Abstract
The contamination of industrial water sources with synthetic dyes, such as methylene blue (MB), remains a persistent environmental concern, demanding effective remediation techniques. In response, this research centers on the utilization of trimetallic nanoparticles (TMNPs) composed of Fe-Ni-Cr, Fe-Ni-Cd and Fe-Ni-Cu as a promising solution to address color-related pollution in aquatic ecosystems. These nanoparticles were synthesized using the wet chemical precipitation method and rigorously characterized using Fourier transform infrared (FT-IR), energy-dispersive X-rays (EDX), and scanning electron microscopy (SEM). Armed with these trimetallic nanoparticles, our primary objective was to harness their photocatalytic prowess when exposed to direct sunlight in aqueous environments for the degradation of MB. The progress of photodegradation was meticulously monitored using a reliable visible spectrophotometer, providing insights into the degradation kinetics. Remarkably, within just six hours of solar irradiation, the TMNPs exhibited a remarkable capacity to degrade MB, achieving an impressive degradation rate ranging from 77.5% to 79.4%. In our relentless pursuit of optimization, we conducted a comprehensive examination of various parameters including catalyst dosage, dye dosage, and pH levels, focusing specifically on the Fe-Ni-Cr TMNPs. Through systematic experimentation, a trifecta of optimal conditions emerged: a pH level of 10 (resulting in a 79.35% degradation after 1.5 h), a catalyst amount of 0.005 g (yielding 43.5% degradation after 1.5 h), and a dye concentration of 40.0 ppm (culminating in a 42.54% degradation after 1.5 h). The study also extended its scope to explore the regeneration potential of the catalyst, shedding light on its sustainability in long-term applications. Amidst the vibrant interplay of color and water, TMNPs emerged as a symbol of optimism, offering a promising avenue for the removal of synthetic dyes from the water system. With each experiment and investigation, we inch closer to realizing clearer waters and brighter environmental horizons.
1. Introduction
Water stands as a fundamental element supporting all life on our planet, underscoring its unparalleled importance [1]. Nonetheless, the escalating challenges of global water contamination have become increasingly urgent, fueled by rapid industrialization, population growth, and climate change [2]. A stark forecast suggests that by 2025, freshwater scarcity could affect up to 50% of the world’s population, surging to a staggering 75% by 2075 [3]. These statistics underscore the pivotal role of water in sustaining economies worldwide [4]. While water conservation efforts are undeniably crucial, they alone have not been sufficient to avert the persistent specter of global water shortages [5].
The significance of wastewater treatment looms large, particularly in sectors like textiles, notorious for their substantial water consumption and pollution footprint. To put it into perspective, a mere 0.5 kg of textile products demands a staggering 80 L of water [6]. Disturbingly, the textile industry alone accounts for the direct release of 2% of all dyes into aquatic ecosystems, posing both health and environmental risks [7,8]. Within this complex tapestry of pollutants, organic dyes originating from various sectors, such as textile dyeing, paper manufacturing, paint, cosmetics, and food processing, have garnered heightened attention due to their alarming lack of biodegradability and the potentially harmful byproducts they generate during wastewater treatment process [9]. Dyes can be broadly categorized into two main groups: natural and synthetic. Among synthetic dyes, a crucial subdivision exists between azo and non-azo dyes, with azo dyes dominating the landscape, constituting more than half of all dyes used across various industries. They exhibit amphoteric properties, manifesting as cations, anions, or nonionic compounds based on the prevailing pH conditions [10]. Within the realm of synthetic dyes, one prominent example is methylene blue (MB), renounced for its widespread applications in dyeing textiles, paper, and even medical treatments [11]. MB finds application in the treatment of various medical conditions including neuro-inflammation, oxidative stress, mitochondrial dysfunction, and Alzheimer’s, among other therapeutic uses [12]. However, it is imperative to acknowledge that MB also bears the ominous label of being a highly carcinogenic pollutant. Its presence in water sources poses several health risks, manifesting as symptoms such as respiratory problems, nausea, and gastrointestinal infections. Consequently, the development of cutting-edge methods for eliminating such hazardous pollutants from water bodies becomes a matter of utmost importance [13]. A plethora of techniques have been devised to address the issue of synthetic dye removal from water bodies, including adsorption [14,15,16,17,18], Fenton-like reactions, activated sludge, membrane filtration, and photocatalysis [19,20]. Photocatalysis, which harnesses semiconductor nanoparticles to convert solar energy into a driving force for dye degradation, has gained significant attention due to its effectiveness and potential for the complete mineralization of dyes [21]. The fundamental process of photocatalysis revolves around the absorption of photons possessing energy greater than the semiconductor’s band gap. This absorption leads to the generation of an electron–hole pair, subsequently triggering redox reactions on the photocatalyst’s surface. The resulting superoxide ions and hydroxyl radicals engage in oxidative attack on organic molecules, leading to dye degradation [22]. The choice of nanoparticles as photocatalysts has gained popularity due to their chemical stability, high surface area, and consistent pore size [23].
In a noteworthy extension of this technology, trimetallic catalysts like Ni-Fe-Ce(/Mn) O2 have found utility in diverse applications, including their role as a cement-based anode in Fe-air rechargeable batteries and as catalysts in amorphous molybdenum sulfide sheets during the hydrogenation evolution reaction [24]. These advancements underscore the versatility and potential of trimetallic catalysts in various domains.
A novel research avenue has emerged, dedicated specifically to the study of TMNPs in the realm of catalysis. This exploration was prompted by the remarkable advantages associated with introducing a second metal into nanoparticle (NP) catalysts, exemplified by systems like Pd to Au NPs supported on high-surface-area metal oxides [25]. Due to their distinctive structural arrangements and composition, trimetallic catalysts performed noticeably better than bimetallic ones [26]. Numerous studies have been conducted on the comparative evaluation of trimetallic nanoparticles for the photocatalytic degradation of methylene blue, but noteworthy research gaps remain to be addressed. These gaps could be effectively filled by investigating a range of aspects, including the photocatalytic activity of trimetallic nanoparticles with varying compositions and shapes for MB degradation [27,28,29]. Additionally, assessing their performance relative to other types of photocatalysts in MB degradation is essential [30,31]. Furthermore, it is crucial to delve into the influence of several factors, including temperature, concentration, and pH, on the photocatalytic activity of trimetallic nanoparticles for MB degradation [32]. Lastly, conducting a comprehensive analysis of the mechanism that governs photocatalytic degradation of MB is a pivotal step [33]. These research avenues hold the promise of providing deeper insights into the photocatalytic performance of trimetallic nanoparticles for MB degradation and, in turn, could contribute to the development of more efficient photocatalysts for environmental remediation.
In the current study, wet chemical precipitation was used to create trimetallic nanoparticles (Fe-Ni-Cr, Fe-Ni-Cd, and Fe-Ni-Cu oxide nanoparticles). These nanoparticles played a pivotal role as catalysts in the photodegradation of MB in an aqueous medium. The photodegradation of a dye with TMNPs as a photocatalyst was carried out under direct sunlight irradiation at various time intervals. Fe-Ni-Cr TMNPs were used to evaluate the photodegradation of methylene blue dye in detail by investigating various parameters such as catalyst dosage, dye concentration, and pH level. Furthermore, an innovative facet of our study involved the recovery and subsequent reutilization of the original Fe-Ni-Cr catalyst for further photodegradation of methylene blue. It is worth noting that our expectations are anchored in the anticipation that these TMPNs will exhibit enhanced degradation capabilities, primarily attributable to the narrowing of the band gap of these nanoparticles.
2. Materials and Methods
2.1. Materials
All of the apparatus and chemicals used in the synthesis of trimetallic nanoparticles were obtained from the chemistry lab of GDC Kanpur.
The chemicals, including sodium hydroxide (NaOH), Nickel chloride (NiCl2), and Ferric Chloride (FeCl3), were purchased from Sigma Aldrich (Darmstadt, Germany). Cadmium chloride (CdCl2.H2O) was purchased from MERCK (Darmstadt, Germany), methylene blue hydrate (C16H16ClN3S.H2O) was purchased from Sigma Aldrich (Germany), and copper chloride 2-hydrate (CuCl2.2H2O) was purchased from Riedel-de-Hean scientific laboratory supplies (North Lanarkshire, UK).
2.2. Synthesis of Trimetallic Nanoparticles
The TMNPs were synthesized using the wet chemical precipitation method [34]. For the synthesis of Fe-Ni-Cr, Fe-Ni-Cd and Fe-Ni-Cu, 1.622 g of FeCl3 (0.1 M), 1.296 g of NiCl2 (0.1 M), and 2.664 g of CrCl3.6H2O (0.1 M), 2.013 g of CdCl2.H2O (0.1 M), or 1.705 g of CuCl2.2H2O (0.1 M) was, respectively, dissolved in 100 mL of distilled water in three different beakers. The obtained solutions were combined into a 500 mL beaker, resulting in the formation of a mixture.
Subsequently, the NaOH solution was added drop-wise to the metal ion mixture until the pH reached a basic level. This alkaline environment facilitated the reduction of the metal ions. The homogenous mixture was subjected to continuous stirring for a duration of two hours within a temperature range of 60–70 °C. Afterward, the mixture was allowed to cool, leading to the formation of precipitates that were isolated from the mixture via filtration. The obtained precipitates were washed several times with distilled water to eliminate any residual un-reacted chemicals. They were then dried at 100 °C in an electrical oven for two hours. The resultant product obtained after drying was the required TMNP (as depicted in Scheme 1).
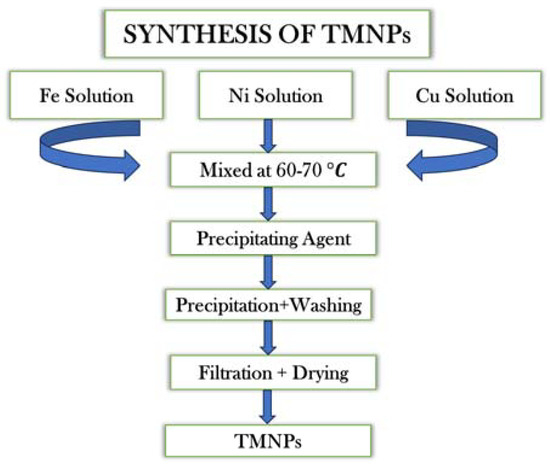
Scheme 1.
Synthetic procedure of TMNPs.
2.3. Photodegradation of Methylene Blue Dye Using Trimetallic Nanoparticles (TMNPs)
To assess the photocatalytic performance of the synthesized TMNPs, we conducted experiments to measure the photocatalyzed degradation rate of the MB solution. For this investigation, an experimental apparatus was set up in accordance with established protocols, aiming to study the photodegradation of the MB solution in the presence of sunlight, which serves as a source of UV-Visible light. Samples of degraded MB solutions were taken at regular intervals of every 30 min, and the absorbance was measured using the UV-Vis spectrometer to estimate changes in concentration, i.e., degradation rates of MB.
In the experimental set-up, initially, 100 mL of the MB solution was prepared with a concentration of 10 ppm from the stock solution. These solutions were placed individually in three separate beakers. Subsequently, 0.0135 g of each chemically synthesized TMNP was introduced into the beakers containing the MB dye solution. Additionally, a control sample was maintained without the presence of TMNPs. The beakers were sealed with transparent plastic sheets to reduce the process of evaporation and allow light to pass through them. The solution was thoroughly mixed using a magnetic stirrer and was placed in darkness for 30 min to establish an adsorption–desorption equilibrium between MB and TMNP in the solution. Following this equilibration period, this solution was exposed to sunlight, and the degradation process was monitored.
After every 30 min, 3–5 mL of samples were withdrawn from the solutions and filtered. These samples were used to examine the degradation of MB. The absorption spectra for each sample were determined with a UV-Visible spectrometer at various wavelengths. For the measurement of the maximum absorption value at 663 nm for the duration of the degradation process, the concentration of MB was determined.
The percentage of MB degradation was quantified using Equation (1)
where is the initial absorbance of the MB solution before irradiation and A is the final absorbance after irradiation.
2.4. Instruments
The infrared spectra of the synthesized nanoparticles were obtained using a Bio-Rad Win-IR instrument. Potassium bromide (KBr) was used as a salt in combination with the sample to form a pellet transparent for IR radiation The morphological study of the nanoparticles was performed by using SEM Model No. JEOL-Jsm-5910, JEOL Company, Tokyo, Japan. The EDX study was performed by using an EDX spectrometer Model Inea 200, UK, a company in Oxford, UK. The photodegradation study was performed by using a UV-visible spectrophotometer (Shimadzu 1900, Tokyo, Japan).
3. Results and Discussion
3.1. FT-IR Study
In order to study the changes in the functional groups on TMNP catalysts, they were characterized by using FT-IR [35] (as shown in Figure 1 and Figure 2).
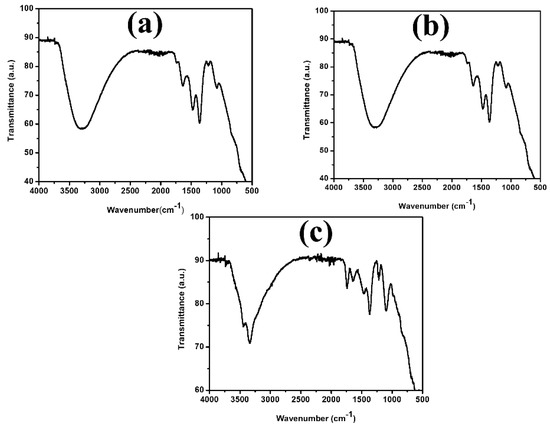
Figure 1.
FT-IR spectra of (a) Fe-Ni-Cr, (b) Fe-Ni-Cd, and (c) Fe-Ni-Cu TMNPs.
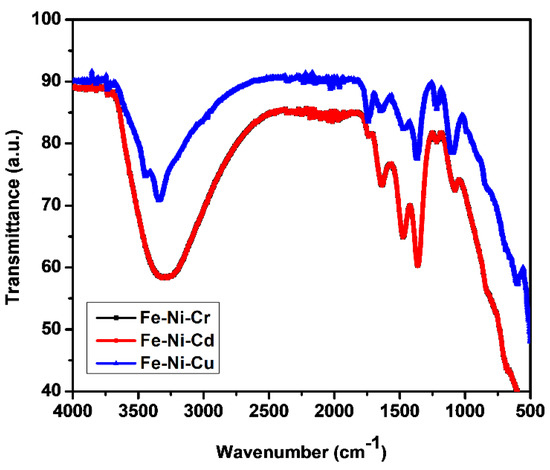
Figure 2.
Comparative FT-IR spectra of TMNPs.
In the FTIR analysis, several key absorption bands were observed that provide valuable insights into the composition of the synthesized nanoparticles. Initially, a broad absorption band was evident in the range of 3650 and 3250 cm−1, which is attributed to the OH stretching. This band serves as a confirmation of the presence of OH groups [36]. Following this, a medium peak was noted centered at around 1630 cm−1, arising from the OH bending vibrations of water molecules [37]. In addition to the above peaks present in all three kinds of nanoparticles, some additional peaks were also observed. For the Fe-Ni-Cr nanoparticle, weak absorption bands were observed in the range of 1200–550 cm−1, indicating the formation of Fe-O (577 cm−1, 631 cm−1), Ni-O (610 cm−1), and Cr-O (569.30 cm−1 and 632.61 cm−1) bonds [38,39,40]. In the case of Fe-Ni-Cd, a few medium peaks were observed in the range of 1400–550 cm−1, which serve as a confirmation of the formation of Fe-O (577 cm−1, 631 cm−1), Ni-O (610 cm−1), and CdO (1400 cm−1) [38,39,40]. Lastly, for the Fe-Ni-Cu nanoparticles, weak peaks in the range between 1200 cm−1 and 950 cm−1 were identified, attributed to vibrations exhibited by Fe-O (577 cm−1, 631 cm−1), Ni-O (610 cm−1), and Cu-O (437.84 cm−1, 516.92 cm−1, and 590.22 cm−1) particles. These findings suggest that the particles underwent oxidation, forming oxide nanoparticles [38,39,41].
3.2. Morphological Study
The morphological study of the TMNPs was investigated by using scanning electron microscopy (SEM) and energy dispersive X-rays (EDX). The SEM images of all three kinds of TMNPs, as depicted in Figure 3, revealed their distribution in both agglomerated and dispersed forms. Spherical particles were observed within the encircled areas, while clustered agglomeration was present within the rectangles as shown in Figure 3c. Confirmation of the elemental composition in the TMNPs was obtained through EDX analysis. In Fe-Ni-Cr, Fe (19.32%), Ni (9.32%) and Cr (20.0%) were present. In Fe-Ni-Cd, Fe (19.08%), Ni (9.64%) and Cd (19.08%) were present, while in the case of Fe-Ni-Cu, Fe (20.43%), Ni (9.80%), and Cu (22.75%) were present. Besides the basic elements of Fe, Ni, Cr, Cd, and Cu, there was a sufficient percentage of oxygen, which confirms that the nanoparticles were in their oxide forms (as shown in Figure 4).
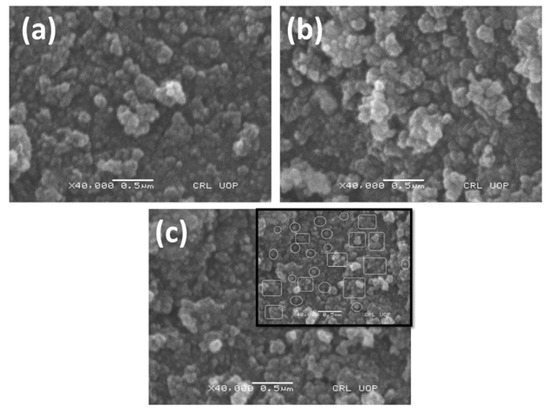
Figure 3.
SEM images of (a) Fe-Ni-Cr, (b) Fe-Ni-Cd, and (c) Fe-Ni-Cu TMNPs.
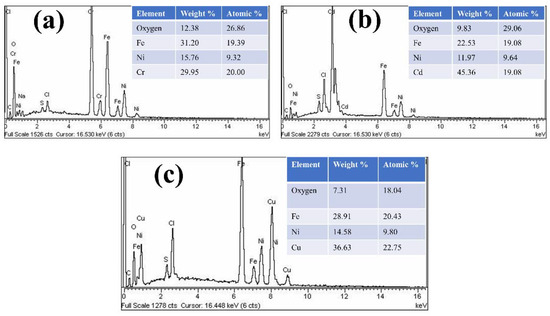
Figure 4.
EDX spectrum of (a) Fe-Ni-Cr, (b) Fe-Ni-Cd, and (c) Fe-Ni-Cu TMNPs.
3.3. Photodegradation Study of Methylene Blue
The photocatalytic activity of synthesized Fe-Ni-Cr, Fe-Ni-Cd, and Fe-Ni-Cu photocatalysts was evaluated by examining their ability to degrade a solution of MB in the presence of sunlight. The UV–Visible spectra for the methylene blue solution were captured before as well as during exposure to the ultraviolet (UV) radiation in an environment of Fe-Ni-Cr, Fe-Ni-Cd, and Fe-Ni-Cu oxide TMNPs (as shown in Figure 5). Using UV–Visible spectra, photodegradation rates of methylene blue were calculated, which show the highest absorption peak at 663 nm. The obtained spectra show that by extending the time of UV irradiation, there is a gradual increase in the photodegradation rate of methylene blue. The results were as shown in Figure 5.
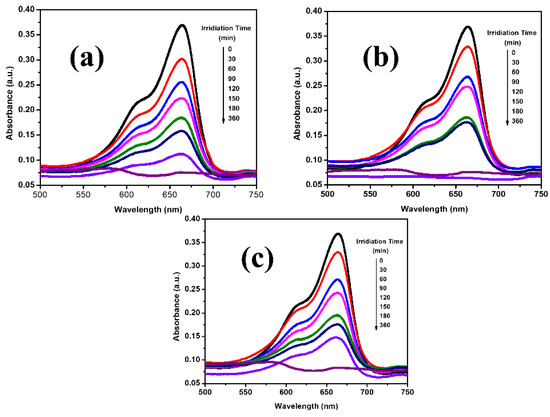
Figure 5.
UV-Visible spectrum of methylene blue dye degradation with (a) Fe-Ni-Cr, (b) Fe-Ni-Cd, and (c) Fe-Ni-Cu.
The comparative investigation of % degradation for the photodegradation of methylene blue with synthesized Fe-Ni-Cr, Fe-Ni-Cd, and Fe-Ni-Cu photocatalysts is shown in Figure 6a. The TMNPs degraded 78% to 83% of the dye within 6 h.
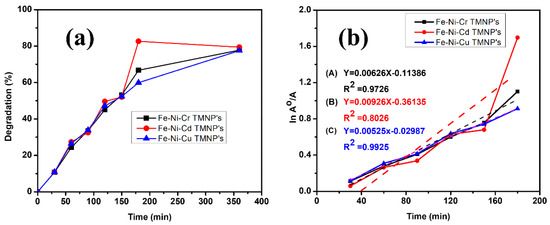
Figure 6.
(a) Comparative % degradation of MB with different TMNPs. (b) Kinetic study of methylene blue dye degradation reaction in the presence of TMNPs.
Kinetic study [42,43] of methylene blue degradation reaction with different TMNPs was investigated by applying a pseudo-first-order kinetic model according to Equation (2) by plotting vs. t. All three TMNPs show almost straight lines with acceptable R2 values (as shown in Figure 6b). The data show that the kinetics is pseudo-first-order.
3.4. Photodegradation Mechanism
Ultraviolet (UV) light plays a crucial role in the photodegradation of dyes, where it serves to photoexcite the valence electrons (e-) within trimetallic nanoparticles (TMNPs). This stimulation prompts the transfer of these valence electrons from the valence band (VB) to the conduction band (CB), instigating the generation of positively charged holes (h+) as well as electron-deficient regions within the VB. This separation holds significance as both electrons and holes become active participants in the creation of highly reactive radicals. To delve deeper, the interaction between positive holes (h+) and water (H2O) molecules, or hydroxide ions (OH−), leads to the production of hydroxyl radicals (•OH). Similarly, a parallel reaction between electrons and oxygen (O2) results in the formation of superoxide radical ions. These exceptionally reactive radicals are indispensable to the photodegradation process of dyes due to their efficient dismantling of the molecular structures involved [21]. The mechanism of photodegradation in a more general form is shown in Figure 7.
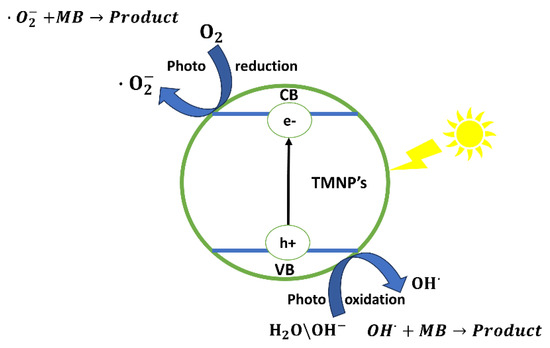
Figure 7.
Photodegradation mechanism.
3.5. Photocatalytic Activity of Recovered Fe-Ni-Cr TMNPs
The photocatalytic activity of the recovered Fe-Ni-Cr TMNPs was evaluated in comparison with the original photocatalyst. The original catalyst after first time use was recovered carefully by filtering with repeated washing with distilled water in order to remove the maximum amount of dye. After complete washing, the recovered catalyst was used for the photodegradation of MB with the same experimental conditions. The results were as shown in Figure 8, which reveals that the recovered catalyst showed less photocatalytic activity as compared to the original catalyst. This decrease in photocatalytic activity of the recovered catalyst might be due to fewer available active sites on the surface of the photocatalyst. As shown in Figure 8a, the original catalyst degraded almost 77.74% of methylene blue dye after 6.0 h, while the recovered catalyst degraded 71.57% in the same course of time. Figure 8b shows the kinetics of the original and recovered Fe-Ni-Cr TMNP photocatalysts.
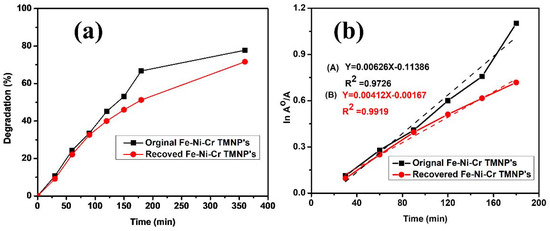
Figure 8.
(a) Degradation (%) of MB with original Fe-Ni-Cr and recovered photocatalyst. (b) Kinetic study of MB degradation reaction with original Fe-Ni-Cr and recovered photocatalyst.
3.6. Effect of the Photocatalyst Dosage
The effect of photocatalyst dosage on the degradation of methylene blue dye was investigated, keeping the dye concentration constant throughout the course of the reaction. The catalyst dosage used varied from 0.005 g to 0.1 g per 10 mL of dye solution at a constant 1.5 h irradiation time. The UV-Visible spectrum of the methylene blue before and after irradiation for different amounts of catalyst is as shown in Figure 9a. As shown, the absorbance decreased with decreasing amount of the catalyst. It is shown that the catalyst had maximum degradation ability at 0.005 g, while degradation % decreased as we increased the catalyst (as shown in Figure 9b). The decrease in the degradation % of the dye with an increase in the catalyst amount might be explained on the basis of a decrease in the active sites on the catalyst surface. The catalyst showed a maximum degradation of 43.47% at 0.005 g of the catalyst, while there was a decrease in degradation and the minimum was 17.39% at a 0.1 g catalyst amount at a constant 1.5 h irradiation time.
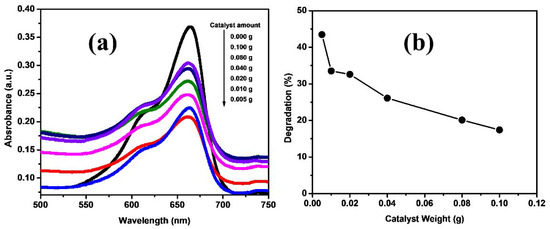
Figure 9.
(a) UV-Visible absorbance spectra of MB degradation with Fe-Ni-Cr photocatalyst having different catalyst amounts. (b) Degradation (%) of MB with different amounts of Fe-Ni-Cr photocatalyst.
3.7. Effect of the Dye Concentration
We also explored how varying the dye concentration impacted the photodegradation of the dye while maintaining a constant amount of catalyst and a consistent irradiation time of 1.5 h. Initially, with increased dye concentration, there was a corresponding increase in dye degradation, with the optimal degradation occurring at 40.0 ppm as illustrated in Figure 10. However, as the dye concentration continued to increase beyond 40.0 ppm, we noticed a decline in dye degradation. Specifically, at 10 ppm of dye concentration, 33.53% of the dye was degraded, while the maximum degradation of 42.53% was achieved at 40 ppm. Subsequently, with a further increase in dye concentration up to 80 ppm, the degradation rate decreased to 15.47%. This phenomenon can be explained by the initial stages where the dye molecule does not fully occupy the active sites on the catalyst, resulting in enhanced degradation. However, as the dye concentration increases, the active sites on the catalyst become fully occupied, leading to a decrease in the production of hydroxyl radicals () and, consequently, a reduction in the degradation rate [44].
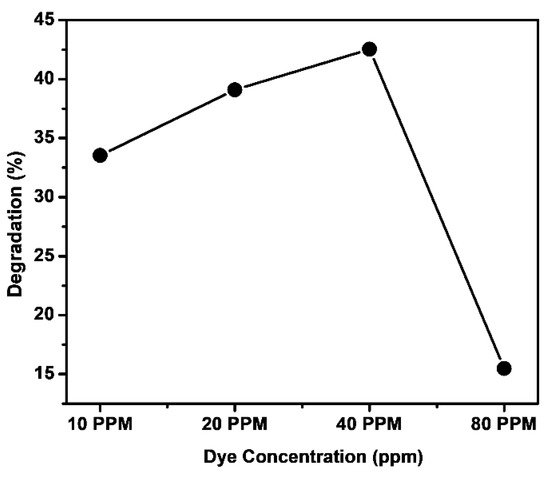
Figure 10.
Effect of MB dye concentration on photodegradation with Fe-Ni-Cr TMNPs, keeping catalyst amount constant for 1.5 h of sunlight irradiation time.
3.8. Effect of pH
The pH level plays a significant role in the degradation of dyes, and its impact is particularly pronounced. Industries such as textile, paints, and dye manufacturing release effluents with different pH levels. It is crucial to thoroughly investigate how pH influences the degradation of dyes. The pH not only affects the generation of hydroxyl radicals but also plays a crucial role in modifying the catalyst surface charge, thereby influencing the degradation process, as well as the conductance and valence bands [45,46]. In the present study, we examined the photodegradation of methylene blue dye under various pH of 4, 7, and 10 using the Fe-Ni-Cr photocatalyst (as shown in Figure 11). The results revealed that photodegradation was less pronounced at pH 4 but significantly improved as the pH became more basic, reaching its highest efficiency at pH 10. The degradation rates at pH levels 4, 7, and 10 were 21.19%, 39.13%, and 79.34%, respectively. The maximum degradation of methylene blue observed at pH 10 can be attributed to the increased formation of hydroxyl radicals and the presence of strong oxidizing species, both of which play a pivotal role in the photodegradation process [47].
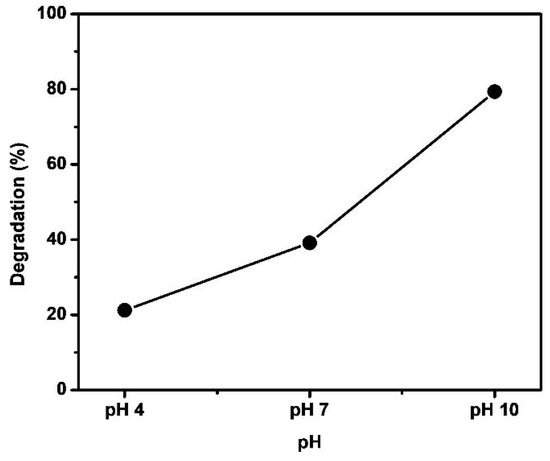
Figure 11.
Effect of pH on photodegradation of MB with Fe-Ni-Cr TMNPs, keeping dye and catalyst amount constant for 1.5 h of sunlight irradiation time.
The results obtained from our study were compared with the literature data for methylene blue photodegradation (as shown in Table 1).

Table 1.
Comparison of methylene blue dye degradation with previous literature studies.
4. Conclusions
In this research, the synthesis of Fe-Ni-Cr, Fe-Ni-Cd, and Fe-Ni-Cu TMNPs was accomplished through the utilization of the wet chemical precipitation method. The characterization process involved employing various techniques, such as FT-IR, SEM, and EDX. The FT-IR analyses confirmed the existence of functional groups within the synthesized TMNPs. Examination of their morphology via SEM demonstrated that the TMNPs exhibited a spherical shape. Furthermore, the SEM analysis revealed the presence of TMNPs in both dispersed and agglomerated states. In the context of photodegradation, it was observed that the degradation of methylene blue dye increased with prolonged irradiation time, ultimately reaching a peak degradation rate of 77.5% to 79.4% within a span of six hours. Investigation into the photodegradation of Fe-Ni-Cr as recovered catalyst indicated that both the original and recovered catalysts retained their effectiveness in degrading methylene blue dye. The influence of varying catalyst amounts on the photodegradation of MB was also examined, revealing that the highest degradation rate of 43.47% was achieved at a catalyst quantity of 0.005 g, with a decline in degradation observed at higher catalyst concentrations, reaching a minimum of 17.39% at 0.1 g of catalyst. Furthermore, the impact of varying dye concentrations on the degradation process was explored, showing that the maximum degradation of 42.53% occurred at a dye concentration of 40 ppm, with degradation decreasing beyond this concentration. The pH level’s effect on the degradation process was investigated as well, and it was determined that an increase in the pH of the medium led to an increase in degradation. Notably, the highest degradation rate of 79.3% was achieved at a specific pH level of 10.0. To further enhance the comprehensiveness of this study, future research endeavors could encompass comparative investigations involving the synthesized TMNPs with monometallic and bimetallic nanoparticles of similar compositions, as well as differing compositions. Such comparative analyses could yield valuable insights.
Author Contributions
Z.A. designed, executed, and supervised the project; R.T., F.B. and N.S. performed the practical work; and N.Z. and H.U. guided the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The study’s data are available from the corresponding author by request.
Acknowledgments
The writers gratefully acknowledge the financial and moral support for this research by the principal Govt: Degree College Khanpur Haripur, Higher Education, Archives and Libraries Department Government of Khyber Pakhtunkhwa, Pakistan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sarsenov, S. Hollow CuO Microparticles for the Efficient Degradation of Model Pollutant Dyes. Master’s Thesis, Nazarbayev University, Astana, Kazakhisthan, 2022. [Google Scholar]
- Nguyen, T.B.; Dong, C.-D.; Huang, C.; Chen, C.-W.; Hsieh, S.-L.; Hsieh, S. Fe-Cu bimetallic catalyst for the degradation of hazardous organic chemicals exemplified by methylene blue in Fenton-like reaction. J. Environ. Chem. Eng. 2020, 8, 104139. [Google Scholar] [CrossRef]
- Gupta, A.; Khosla, N.; Govindasamy, V.; Saini, A.; Annapurna, K.; Dhakate, S. Trimetallic composite nanofibers for antibacterial and photocatalytic dye degradation of mixed dye water. Appl. Nanosci. 2020, 10, 4191–4205. [Google Scholar]
- de Oliveira Guidolin, T.; Possolli, N.M.; Polla, M.B.; Wermuth, T.B.; de Oliveira, T.F.; Eller, S.; Montedo, O.R.K.; Arcaro, S.; Cechinel, M.A.P. photocatalytic pathway on the degradation of methylene blue from aqueous solutions using magnetite nanoparticles. J. Clean. Prod. 2021, 318, 128556. [Google Scholar]
- Zhang, L. Photocatalysts with Adsorption Property for Dye-Contaminated Water Purification. Bachlor’s Thesis, The University of Queensland, Brisbane, Australia, 2017. [Google Scholar]
- Nuramdhani, I. Towards Environmentally Benign Wastewater Treatment-Photocatalytic Study of Degradation of Industrial Dyes. Master’s Thesis, University of Canterbury, Christchurch, New Zealand, 2011. [Google Scholar]
- Mohamed, R.; Mkhalid, I.; Baeissa, E.; Al-Rayyani, M. Photocatalytic degradation of methylene blue by Fe/ZnO/SiO2 nanoparticles under visiblelight. J. Nanotechnol. 2012, 2012, 329082. [Google Scholar]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Jarvin, M.; Inbanathan, S.; Umar, A.; Lalla, N.; Dzade, N.Y.; Algadi, H.; Rahman, Q.I.; Baskoutas, S. Facile green synthesis of magnesium oxide nanoparticles using tea (Camellia sinensis) extract for efficient photocatalytic degradation of methylene blue dye. Environ. Technol. Innov. 2022, 28, 102746. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.; Idriss, H.; Nadeem, M. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Kayabaşı, Y.; Erbaş, O. Methylene blue and its importance in medicine. Demiroglu Sci. Univ. Florence Nightingale J. Med. 2020, 6, 136–145. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies-a critical review. J. Clean. Prod. 2021, 298, 126567. [Google Scholar]
- Yu, H.; Zhu, J.; Qiao, R.; Zhao, N.; Zhao, M.; Kong, L. Facile Preparation and Controllable Absorption of a Composite Based on PMo12/Ag Nanoparticles: Photodegradation Activity and Mechanism. Chem. Sel. 2022, 7, e202103668. [Google Scholar] [CrossRef]
- Feng, X.; Wang, B.; Gao, G.; Gao, S.; Xie, C.; Shi, J.-W. MnyCo3−yOx bimetallic oxide prepared by ultrasonic technology for significantly improved catalytic performance in the reduction of NOx with NH3. J. Fuels 2023, 352, 129159. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, A.; Huang, G.; Yang, M.; Li, D.; Teng, M.; Han, D. Enhanced efficiency with CDCA co-adsorption for dye-sensitized solar cells based on metallosalophen complexes. J. Sol. Energy 2020, 209, 316–324. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, L.; Luo, J.; Gong, H.; Zhu, N. A sustainable reuse strategy of converting waste activated sludge into biochar for contaminants removal from water: Modifications, applications and perspectives. J. Hazard. Mater. 2022, 438, 129437. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, L.; Yao, J.; Guo, T.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Enhanced adsorption and reduction performance of nitrate by Fe–Pd–Fe3O4 embedded multi-walled carbon nanotubes. Chemosphere 2021, 281, 130718. [Google Scholar] [CrossRef]
- Yasin, A.; Fatima, U.; Shahid, S.; Mansoor, S.; Inam, H.; Javed, M.; Iqbal, S.; Alrbyawi, H.; Somaily, H.H.; Pashameah, R.A.; et al. Fabrication of Copper Oxide Nanoparticles Using Passiflora edulis Extract for the Estimation of Antioxidant Potential and Photocatalytic Methylene Blue Dye Degradation. Agronomy 2022, 10, 2315. [Google Scholar] [CrossRef]
- Iqbal, S.; Amjad, A.; Jave, M.A.M.; Mushtaq, M.; Rabea, S.; Elkaeed, E.B.; Pashmeah, R.H.; Alzahrani, E.; Farouk, A.-E.A. Boosted spatial charge carrier separation of binary ZnFe2O4/S-g-C3N4 heterojunction for visible-light-driven photocatalytic activity and antimicrobial performance. Front. Chem. 2022, 10, 975355. [Google Scholar] [CrossRef]
- Moosavi, S.; Li, R.Y.M.; Lai, C.W.; Yusof, Y.; Gan, S.; Akbarzadeh, O.; Chowhury, Z.Z.; Yue, X.-G.; Johan, M.R. Methylene blue dye photocatalytic degradation over synthesised Fe3O4/AC/TiO2 nano-catalyst: Degradation and reusability studies. J. Nanomater. 2020, 10, 2360. [Google Scholar] [CrossRef]
- Samsudin, E.M.; Goh, S.N.; Wu, T.Y.; Ling, T.T.; Hamid, S.B.A.; Juan, J.C. Evaluation on the photocatalytic degradation activity of reactive blue 4 using pure anatase nano-TiO2. Sains Malays. 2015, 44, 1011–1019. [Google Scholar] [CrossRef]
- Saeed, K.; Zada, N.; Khan, I.; Sadiq, M. Synthesis, characterization and photodegradation application of Fe-Mn and F-MWCNTs supported Fe-Mn oxides nanoparticles. Desalin. Water Treat. 2018, 108, 362–368. [Google Scholar] [CrossRef]
- Allaedini, G.; Tasirin, S.M.; Aminayi, P. Synthesis of Fe–Ni–Ce trimetallic catalyst nanoparticles via impregnation and co-precipitation and their application to dye degradation. Chem. Pap. 2016, 70, 231–242. [Google Scholar] [CrossRef]
- Crawley, J.W.; Gow, I.E.; Lawes, N.; Kowalec, I.; Kabalan, L.; Catlow, C.R.A.; Logsdail, A.J.; Taylor, S.H.; Dummer, N.F.; Hutchings, G.J. Heterogeneous trimetallic nanoparticles as catalysts. Chem. Rev. 2022, 122, 6795–6849. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kumar, D.; Kumar, A.; Al-Muhtaseb, A.H.; Pathania, D.; Naushad, M.; Mola, G.T. Revolution from monometallic to trimetallic nanoparticle composites, various synthesis methods and their applications: A review. Mater. Sci. Eng. C 2017, 71, 1216–1230. [Google Scholar] [CrossRef]
- Cai, X.-L.; Liu, C.-H.; Liu, J.; Lu, Y.; Zhong, Y.-N.; Nie, K.-Q.; Xu, J.-L.; Gao, X.; Sun, X.-H.; Wang, S.-D. Synergistic effects in CNTs-PdAu/Pt trimetallic nanoparticles with high electrocatalytic activity and stability. Nano-Micro Lett. 2017, 9, 48. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Malik, M.A. Facile one-pot biogenic synthesis of Cu-Co-Ni trimetallic nanoparticles for enhanced photocatalytic dye degradation. Catalyst 2020, 10, 1138. [Google Scholar] [CrossRef]
- Paredes, P.; Rauwel, E.; Wragg, D.S.; Rapenne, L.; Estephan, E.; Volobujeva, O.; Rauwel, P. Sunlight-Driven Photocatalytic Degradation of Methylene Blue with Facile One-Step Synthesized Cu-Cu2O-Cu3N Nanoparticle Mixtures. J. Nanomater. 2023, 13, 1311. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Jatrana, I.; Khan, A.U.; Khan, A.A.; Satiya, H.; Khan, M.; Moon, I.S.; Alam, H. Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study. Nanotechnol. Rev. 2021, 10, 1912–1925. [Google Scholar] [CrossRef]
- Soltani, T.; Entezari, M.H. Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation. J. Mol. Catal. A Chem. 2013, 377, 197–203. [Google Scholar] [CrossRef]
- Alomar, T.S.; Al Masoud, N.; Sharma, G.; Al Othman, Z.A.; Naushad, M. Incorporation of trimetallic nanoparticles to the SiO2 matrix for the removal of methylene blue dye from aqueous medium. J. Mol. Liq. 2021, 336, 116274. [Google Scholar] [CrossRef]
- Althomali, R.H.; Adeosun, W.A. Wet chemically synthesized metal oxides nanoparticles, characterization and application in electrochemical energy storage: An updated review. Synth. Met. 2023, 298, 117424. [Google Scholar] [CrossRef]
- Mishra, S.; Chakinala, N.; Chakinala, A.G.; Surolia, P.K. Photocatalytic degradation of methylene blue using monometallic and bimetallic Bi-Fe doped TiO2. Catal. Commun. 2022, 171, 106518. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Jadhav, P.; Khalid, Z.B.; Krishnan, S.; Bhuyar, P.; Zularisam, A.; Razak, A.S.A.; Nasrullah, M. Application of iron-cobalt-copper (Fe-Co–Cu) trimetallic nanoparticles on anaerobic digestion (AD) for biogas production. Biomass Convers. Biorefin. 2022, 1–11. [Google Scholar] [CrossRef]
- Hwang, S.; Umar, A.; Dar, G.; Kim, S.; Badran, R. Synthesis and characterization of iron oxide nanoparticles for phenyl hydrazine sensor applications. Sensor Lett. 2014, 12, 97–101. [Google Scholar] [CrossRef]
- Sharma, A.K.; Desnavi, S.; Dixit, C.; Varshney, U.; Sharma, A. Extraction of nickel nanoparticles from electroplating waste and their application in production of bio-diesel from biowaste. Int. J. Chem. Eng. Appl. 2015, 6, 156. [Google Scholar] [CrossRef]
- Jaswal, V.S.; Arora, A.K.; Kinger, M.; Gupta, V.; Singh, J. Synthesis and characterization of chromium oxide nanoparticles. Orient J. Chem. 2014, 30, 559–566. [Google Scholar] [CrossRef]
- Arun, K.; Kumar, K.S.; Batra, A.; Aggarwal, M.; Francis, P. Surfactant free hydrothermal synthesis of CdO nanostructure and its characterization. Adv. Sci. Eng. Med. 2015, 7, 771–775. [Google Scholar] [CrossRef]
- Elango, M.; Deepa, M.; Subramanian, R.; Musthafa, A.M. Synthesis, Characterization, and Antibacterial Activity of Polyindole/Ag–Cuo Nanocomposites by Reflux Condensation Method. Polym. Plast. Technol. Eng. 2018, 57, 1440–1451. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Guo, X.; Chen, Z.; Zhang, W.; Wang, Y.; Tang, X.; Zhang, Y.; Zhao, Y. Sulfur-doped g-C3N4/rGO porous nanosheets for highly efficient photocatalytic degradation of refractory contaminants. J. Mater. Sci. Technol. 2020, 41, 117–126. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, L.; Zhao, M.; Dai, L.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Bamboo charcoal fused with polyurethane foam for efciently removing organic solvents from wastewater: Experimental and simulation. Biochar 2022, 4, 28. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.S.W.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Neppolian, B.; Choi, H.; Sakthivel, S.; Arabindoo, B.; Murugesan, V. Solar/UV-induced photocatalytic degradation of three commercial textile dyes. J. Hazard. Mater. 2002, 89, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Giva, A.; Nkeonye, P.O.; Bello, K.A.; Kolawole, K.A. Photocatalytic decolourization and degradation of CI Basic Blue 41 using TiO2 nanoparticles. J. Environ. Prot. 2012, 2012, 22707. [Google Scholar]
- Saeed, K.; Khan, I.; Park, S.-Y. TiO2/amidoxime-modified polyacrylonitrile nanofibers and its application for the photodegradation of methyl blue in aqueous medium. Desalin. Water Treat. 2015, 54, 3146–3151. [Google Scholar] [CrossRef]
- Ruifen, W.; Fuming, W.; Shengli, A.; Jinling, S.; Zhang, Y. Y/Eu co-doped TiO2: Synthesis and photocatalytic activities under UV-light. J. Rare Earths 2015, 33, 154–159. [Google Scholar]
- Tan, H.; Zhang, Y.; Li, B.; Yang, H.; Hou, H.; Huang, Q. Preparation of TiO2-coated glass flat membrane and its photocatalytic degradation of methylene blue. Ceram. Int. 2023, 49, 17236–17244. [Google Scholar] [CrossRef]
- Alenad, A.M.; Waheed, M.S.; Aman, S.; Ahmad, N.; Khan, A.R.; Khosa, R.Y.; Ansari, M.Z.; Khan, S.A.; Farid, H.M.T.; Taha, T.A. Met. Visible light driven Ni doped hematite for photocatalytic reduction of noxious methylene blue. Mater. Res. Bull. 2023, 165, 112306. [Google Scholar] [CrossRef]
- Ma, S.; Shi, Y.; Xia, X.; Song, Q.; Yang, J. Cerium-cobalt bimetallic metal–organic frameworks with the mixed ligands for photocatalytic degradation of methylene blue. Inorg. Chem. Commun. 2023, 152, 110664. [Google Scholar] [CrossRef]
- Ata, S.; Shaheen, I.; Aslam, H.; Mohsin, I.U.; Alwadai, N.; Al Huwayz, M.; Iqbal, M.; Youna, U. Barium and strontium doped La-based perovskite synthesis via sol-gel route and photocatalytic activity evaluation for methylene blue. Results Phys 2023, 45, 106235. [Google Scholar] [CrossRef]
- Bhapkar, A.; Prasad, R.; Jaspal, D.; Shirolkar, M.; Gheisari, K.; Bhame, S. Visible light driven photocatalytic degradation of methylene blue by ZnO nanostructures synthesized by glycine nitrate auto combustion route. Inorg. Chem. Commun. 2023, 148, 110311. [Google Scholar] [CrossRef]
- Zaid, E.H.A.; Sin, J.-C.; Lam, S.-M.; Mohamed, A.R. Fabrication of La, Ce co-doped ZnO nanorods for improving photodegradation of methylene blue. J. Rare Earths 2023. [Google Scholar] [CrossRef]
- Husna, R.A.; Natsir, T.A. Enhancing photocatalytic degradation of methylene blue by mixed oxides TiO2/SnO2/CeO2 under visible light. Results Eng. 2023, 19, 101253. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).