Pollution Characteristics and Risk Assessment of Typical Antibiotics and Persistent Organic Pollutants in Reservoir Water Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standard Reagents
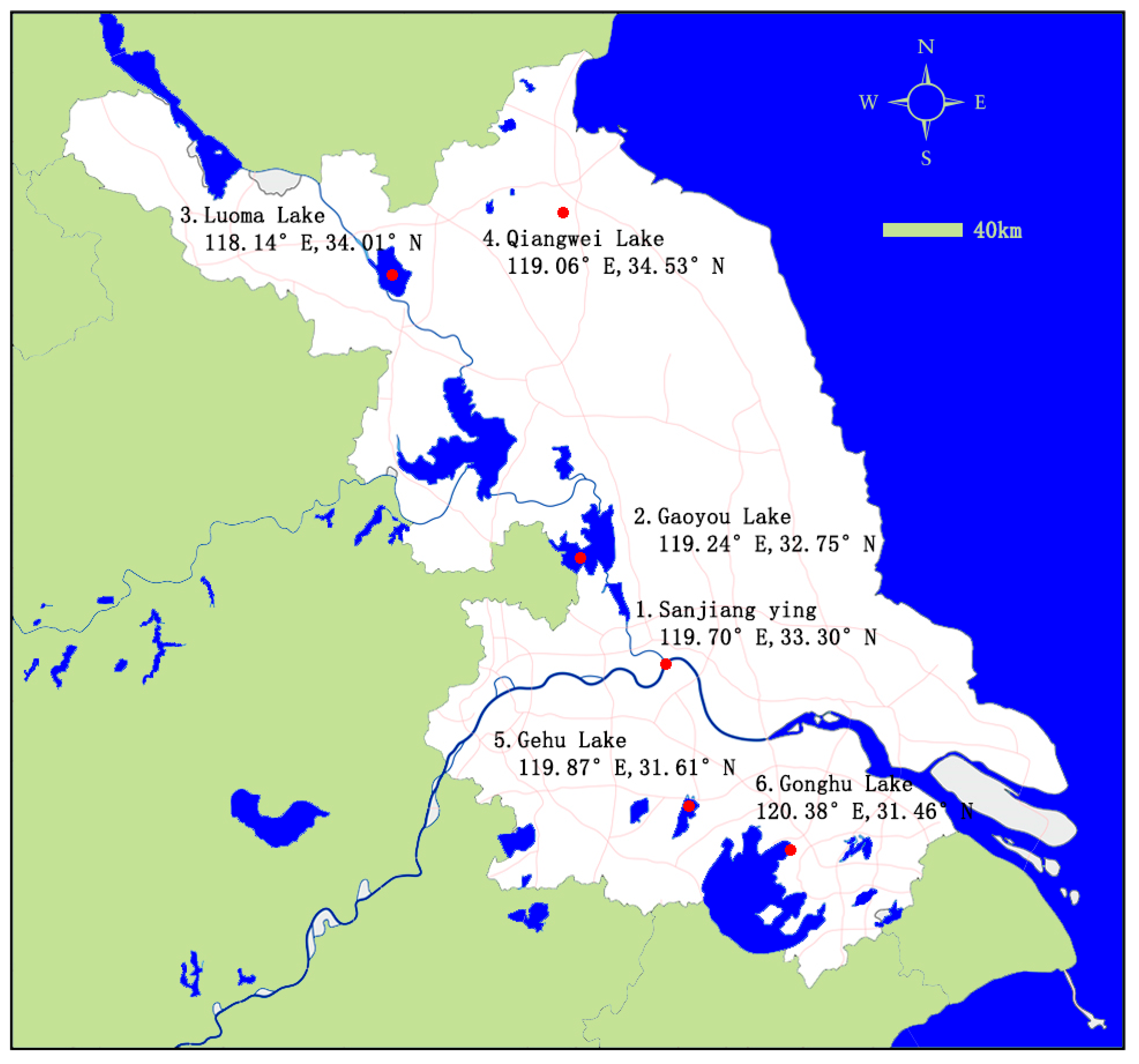
2.2. Sample Collection
2.3. Pretreatment and Pollutant Detection
2.3.1. Pretreatment and Detection Protocols for Antibiotics
2.3.2. Pretreatment and Detection Protocols for POPs
3. Results and Discussion
3.1. Water Quality Analysis of Water Sources
3.2. Distribution Characteristics of Target Antibiotics
3.3. POPs Distribution Characteristics and Source Analysis
3.3.1. OCPs Distribution Characteristics and Sources
3.3.2. PCBs Distribution Characteristics and Source Analysis
3.3.3. PBDEs Distribution Characteristics and Sources
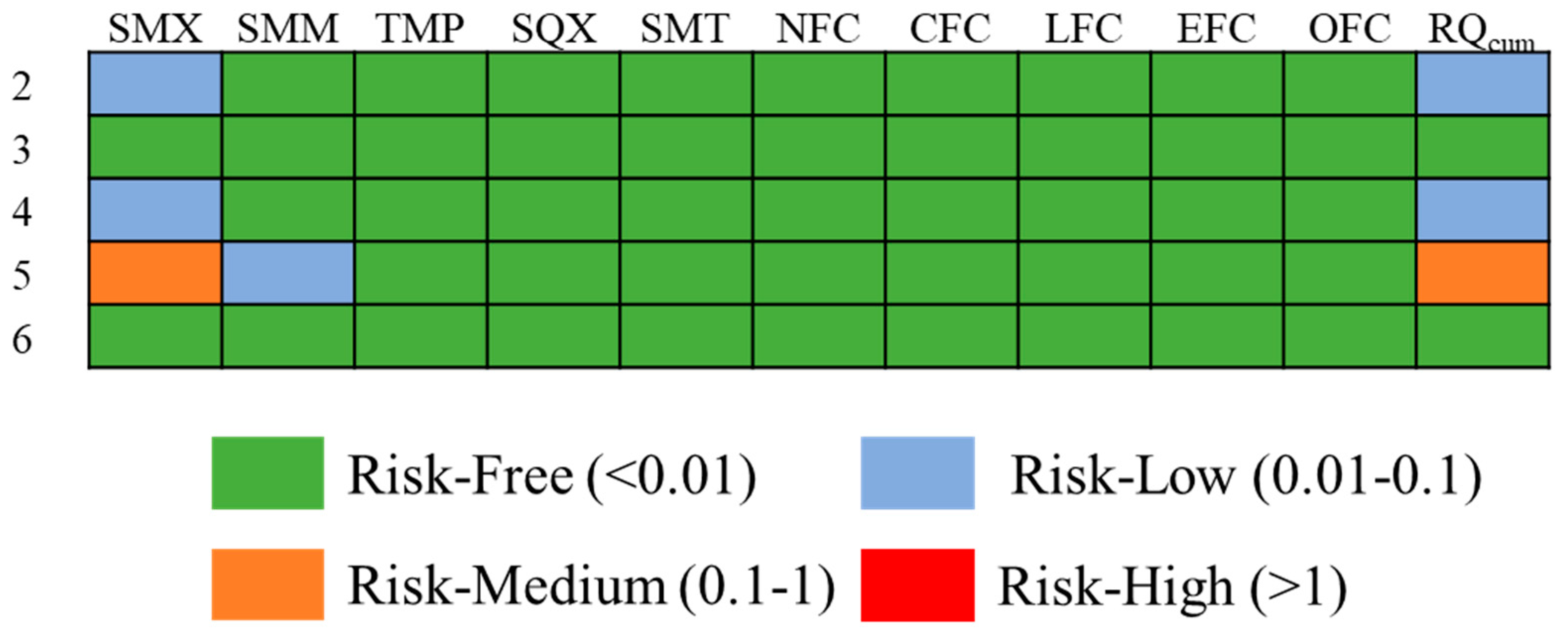
3.4. Ecological Risk Assessment of Antibiotics
3.5. Ecological Risk Assessment of POPs in Representative Water Sources
3.5.1. OCPs Ecological Risk Assessment in Sediments
3.5.2. PCBs Ecological Risk Assessment in Sediments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jose, P.A.; Jebakumar, S.R.D. Non-streptomycete actinomycetes nourish the current microbial antibiotic drug discovery. Front. Microbiol. 2013, 4, 240. [Google Scholar] [CrossRef] [Green Version]
- Chandan, R.C.; Gandhi, A.; Shah, N.P. Yogurt: Historical background, health benefits, and global trade. In Yogurt in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2017; pp. 3–29. [Google Scholar]
- Horrigan, L.; Lawrence, R.S.; Walker, P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ. Health Perspect. 2002, 110, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Serwecińska, L. Antimicrobials and antibiotic-resistant bacteria: A risk to the environment and to public health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef]
- Almakki, A.; Jumas-Bilak, E.; Marchandin, H.; Licznar-Fajardo, P. Antibiotic resistance in urban runoff. Sci. Total Environ. 2019, 667, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Sanganyado, E.; Gwenzi, W. Antibiotic resistance in drinking water systems: Occurrence, removal, and human health risks. Sci. Total Environ. 2019, 669, 785–797. [Google Scholar] [CrossRef]
- Paritala, H.; Carroll, K.S. New targets and inhibitors of mycobacterial sulfur metabolism. Infect. Disord.-Drug Targets. 2013, 13, 85–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, L.; Vanderbeck, R.M.; Valentine, G.; Zhang, M.; Diprose, K.; McQuaid, K.A. Chinese route to sustainability: Postsocialist transitions and the construction of ecological civilization. Sustain. Dev. 2018, 26, 741–748. [Google Scholar] [CrossRef]
- Yurui, L.; Xuanchang, Z.; Zhi, C.; Zhengjia, L.; Zhi, L.; Yansui, L. Towards the progress of ecological restoration and economic development in China’s Loess Plateau and strategy for more sustainable development. Sci. Total Environ. 2021, 756, 143676. [Google Scholar] [CrossRef]
- Ding, X.; Chong, X.; Bao, Z.; Xue, Y.; Zhang, S. Fuzzy comprehensive assessment method based on the entropy weight method and its application in the water environmental safety evaluation of the Heshangshan drinking water source area, three gorges reservoir area, China. Water 2017, 9, 329. [Google Scholar] [CrossRef] [Green Version]
- Voigt, A.M.; Ciorba, P.; Döhla, M.; Exner, M.; Felder, C.; Lenz-Plet, F.; Sib, E.; Skutlarek, D.; Schmithausen, R.M.; Faerber, H.A. The investigation of antibiotic residues, antibiotic resistance genes and antibiotic-resistant organisms in a drinking water reservoir system in Germany. Int. J. Hyg. Environ. Health 2020, 224, 113449. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Wu, Y.; Hu, J.; Zhang, Y.; Sun, Q.; Sun, W.; Geng, J.; Liu, X.; Jia, D.; et al. Antibiotics in global rivers. Natl. Sci. Open 2022, 1, 20220029. [Google Scholar] [CrossRef]
- Guo, F.; Wang, Y.; Peng, J.; Huang, H.; Tu, X.; Zhao, H.; Zhan, N.; Rao, Z.; Zhao, G.; Yang, H. Occurrence, Distribution, and Risk Assessment of Antibiotics in the Aquatic Environment of the Karst Plateau Wetland of Yangtze River Basin, Southwestern China. Int. J. Environ. Res. Public Health 2022, 19, 7211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Li, J.; Zhang, Y.; Kong, L.; Jin, M.; Yang, X.; Wu, Q.L. Trends in the occurrence and risk assessment of antibiotics in shallow lakes in the lower-middle reaches of the Yangtze River basin, China. Ecotoxicol. Environ. Saf. 2019, 183, 109511. [Google Scholar] [CrossRef]
- Li, S.; Shi, W.; Liu, W.; Li, H.; Zhang, W.; Hu, J.; Ke, Y.; Sun, W.; Ni, J. A duodecennial national synthesis of antibiotics in China’s major rivers and seas (2005–2016). Sci. Total Environ. 2018, 615, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, J.L. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 2014, 95, 604–612. [Google Scholar] [CrossRef]
- Huang, F.; An, Z.; Moran, M.J.; Liu, F. Recognition of typical antibiotic residues in environmental media related to groundwater in China (2009–2019). J. Hazard. Mater. 2020, 399, 122813. [Google Scholar] [CrossRef]
- Xu, W.; Yan, W.; Li, X.; Zou, Y.; Chen, X.; Huang, W.; Miao, L.; Zhang, R.; Zhang, G.; Zou, S. Antibiotics in riverine runoff of the Pearl River Delta and Pearl River Estuary, China: Concentrations, mass loading and ecological risks. Environ. Pollut. 2013, 182, 402–407. [Google Scholar] [CrossRef]
- Guo, X.; Feng, C.; Gu, E.; Tian, C.; Shen, Z. Spatial distribution, source apportionment and risk assessment of antibiotics in the surface water and sediments of the Yangtze Estuary. Sci. Total Environ. 2019, 671, 548–557. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, C.; Huang, D.; Wang, R.; Zeng, G.; Yan, M.; Xiong, W.; Zhou, C.; Cheng, M.; Xue, W.; et al. Recent progress in sustainable technologies for adsorptive and reactive removal of sulfonamides. Chem. Eng. J. 2020, 389, 123423. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Li, L.; Fu, C.; Tu, C.; Huang, Y.; Wu, L.; Tang, J.; Luo, Y.; Christie, P. Levels, distributions and sources of veterinary antibiotics in the sediments of the Bohai Sea in China and surrounding estuaries. Mar. Pollut. Bull. 2016, 109, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Cheng, Y.; Zhang, Y.; Li, Z.; Yu, Y.; Feng, L.; Zhang, S.; Xu, L. Distribution and human health risk assessment of antibiotic residues in large-scale drinking water sources in Chongqing area of the Yangtze River. Environ. Res. 2020, 185, 109386. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Hong, H.S.; Zhou, J.L.; Huang, J.; Yu, G. Fate and assessment of persistent organic pollutants in water and sediment from Minjiang River Estuary, Southeast China. Chemosphere 2003, 52, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A. Persistent organic pollutants (POPs): A global issue, a global challenge. Environ. Sci. Pollut. Res. 2017, 24, 4223–4227. [Google Scholar] [CrossRef]
- Jaikanlaya, C.; Settachan, D.; Denison, M.S.; Ruchirawat, M.; van den Berg, M. PCBs contamination in seafood species at the Eastern Coast of Thailand. Chemosphere 2009, 76, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Zhu, L.; Kong, Q. Persistent chlorinated pesticides in fish species from Qiantang River in East China. Chemosphere 2007, 68, 838–847. [Google Scholar] [CrossRef]
- Humphries, M.S.; Myburgh, J.G.; Campbell, R.; Buah-Kwofie, A.; Combrink, X. Organochlorine pesticide bioaccumulation in wild Nile crocodile (Crocodylus niloticus) fat tissues: Environmental influences on changing residue levelsand contaminant profiles. Sci. Total Environ. 2021, 753, 142068. [Google Scholar] [CrossRef]
- Guo, L.L.; Qiu, Y.W.; Zhang, G.; Zheng, G.J.; Lam, P.K.; Li, X. Levels and bioaccumulation of organochlorine pesticides (OCPs) and polyb-rominated diphenyl ethers (PBDEs) in fishes from the Pearl River estuary and Daya Bay, South China. Environ. Pollut. 2008, 152, 604–611. [Google Scholar] [CrossRef]
- Liao, L.N.; Zhang, Z.F.; Liu, L.L.; Song, W.W.; Ma, W.L.; Zhu, N.Z.; Li, Y.F. Measurement and modeling the gas/particle partitioning oforganochlorine pesticides (OCPs) in atmosphere at low temperatures. Sci. Total Environ. 2019, 667, 318–324. [Google Scholar]
- Ma, Y.; Yun, X.; Ruan, Z.; Lu, C.; Shi, Y.; Qin, Q.; Men, Z.; Zou, D.; Du, X.; Xing, B.; et al. Review of hexachlorocyclohexane (HCH) and dichlorodiphenyltrichloroethane (DDT) contamination in Chinese soils. Sci. Total Environ. 2020, 749, 141212. [Google Scholar] [CrossRef]
- Nadal, M.; Rovira, J.; Díaz-Ferrero, J.; Schuhmacher, M.; Domingo, J.L. Human exposure to environmental pollutants after a tire landfill fire in Spain: Health risks. Environ. Int. 2016, 97, 37–44. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Ji, D.; Wang, T.; Wang, Y.; Wang, P.; Ding, L.; Jiang, G. Levels and Vertical Distributions of PCBs, PBDEs, and OCPs in the Atmospheric Boundary Layer: Observation from the Beijing 325-m Meteorological Tower. Environ. Sci. Technol. 2009, 43, 1030. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Breivik, K.; Liu, G.; Zheng, M.; Jones, K.C.; Sweetman, A.J. Long-term temporal trends of polychlorinated biphenyls and their controlling sources in China. Environ. Sci. Technol. 2017, 51, 2838–2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhao, Y.; Li, M.; Du, M.; Li, X.; Li, Y. A review of a class of emerging contaminants: The classification, distribution, intensity of consumption, synthesis routes, environmental effects and expectation of pollution abatement to organophosphate flame retardants (OPFRs). Int. J. Mol. Sci. 2019, 20, 2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, M. Hyperthyroidism in cats: What’s causing this epidemic of thyroid disease and can we prevent it? J. Feline Med. Surg. 2012, 14, 804–818. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Endocrine disrupting compounds in drinking water supply system and human health risk implication. Environ. Int. 2017, 106, 207–233. [Google Scholar] [CrossRef]
- Yang, J.; Qadeer, A.; Liu, M.; Zhu, J.M.; Huang, Y.P.; Du, W.N.; Wei, X.Y. Occurrence, source, and partition of PAHs, PCBs, and OCPs in the multiphase system of an urban lake, Shanghai. Appl. Geochem. 2019, 106, 17–25. [Google Scholar] [CrossRef]
- Chen, K.; Cai, M.; Wang, Y.; Chen, B.; Li, X.; Qiu, C.; Huang, S.; Sun, J.; Liu, X.; Qian, B.; et al. Organochlorine Pesticides in Sediment of Zhang River Estuary Mangrove National Natural Reserve: The Implication of Its Source Change in China’s Mangroves. Sustainability 2020, 12, 3016. [Google Scholar] [CrossRef] [Green Version]
- Tsai, W.T. A review on environmental exposure and health risks of herbicide paraquat. Toxicol. Environ. Chem. 2013, 95, 197–206. [Google Scholar] [CrossRef]
- Gao, H.; Ji, H.; Yu, R.; Zhu, G. Effects of ozonation on disinfection by-product formation potentials and biostability in a pilot-scale drinking water treatment plant with micro-polluted water. Environ. Technol. 2021, 42, 3254–3265. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.; Singh, V.P.; Zhu, J.; Jiang, L.; Zeng, D.; Liu, D.; Zeng, X.; Wu, J.; Wang, L.; et al. Ecological and health risk assessment of PAHs, OCPs, and PCBs in Taihu Lake basin. Ecol. Indic. 2018, 92, 171–180. [Google Scholar] [CrossRef]
- Sun, P.; Li, Y.; Meng, T.; Zhang, R.; Song, M.; Ren, J. Removal of sulfonamide antibiotics and human metabolite by biochar and biochar/H2O2 in synthetic urine. Water Res. 2018, 147, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Fu, L.; Tang, B.; Bin, L.; Li, P.; Huang, S.; Fu, F. Occurrence, ecotoxicological risks of sulfonamides and their acetylated metabolites in the typical wastewater treatment plants and receiving rivers at the Pearl River Delta. Sci. Total Environ. 2020, 709, 136192. [Google Scholar] [CrossRef]
- Niu, Z.G.; Zhang, K.; Zhang, Y. Occurrence and distribution of antibiotic resistance genes in the coastal area of the Bohai Bay, China. Mar. Pollut. Bull. 2016, 107, 245–250. [Google Scholar] [CrossRef]
- Yan, M.; Xu, C.; Huang, Y.; Nie, H.; Wang, J. Tetracyclines, sulfonamides and quinolones and their corresponding resistance genes in the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 631, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, Y.; He, Y.; Zhang, B.; Huang, Y.; Yuan, Q.; Chen, Y. Occurrence and fate of antibiotic residues and antibiotic resistance genes in a reservoir with ecological purification facilities for drinking water sources. Sci. Total Environ. 2020, 707, 135276. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Nguyen, V.B.C.; Boroznjak, R.; Syritski, V. Advances in Detection of Antibiotic Pollutants in Aqueous Media Using Molecular Imprinting Technique—A Review. Biosensors 2022, 12, 441. [Google Scholar] [CrossRef]
- Lu, J.; Ji, Y.; Chovelon, J.M.; Lu, J. Fluoroquinolone antibiotics sensitized photodegradation of isoproturon. Water Res. 2021, 198, 117136. [Google Scholar] [CrossRef]
- Tasho, R.P.; Cho, J.Y. Veterinary antibiotics in animal waste, its distribution in soil and uptake by plants: A review. Sci. Total Environ. 2016, 563, 366–376. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, C.; Liu, Y.; Feng, Z.; Ji, C.; Zhang, H. Study on the raw water allocation and optimization in Shenzhen city, China. Water 2019, 11, 1426. [Google Scholar] [CrossRef]
- Silva, H.F.; Silva, N.F.; Oliveira, C.M.; Matos, M.J. Heavy metals contamination of urban soils—A decade study in the city of lisbon, portugal. Soil Syst. 2021, 5, 27. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, Y.; Li, Q.; Cai, Y.; Yin, H.; Zhang, L.; Zhang, J. Spatial correlation analysis of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in sediments between Taihu Lake and its tributary rivers. Ecotoxicol. Environ. Saf. 2017, 142, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Pan, L.; Yang, X.Y.; Liu, X.L.; Tao, S.Y.; Zhao, L.; Qin, X.P.; Sun, Z.J.; Hou, H.; Zhou, Y.Z. DDT, DDD, and DDE in soil of Xiangfen County, China: Residues, sources, spatial distribution, and health risks. Chemosphere 2016, 163, 578–583. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Qi, S.; Li, X.; Peng, X. Concentrations, enantiomeric compositions, and sources of HCH, DDT and chlordane in soils from the Pearl River Delta, South China. Sci. Total Environ. 2006, 372, 215–224. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, H.; Luo, Y.; Teng, Y.; Song, J.; Chen, M. Levels and potential health hazards of PCBs in shallow groundwater of an e-waste recycling area, China. Environ. Earth Sci. 2015, 74, 4431–4438. [Google Scholar] [CrossRef]
- Besis, A.; Botsaropoulou, E.; Balla, D.; Voutsa, D.; Samara, C. Toxic organic pollutants in Greek house dust: Implications for human exposure and health risk. Chemosphere 2021, 284, 131318. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al, A.M.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci. Total Environ. 2012, 430, 109–118. [Google Scholar] [CrossRef]
- EC (European Commission). Technical Guidance Docu Ment in Support of Commission Directive 93/7677EECon Risk Assessment for New Notified Substances Andcommission Regulation (EC) No.1488/94 on Risk Assessment for Existing Substances; Office for Official Publications of the European Communities: Luxembourg, 2003. [Google Scholar]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Zioris, I.; Danis, T.; Bikiaris, D.; Lambropoulou, D. Comprehensive investigation of a wide range of pharmaceuticals and personal care products in urban and hospital wastewaters in Greece. Sci. Total Environ. 2019, 694, 133565. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Liao, Z.; Wang, L.; Li, Y.; Zhang, W.; Ji, Y.; Chen, J. The distribution and ecological risks of antibiotics in the sediments from a diverging area of the bifurcated river: Effects of hydrological properties. J. Environ. Manag. 2022, 320, 115787. [Google Scholar] [CrossRef]
| Compound | Retention Tim e/min | Parention (m/z) | Daughterion (m/z) | Residence Time/s | Cone/V | Collision/V |
|---|---|---|---|---|---|---|
| Sulfamethoxazole | 2.98 | 254.0 | 92.0 156.0 | 0.050 | 25 | 26 16 |
| Sulfamonomethoxine | 2.69 | 281.0 | 92.0 156.0 | 0.078 | 27 | 35 22 |
| Trimethoprim | 2.06 | 291.0 | 123.0 230.0 | 0.022 | 35 | 27 25 |
| Sulphaquinoxaline | 3.44 | 301.1 | 92.2 156.1 | 0.050 | 23 | 30 16 |
| Sulfadimethoxine | 3.43 | 311.1 | 92.0 156.0 | 0.050 | 28 | 32 20 |
| Norfloxacin | 2.08 | 320.1 | 233.0 276.1 | 0.022 | 32 | 25 20 |
| Ciprofloxacin | 2.12 | 332.1 | 288.1 314.1 | 0.022 | 32 | 18 22 |
| Lomefloxacin | 2.22 | 352.1 | 265.1 308.1 | 0.022 | 31 | 22 16 |
| Enrofloxacin | 2.29 | 360.2 | 245.0 316.1 | 0.022 | 32 | 20 22 |
| Ofloxacin | 2.08 | 362.1 | 261.1 368.1 | 0.022 | 31 | 25 20 |
| Sites | Longitude | Latitude | Sampling Time | Water Quality Testing Indicators | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T °C | pH | DO mg/L | Conductivity μs/cm | TDS mg/L | Turbidity NTU | Chla μg/L | NH3-N mg/L | ||||
| 1 | 119.70° | 33.30° | Dec 2018 | 19.1 ± 0.57 | 9.0 ± 0.27 | 11.49 ± 0.34 | 271.50 ± 8.15 | 55.7 ± 1.67 | 16.1 ± 0.48 | 1.0 ± 0.03 | 0.55 ± 0.02 |
| Mar 2019 | 15.7 ± 0.63 | 8.8 ± 0.35 | 11.26 ± 0.45 | 287.30 ± 11.49 | — | 66.0 ± 2.64 | 18.6 ± 0.74 | 0.24 ± 0.01 | |||
| June 2019 | 28.5 ± 1.43 | 8.6 ± 0.43 | 9.42 ± 0.47 | 345.20 ± 17.26 | 257.9 ± 12.90 | 13.8 ± 0.69 | 8.1 ± 0.41 | 0.4 ± 0.02 | |||
| 2 | 119.24° | 32.75° | Dec 2018 | 14.8 ± 0.89 | 9.3 ± 0.56 | 14.54 ± 0.87 | 310.00 ± 18.60 | 43.0 ± 2.58 | 26.6 ± 1.60 | 7.1 ± 0.43 | 0.61 ± 0.04 |
| Mar 2019 | 13.5 ± 0.95 | 9.3 ± 0.65 | 12.78 ± 0.89 | 320.00 ± 22.40 | — | 31.8 ± 2.23 | 19.6 ± 1.37 | 0.23 ± 0.02 | |||
| June 2019 | 28.7 ± 0.86 | 9.2 ± 0.28 | 8.98 ± 0.27 | 596.20 ± 17.89 | 219.6 ± 6.59 | 45.8 ± 1.37 | 5.3 ± 0.16 | 0.33 ± 0.01 | |||
| 3 | 118.14° | 34.01° | Dec 2018 | 13.0 ± 0.52 | 8.6 ± 0.34 | 10.73 ± 0.43 | 484.40 ± 19.38 | 60.4 ± 2.42 | 6.3 ± 0.25 | 5.8 ± 0.23 | 0.47 ± 0.02 |
| Mar 2019 | 12.3 ± 0.62 | 8.42 ± 0.42 | 12.31 ± 0.62 | 493.00 ± 24.65 | — | 19.0 ± 0.95 | 3.6 ± 0.18 | 0.04 ± 0.00 | |||
| June 2019 | 29.8 ± 1.79 | 8.79 ± 0.53 | 10.63 ± 0.64 | 777.30 ± 46.64 | 241.5 ± 14.49 | 9.0 ± 0.54 | 7.6 ± 0.46 | 0.24 ± 0.01 | |||
| 4 | 119.06° | 34.53° | Dec 2018 | 13.9 ± 0.97 | 8.23 ± 0.58 | 9.91 ± 0.69 | 518.30 ± 36.28 | 34.4 ± 2.41 | 13.8 ± 0.97 | 46.4 ± 3.25 | 0.42 ± 0.03 |
| Mar 2019 | 10.9 ± 0.33 | 8.74 ± 0.26 | 10.29 ± 0.31 | 34.88 ± 1.05 | — | 22.0 ± 0.66 | 16.6 ± 0.50 | 0.59 ± 0.02 | |||
| June 2019 | 25.5 ± 1.02 | 7.81 ± 0.31 | 6.72 ± 0.27 | 71.75 ± 2.87 | 316.8 ± 12.67 | 16.9 ± 0.68 | 10.8 ± 0.43 | 0.16 ± 0.01 | |||
| 5 | 119.87° | 31.61° | Dec 2018 | 13.4 ± 0.67 | 8.67 ± 0.43 | 9.78 ± 0.49 | 415.8 ± 20.79 | 133.5 ± 6.68 | 76. ±3.80 | 45.7 ± 2.29 | 0.48 ± 0.02 |
| Mar 2019 | 9.7 ± 0.58 | 8.74 ± 0.52 | 10.29 ± 0.62 | 34.9 ± 2.09 | — | 22.0 ± 1.32 | 16.6 ± 1.00 | 0.15 ± 0.01 | |||
| June 2019 | 29.4 ± 2.06 | 8.09 ± 0.57 | 7.97 ± 0.56 | 512.1 ± 35.85 | 257.2 ± 18.00 | 82.6 ± 5.78 | 22.0 ± 1.54 | 0.32 ± 0.02 | |||
| 6 | 120.38 | 31.46° | Dec 2018 | 15.0 ± 0.45 | 8.47 ± 0.25 | 9.27 ± 0.28 | 386 ± 11.58 | 84.3 ± 2.53 | 15.7 ± 0.47 | 29.6 ± 0.89 | 0.53 ± 0.02 |
| Mar 2019 | 10.4 ± 0.42 | 8.95 ± 0.36 | 10.71 ± 0.43 | 362.8 ± 14.51 | — | 12.8 ± 0.51 | 15.5 ± 0.62 | 0.10 ± 0.00 | |||
| June 2019 | 25.6 ± 1.28 | 7.96 ± 0.40 | 6.96 ± 0.35 | 617.6 ± 30.88 | 210.2 ± 10.51 | 18.6 ± 0.93 | 14.8 ± 0.74 | 0.48 ± 0.02 | |||
| Samples | Sites | SMX | SMM | TMP | SQX | SMT | NFC | CFC | LFC | EFC | OFC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water (ng/L) | 2 | 9.8 ± 0.29 ~15.5 ± 0.47 | 4.0 ± 0.12 ~12.6 ± 0.38 | NF | NF | NF | NF | NF | NF | NF | NF ~1.1 ± 0.03 |
| 3 | 1.7 ± 0.07 ~1.9 ± 0.08 | NF ~6.0 ± 0.24 | NF | NF | NF | NF | NF | NF | NF ~0.4 ± 0.02 | NF ~3.4 ± 0.14 | |
| 4 | 11.2 ± 0.56 ~21.2 ± 1.06 | 10.0 ± 0.50 ~13.3 ± 0.67 | NF | NF | NF | NF ~5.7 ± 0.29 | NF | NF | NF | NF ~1.7 ± 0.09 | |
| 5 | 5.7 ± 0.34 ~78.1 ± 4.69 | 8.8 ± 0.53 ~56.7 ± 3.40 | 13.3 ± 0.80 ~21.4 ± 1.28 | NF ~8.1 ± 0.49 | NF ~3.1 ± 0.19 | NF | NF ~3.5 ± 0.25 | NF ~2.3 ± 0.14 | NF ~2.6 ± 0.16 | NF ~3.1 ± 0.19 | |
| 6 | 1.0 ± 0.07 ~2.6 ± 0.18 | NF | NF | NF | NF | NF ~3.8 ± 0.27 | NF | NF | 0.7 ± 0.05 ~1.6 ± 0.11 | 2.8 ± 0.20 ~6.9 ± 0.48 | |
| Sediment (ng/g) | 2 | NF ~15.4 ± 0.46 | 5.9 ± 0.1 8~25.3 ± 0.76 | 14.0 ± 0.42 ~87.0 ± 2.61 | NF ~1.9 ± 0.06 | NF ~3.8 ± 0.11 | NF | NF ~0.7 ± 0.02 | 9.0 ± 0.27 ~31.0 ± 0.93 | NF ~0.3 ± 0.01 | 0.1 ± 0.01 ~0.3 ± 0.01 |
| 3 | 1.4 ± 0.06 ~4.2 ± 0.17 | 3.4 ± 0.14 ~8.1 ± 0.32 | 8.2 ± 0.33 ~32.6 ± 1.30 | 0.7 ± 0.03 ~1.1 ± 0.04 | 0.7 ± 0.03 ~3.8 ± 0.15 | NF | NF | 2.2 ± 0.09 ~4.6 ± 0.18 | 0.1 ± 0.02 ~0.4 ± 0.04 | NF ~0.1 ± 0.01 | |
| 4 | 1.7 ± 0.09 ~6.0 ± 0.30 | 5.9 ± 0.30 ~9.4 ± 0.47 | 2.6 ± 0.13 ~12.4 ± 0.62 | 0.6 ± 0.03 ~1.2 ± 0.06 | NF ~0.8 ± 0.04 | NF | 0.1 ± 0.01 ~0.2 ± 0.01 | NF ~3.0 ± 0.15 | 0.7 ± 0.0 8~1.6 ± 0.13 | NF ~0.1 ± 0.01 | |
| 5 | 1.4 ± 0.0 8~13.9 ± 0.83 | 1.7 ± 0.10 ~15.4 ± 0.92 | 8.0 ± 0.4 8~41.4 ± 2.48 | NF ~7.9 ± 0.47 | NF ~10.7 ± 0.64 | 0.2 ± 0.01 ~0.7 ± 0.04 | 2.1 ± 0.13 ~3.8 ± 0.23 | 2.0 ± 0.12 ~93.2 ± 5.59 | 2.1 ± 2.33 ~38.8 ± | 0.1 ± 0.01 ~0.8 ± 0.05 | |
| 6 | 1.1 ± 0.0 8~2.4 ± 0.17 | 4.7 ± 0.33 ~6.2 ± 0.43 | 3.6 ± 0.25 ~97.8 ± 6.85 | 1.2 ± 0.0 8~1.6 ± 0.11 | 0.3 ± 0.02 ~1.1 ± 0.08 | NF | NF ~0.1 ± 0.01 | NF | NF ~0.1 ± 0.01 | NF ~0.1 ± 0.01 |
| Sites No. | Sampling Time | p,p’-DDT ng/g | p,p’-DDE ng/g | Endosulfan Sulfate ng/g | Beta-Endosulfan ng/g | Methoxychlor ng/g |
|---|---|---|---|---|---|---|
| 1 | Dec 2018 | 12.6 ± 0.38 | 21.8 ± 0.65 | NF | NF | NF |
| Mar 2019 | 3.9 ± 0.16 | 21.1 ± 0.84 | NF | NF | NF | |
| June 2019 | — | — | — | — | — | |
| 2 | Dec 2018 | 13.2 ± 0.66 | 22.4 ± 1.12 | NF | NF | NF |
| Mar 2019 | — | — | — | — | — | |
| June 2019 | 9.7 ± 0.68 | 23.7 ± 1.66 | 10.6 ± 0.74 | NF | 9.2 ± 0.64 | |
| 3 | Dec 2018 | NF | 0.4 ± 0.01 | NF | NF | NF |
| Mar 2019 | 1.9 ± 0.08 | 11.6 ± 0.46 | NF | NF | NF | |
| June 2019 | — | — | — | — | — | |
| 4 | Dec 2018 | NF | 0.9 ± 0.03 | NF | NF | NF |
| Mar 2019 | 1.8 ± 0.13 | 10.2 ± 0.71 | NF | NF | NF | |
| June 2019 | — | — | — | — | — | |
| 5 | Dec 2018 | NF | 9.5 ± 0.29 | NF | NF | NF |
| Mar 2019 | — | — | — | — | — | |
| June 2019 | NF | 25 ± 0.75 | 9.0 ± 0.27 | NF | NF | |
| 6 | Dec 2018 | 2.0 ± 0.14 | 14.8 ± 1.04 | NF | NF | NF |
| Mar 2019 | 69.6 ± 2.09 | 19.6 ± 0.59 | NF | NF | NF | |
| June 2019 | 8.6 ± 0.34 | NF | NF | 14.1 ± 0.56 | NF |
| Sites No. | Sampling Time | PCB-52 ng/g |
|---|---|---|
| 1 | Dec 2018 | 0.9 ± 0.03 |
| Mar 2019 | 18.4 ± 0.74 | |
| June 2019 | — | |
| 2 | Dec 2018 | 0.8 ± 0.05 |
| Mar 2019 | — | |
| June 2019 | NF | |
| 3 | Dec 2018 | 1.1 ± 0.04 |
| Mar 2019 | 47.4 ± 2.37 | |
| June 2019 | — | |
| 4 | Dec 2018 | 0.9 ± 0.06 |
| Mar 2019 | 10.9 ± 0.33 | |
| June 2019 | — | |
| 5 | Dec 2018 | 9.7 ± 0.49 |
| Mar 2019 | — | |
| June 2019 | NF | |
| 6 | Dec 2018 | 21.7 ± 0.65 |
| Mar 2019 | 36.0 ± 1.44 | |
| June 2019 | NF |
| Antibiotics | Toxicity | Assessment Factors | LC50 or EC50 (mg·L−1) | PNEC (ng·L−1) |
|---|---|---|---|---|
| SMX | acute | 1000 | 0.27 | 270 |
| SMM | acute | 1000 | 1.277 | 1277 |
| TMP | acute | 1000 | 16 | 16,000 |
| SQX | acute | 1000 | 84.46 | 84,500 |
| SMT | acute | 1000 | 1.74 | 17,400 |
| NFC | acute | 1000 | 22 | 22,000 |
| CFC | acute | 1000 | 5 | 5000 |
| LFC | acute | 1000 | 4.137 | 4137 |
| EFC | acute | 1000 | 49 | 49,000 |
| OFC | acute | 1000 | 11.3 | 11,300 |
| OCPs | ERL | ERM | TEL | PEL |
|---|---|---|---|---|
| p,p’-DDE | 2.2 | 27 | 2.07 | 3.74 |
| p,p’-DDT | 1 | 7 | 1.19 | 4.77 |
| DDTs | 3.0 | 46.1 | 3.89 | 51.7 |
| PCB | ERL ng/g | ERM ng/g |
|---|---|---|
| PCB52 | 2.2 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Xu, Y.; Song, W. Pollution Characteristics and Risk Assessment of Typical Antibiotics and Persistent Organic Pollutants in Reservoir Water Sources. Water 2023, 15, 259. https://doi.org/10.3390/w15020259
Li C, Xu Y, Song W. Pollution Characteristics and Risk Assessment of Typical Antibiotics and Persistent Organic Pollutants in Reservoir Water Sources. Water. 2023; 15(2):259. https://doi.org/10.3390/w15020259
Chicago/Turabian StyleLi, Chunwei, Yuan Xu, and Weiwei Song. 2023. "Pollution Characteristics and Risk Assessment of Typical Antibiotics and Persistent Organic Pollutants in Reservoir Water Sources" Water 15, no. 2: 259. https://doi.org/10.3390/w15020259
APA StyleLi, C., Xu, Y., & Song, W. (2023). Pollution Characteristics and Risk Assessment of Typical Antibiotics and Persistent Organic Pollutants in Reservoir Water Sources. Water, 15(2), 259. https://doi.org/10.3390/w15020259





