Characteristics of Dissolution Changes in Carbonate Rocks and Their Influencing Factors in the Maocun Basin, Guilin, China
Abstract
:1. Introduction
2. Overview of the Study Area
3. Research Methods
4. Results and Analysis
4.1. Characteristics of Physico-Chemical Changes in the Water Column at the Study Site
4.2. Carbonate Dissolution Rates at Typical Study Sites
4.3. Factors Influencing the Rate of Dissolution of Carbonate Rocks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, D.X.; Zhang, C. Theoretical exploration and practice of karst dynamics. Acta Geosci. Sin. 2008, 29, 355–365. [Google Scholar]
- Liu, Z.H. Recent advances and perspectives in the study of weathering carbon sinks in rocks. Chin. Sci. Bull. 2012, 57, 95–102. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dreybrodt, W.; Liu, H. Atmospheric CO2 sink: Silicate weathering or carbonate weathering. Appl. Geochem. 2011, 26, S292–S294. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, Q.; Wu, Z.Y.; Knez, M. Ecosystem-driven karst carbon cycle and carbon sink effects. J. Groundw. Sci. Eng. 2022, 10, 99–112. [Google Scholar]
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, N.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; et al. The global carbon cycle: A test of our knowledge of earth as a system. Science 2000, 290, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Gaillardet, J.; Calmels, D.; Romero Mujalli, G.; Zakharova, E.; Hartmann, J. Global climate control on carbonate weathering intensity. Chem. Geol. 2019, 527, 118762. [Google Scholar] [CrossRef]
- Zeng, J.; Yue, F.J.; Xiao, M.; Wang, Z.; Qin, C. Dissolved organiccarbon in rainwater from a karst agricultural area of Southwest China: Variations, sources, and wet deposition fluxes. Atmos. Res. 2020, 245, 105140. [Google Scholar] [CrossRef]
- Zhao, M.; Zeng, C.; Liu, Z.H.; Wang, S.J. Effect of different land use/land cover on karst hydrogeochemistry: A paired catchment study of Chenqi and Dengzhanhe, Puding, Guizhou, SW China. J. Hydrol. 2010, 388, 121–130. [Google Scholar] [CrossRef]
- Yang, R.; Chen, B.; Liu, H.; Liu, Z.; Yan, H. Carbon sequestration and decreased CO2 emission caused by terrestrial aquatic photosynthesis: Insights from diel hydrochemical variations in an epikarst spring and two spring-fed ponds in different seasons. Appl. Geochem. 2015, 63, 248–260. [Google Scholar] [CrossRef]
- Liu, Z.; Dreybrodt, W. Significance of the carbon sink produced by H2O–carbonate–CO2–aquatic phototroph interaction on land. Sci. Bull. 2015, 60, 182–191. [Google Scholar] [CrossRef]
- Zhang, Z.; Lian, B.; Hou, W.; Chen, M.; Xin, L.; Yan, L. Bacillus mucilaginosus can capture atmospheric CO2 by carbonic anhydrase. Afr. J. Microbiol. Res. 2011, 5, 106–112. [Google Scholar]
- Liu, Z.; Macpherson, G.; Groves, C.; Martini, J.B.; Yuan, D.; Zeng, S. Large and Active CO2 Uptake by Coupled Carbonate Weathering. Earth-Sic. Rev. 2018, 182, 42–49. [Google Scholar] [CrossRef]
- Li, N.Y.; Feng, Y.S.; Liu, P.L.; Luo, Z.F.; Zhao, L.Q. Study of Acid–Rock Reaction Kinetics Under High Temperature and Pressure Conditions Based on the Rotating Disk Instrument. Arab. J. Sci. Eng. 2015, 40, 135–142. [Google Scholar] [CrossRef]
- Li, Q.; Yi, X.Y.; Li, G.S.; Chen, W.L. The law of the hydrogen ion diffusion coefficient in acid rock reaction. J. Pet. Sci. Eng. 2016, 146, 694–701. [Google Scholar] [CrossRef]
- Hyunsang, Y.; Youngmin, K.; Wonsuk, L.; Jeonghwan, L. An experimental study on acid–rock reaction kinetics using dolomite in carbonate acidizing. J. Pet. Sci. Eng. 2018, 168, 478–494. [Google Scholar]
- Larson, E.B.; Emmons, R.V. Dissolution of Carbonate Rocks in a Laboratory Setting: Rates and Textures. Minerals 2021, 11, 605. [Google Scholar] [CrossRef]
- Plummer, L.N.; Wigley, T.M.L.; Parkhurst, D.L. The kinetics of calcite dissolution in CO2–water systems at 5 degrees to 60 degrees C and 0.0 to 1.0 atm CO2. Am. J. Sci. 1978, 278, 179–216. [Google Scholar] [CrossRef]
- Yu, S.; Yan, Y.P.; Zhang, C.L.; Wang, X.Y.; Liu, Q.; Li, Y.L. Experimental study on carbonate dissolution rate influence by acid rain. J. Guilin Univ. Technol. 2011, 31, 539–544. [Google Scholar]
- Shao, D.M. Effect of temperature on the dissolution rate of Ordovician carbonates at different water flow rates. Coalf. Geol. Explor. 2012, 40, 62–65. [Google Scholar]
- Zeng, S.B.; Liu, Z.H.; Goldscheider, N.; Frank, S.; Goeppert, N.; Kaufmann, G.; Zeng, C.; Zeng, Q.R.; Sun, H.L. Compari–sons on the effects of temperature, runoff, and land–cover on carbonate weathering in different karstcatchments: Insights into the future global carbon cycle. Hydrogeol. J. 2021, 29, 331–345. [Google Scholar] [CrossRef]
- Luo, M.M.; Zhou, H.; Liang, Y.P.; Chen, Z.H.; Chen, R.B.; Li, X.L.; Jakada, H. Horizontal and vertical zoning of carbonate dissolution in China. Geomorphology 2018, 322, 66–75. [Google Scholar] [CrossRef]
- Zeng, Q.R.; Liu, Z.H.; Chen, B.; Hu, Y.D.; Zeng, S.B.; Zeng, C.; Yang, R.; He, H.B.; Zhu, H.; Cai, X.L.; et al. Car–bonate weathering–related carbon sink fluxes under different land uses: A case study from the Shawan Simulation TestSite, Puding, Southwest China. Chem. Geol. 2017, 474, 58–71. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Q.; Jia, Y.N. The influence of meteorological factors and soil physicochemical properties on Karst processes in six land-use patterns in summer and winter in a typical Karst valley. Acta Ecol. Sin. 2014, 34, 1418–1428. [Google Scholar]
- Zeng, S.B.; Liu, Z.H.; Kaufmann, G. Sensitivity of the global carbonate weathering carbon–sink flux to climate and land–use changes. Nat. Commun. 2019, 10, 5749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Carbonate rock dissolution rates indifferent landuses and their carbon sink effect. Sci. Bull. 2011, 56, 3759–3765. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.Z.; Ju, X.T.; Chen, Z.J.; Zhou, J.B.; Kuzyakov, Y. Dramatic loss of inorganic carbon by nitro–gen–induced soil acidification in Chinese croplands. Glob. Chang. Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Abril, G.; Etcheber, H.; Delille, B.; Frankignoulle, M.; Borges, A.V. Carbonate dissolution in the turbid and eutrophic Loire estuary. Mar. Ecol. Prog. Ser. 2003, 259, 129–138. [Google Scholar] [CrossRef]
- Plan, L. Factors controlling carbonate dissolution rates quantified in a field test in the Austrian alps. Geomorphology 2005, 68, 201–212. [Google Scholar] [CrossRef]
- Li, Q.; Song, A.; Peng, W.J.; Jin, Z.J.; Müller Werner, E.G.; Wang, X.H. Contribution of aerobic anoxygenic phototrophic bacteria to total organic carbon pool in aquatic system of subtropical karst catchments, Southwest China: Evidence from hydrochemical and microbiological study. Resour. FEMS Microbiol. Ecol. 2017, 6, fix065. [Google Scholar] [CrossRef]
- Liu, Z.H. “Method of maximum potential dissolution” to calculate the intensity of karst process and the relevant carbon sink: With discussions on methods of maximum potential dissolution process and the relevant carbon sink: With discussions on methods of solute load and carbonate-rock-tablet test. Garsologica Sin. 2011, 30, 379–382. [Google Scholar]
- Liu, Z.H.; Groves, C.; Yuan, D.X.; Meiman, J.; Jiang, G.H.; He, S.Y. Study on the hydrochemical variations caused by the water-rock-gas interaction-an example from the Guilin Karst Experimental Site. Hydrogeol. Eng. Geol. 2003, 30, 13–18. [Google Scholar]
- Chen, R.B.; Luo, M.M.; Luo, Z.H.; Chen, Z.H.; Zhou, H. Response relationship between chemical composition and dissolution rate of carbonate rocks in the Gorges Area. Garsologica Sin. 2019, 38, 258–264. [Google Scholar]
- Liu, Z.H.; Dreybrodt, W.; Li, H.J. Comparison of dissolution rate–determinig mechanisms in between limestone and dolomite. Earth Sci. J. China Univ. Geosci. 2006, 31, 411–416. [Google Scholar]
- Wu, J.Q.; Gu, C.S.; Xu, S.G.; Zhao, X.F.; Huang, G.M. Corrosion analysis of carbonate rocks in southern Jiangsu Province. Garsologica Sin. 2021, 40, 565–571. [Google Scholar]
- Tang, W.; Cao, J.H.; Yang, H.; Wang, H.; Tu, L.L.; Ying, Q.H. Research on carbonate rock corrosion rate by allogenic water as exemplified by the Maocun subterranean river in Guilin. Earth Environ. 2014, 42, 207–212. [Google Scholar]
- Huang, F.; Tang, W.; Wang, J.L.; Cao, J.H.; Yin, J.J. The influence of allogenic water on karst carbon sink: A case study in the Maocun Subterranean River in Guilin, Guilin. Carsologica Sin. 2011, 30, 417–421. [Google Scholar]
- Fu, Y.C.; Lang, Y.C.; Wang, Z.J.; Li, S.L.; Ding, H. Diurnal dynamics and constraints of soil CO2 concentration in a limestone site during summer. Chin. J. Ecol. 2018, 37, 3315–3322. [Google Scholar]
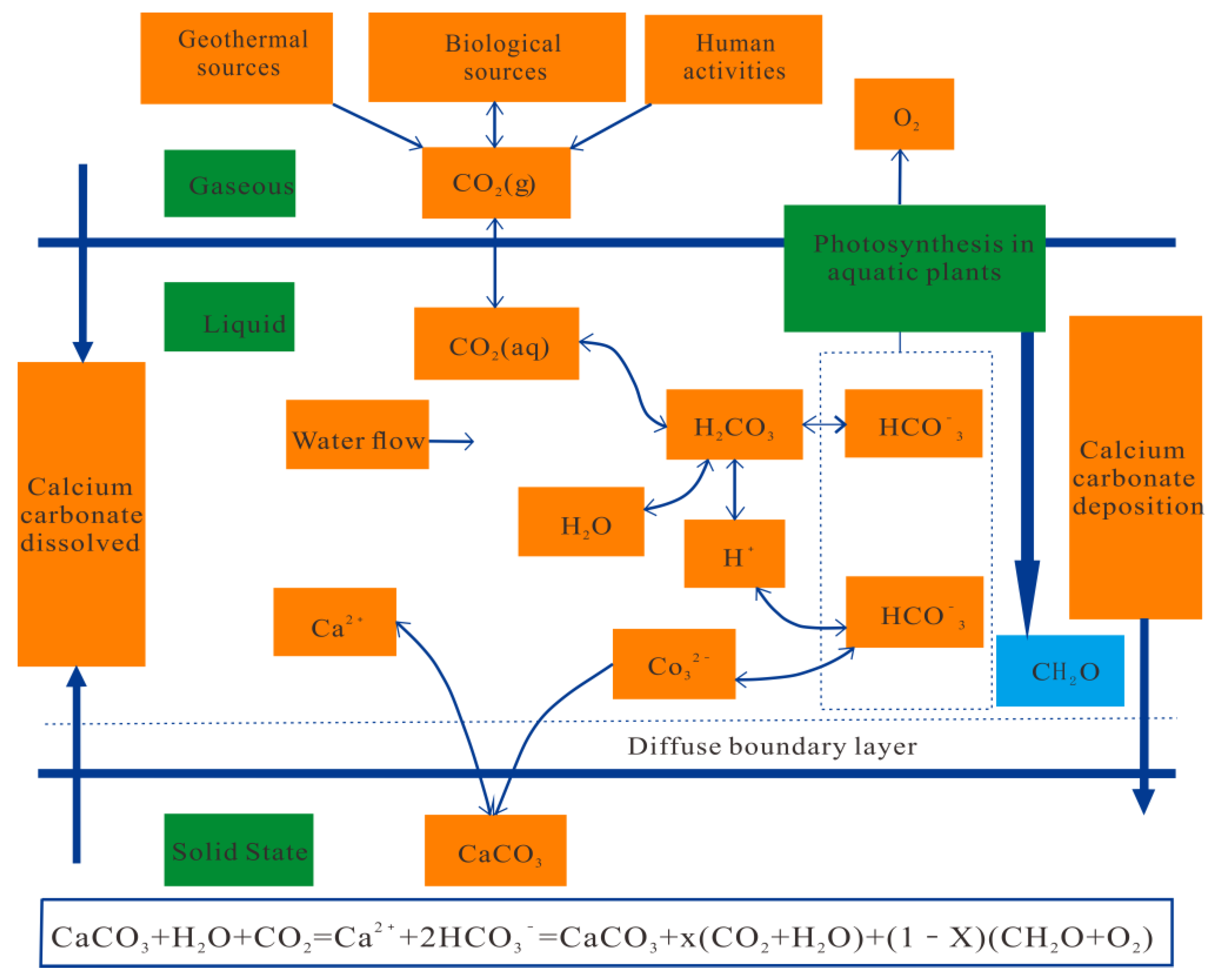





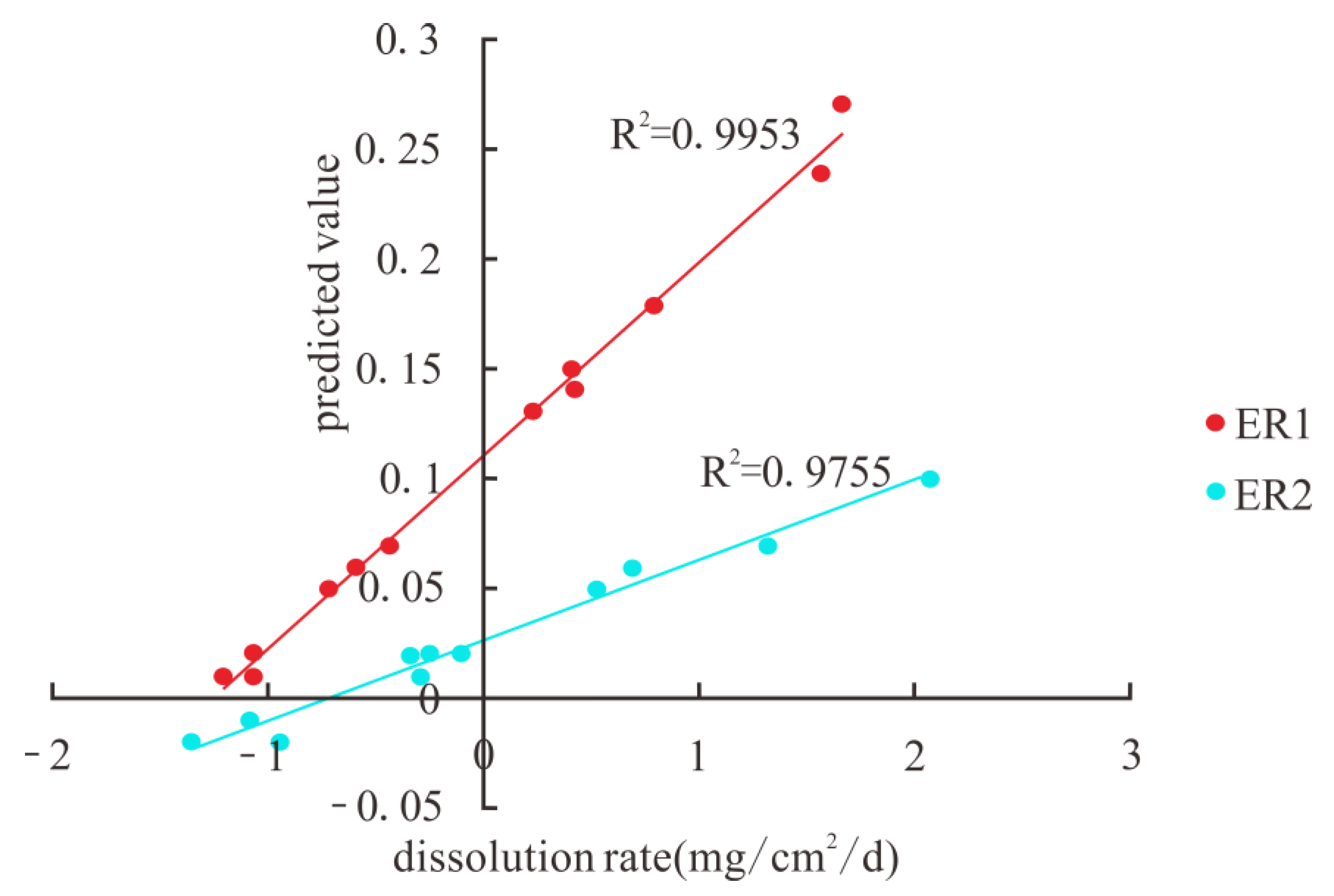

| Rock Samples | SiO2 | Al2O3 | Fe2O3 | K2O | CaO | MgO | P2O5 | Loss on Ignition | Acid Insoluble Matter | CO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | ||||||||||
| Dolomite | 0.012 | 0.001 | 0.021 | 0.01 | 30.74 | 21.05 | 0.004 | 46.76 | 0.45 | 48.82 |
| Limestone | 0.02 | 0.024 | 0.071 | 0.006 | 55.52 | 0.42 | 0.003 | 43.48 | 0.35 | 45.41 |
| Rate of Dissolutionmg/cm2/d | Spring | Summer | Autumn | Winter | ||||
|---|---|---|---|---|---|---|---|---|
| Limestone | Dolomite | Limestone | Dolomite | Limestone | Dolomite | Limestone | Dolomite | |
| XLB | 0.146 | 0.018 | 0.258 | 0.103 | 0.180 | 0.075 | 0.131 | 0.017 |
| BY | 0.071 | 0.016 | 0.249 | 0.046 | 0.147 | 0.052 | 0.057 | 0.023 |
| BDP | 0.015 | −0.008 | 0.046 | 0.014 | 0.015 | −0.023 | 0.002 | −0.013 |
| T | pH | EC | Ca2+ | HCO3− | K+ | Na+ | Mg2+ | Cl− | SO42− | SIc | pCO2 | Q | ER1 | ER2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | 1 | ||||||||||||||
| pH | 0.006 | 1 | |||||||||||||
| EC | 0.103 | 0.866 ** | 1 | ||||||||||||
| Ca2+ | 0.109 | 0.895 ** | 0.990 ** | 1 | |||||||||||
| HCO3− | 0.126 | 0.892 ** | 0.995 ** | 0.996 ** | 1 | ||||||||||
| K+ | −0.073 | 0.3 | 0.486 | 0.473 | 0.466 | 1 | |||||||||
| Na+ | 0.116 | 0.794 ** | 0.929 ** | 0.939 ** | 0.924 ** | 0.571 | 1 | ||||||||
| Mg2+ | 0.169 | 0.880 ** | 0.983 ** | 0.979 ** | 0.987 ** | 0.389 | 0.918 ** | 1 | |||||||
| Cl− | −0.591 * | −0.056 | −0.265 | −0.231 | −0.265 | −0.181 | −0.099 | −0.264 | 1 | ||||||
| SO42− | 0.379 | 0.327 | 0.562 | 0.536 | 0.546 | 0.367 | 0.610 * | 0.567 | 0.023 | 1 | |||||
| SIc | 0.099 | 0.874 ** | 0.964 ** | 0.941 ** | 0.964 ** | 0.453 | 0.836 ** | 0.959 ** | −0.369 | 0.452 | 1 | ||||
| pCO2 | −0.107 | −0.846 ** | −0.911 ** | −0.910 ** | −0.922 ** | −0.519 | −0.845 ** | −0.914 ** | 0.249 | −0.543 | −0.902 ** | 1 | |||
| Q | 0.505 | −0.775 ** | −0.610 * | −0.655 * | −0.628 * | −0.47 | −0.596 * | −0.555 | −0.277 | −0.063 | −0.593 * | 0.567 | 1 | ||
| ER1 | 0.349 | −0.865 ** | −0.735 ** | −0.765 ** | −0.746 ** | −0.563 | −0.719 ** | −0.687 * | −0.176 | −0.254 | −0.722 ** | 0.743 ** | 0.953 ** | 1 | |
| ER2 | 0.417 | −0.750 ** | −0.676 * | −0.701 * | −0.701 * | −0.557 | −0.681 * | −0.652 * | −0.224 | −0.228 | −0.672 * | 0.713 ** | 0.883 ** | 0.874 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, C.; Xin, S.; Huang, F.; Cao, J.; Xiao, J. Characteristics of Dissolution Changes in Carbonate Rocks and Their Influencing Factors in the Maocun Basin, Guilin, China. Water 2023, 15, 3285. https://doi.org/10.3390/w15183285
Mo C, Xin S, Huang F, Cao J, Xiao J. Characteristics of Dissolution Changes in Carbonate Rocks and Their Influencing Factors in the Maocun Basin, Guilin, China. Water. 2023; 15(18):3285. https://doi.org/10.3390/w15183285
Chicago/Turabian StyleMo, Chunmeng, Shenglin Xin, Fen Huang, Jianhua Cao, and Junbo Xiao. 2023. "Characteristics of Dissolution Changes in Carbonate Rocks and Their Influencing Factors in the Maocun Basin, Guilin, China" Water 15, no. 18: 3285. https://doi.org/10.3390/w15183285
APA StyleMo, C., Xin, S., Huang, F., Cao, J., & Xiao, J. (2023). Characteristics of Dissolution Changes in Carbonate Rocks and Their Influencing Factors in the Maocun Basin, Guilin, China. Water, 15(18), 3285. https://doi.org/10.3390/w15183285





