Abstract
Karstification and the global carbon cycle are closely related. Understanding the features of dissolution variations in carbonate rocks and the variables influencing carbonate dissolution is crucial for producing reliable estimates of karst carbon sinks. The seasonal variations in carbonate dissolution rates and the primary factors affecting carbonate dissolution in the Maocun watershed, Guilin, are examined under external source water (Xiaolongbei), karst water (Beidiping), and the mixed external source water and karst water (Bianyan) conditions. In this work, the characteristics of carbonate dissolution rates in several water bodies are investigated using field sampling and indoor experimental measurements. A correlation analysis is performed to analyze the key environmental factors impacting carbonate dissolution. The findings demonstrate that there is a clear seasonal and regional variation in the rate of the dissolution of carbonate rocks. The seasonal characteristics of the carbonate dissolution rate are summer > autumn > spring > winter. The carbonate dissolution rate ranges from −0.023 to 0.258 mg/cm2/d, with a mean value of 0.068 mg/cm2/d. The variation in carbonate dissolution rates on a spatial scale is characterized by exogenous water (Xiaolongbei) > exogenous water mixed with karst water (Bianyan) > karst water (Beidiping). As the rate of carbonate erosion in the Maocun basin is influenced by many factors, the correlation analysis shows that the main controlling factors for the rate of carbonate erosion in the Maocun basin are flow, the saturation index, pH, and pCO2. Seasonal variations in carbonate dissolution rates are mainly influenced by pCO2 and the flow rate, and the spatial variations are mainly influenced by pH and the saturation index. The results of this study are important for the scientific assessment of karst development in the study area and the accurate estimation of karst carbon sinks.
1. Introduction
The Global Greenhouse Gas Bulletin 2021, published by the World Meteorological Organization (WMO), states that global CO2 concentrations continue to rise. In response to severe climate change, the investigation of carbon cycle mechanisms, processes, and karst carbon sinks has become one of the hotspots of research. The conceptual model of the karst dynamics system was first proposed by Daoxian Yuan, and the research field of the atmospheric CO2 source sink began to involve the geological process of the karst carbon cycle [1]. Since then, the carbonate weathering carbon sink model of the water–rock–gas–bio project (see Figure 1) has been proposed [2]. Rivers can transport dissolved inorganic carbon from karst to the ocean, where it is fixed by marine creatures, seawater, terrestrial water organisms, or soil organisms to create soil organic carbon, creating a carbon sink effect [3]. Karst processes contributed 1.1–6.08 × 108 t C/a to the global carbon sink, which makes up 15–30% of the missing sink [4]. This shows that karst plays an important role in the carbon sink effect. Carbon is distributed in the atmosphere, hydrosphere, soil, biosphere, and lithosphere, of which the lithosphere is the largest carbon reservoir [5]. Rock weathering continuously consumes CO2 from the atmosphere and the soil, while the water cycle continuously transports the weathering byproducts to the ocean. Climate, soil/atmospheric CO2, aquatic plants, and microbes all have an impact on the karst process. Karst may significantly shift and be impacted as a result of climate change [6]. While temperature indirectly affects karst by increasing the dissolution of CO2, rainfall can enhance the surface water flow, which increases the solubility of CO2 in soils and promotes the transit of inorganic carbon and karst [7]. Soil CO2 is directly involved in carbonate lithification, and the rate of subsoil lithification follows the same trend as the soil CO2 concentration [8]. During photosynthesis, aquatic plants can transform unstable inorganic carbon into stable organic carbon, a mechanism that can promote karstogenesis [9,10]. The involvement of microorganisms in karst is evident not only in their direct impact on the dissolution of carbonates but also in their use of inorganic carbon to produce organic carbon during photosynthesis [11,12].
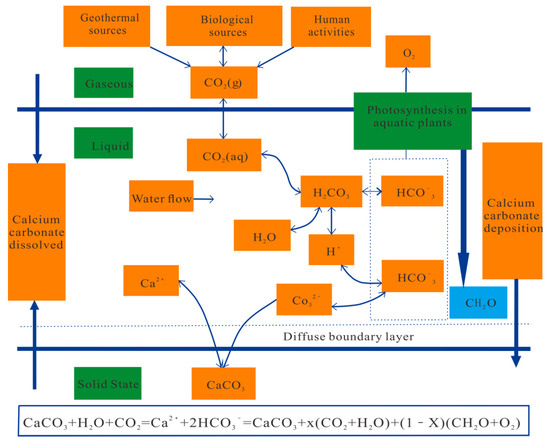
Figure 1.
A diagram of the water–rock–gas–bio interaction weathering carbon sink model for carbonate rocks [2].
Karst has received more and more attention from international scholars, and some research results have been achieved on carbonate rock dissolution experiments, which are mainly focused on indoor simulation experiments. For example, Li, N.Y. et al. [13], Li, Q. et al. [14], and Hyunsang et al. [15] simulated the dissolution reaction indoors by using a rock disk rotating and moving relative to the solution; Erik B. Larson et al. [16], in an indoor study of carbonate dissolution rates, found that the petrography and mineralogy of carbonate rocks affect their dissolution rates in a laboratory setting; Plummer et al. [17] found large differences in calcite dissolution rates and their mechanisms of change under different pCO2 conditions by studying the kinetic mechanisms of calcite dissolution indoors; Yu, S. et al. [18] showed that the dissolution rate of carbonate rocks was affected by rainwater acidity and rainfall via an experimental indoor dissolution study of carbonate rocks by acid rain in Guilin, China. In addition, Shao, D.M. [19] showed that the effect of temperature on the dissolution of carbonate rocks under different water flow conditions varies widely, and when the water flow rate is low, the effect of temperature on the dissolution of carbonate rocks is small, while when the water flow is large, the effect of temperature on the dissolution of carbonate rocks is large. Of course, some researchers have carried out outdoor experimental research on the dissolution of carbonate rocks in karst areas, and the research mainly focuses on the association between the dissolution of carbonate rocks and the type of vegetation cover [20,21], land-use modes [22,23], climate [24], and the carbon sink effect, etc. [25], as well as the influence of soil environmental factors on the dissolution of carbonate rocks under different land-use modes [26]. Researchers have made a number of achievements in carbonate rock dissolution experiments [27,28]. However, there are relatively few studies on the dynamic characteristics of spatial and seasonal dissolution volumes for carbonate rocks in the same watershed. Therefore, it is of great scientific significance to examine the impact of various factors on carbonate dissolution and quantify the dissolution rate of carbonate rocks in the Maocun Basin under the conditions of mixing three different exogenous water sources and karst water at three different sites in the Maocun Basin.
In this study, we investigate the seasonal dissolution characteristics of carbonate rocks in the Maocun basin via the dissolution test piece method combined with environmental parameters of the water body. It aims to reveal (1) the change rule of the seasonal dissolution rate of carbonate rocks in the Maocun basin; and (2) systematically analyze the main controlling factors affecting the dissolution rate of carbonate rocks in the Maocun basin.
2. Overview of the Study Area
The Guilin Maocun basin is located in Chaotian Township, Lingchuan County, Guilin City, and the Guangxi Zhuang Autonomous Region. The basin belongs to the subtropical humid monsoon climate with an average annual temperature of 19 °C, and abundant precipitation with an annual precipitation of about 1949.5 mm, of which 60–80% of the precipitation occurs from April to July each year, where the least rainfall occurs from December of the year to January of the following year. The rainfall is unevenly distributed throughout the year. The strata exposed in the basin are the Upper Palaeozoic Lower Middle Devonian (D21), which is composed of mixed-colored quartz sandstone and siltstone interbedded with shale; the Donggangling Formation (D2d), which is composed of gray and dark gray laminated porestone mud crystal limestone interbedded with dolomite and gray and black brecciated dolomite; the Upper Devonian Rongxian Formation (D3r), which is composed of light gray and gray-white thickly bedded massive mud leucogranular oolitic sandstone limestone and the Quaternary sediments (Q). The Rongxian Formation (D3r) limestone and the Donggangling Formation (D2d) dolomitic limestone are the principal rock formations in the basin that contain water.
The Maocun underground river system has both endogenous and exogenous recharge. The endogenous recharge is the precipitation from karst areas and the exogenous recharge has two sources: one is the surface river water of Xiaolongbei that flows through the ground for some distance into an underground pipe in the karst area and finally joins at Bianyan, and the other is the water of the Maodaojiang River that flows through an underground pipe in the karst area and joins at Shegengyan. These two exogenous water streams come from Beidiping karst water in the vicinity of the dolomites, merge at the foot of Zhangshan, travel through Dayanqian, and finally discharge at the outlet of the Maocun underground river.
Three typical study locations were chosen, including Xiaolongbei (XLB), where external source water flows through; Bianyan (BY), where karst water and external source water mix; and Beidiping (BDP), a karst spring site recharged by dolomite. Figure 2 shows the exact study locations.
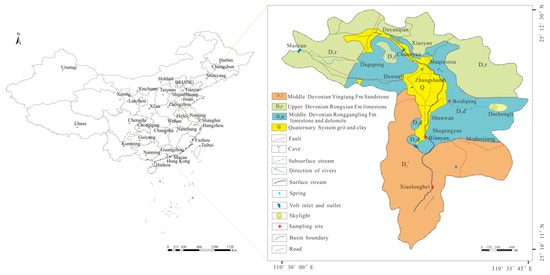
Figure 2.
Schematic diagram of the sampling point locations [29].
3. Research Methods
In order to understand the dissolution of carbonate rocks in the study area, a standard circular specimen with a diameter of (40 ± 2) mm and a thickness of (4 ± 0.4) mm [30] was processed into a core from Maocun, Chaotian Township, Lingchuan County, Guilin City, as an experimental specimen. Carbonate rock specimens were tested and analyzed by China Nonferrous Metals (Guilin) Geology And Mining Co., Ltd., and the determination of SiO2 content was performed via the animal glue coagulation weight method, and the ignition temperature of this method was 1000 °C; the content of Al2O3, CaO, and MgO was determined by taking the filtrate after separating silica; the content of P2O5 was determined via the phosphorus-vanadium-molybdenum yellow photometric method; and the content of K2O was determined via the flame photometric method. The caustic asbestos absorption gravimetric method was used for the determination of carbon content in carbonate. The contents of the main compounds in the carbonate rock specimens are shown in Table 1.

Table 1.
The content of major compounds in carbonate specimens (wt%).
Prior to the experiment, the carbonate specimens were cleaned with distilled water, dried at 105 °C for 10 h, and then numbered and weighed to record the pre-dissolution weights of the samples. The carbonate specimens were placed between 13 October 2017 and 15 October 2018. A total of 76 specimens were placed in the water column at Xiaolongbei, Bianyan, and Beidiping, with 12 limestone specimens and 13 dolomite specimens placed at Xiaolongbei; 13 limestone specimens and 13 dolomite specimens placed at Beidiping; and 12 limestone specimens and 13 dolomite specimens placed at Bianyan. The following equation can be used to determine the rate of carbonate rock dissolution using the specimen’s weight before and after dissolution, along with its surface area [21]:
where ER is the amount of dissolution per unit area mg/cm2/d; W1 is the weight of the rock specimen before dissolution (g); W2 is the weight of the rock specimen after dissolution (g); T is the dissolution time (d); and S is the surface area of the rock specimen (cm2).
ER = (W1 − W2) × 1000/T/S
This experiment was sampled over a hydrological year, and the study sites were sampled and tested monthly. Water temperature, pH, and conductivity were measured at the study site using a Multi350i portable water chemistry analyzer (WTW, Suburb of Munich, Germany) with an accuracy of 0.1 °C, 0.01 pH, and 1 μS/cm, respectively. Calcium ion kits (Aquanmerck, Darmstadt, Germany) were used to determine Ca2+ and HCO3− at the study sites with an accuracy of 2 mg/L and 0.1 mmol/L, respectively. Two bottles of water samples were collected at each study site for the determination of anions and cations. Cations were measured using a PerkinElmer (Waltham, MA, USA) Optima 2100 ICP-OES spectrometer, and anions were measured using a V-2450 UV and visible spectrophotometer, both with a ±5% measurement error. Within 12 h, all collected water samples were delivered to the lab and chilled at 4 °C. The workflow of the study is shown in Figure 3.
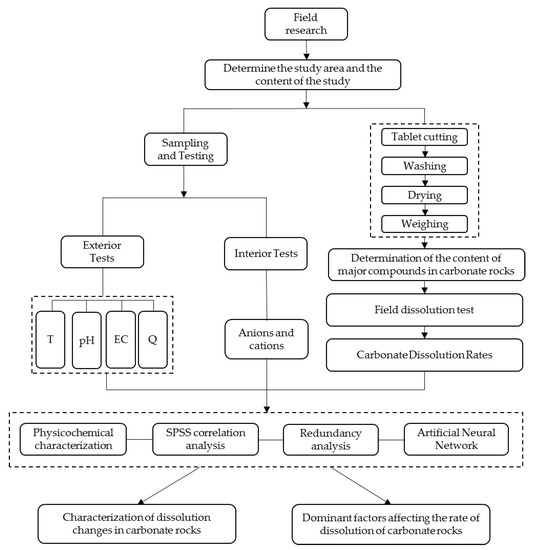
Figure 3.
Work flowchart for this study.
4. Results and Analysis
4.1. Characteristics of Physico-Chemical Changes in the Water Column at the Study Site
The patterns of changes in water temperature, pH, electrical conductivity (EC), Ca2+, HCO3−, flow, SIc, and pCO2 in Xiaolongbei, Bianyan, and Beidiping during the monitoring period from October 2017 to October 2018 are shown in Figure 4. The water temperature at Xiaolongbei is highly variable, reaching 24.18 °C in summer with a maximum difference of 18.4 °C during the year. Meanwhile, the water temperature at Bianyan and Beidiping is more stable each month, with a monthly average of 19 °C and 18.8 °C, respectively. The pH of Xiaolongbei, Bianyan, and Beidiping is higher in winter and spring than in summer and autumn. This is because wet seasons like summer and autumn dissolve a lot of CO2 into the water through the soil, increasing the H+ content and decreasing pH, whereas winter and fall do the opposite. The conductivity varied from 97.86 to 246.6 μS/cm and 360 to 482.7 μS/cm in Bianyan and Beidiping, respectively, while the conductivity in Xiaolongbei is more stable, with a difference of 1.5% between its highest and lowest values. The conductivity values in the dry season are higher than those in the rainy season because the precipitation in the rainy season is high and the concentration of solutes in the water is more significantly diluted by the rain, while in the dry season, the dilution effect is relatively weaker due to the low precipitation, and the concentration of solutes in the water is instead elevated. The order of magnitude of Ca2+ and HCO3− concentrations in Xiaolongbei, Bianyan, and Beidiping is Xiaolongbei < Bianyan < Beidiping. The maximum Ca2+ and HCO3− concentrations were found in Beidiping, whose annual mean values are 92 mg/L and 4.69 mg/L, respectively, while the minimum Ca2+ and HCO3− concentrations were found in Xiaolongbei, whose annual mean values are 5.29 mg/L and 0.19 mg/L, respectively. The Ca2+ and HCO3− concentrations in Beidiping are 17 and 25 times higher than those in Xiaolongbei. Beidiping is not recharged by external water sources, which corresponds to the largest concentrations of Ca2+ and HCO3−, whose annual average values are 92 mg/L and 4.69 mg/L, respectively. Xiaolongbei is directly recharged by atmospheric precipitation and is located in the clastic zone, so the Ca2+ and HCO3− concentrations are the smallest, with annual averages of 5.29 mg/L and 0.19 mg/L, respectively. As Ca2+ and HCO3− in the water of the Maocun basin are affected by rain dilution and water–rock interaction [31], the Ca2+ and HCO3− concentrations in the water bodies of Bianyan and Beidiping become larger in summer when it is hot and rainy, and the corresponding Ca2+ and HCO3− concentrations become smaller in winter when it is cold and less rainy. The three study sites’ flows are in the following order: Xiaolongbei > Bianyan > Beidiping. The flow at Bianyan and Beidiping is rather consistent; however, the flow at Xiaolongbei is more variable and increased in March and July due to concentrated rainfall. The order of magnitude of the calcite saturation indices at the three study sites is Beidiping > Bianyan > Xiaolongbei. The annual mean SIc values for Xiaolongbei, Bianyan, and Beidiping are −4.56, −3.01, and −1.76, respectively, all of which are unsaturated and erosive. Xiaolongbei is recharged by an external source of water, and its saturation index is low. In the downstream direction, the carbonate rocks dissolve in the water, and the concentration of calcium and magnesium ions in the water becomes continuously larger, corresponding to a larger saturation index of calcite. The SIc was greater in the dry season than in the wet season under different seasons, especially in the water samples in June 2018, where the SIc value was the smallest, indicating that the water bodies in the study area were affected by the dilution effect of rainfall in the wet season, which increased the erosion capacity of the water bodies and was more pronounced in June. The pCO2 sizes at the three study sites are ranked as follows: Xiaolongbei > Bianyan > Beidiping. Compared to other seasons, pCO2 is relatively low in the summer. The temperature of the water has a greater impact on pCO2. As the temperature rises, the solubility of CO2 in the river water is small, and CO2 diffuses to the atmosphere, making the pCO2 of the water body smaller. On the contrary, as the water temperature decreases, the pCO2 becomes larger. Secondly, changes in water temperature can affect the growth of phytoplankton in the water body by controlling the photosynthesis and respiration of aquatic plants, and thus, affects the pCO2 distribution.
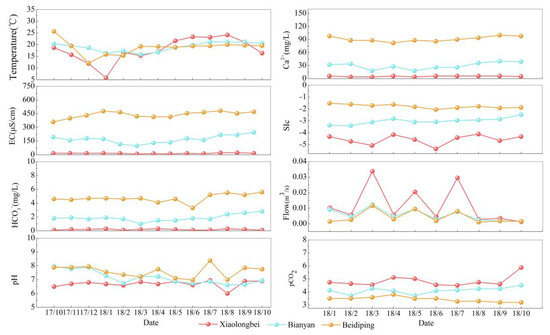
Figure 4.
Seasonal variation in water chemistry parameters at the study site.
4.2. Carbonate Dissolution Rates at Typical Study Sites
The rates of carbonate dissolution under exogenous water (Xiaolongbei), karst water (Beidiping), and the mixed exogenous and karst water (Bianyan) conditions are shown in Table 2 and Figure 5. From Table 2, it can be seen that the dissolution rate of limestone and dolomite varies greatly under different conditions. The dissolution of limestone occurs in every season. The maximum dissolution rate of limestone is 0.258 mg/cm2/d, which occurs at Xiaolongbei in summer, and the minimum dissolution rate is 0.002 mg/cm2/d, which occurs at Beidiping in winter. The maximum dissolution rate of limestone is about 129 times the minimum dissolution rate. Deposition of the dolomite at Beidiping occurred during the spring, autumn, and winter seasons with a maximum deposition rate of 0.023 mg/cm2/d, and dissolution occurred at all other study sites with a maximum dissolution rate of 0.103 mg/cm2/d. The main reasons for the above phenomena are the following: (1) the lattice energy of dolomite is greater than that of limestone; the greater the lattice energy, the more stable the crystal and the smaller the corresponding rate of dissolution; and (2) the acid insoluble matter content of dolomite and limestone in this experiment is 0.45% and 0.35%, respectively. The amount of dolomite that is acid-insoluble is higher than the amount of limestone that is acid-insoluble. The acid insoluble content is higher, which reduces the amount of carbonate material that reacts, and at the same time, it also reduces the contact area between the soluble material in the carbonate rock and the erosive fluid, which inhibits the dissolution of carbonate rock [32]; (3) the rate of dissolution of limestone is more sensitive to the hydrodynamic conditions than that of dolomite [18,33]; and (4) the calcium content of limestone is greater than that of dolomite, and rocks with high calcium content are highly soluble [34].

Table 2.
Rates of dissolution of carbonate rocks in the study area.
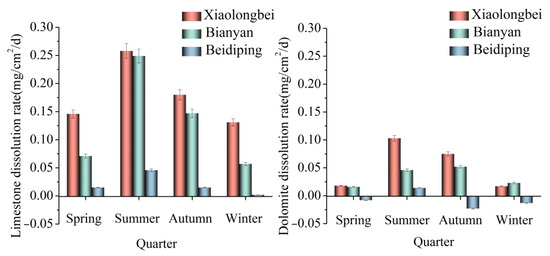
Figure 5.
Variation in carbonate dissolution rates at the study site.
The dissolving rate of carbonate rocks varies significantly over both time and space, as shown in Figure 5. The same lithology dissolves at different rates in Xiaolongbei, Bianyan, and Beidiping due to the exogenous nature of Xiaolongbei’s water and the fact that its hardness, pH, and saturation index are lower than those of the karst water in Beidiping and the karst water in Bianyan with exogenous water confluence [35,36]. At the same study site, the limestone dissolving rate was greatest in the summer, followed by autumn, spring, and winter. The relationship between temperature and the soil CO2 concentration is significant and positively correlated [37], and high summertime temperatures are associated with higher soil temperatures, increased soil microbial activity, larger microbial respiration, and larger plant root respiration, while high summertime rainfall introduces CO2 from the soil surrounding the water body into the water, increasing the water body’s pCO2. Due to the summer’s heavy precipitation, the water body’s flow rate is high and quick, the diffusion boundary layer is thin, the dissolved CO2 flux increases, and the dissolution effect is strengthened.
4.3. Factors Influencing the Rate of Dissolution of Carbonate Rocks
To investigate the influence of environmental factors on the rate of carbonate dissolution in the Maocun basin, SPSS 24.0 and redundancy analyses were used to analyze the correlation between environmental factors and the rate of carbonate dissolution. The results of the analysis are shown in Table 3 and Figure 6. The rate of limestone dissolution was highly significantly negatively correlated with pH, EC, and SIc (p < 0.01) and positively correlated with pCO2 (p < 0.01). Dolomite dissolution rates were highly significantly negatively correlated with pH (p < 0.01), significantly negatively correlated with EC and SIc (p < 0.05), and highly significantly positively correlated with pCO2 (p < 0.01). Carbonate dissolution is mainly influenced by pH, with low pH, high hydrogen ion concentrations, and aggressive solutions corresponding to the high rates of carbonate dissolution and vice versa.

Table 3.
Correlation between dissolution rates of carbonate rocks and environmental factors.
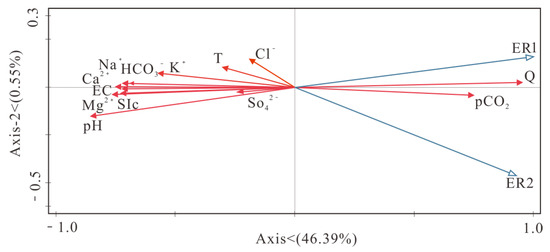
Figure 6.
Redundancy analysis of carbonate dissolution rates and environmental factors.
The chemical reaction equation in a karst water body is as follows:
Chemical Equation (2) moves to the right when Ca2+, HCO3−, and CO2 concentrations rise and carbonate dissolution rises. Ca2+, HCO3−, and conductivity all have a significant impact on the rate of carbonate dissolution. In addition, rising ion concentrations enhance the water column’s conductivity. An increase in the flow rate of the water body will accelerate the convection and diffusion of solution salt ions, thus accelerating the dissolution of minerals, resulting in an increase in the dissolution rate of minerals with the increase in the flow rate of the water, i.e., the higher the flow rate, the greater the dissolution rate. The above indicates that multiple environmental factors synergistically influence carbonate dissolution rates, and the synergistic effects are complex.
An artificial neural network was used for analysis in this paper to anticipate the evolution of carbonate dissolution rates and to elucidate the relationship between environmental parameters and carbonate dissolution rates. The results are displayed in Figure 7 and Figure 8. The analysis considers 12 pieces of data for each environmental factor, with 70% of the data used for training and 30% of the data used for prediction. Fifteen analyses were carried out using artificial neural networks, and the results of one particular analysis are shown in Figure 7. The R2 values of the measured and predicted rates of dissolution in the limestone ranged from 0.901 to 0.998, and the R2 values of the measured and predicted rates of dissolution in the dolomite ranged from 0.863 to 0.96, with a mean value of 0.936. The analysis of environmental factors and carbonate dissolution rates based on artificial neural networks has good results. The weights of the factors influencing the rate of carbonate dissolution in the basin are shown in Figure 8. The results show that flow, the saturation index, pH, and pCO2 have higher weights than the other factors, indicating that these four factors are the dominant factors influencing the rate of dissolution of carbonate rocks.
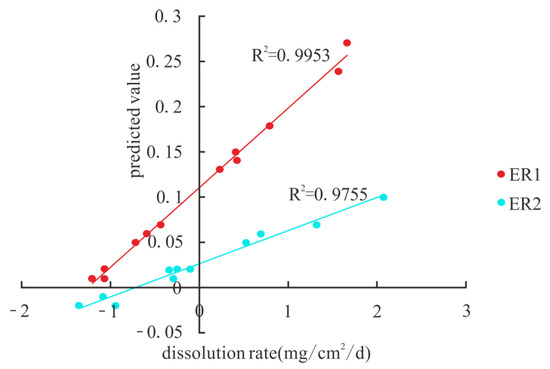
Figure 7.
Evolution of carbonate dissolution rates in the Maocun basin.
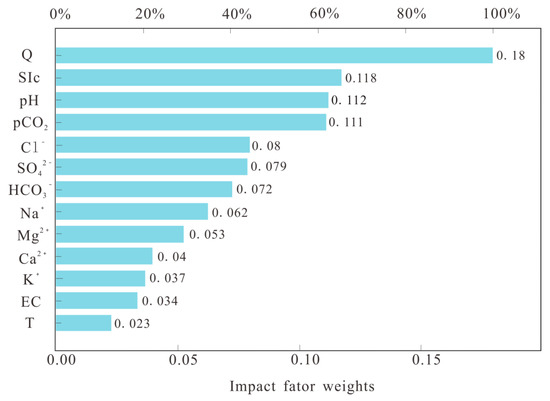
Figure 8.
Predicted trend of carbonate dissolution rate evolution in the Maocun basin.
5. Conclusions
We have investigated the spatial variation and the temporal patterns of carbonate dissolution in the Maocun basin. We have used correlation analysis and artificial neural networks to analyze the influence of the physicochemical properties of the water bodies in the Maocun basin on the dissolution intensity of carbonate rocks. The main conclusions reached were as follows: (1) The limestones at the study sites undergo dissolution throughout the year, with a maximum dissolution rate of 0.258 mg/cm2/d and a minimum dissolution rate of 0.002 mg/cm2/d. The dolomite at Xiaolongbei and Bianyan undergoes dissolution throughout the year, but the dolomite at Beidiping undergoes dissolution only in summer with deposition occurring in the other seasons, with a maximum dissolution rate of 0.103 mg/cm2/d and a maximum deposition rate of 0.023 mg/cm2/d. (2) The dissolution rate of carbonate rocks in the Maocun basin varies greatly at different time scales and has obvious seasonal variations. Broadly speaking, it shows summer > autumn > spring > winter. Seasonal variation is mainly influenced by the pCO2 of the water column and the flow rate of the water column. (3) The rate of carbonate dissolution in the Maocun basin is characterized by significant spatial variability. The magnitude of the carbonate dissolution rate at each study site is Xiaolongbei > Bianyan > Beidiping. The main controls on the variation in carbonate dissolution rates at the spatial scale are the pH and saturation index of the water column.
Author Contributions
C.M. and F.H. conceived and designed the experiments; C.M. and S.X. performed the experiments, analyzed the data, and prepared the manuscript; J.C. and J.X. optimized the experiment and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Natural Science Foundation of Gunagxi (No. 2020GXNSFBA297035; No. 2023GXNSFBA026306); the General Program of Natural Science Foundation of Guangxi (No. 2022GXNSFAA035569); the Science and Technology Base and Talent Project of Guangxi (Grant/Award Number: Guike-AD21196001); and the Survey and China Geological Survey (grant No. DD20230547).
Data Availability Statement
The data are not publicly available due to further research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, D.X.; Zhang, C. Theoretical exploration and practice of karst dynamics. Acta Geosci. Sin. 2008, 29, 355–365. [Google Scholar]
- Liu, Z.H. Recent advances and perspectives in the study of weathering carbon sinks in rocks. Chin. Sci. Bull. 2012, 57, 95–102. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dreybrodt, W.; Liu, H. Atmospheric CO2 sink: Silicate weathering or carbonate weathering. Appl. Geochem. 2011, 26, S292–S294. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, Q.; Wu, Z.Y.; Knez, M. Ecosystem-driven karst carbon cycle and carbon sink effects. J. Groundw. Sci. Eng. 2022, 10, 99–112. [Google Scholar]
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, N.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; et al. The global carbon cycle: A test of our knowledge of earth as a system. Science 2000, 290, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Gaillardet, J.; Calmels, D.; Romero Mujalli, G.; Zakharova, E.; Hartmann, J. Global climate control on carbonate weathering intensity. Chem. Geol. 2019, 527, 118762. [Google Scholar] [CrossRef]
- Zeng, J.; Yue, F.J.; Xiao, M.; Wang, Z.; Qin, C. Dissolved organiccarbon in rainwater from a karst agricultural area of Southwest China: Variations, sources, and wet deposition fluxes. Atmos. Res. 2020, 245, 105140. [Google Scholar] [CrossRef]
- Zhao, M.; Zeng, C.; Liu, Z.H.; Wang, S.J. Effect of different land use/land cover on karst hydrogeochemistry: A paired catchment study of Chenqi and Dengzhanhe, Puding, Guizhou, SW China. J. Hydrol. 2010, 388, 121–130. [Google Scholar] [CrossRef]
- Yang, R.; Chen, B.; Liu, H.; Liu, Z.; Yan, H. Carbon sequestration and decreased CO2 emission caused by terrestrial aquatic photosynthesis: Insights from diel hydrochemical variations in an epikarst spring and two spring-fed ponds in different seasons. Appl. Geochem. 2015, 63, 248–260. [Google Scholar] [CrossRef]
- Liu, Z.; Dreybrodt, W. Significance of the carbon sink produced by H2O–carbonate–CO2–aquatic phototroph interaction on land. Sci. Bull. 2015, 60, 182–191. [Google Scholar] [CrossRef]
- Zhang, Z.; Lian, B.; Hou, W.; Chen, M.; Xin, L.; Yan, L. Bacillus mucilaginosus can capture atmospheric CO2 by carbonic anhydrase. Afr. J. Microbiol. Res. 2011, 5, 106–112. [Google Scholar]
- Liu, Z.; Macpherson, G.; Groves, C.; Martini, J.B.; Yuan, D.; Zeng, S. Large and Active CO2 Uptake by Coupled Carbonate Weathering. Earth-Sic. Rev. 2018, 182, 42–49. [Google Scholar] [CrossRef]
- Li, N.Y.; Feng, Y.S.; Liu, P.L.; Luo, Z.F.; Zhao, L.Q. Study of Acid–Rock Reaction Kinetics Under High Temperature and Pressure Conditions Based on the Rotating Disk Instrument. Arab. J. Sci. Eng. 2015, 40, 135–142. [Google Scholar] [CrossRef]
- Li, Q.; Yi, X.Y.; Li, G.S.; Chen, W.L. The law of the hydrogen ion diffusion coefficient in acid rock reaction. J. Pet. Sci. Eng. 2016, 146, 694–701. [Google Scholar] [CrossRef]
- Hyunsang, Y.; Youngmin, K.; Wonsuk, L.; Jeonghwan, L. An experimental study on acid–rock reaction kinetics using dolomite in carbonate acidizing. J. Pet. Sci. Eng. 2018, 168, 478–494. [Google Scholar]
- Larson, E.B.; Emmons, R.V. Dissolution of Carbonate Rocks in a Laboratory Setting: Rates and Textures. Minerals 2021, 11, 605. [Google Scholar] [CrossRef]
- Plummer, L.N.; Wigley, T.M.L.; Parkhurst, D.L. The kinetics of calcite dissolution in CO2–water systems at 5 degrees to 60 degrees C and 0.0 to 1.0 atm CO2. Am. J. Sci. 1978, 278, 179–216. [Google Scholar] [CrossRef]
- Yu, S.; Yan, Y.P.; Zhang, C.L.; Wang, X.Y.; Liu, Q.; Li, Y.L. Experimental study on carbonate dissolution rate influence by acid rain. J. Guilin Univ. Technol. 2011, 31, 539–544. [Google Scholar]
- Shao, D.M. Effect of temperature on the dissolution rate of Ordovician carbonates at different water flow rates. Coalf. Geol. Explor. 2012, 40, 62–65. [Google Scholar]
- Zeng, S.B.; Liu, Z.H.; Goldscheider, N.; Frank, S.; Goeppert, N.; Kaufmann, G.; Zeng, C.; Zeng, Q.R.; Sun, H.L. Compari–sons on the effects of temperature, runoff, and land–cover on carbonate weathering in different karstcatchments: Insights into the future global carbon cycle. Hydrogeol. J. 2021, 29, 331–345. [Google Scholar] [CrossRef]
- Luo, M.M.; Zhou, H.; Liang, Y.P.; Chen, Z.H.; Chen, R.B.; Li, X.L.; Jakada, H. Horizontal and vertical zoning of carbonate dissolution in China. Geomorphology 2018, 322, 66–75. [Google Scholar] [CrossRef]
- Zeng, Q.R.; Liu, Z.H.; Chen, B.; Hu, Y.D.; Zeng, S.B.; Zeng, C.; Yang, R.; He, H.B.; Zhu, H.; Cai, X.L.; et al. Car–bonate weathering–related carbon sink fluxes under different land uses: A case study from the Shawan Simulation TestSite, Puding, Southwest China. Chem. Geol. 2017, 474, 58–71. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Q.; Jia, Y.N. The influence of meteorological factors and soil physicochemical properties on Karst processes in six land-use patterns in summer and winter in a typical Karst valley. Acta Ecol. Sin. 2014, 34, 1418–1428. [Google Scholar]
- Zeng, S.B.; Liu, Z.H.; Kaufmann, G. Sensitivity of the global carbonate weathering carbon–sink flux to climate and land–use changes. Nat. Commun. 2019, 10, 5749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Carbonate rock dissolution rates indifferent landuses and their carbon sink effect. Sci. Bull. 2011, 56, 3759–3765. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.Z.; Ju, X.T.; Chen, Z.J.; Zhou, J.B.; Kuzyakov, Y. Dramatic loss of inorganic carbon by nitro–gen–induced soil acidification in Chinese croplands. Glob. Chang. Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Abril, G.; Etcheber, H.; Delille, B.; Frankignoulle, M.; Borges, A.V. Carbonate dissolution in the turbid and eutrophic Loire estuary. Mar. Ecol. Prog. Ser. 2003, 259, 129–138. [Google Scholar] [CrossRef]
- Plan, L. Factors controlling carbonate dissolution rates quantified in a field test in the Austrian alps. Geomorphology 2005, 68, 201–212. [Google Scholar] [CrossRef]
- Li, Q.; Song, A.; Peng, W.J.; Jin, Z.J.; Müller Werner, E.G.; Wang, X.H. Contribution of aerobic anoxygenic phototrophic bacteria to total organic carbon pool in aquatic system of subtropical karst catchments, Southwest China: Evidence from hydrochemical and microbiological study. Resour. FEMS Microbiol. Ecol. 2017, 6, fix065. [Google Scholar] [CrossRef]
- Liu, Z.H. “Method of maximum potential dissolution” to calculate the intensity of karst process and the relevant carbon sink: With discussions on methods of maximum potential dissolution process and the relevant carbon sink: With discussions on methods of solute load and carbonate-rock-tablet test. Garsologica Sin. 2011, 30, 379–382. [Google Scholar]
- Liu, Z.H.; Groves, C.; Yuan, D.X.; Meiman, J.; Jiang, G.H.; He, S.Y. Study on the hydrochemical variations caused by the water-rock-gas interaction-an example from the Guilin Karst Experimental Site. Hydrogeol. Eng. Geol. 2003, 30, 13–18. [Google Scholar]
- Chen, R.B.; Luo, M.M.; Luo, Z.H.; Chen, Z.H.; Zhou, H. Response relationship between chemical composition and dissolution rate of carbonate rocks in the Gorges Area. Garsologica Sin. 2019, 38, 258–264. [Google Scholar]
- Liu, Z.H.; Dreybrodt, W.; Li, H.J. Comparison of dissolution rate–determinig mechanisms in between limestone and dolomite. Earth Sci. J. China Univ. Geosci. 2006, 31, 411–416. [Google Scholar]
- Wu, J.Q.; Gu, C.S.; Xu, S.G.; Zhao, X.F.; Huang, G.M. Corrosion analysis of carbonate rocks in southern Jiangsu Province. Garsologica Sin. 2021, 40, 565–571. [Google Scholar]
- Tang, W.; Cao, J.H.; Yang, H.; Wang, H.; Tu, L.L.; Ying, Q.H. Research on carbonate rock corrosion rate by allogenic water as exemplified by the Maocun subterranean river in Guilin. Earth Environ. 2014, 42, 207–212. [Google Scholar]
- Huang, F.; Tang, W.; Wang, J.L.; Cao, J.H.; Yin, J.J. The influence of allogenic water on karst carbon sink: A case study in the Maocun Subterranean River in Guilin, Guilin. Carsologica Sin. 2011, 30, 417–421. [Google Scholar]
- Fu, Y.C.; Lang, Y.C.; Wang, Z.J.; Li, S.L.; Ding, H. Diurnal dynamics and constraints of soil CO2 concentration in a limestone site during summer. Chin. J. Ecol. 2018, 37, 3315–3322. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).