Zeolite Adsorbents for Selective Removal of Co(II) and Li(I) from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Kinetic Experiments
2.3. Adsorption Equilibrium Experiments
3. Results and Discussion
3.1. Adsorbent Characterization
3.1.1. X-ray Diffraction
3.1.2. X-ray Fluorescence
3.1.3. Isotherms Adsorption–Desorption of N2
3.1.4. pH Evolution in Aqueous Suspension of Zeolite Samples
3.2. Influence of the Exchanged Cation
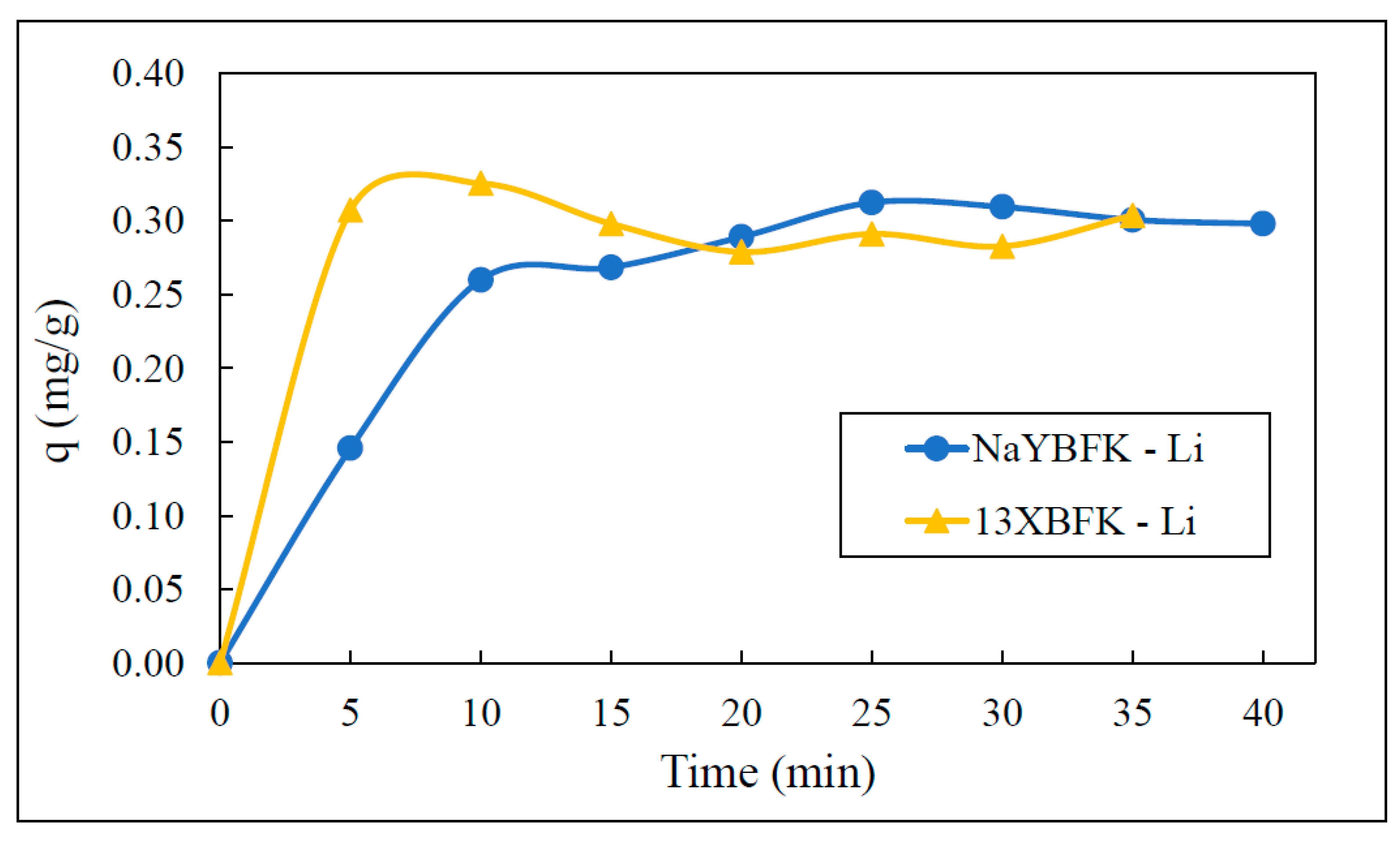
3.3. Kinetic Adsorption Experiments
3.4. Kinetic Equilibrium Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lu, S.; Song, W.; Chen, T.; Hayat, T.; Alsaedi, N.S.; Chen, C.; Liu, H. Competitive adsorption of U(VI) and Co(II) on montmorillonite: A batch and spectroscopic approach. Appl. Clay Sci. 2018, 157, 121–129. [Google Scholar] [CrossRef]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Pramanik, B.K.; Mainali, B.; Angove, M.J. Opportunities and constraints of using the innovative adsorbents for the removal of cobalt(II) from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2018, 10, 435–456. [Google Scholar] [CrossRef]
- Lemaire, J.; Svecova, L.; Lagallarde, F.; Laucournet, R.; Thivel, P.X. Lithium recovery from aqueous solution by sorption/desorption. Hydrometallurgy 2014, 143, 1–11. [Google Scholar] [CrossRef]
- Coman, V.; Robotin, B.; Ilea, P. Nickel recovery/removal from industrial wastes: A review. Resour. Conserv. Recycl. 2013, 73, 229–238. [Google Scholar] [CrossRef]
- Chivot, J.; Mendoza, L.; Mansour, C.; Pauporté, T.; Cassir, M. New insight in the behaviour of Co-H2O system at 25–150 °C, based on revised Pourbaix diagrams. Corros. Sci. 2008, 50, 62–69. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Activated carbons produced by pyrolysis of waste potato peels: Cobaltions removal by adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 74–83. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Y.; Sun, S.; Yu, J. Adsorption of lithium ions on lithium-aluminum hydroxides: Equilibrium and kinetics. Can. J. Chem. Eng. 2020, 98, 544–555. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Kesuma, E.P.; Perdana, I.; Aziz, M. Lithium recovery from spent Li-ion batteries using coconut shell activated carbon. Waste Manag. 2018, 79, 454–461. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Motamedi, M. Adsorption of nickel, Ni(ii), in aqueous solution by modified zeolite as a cation-exchange adsorbent. J. Chem. Eng. Data 2020, 65, 185–197. [Google Scholar] [CrossRef]
- Blengini, G.A.; Latunussa, C.E.L.; Eynard, U. Study on the EU’s List of Critical Raw Materials—Critical Raw Materials Factsheets. European Commission: Brussels, Belgium, 2020; Available online: https://op.europa.eu/en/publication-detail/-/publication/c0d5292a-ee54-11ea-991b-01aa75ed71a1/language-en (accessed on 7 December 2022).
- Xu, J.; Thomas, H.R.; Francis, R.W.; Lum, K.R.; Wang, J.; Liang, B. A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources 2008, 177, 512–527. [Google Scholar] [CrossRef]
- Karate, V.D.; Marathe, K.V. Simultaneous removal of nickel and cobalt from aqueous stream by cross flow micellar enhanced ultrafiltration. J. Hazard. Mater. 2008, 157, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Assaad, E.; Azzouz, A.; Nistor, D.; Ursu, A.V.; Sajin, T.; Miron, D.N.; Monette, F.; Niquette, P.; Hausler, R. Metal removal through synergic coagulation-flocculation using an optimized chitosan-montmorillonite system. Appl. Clay Sci. 2007, 37, 258–274. [Google Scholar] [CrossRef]
- Demirbaş, E. Adsorption of cobalt(II) ions from aqueous solution onto activated carbon prepared from hazelnut shells. Adsorpt. Sci. Technol. 2003, 21, 951–963. [Google Scholar] [CrossRef] [Green Version]
- Sari, A.; Tüzen, M. Adsorption of silver from aqueous solution onto raw vermiculite and manganese oxide-modified vermiculite. Microporous Mesoporous Mater. 2013, 170, 155–163. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Cd(II) adsorption from aqueous solution by raw and modified kaolinite. Appl. Clay Sci. 2014, 88–89, 63–72. [Google Scholar] [CrossRef]
- Saleh, T.A.; Sari, A.; Tuzen, M. Chitosan-modified vermiculite for As(III) adsorption from aqueous solution: Equilibrium, thermodynamic and kinetic studies. J. Mol. Liq. 2016, 219, 937–945. [Google Scholar] [CrossRef]
- Saleh, T.A.; Sarı, A.; Tuzen, M. Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chem. Eng. J. 2017, 307, 230–238. [Google Scholar] [CrossRef]
- Tuzen, M.; Saleh, T.A.; Sarı, A. Naeemullah Interfacial polymerization of trimesoyl chloride with melamine and palygorskite for efficient uranium ions ultra-removal. Chem. Eng. Res. Des. 2020, 159, 353–361. [Google Scholar] [CrossRef]
- Prabakaran, R.; Arivoli, S. Removal of cobalt (II) from aqueous solutions by adsorption on low cost activated carbon. Int. J. Sci. Eng. Technol. Res. 2013, 2, 271–283. [Google Scholar]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Deravanesiyan, M.; Beheshti, M.; Malekpour, A. Alumina nanoparticles immobilization onto the NaX zeolite and the removal of Cr (III) and Co(II) ions from aqueous solutions. J. Ind. Eng. Chem. 2015, 21, 580–586. [Google Scholar] [CrossRef]
- Wang, H.; Huang, K.; Zhang, Y.; Chen, X.; Jin, W.; Zheng, S.; Zhang, Y.; Li, P. Recovery of Lithium, Nickel, and Cobalt from Spent Lithium-Ion Battery Powders by Selective Ammonia Leaching and an Adsorption Separation System. ACS Sustain. Chem. Eng. 2017, 5, 11489–11495. [Google Scholar] [CrossRef]
- Díez, E.; Gómez, J.M.; Rodríguez, A.; Bernabé, I.; Sáez, P.; Galán, J. A new mesoporous activated carbon as potential adsorbent for effective indium removal from aqueous solutions. Microporous Mesoporous Mater. 2020, 295, 109984. [Google Scholar] [CrossRef]
- Li, H.; Zheng, F.; Wang, J.; Zhou, J.; Huang, X.; Chen, L.; Hu, P.; Gao, J.M.; Zhen, Q.; Bashir, S.; et al. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chem. Eng. J. 2020, 390, 124513. [Google Scholar] [CrossRef]
- Iftekhar, S.; Heidari, G.; Amanat, N.; Zare, E.N.; Asif, M.B.; Hassanpour, M.; Lehto, V.P.; Sillanpaa, M. Porous materials for the recovery of rare earth elements, platinum group metals, and other valuable metals: A review. Environ. Chem. Lett. 2022, 20, 3697–3746. [Google Scholar] [CrossRef]
- Araissi, M.; Elaloui, E.; Moussaou, Y. The removal of Cadmium, Cobalt, and Nickel by adsorption with Na-Y zeolite. Iran. J. Chem. Chem. Eng. 2020, 39, 169–179. [Google Scholar]
- Rodríguez, A.; Sáez, P.; Díez, E.; Gómez, J.M.; García, J.; Bernabé, I. Highly efficient low-cost zeolite for cobalt removal from aqueous solutions: Characterization and performance. Environ. Prog. Sustain. Energy 2019, 38, S352–S365. [Google Scholar] [CrossRef]
- Conte, N.; Gómez, J.M.; Díez, E.; Sáez, P.; Monago, J.I.; Espinosa, A.; Rodríguez, A. Sequential separation of cobalt and lithium by sorption: Sorbent set selection. Sep. Purif. Technol. 2022, 303, 122199. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 5th ed.Elsevier Science: Amsterdam, The Netherland, 2007; ISBN 0144-2449. [Google Scholar]
- Kennedy, D.A.; Tezel, F.H. Cation exchange modification of clinoptilolite—Screening analysis for potential equilibrium and kinetic adsorption separations involving methane, nitrogen, and carbon dioxide. Microporous Mesoporous Mater. 2018, 262, 235–250. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Lanzafame, P.; Papanikolaou, G.; Perathoner, S.; Centi, G.; Migliori, M.; Catizzone, E.; Giordano, G. Reassembly mechanism in Fe-Silicalite during NH4OH post-treatment and relation with the acidity and catalytic reactivity. Appl. Catal. A Gen. 2019, 580, 186–196. [Google Scholar] [CrossRef]
- Garcia-Basabe, Y.; Rodriguez-Iznaga, I.; De Menorval, L.C.; Llewellyn, P.; Maurin, G.; Lewis, D.W.; Binions, R.; Autie, M.; Ruiz-Salvador, A.R. Step-wise dealumination of natural clinoptilolite: Structural and physicochemical characterization. Microporous Mesoporous Mater. 2010, 135, 187–196. [Google Scholar] [CrossRef]
- Arshadi, M.; Amiri, M.J.; Mousavi, S. Kinetic, equilibrium and thermodynamic investigations of Ni(II), Cd(II), Cu(II) and Co(II) adsorption on barley straw ash. Water Resour. Ind. 2014, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Füger, A.; Konrad, F.; Leis, A.; Dietzel, M.; Mavromatis, V. Effect of growth rate and pH on lithium incorporation in calcite. Geochim. Cosmochim. Acta 2019, 248, 14–24. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Allred, A.L. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 1961, 17, 215–221. [Google Scholar] [CrossRef]
- Monroe, C.W. Ionic Mobility and Diffusivity. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Savinell, R.F., Ota, K., Eds.; Springer: New York, NY, USA, 2014; pp. 1125–1130. ISBN 9781441969958. [Google Scholar]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, M. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Vuono, D.; Catizzone, E.; Aloise, A.; Policicchio, A.; Agostino, R.G.; Migliori, M.; Giordano, G. Modelling of adsorption of textile dyes over multi-walled carbon nanotubes: Equilibrium and kinetic. Chin. J. Chem. Eng. 2017, 25, 523–532. [Google Scholar] [CrossRef]
- Webber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Hinz, C. Description of sorption data with isotherm equations. Geoderma 2001, 99, 225–243. [Google Scholar] [CrossRef]
- Abou-Mesalam, M.M. Applications of inorganic ion exchangers: II—Adsorption of some heavy metal ions from their aqueous waste solution using synthetic iron(III) titanate. Adsorption 2004, 10, 87–92. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Simultaneous removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Kuwer, P.; Yadav, A.; Labhasetwar, P.K. Adsorption of cupric, cadmium and cobalt ions from the aqueous stream using the composite of iron (II,III) oxide and zeolitic imidazole framework-8. Water Sci. Technol. 2021, 84, 2288–2303. [Google Scholar] [CrossRef]
- Xu, C.; Yu, T.; Peng, J.; Zhao, L.; Li, J.; Zhai, M. Efficient adsorption performance of lithium ion onto cellulose microspheres with sulfonic acid groups. Quantum Beam Sci. 2020, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Wajima, T.; Munakata, K.; Uda, T. Adsorption behavior of lithium from seawater using manganese oxide adsorbent. Plasma Fusion Res. 2012, 7, 5–8. [Google Scholar] [CrossRef]







| 13XBFK | 13XBFK(H) | NaYBFK | NaYBFK(H) | |
|---|---|---|---|---|
| SiO2 | 57.48 | 61.22 | 74.23 | 74.87 |
| Al2O3 | 23.37 | 23.36 | 13.82 | 13.50 |
| Na2O | 16.88 | 12.34 | 9.81 | 7.59 |
| MgO | 1.57 | 1.58 | 1.15 | 2.30 |
| K2O | 0.07 | 0.06 | 0.05 | 0.05 |
| CaO | 0.00 | 0.14 | 0.50 | 0.56 |
| Si/Al molar ratio | 1.23 | 1.31 | 2.69 | 2.77 |
| Cations/Al molar ratio | 0.79 | 0.60 | 0.83 | 0.78 |
| Protonation degree (%) | 21 | 40 | 17 | 22 |
| Adsorbent | SBET (m2/g) | C·10−3 | SINT (m2/g) | SEXT (m2/g) | Micropore Volume (cm3/g) | Average Pore Size (Å) |
|---|---|---|---|---|---|---|
| 13XBFK | 910 | 18.5 | 860 | 50 | 0.319 | 16.2 |
| 13XBFK(H) | 845 | 20.0 | 805 | 40 | 0.297 | 16.5 |
| NaYBFK | 875 | 21.2 | 825 | 50 | 0.306 | 16.6 |
| NaYBFK(H) | 860 | 22.7 | 810 | 50 | 0.301 | 16.8 |
| 13XBFK | 13XBFK(H) | NaYBFK | NaYBFK(H) | |
|---|---|---|---|---|
| Cobalt Adsorption | ||||
| q (mg/g) | 23.0 | 17.6 | 24.2 | 23.4 |
| % Removed | 96.4 | 99.1 | 99.3 | 99.5 |
| Lithium adsorption | ||||
| q (mg/g) | 0.30 | 0.28 | 0.30 | 0.30 |
| % Removed | 27.4 | 25.6 | 26.9 | 27.4 |
| Hydrated Ionic Radius (Å) [39] | Dehydration Energy (kJ/mol) | Electronegativity [40] | Ionic Mobility (10−5 cm2/s) [41] | |
|---|---|---|---|---|
| Co2+ | 4.23 | 2054 | 1.88 | 0.732 |
| Li+ | 3.82 | 520 | 0.98 | 1.029 |
| NaYBFK Adsorbent | |||||
|---|---|---|---|---|---|
| Pseudo-First Order | |||||
| Cation | qe (mg/g) | k1 (min−1) | R2 | RMSE | F value |
| Co(II) | 24.09 | 0.176 | 0.999 | 0.459 | 1.004 |
| Li(I) | 0.31 | 0.150 | 0.988 | 0.001 | 1.034 |
| Pseudo-second order | |||||
| Cation | qe (mg/g) | k2 (g·min/mg) | R2 | RMSE | F value |
| Co(II) | 27.74 | 0.008 | 0.992 | 3.47 | 0.998 |
| Li(I) | 0.37 | 0.449 | 0.973 | 0.002 | 0.997 |
| 13XBFK adsorbent | |||||
| Pseudo-first order | |||||
| Cation | qe (mg/g) | k1 (min−1) | R2 | RMSE | F value |
| Co(II) | 23.92 | 0.119 | 0.981 | 10.4012 | 1.094 |
| Li(I) | 0.31 | 0.430 | 0.957 | 0.004 | 0.948 |
| Pseudo-second order | |||||
| Cation | qe (mg/g) | k2 (g·min/mg) | R2 | RMSE | F value |
| Co(II) | 30.55 | 0.0037 | 0.966 | 17.72 | 1.081 |
| Li(I) | 0.30 | 5.58.104 | 0.981 | 0.001 | 1.019 |
| Langmuir Model | ||||
|---|---|---|---|---|
| Metal | qsat (mg/g) | b (L/mg) | R2 | RMSE |
| Co(II) | 22.12 | 0.354 | 0.933 | 7.7 |
| Li(I) | 31.12 | 0.019 | 0.987 | 11.1 |
| Freundlich Model | ||||
| Metal | KF (L/mg) | n | R2 | RMSE |
| Co(II) | 11.30 | 6.67 | 0.974 | 14.9 |
| Li(I) | 1.82 | 1.90 | 0.984 | 12.7 |
| Reference | Metal | Adsorbent | [Metal]Initial (mg/L) | Sorbent Dosage (g/L) | qsat (mg/g) |
|---|---|---|---|---|---|
| This work | Co | Zeolite X | 50 | 5 | 22.16 |
| [30] | Co | Clinoptilolite | 10–200 | 12 | 4.2 |
| [49] | Co | FAU type zeolite synthesized from coal fly ash | 100–500 | 5 | 12.2 |
| [50] | Co | Composite of Fe3O4 and zeolitic imidazole framework 8 | 5–100 | 2 | 71.2 |
| This work | Li | Zeolite X | 20 | 5 | 0.30 |
| [8] | Li | Lithium—Aluminum hydroxide | 350 | ≈ 0,1 | 0.6 |
| [51] | Li | Modified cellulose | 140 | 20 | 16.0 |
| [52] | Li | Manganese oxide | 35 | 0.5 | 10.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez, E.; Redondo, C.; Gómez, J.M.; Miranda, R.; Rodríguez, A. Zeolite Adsorbents for Selective Removal of Co(II) and Li(I) from Aqueous Solutions. Water 2023, 15, 270. https://doi.org/10.3390/w15020270
Díez E, Redondo C, Gómez JM, Miranda R, Rodríguez A. Zeolite Adsorbents for Selective Removal of Co(II) and Li(I) from Aqueous Solutions. Water. 2023; 15(2):270. https://doi.org/10.3390/w15020270
Chicago/Turabian StyleDíez, Eduardo, Cinthya Redondo, José María Gómez, Ruben Miranda, and Araceli Rodríguez. 2023. "Zeolite Adsorbents for Selective Removal of Co(II) and Li(I) from Aqueous Solutions" Water 15, no. 2: 270. https://doi.org/10.3390/w15020270
APA StyleDíez, E., Redondo, C., Gómez, J. M., Miranda, R., & Rodríguez, A. (2023). Zeolite Adsorbents for Selective Removal of Co(II) and Li(I) from Aqueous Solutions. Water, 15(2), 270. https://doi.org/10.3390/w15020270












