaviour 2012, 149, 1099–1123. [Google Scholar] [CrossRef]
- EPA. Decision Documents for Atrazine; U.S. Environmental Protection Agency: Washington, DC, USA, 2006.
- Rowland, W.J. Studying visual cues in fish behavior: A review of ethological techniques. Environ. Biol. Fishes 1999, 56, 285–305. [Google Scholar] [CrossRef]
- MacLaren, R.D. The effects of male proximity, apparent size, and absolute size on female preference in the sailfin molly, Poecilia latipinna. Behaviour 2006, 143, 1457–1472. [Google Scholar] [CrossRef] [Green Version]
- MacLaren, R.D. Evidence of an emerging female preference for an artificial male trait and the potential for spread via mate choice copying in Poecilia latipinna. Ethology 2019, 125, 575–586. [Google Scholar] [CrossRef]
- MacLaren, R.D. Social environment affects female preference for male body color during development in artificially selected varieties of Poecilia latipinna. Ethol. Ecol. Evol. 2017, 29, 421–435. [Google Scholar] [CrossRef]
- MacLaren, R.D. Effects of male apparent length on female preference for absolute body size in Xiphophorus helleri. Acta Ethologica 2017, 20, 27–36. [Google Scholar] [CrossRef]
- Wagner, W.E. Measuring female mating preferences. Anim. Behav. 1998, 55, 1029–1042. [Google Scholar] [CrossRef]
- Farr, J.A. Sexual selection and secondary sexual differentiation in poeciliids: Determinants of male mating success and the evolution of female choice. In Ecology and Evolution of Livebearing Fishes; Meffe, G.K., Snelson, F.F., Jr., Eds.; Prentice Hall: Englewood Cliffs, NJ, USA, 1989; pp. 91–124. [Google Scholar]
- Houde, A. Sex, Color, and Mate Choice in Guppies; Princeton University Press: Princeton, NJ, USA, 1997; 210p. [Google Scholar]
- Basolo, A.L. Congruence between the sexes in preexisting receiver responses. Behav. Ecol. 2002, 13, 832–837. [Google Scholar] [CrossRef]
- Brodeur, J.C.; Svartz, G.; Perez-Coll, C.S.; Marino, D.J.G.; Herkovits, J. Comparative susceptibility to atrazine of three developmental stages of Rhinella arenarum and influence on metamorphosis: Non-monotonous acceleration of the time to climax and delayed tail resorption. Aquat. Toxicol. 2009, 91, 161–170. [Google Scholar] [CrossRef]
- Hamlin, H.J.; Guillette, L.J. Embryos as targets of endocrine disrupting contaminants in wildlife. Birth Defects Res. C Embryo Today 2011, 93, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Welshons, W.V.; Thayer, K.A.; Judy, B.M.; Taylor, J.A.; Curran, E.M.; Vom Saal, F.S. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect. 2003, 111, 994–1006. [Google Scholar] [CrossRef] [Green Version]
- Brodeur, J.C.; Sassone, A.; Hermida, G.N.; Codugnello, N. Environmentally-relevant concentrations of atrazine induce non-monotonic acceleration of developmental rate and increased size at metamorphosis in Rhinella arenarum tadpoles. Ecotoxicol. Environ. Saf. 2013, 92, 10–17. [Google Scholar] [CrossRef]
- Marcus, S.R.; Fiumera, A.C. Atrazine exposure affects longevity, development time and body size in Drosophila melanogaster. J. Insect. Physiol. 2016, 91, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCallum, M.L.; Matlock, M.; Treas, J.; Safi, B.; Sanson, W.; McCallum, J.L. Endocrine disruption of sexual selection by an estrogenic herbicide in the mealworm beetle (Tenebrio molitor). Ecotoxicology 2013, 22, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Riffle, B.W.; Klinefelter, G.R.; Cooper, R.L.; Winnik, W.M.; Swank, A.; Jayaraman, S.; Suarez, J.; Best, D.; Laws, S. Novel molecular events associated with altered steroidogenesis induced by exposure to atrazine in the intact and castrate male rat. Reprod. Toxicol. 2014, 47, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Way, G.P.; Ruhl, N.; Snekser, J.L.; Kiesel, A.L.; McRobert, S.P. A comparison of methodologies to test aggression in zebrafish. Zebrafish 2015, 12, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Ariyomo, T.O.; Watt, P.J. The effect of variation in boldness and aggressiveness on the reproductive success of zebrafish. Anim. Behav. 2012, 83, 41–46. [Google Scholar] [CrossRef]
- MacLaren, R.D.; Wisniewski, K.; MacLaren, C. Environmental concentrations of metformin exposure affect aggressive behavior in the Siamese fighting fish, Betta splendens. PLoS ONE 2018, 13, e0197259. [Google Scholar] [CrossRef] [Green Version]
- Maximino, C.; de Brito, T.M.; da Silva Batista, A.W.; Herculano, A.M.; Morato, S.; Gouveia, A., Jr. Measuring anxiety in zebrafish: A critical review. Behav. Brain Res. 2010, 214, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.E.; Lorenz, R.; Spieser, O.H. Effects of atrazine on swimming behavior of zebrafish, Brachydanio rerio. Water Res. 1995, 29, 981–985. [Google Scholar] [CrossRef]
- Volkova, K.; Caspillo, N.R.; Porseryd, T.; Hallgren, S.; Dinnetz, P.; Olsén, H.; Hällström, I.P. Transgenerational effects of 17α-ethinyl estradiol on anxiety behavior in the guppy, (Poecilia reticulata). Gen. Comp. Endocrinol. 2015, 223, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Pink, M.; Abrahams, M.V. In shallow water ecosytems the abiotic environment is more important than prey abundance for foraging terns. Environ. Biol. Fishes 2018, 101, 355–362. [Google Scholar] [CrossRef]
- Rypel, A.L.; Layman, C.A.; Arrington, D.A. Water depth modifies relative predation risk for a motile fish taxon in Bahamian tidal creeks. Estuaries Coasts 2007, 30, 518–525. [Google Scholar] [CrossRef]
- Werner, E.E.; Gilliam, J.F.; Hall, D.J.; Mittelbach, G.G. An experimental test of the effects of predation risk on habitat use in fish. Ecology 1983, 64, 1540–1548. [Google Scholar] [CrossRef]
- Luca, R.M.; Gerlai, R. Animated bird silhouette above the tank: Acute alcohol diminishes fear responses in zebrafish. Behav. Brain Res. 2012, 229, 194–201. [Google Scholar] [CrossRef]
- Pitcher, T.; Lang, S.; Turner, J. A risk-balancing trade off between foraging rewards and predation hazard in a shoaling fish. Behav. Ecol. Sociobiol. 1988, 22, 225–228. [Google Scholar] [CrossRef]
- MacPherson, B.; Mashayekhi, M.; Gras, R.; Scott, R. Exploring the connection between emergent animal personality and fitness using a novel individual-based model and decision tree approach. Ecol. Inform. 2017, 40, 81–92. [Google Scholar] [CrossRef]
- Smith, B.R.; Blumstein, D.T. Fitness consequences of personality: A meta-analysis. Behav. Ecol. 2008, 19, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Biro, P.A.; Stamps, J.A. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 2008, 23, 361–368. [Google Scholar] [CrossRef]
- Wolf, M.; Van Doorn, G.S.; Leimar, O.; Weissing, F.J. Life-history trade-offs favour the evolution of animal personalities. Nature 2007, 447, 581. [Google Scholar] [CrossRef]
- Peterson, E.K.; Buchwalter, D.B.; Kerby, J.L.; LeFauve, M.K.; Varian-Ramos, C.W.; Swaddle, J.P. Integrative behavioral ecotoxicology: Bringing together fields to establish new insight to behavioral ecology, toxicology, and conservation. Curr. Zool. 2017, 63, 185–194. [Google Scholar] [CrossRef] [Green Version]
- EPA. Persistent Organic Pollutants: A Global Issue, A Global Response; United States Environmental protection Agency: Washington, DC, USA, 2009.
- Hayes, T.B.; Stuart, A.A.; Mendoza, M.; Collins, A.; Noriega, N.; Vonk, A.; Johnston, G.; Liu, R.; Kpodzo, D. Characterization of atrazine-induced gonadal malformations in African clawed frogs (Xenopus laevis) and comparisons with effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (17 b-estradiol): Support for the demasculinization/feminization hypothesis. Environ. Health Perspect. 2006, 114, 134–141. [Google Scholar]
- Bartke, A.; Williams, K.I.H.; Dalterio, S. Effects of estrogens on testicular testosterone production in vitro. Biol. Reprod. 1977, 17, 645–649. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Kim, J.H.; Lee, H.J.; Yoon, Y.D. Octylphenol reduces the expressions of steroidogenic enzymes and testosterone production in mouse testis. Environ. Toxicol. 2007, 22, 449–458. [Google Scholar] [CrossRef]
- Ankley, G.T.; Kahl, M.D.; Jensen, K.M.M.; Hornung, M.W.; Korte, J.J.; Makynen, E.; Leino, R. Evaluation of the aromatase inhibitor fadrozole in a short-term reproduction assay with the fathead minnow (Pimephales promelas). Toxicol. Sci. 2002, 67, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Ankley, G.T.; Jensen, K.M.; Durhan, E.J.; Makynen, E.A.; Butterworth, B.C.; Kahl, M.; Villeneuve, D.; Linnum, A.; Gray, E.; Cardon, M.; et al. Effects of two fungicides with multiple modes of action on reproductive endocrine function in the fathead minnow (Pimephales promelas). Toxicol. Sci. 2005, 86, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Toft, G.; Guillette, L.J. Decreased sperm count and sexual behavior in mosquitofish exposed to water from a pesticide-contaminated lake. Ecotoxicol. Environ. Saf. 2005, 60, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Baatrup, E.; Junge, M. Antiandrogenic pesticides disrupt sexual characteristics in the adult male guppy (Poecilia reticulata). Environ. Health Perspect. 2001, 109, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Bayley, M.; Junge, M.; Baatrup, E. Exposure of juvenile guppies to three antiandrogens causes demasculinization and a reduced sperm count in adult males. Aquat. Toxicol. 2002, 56, 227–239. [Google Scholar] [CrossRef]
- Thompson, C.J.; Ross, S.M.; Gaido, K.W. Di(n-butyl)phthalate impairs cholesterol transport and steroidogenesis in the fetal rat testis through a rapid and reversible mechanism. Endocrinology 2004, 145, 1227–1273. [Google Scholar] [CrossRef] [Green Version]
- Gabor, C.R.; Grober, M.S. A potential role of male and female androgen in species recognition in a unisexual–bisexual mating complex. Horm. Behav. 2010, 57, 427–433. [Google Scholar] [CrossRef]
- Friedman, A.S. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod. Toxicol. 2002, 16, 275–279. [Google Scholar] [CrossRef]
- Babic-Gojmerac, T.; Kniewald, Z.; Kniewald, J. Testosterone metabolism in neuroendocrine organs in male rats under atrazine and deethylatrazine influence. J. Steroid Biochem. 1989, 33, 141–146. [Google Scholar] [CrossRef]
- Kniewald, J.; Mildner, P.; Kniewald, Z. Effects of s-triazine herbicides on hormonereceptor complex formation, 5 [alpha]-reductase and 3 [alpha]-hydroxysteroid dehydrogenase activity at the anterior pituitary level. J. Steroid Biochem. 1979, 11, 833–838. [Google Scholar] [CrossRef]
- Bischoff, R.J.; Gould, J.L.; Rubenstein, D.I. Tail size and female choice in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 1985, 17, 253–255. [Google Scholar] [CrossRef]
- Kodric-Brown, A. Female choice of multiple male criteria in guppies: Interacting effects of dominance, coloration and courtship. Behav. Ecol. Sociobiol. 1993, 32, 415–420. [Google Scholar] [CrossRef]
- Forsgren, E. Predation risk affects mate choice in a gobiid fish. Am. Nat. 1992, 140, 1041–1049. [Google Scholar] [CrossRef]
- Berglund, A. Risky sex: Male pipefishes mate at random in the presence of a predator. Anim. Behav. 1993, 46, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Gabor, C. Association patterns of sailfin mollies (Poecilia latipinna): Alternative hypotheses. Behav. Ecol. Sociobiol. 1999, 46, 333–340. [Google Scholar] [CrossRef]
- Ptacek, M.B.; Travis, J. Mate choice in the sailfin molly, Poecilia latipinna. Evolution 1997, 51, 1217–1231. [Google Scholar] [CrossRef]
- Schlupp, I.; Marler, C.; Ryan, M.J. Benefit to male sailfin mollies of mating with heterospecific females. Science 1994, 263, 373–374. [Google Scholar] [CrossRef]

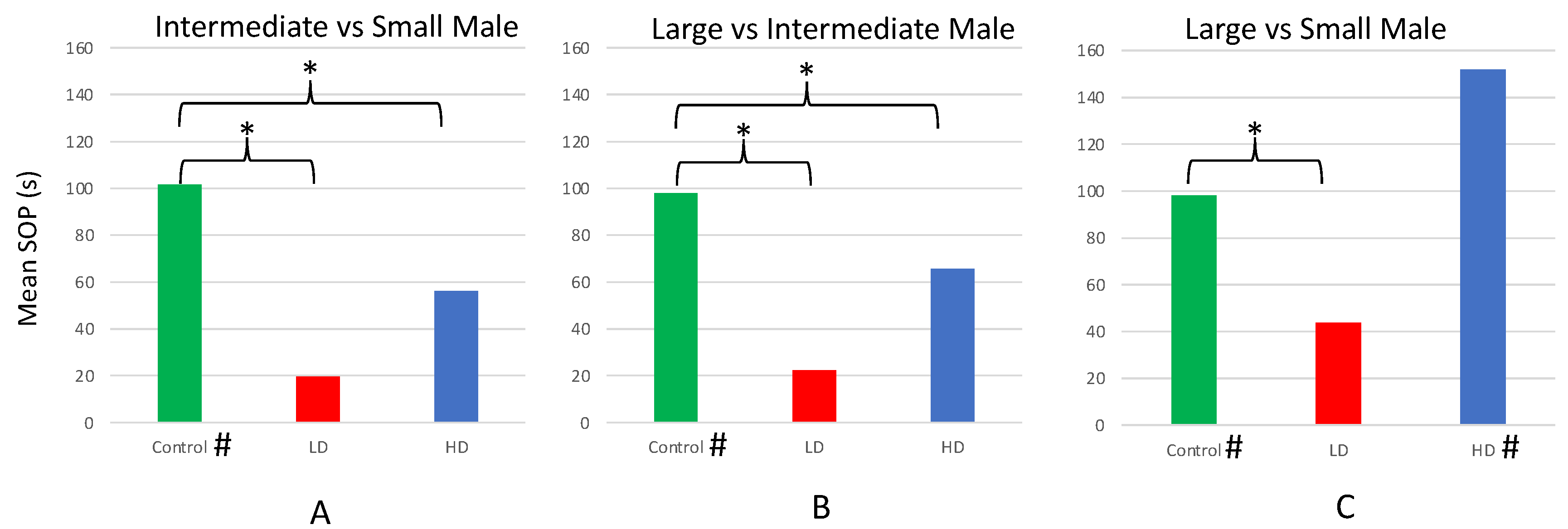
| Treatment | Trial | Time w/ Smaller Male (s) | Time w/ Larger Male (s) | T | df | p |
|---|---|---|---|---|---|---|
| Control * | S vs. I | 144.69 ± 15.08 | 246.38 ± 17.90 | 3.258 | 25 | 0.003 * |
| Control * | S vs. L | 132.23 ± 9.71 | 230.46 ± 11.70 | 5.171 | 25 | <0.001 * |
| Control * | I vs. L | 143.00 ± 12.03 | 240.92 ± 14.69 | 3.893 | 25 | <0.001 * |
| LD | S vs. I | 187.06 ± 34.86 | 206.72 ± 35.54 | 0.293 | 17 | 0.773 |
| LD | S vs. L | 140.28 ± 35.52 | 183.94 ± 34.71 | 0.678 | 17 | 0.507 |
| LD | I vs. L | 143.33 ± 28.15 | 165.78 ± 32.59 | 0.405 | 17 | 0.691 |
| HD | S vs. I | 193.80 ± 31.66 | 249.10 ± 33.16 | 0.896 | 19 | 0.382 |
| HD * | S vs. L | 132.25 ± 18.06 | 284.45 ± 29.23 | 3.451 | 19 | 0.003 * |
| HD | I vs. L | 178.00 ± 28.30 | 227.85 ± 33.18 | 0.846 | 19 | 0.408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacLaren, R.D. Environmentally Realistic Waterborne Atrazine Exposure Affects Behavior in Poecilia latipinna. Water 2023, 15, 306. https://doi.org/10.3390/w15020306
MacLaren RD. Environmentally Realistic Waterborne Atrazine Exposure Affects Behavior in Poecilia latipinna. Water. 2023; 15(2):306. https://doi.org/10.3390/w15020306
Chicago/Turabian StyleMacLaren, R. David. 2023. "Environmentally Realistic Waterborne Atrazine Exposure Affects Behavior in Poecilia latipinna" Water 15, no. 2: 306. https://doi.org/10.3390/w15020306









