Phosphate in Aqueous Solution Adsorbs on Limestone Surfaces and Promotes Dissolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Selection and Preparation
2.2. Static Adsorption Experiments
2.3. Characterization of Materials
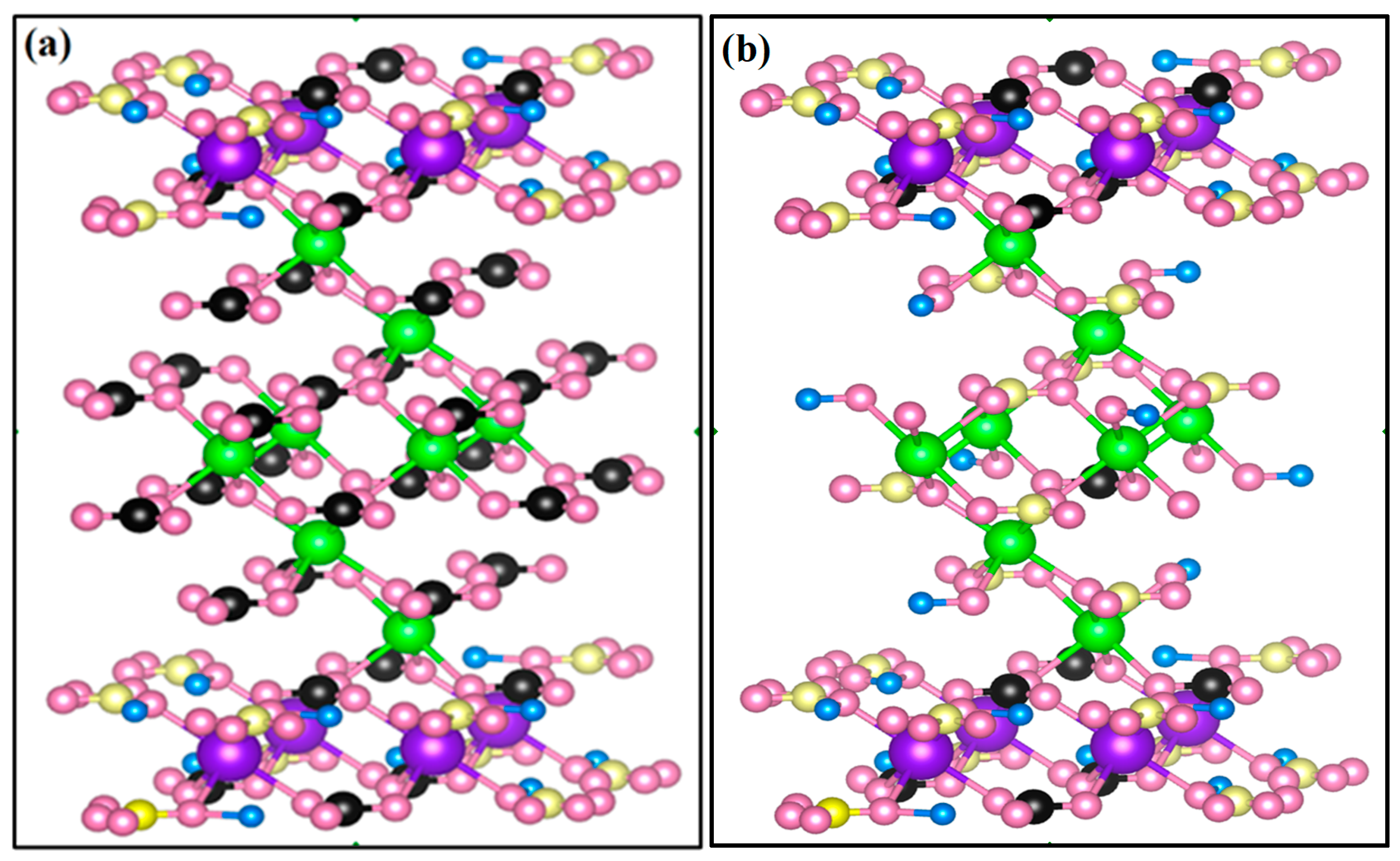
3. Results and Discussion
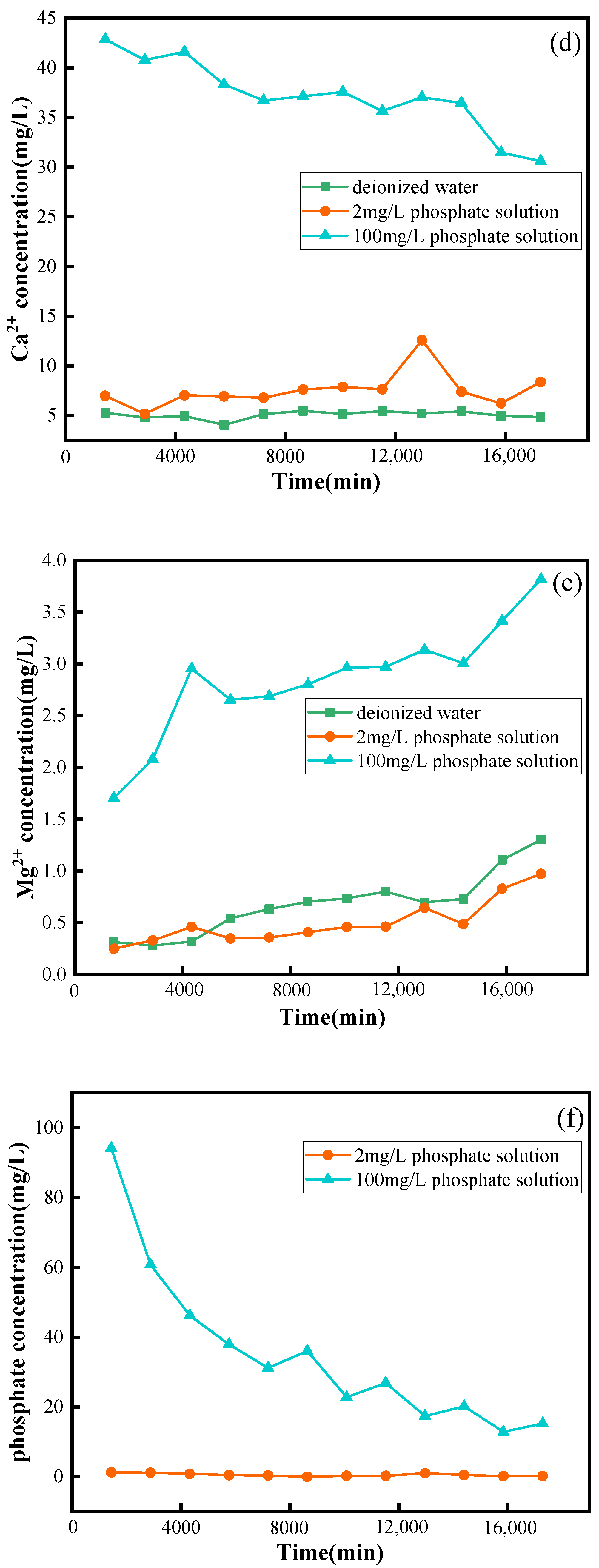
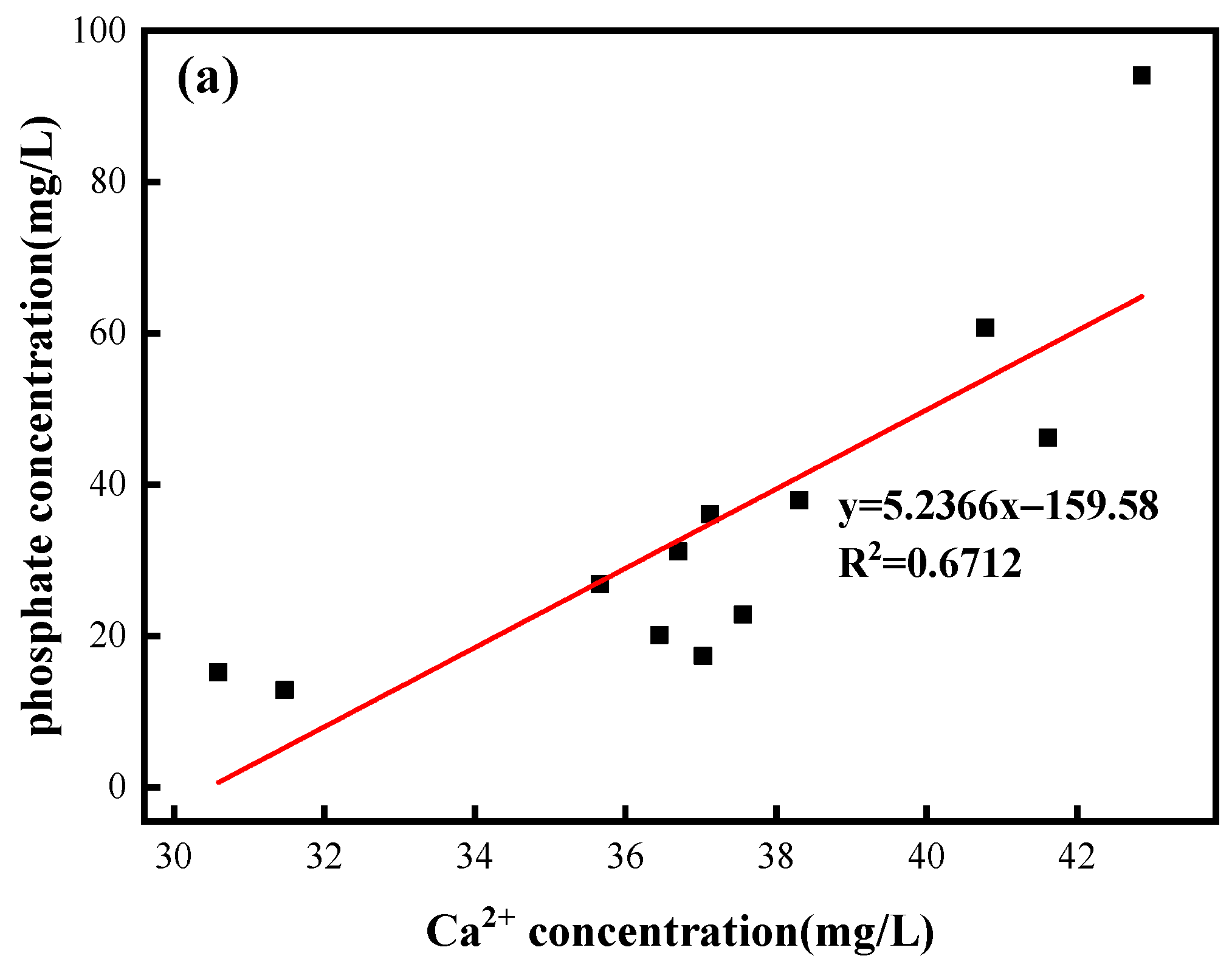
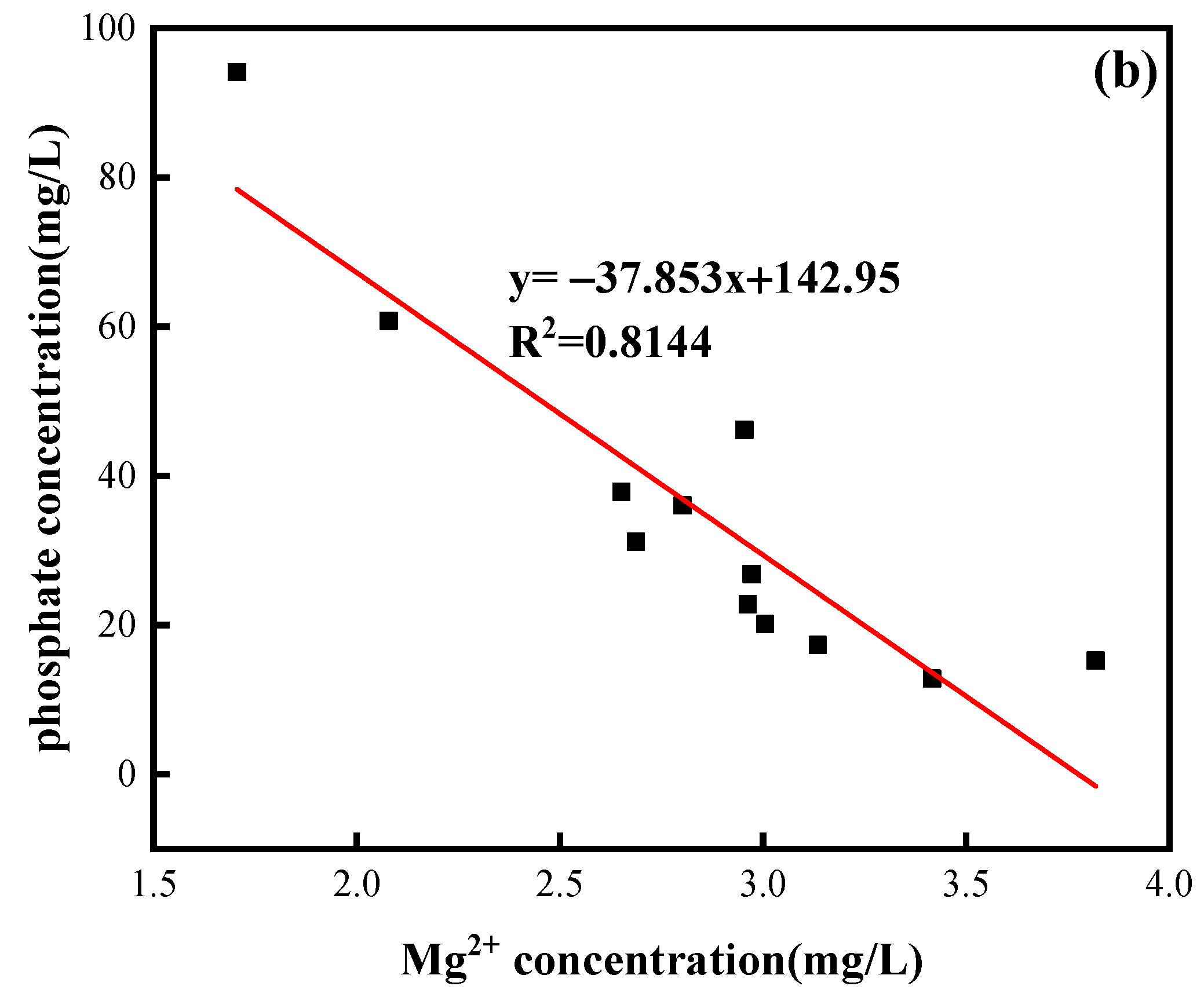
3.1. Phosphate Involvement in the Hydrochemical Processes of Limestone Dissolution
3.2. Characterization of Limestone before and after Dissolution
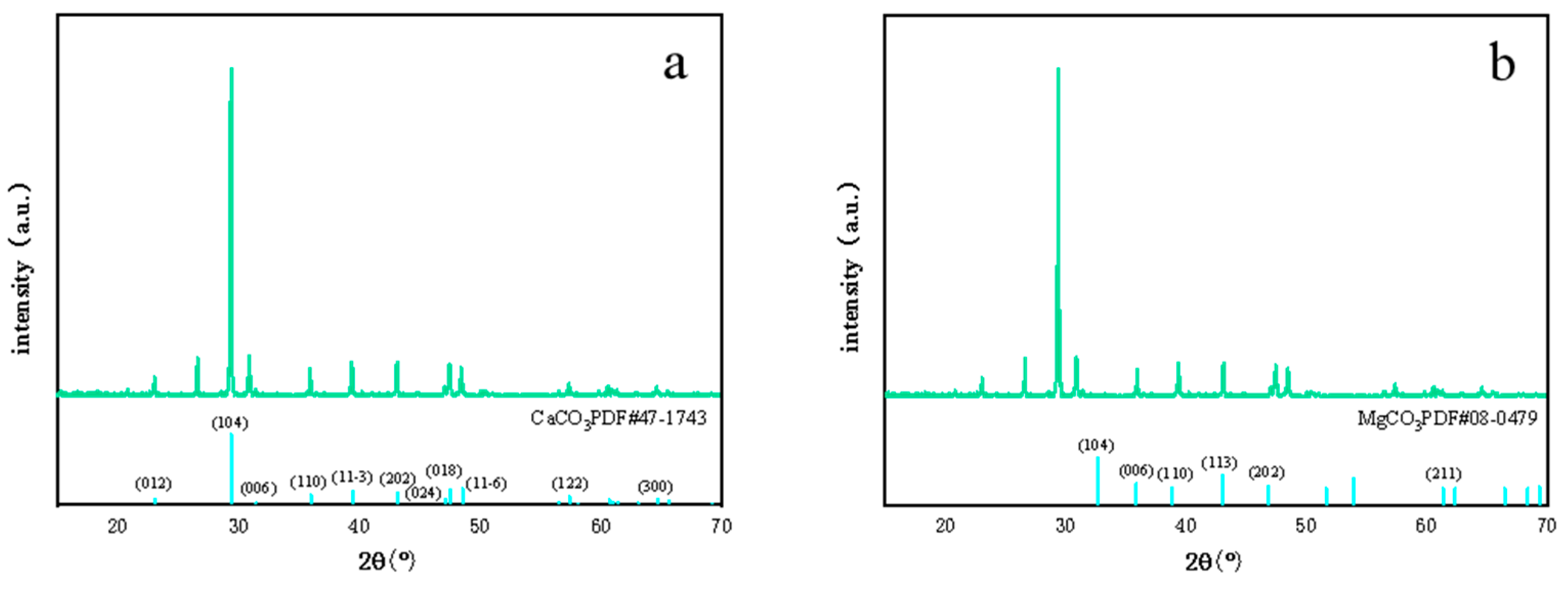
3.2.1. XRD Analysis
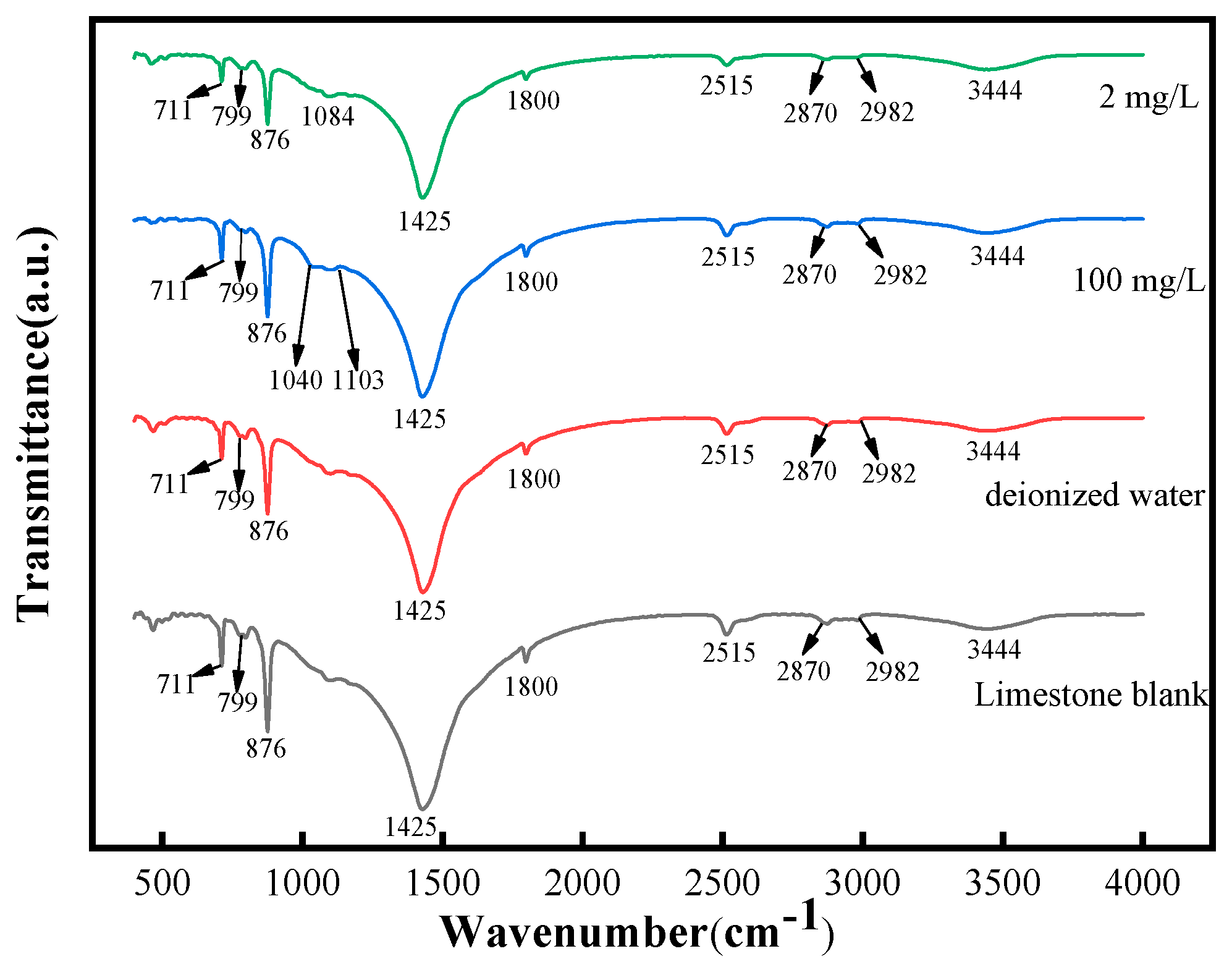
3.2.2. FTIR Analysis
3.2.3. Raman Analysis
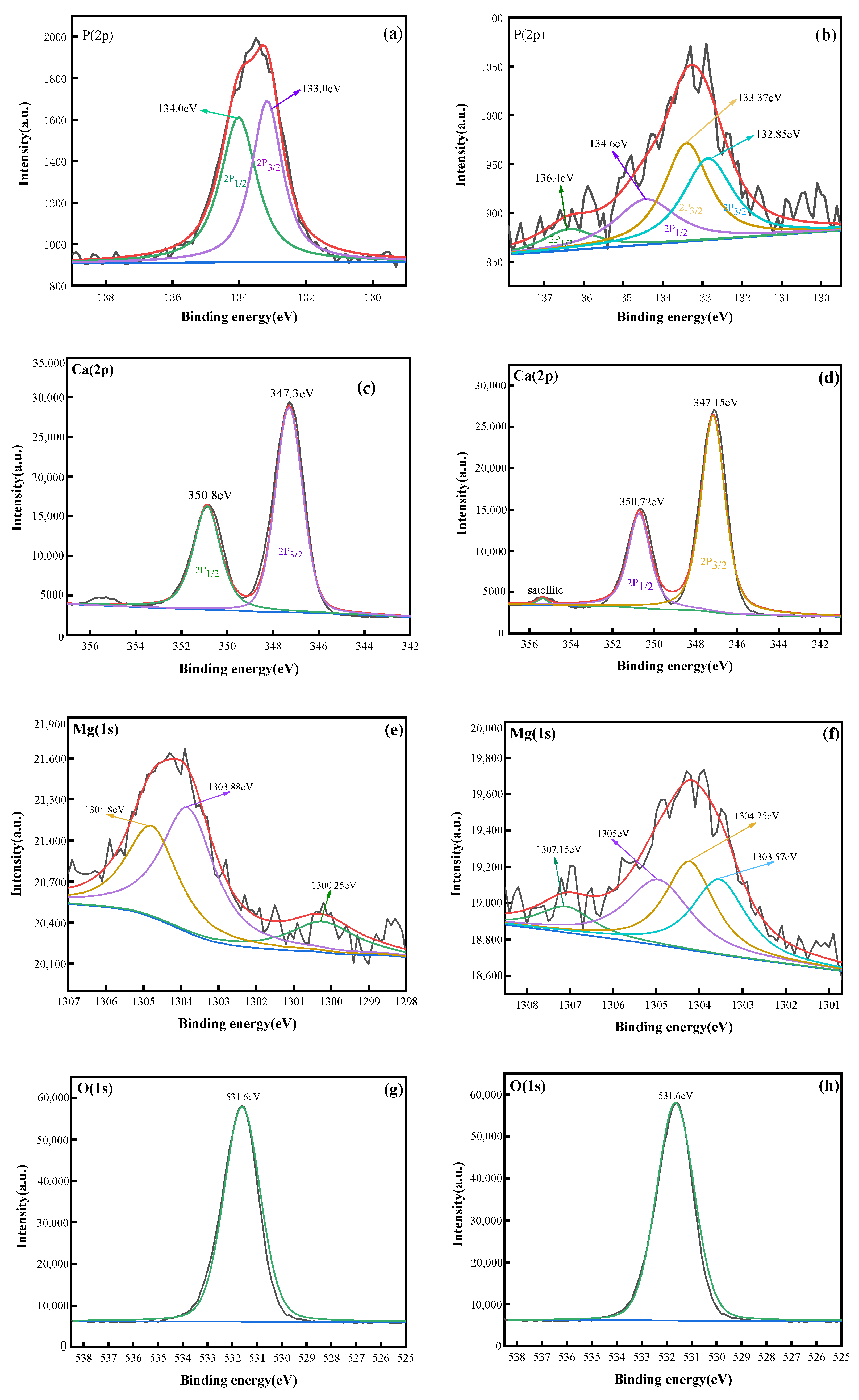
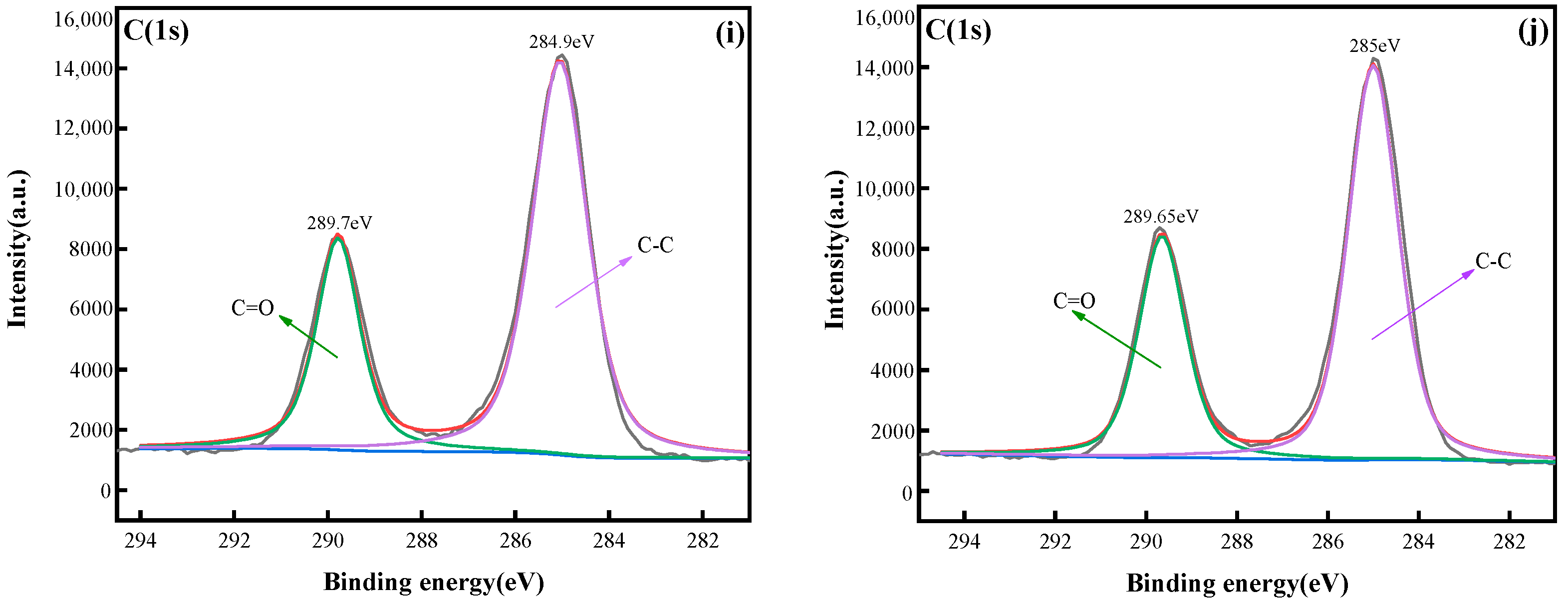
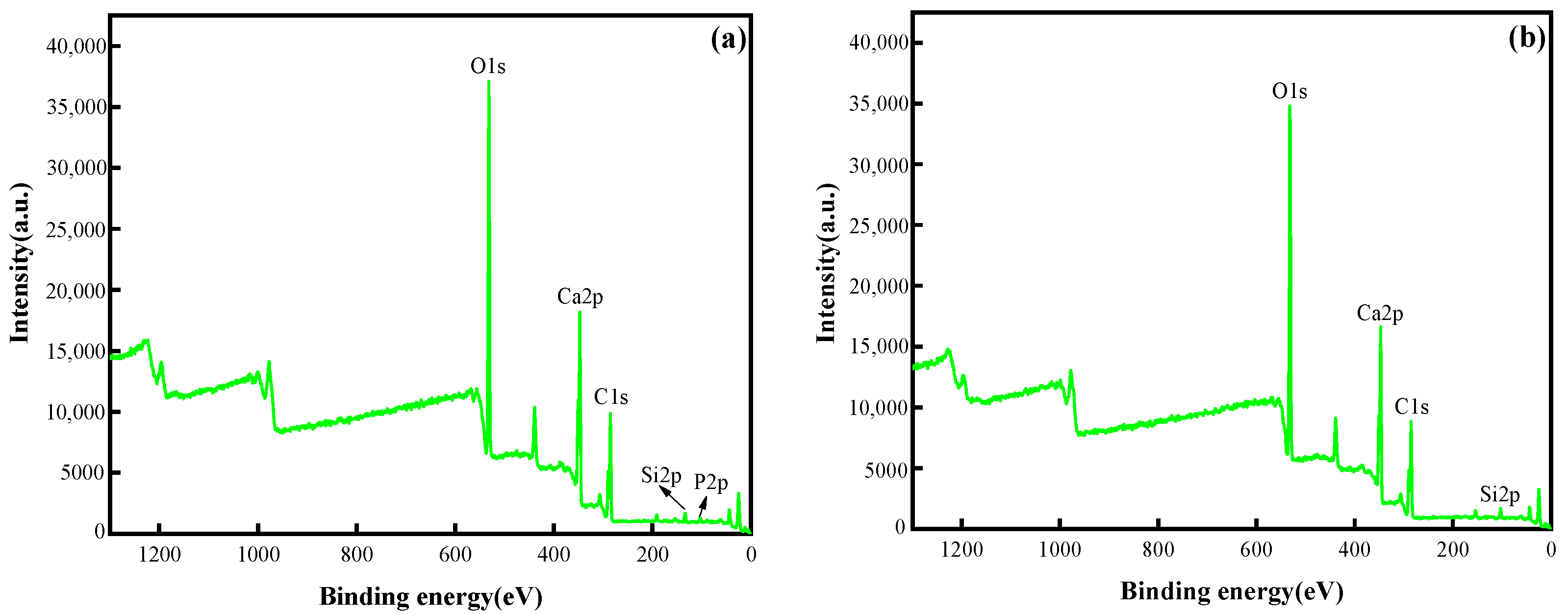
3.2.4. XPS Analysis
3.2.5. SEM/EDS Analysis
3.3. Discussion of Adsorption Model and Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, S.Z. World Climate Conference. World Agric. 1981, 12, 44–45. [Google Scholar]
- Yuan, D. The carbon cycle in karst. Z. Geomorpholo-Gie Neue Folge China 1997, 108, 91–102. [Google Scholar]
- Li, W.L.; Bai, B.; Cao, J.H. The “invisible” carbon sink effect. Pop. Land Resour. Sci. Cult. 2018, 2, 18–23. [Google Scholar]
- Huang, F.; Tang, W.; Wang, J.L. Impact of exogenous water on karst carbon sinks. China Karst 2011, 30, 4. [Google Scholar]
- He, R.X. Research on the Influence of Bacterial Communities in Karst Water Bodies on Karst Carbon Sinks; Southwest University: Chongqing, China, 2017. [Google Scholar]
- Yuan, Y.Q. Effects of Aquatic Photosynthetic Organisms on Water Chemistry and Karst Carbon Sinks in the Li River Basin; Southwest University: Chongqing, China, 2016. [Google Scholar]
- Lan, J.C.; Xiao, S.Z. Research progress on the impact of land use change on soil organic carbon and karst carbon sinks in karst areas. J. Ecol. 2017, 36, 2633–2640. [Google Scholar]
- Zhao, R.Y.; Li, J.H.; Dong, L.L. Characterization of temporal and spatial soil CO2 changes in karst springs under different land use types and analysis of sources. J. Ecol. Environ. 2020, 29, 81. [Google Scholar]
- Zhang, X.B.; Jiang, Y.J.; Qiu, S.L. Impacts of agricultural activities on carbon sinks in karst: A case study of Chongqing Qingmuguan underground river basin. Adv. Earth Sci. 2012, 27, 466. [Google Scholar]
- Song, C.; Liu, C.L.; Wang, J.K. Indoor simulation study on the effect of cow dung fertilizer and compound fertilizer on the erosion of carbonate rock specimens. Earth Environ. 2011, 39, 597–604. [Google Scholar]
- Liu, C.L.; Zhang, Y.; Song, C. Effects of agricultural fertilizer application on karst dissolution and its ecological significance. China Geol. 2015, 6, 205–214. [Google Scholar]
- Li, S.L.; Xu, S.; Wang, T.J. Effects of agricultural activities coupled with karst structures on riverine biogeochemical cycles and environmental quality in the karst region. Agric. Ecosyst. Environ. 2020, 303, 107120. [Google Scholar] [CrossRef]
- Sun, Q.; Ruan, Y.; Chen, P. Effects of mineral-organic fertilizer on the biomass of green Chinese cabbage and potential carbon sequestration ability in karst areas of Southwest China. Acta Geochim. 2019, 38, 430–439. [Google Scholar] [CrossRef]
- El-Mofty, S.E.D.; El-Midany, A. Calcite flotation in potassium oleate/potassium dihydrogen phosphate system. J. Surfactants Deterg. 2015, 18, 905–911. [Google Scholar] [CrossRef]
- Klasa, J.; Ruiz-Agudo, E.; Wang, L.J. An atomic force microscopy study of the dissolution of calcite in the presence of phosphate ions. Geochim. Cosmochim. Acta 2013, 117, 115–128. [Google Scholar] [CrossRef]
- Zou, Z.; Xie, J.; Macias-Sanchez, E. Nonclassical crystallization of amorphous calcium carbonate in the presence of phosphate ions. Cryst. Growth Des. 2020, 21, 414–423. [Google Scholar] [CrossRef]
- Sawyer, C.N.; McCarty, P.L.; Parkin, G.F. Chemistry for Environmental Engineering and Science; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Song, Y.; Weidler, P.G.; Berg, U. Calcite-seeded crystallization of calcium phosphate for phosphorus recovery. Chemosphere 2006, 63, 236–243. [Google Scholar] [CrossRef]
- Sø, H.U.; Postma, D.; Jakobsen, R. Sorption of phosphate onto calcite; results from batch experiments and surface complexation modeling. Geochim. Cosmochim. Acta 2011, 75, 2911–2923. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Putnis, C.V.; Lopez-Jimenez, C.; Navarro, R.C. An atomic force microscopy study of calcite dissolution in saline solutions: The role of magnesium ions. Geochim. Cosmochim. Acta 2009, 73, 3201–3217. [Google Scholar] [CrossRef]
- Perassi, I.; Borgnino, L. Adsorption and surface precipitation of phosphate onto CaCO3–montmorillonite: Effect of pH, ionic strength and competition with humic acid. Geoderma 2014, 232, 600–608. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; Comite, V.; La Russa, M.F.; Belfiore, C.M.; Barca, D.; Bonazza, A.; Crisci, G.M.; Pezzino, A.; Sabbioni, C. An analysis of the black crusts from the Seville Cathedral: A challenge to deepen the understanding of the relationships among microstructure, microchemical features, and pollution sources. Sci. Total Environ. 2015, 502, 157–166. [Google Scholar] [CrossRef]
- Samanta, A.; Chanda, D.K.; Das, P.S.; Ghosh, J.; Mukhopadhyay, A.K.; Dey, A. Synthesis of nano calcium hydroxide in aqueous medium. J. Am. Ceram. Soc 2016, 99, 787–795. [Google Scholar] [CrossRef]
- Wang, L.; Sondi, I.; Matijević, E. Preparation of uniform needle-like aragonite particles by homogeneous precipitation. J. Colloid Interface Sci. 1999, 218, 545–553. [Google Scholar] [CrossRef]
- Destainville, A.; Champion, E.; Bernache-Assollant, D.; Laborde, E. Synthesis, characterization and thermal behavior of apatitic tricalcium phosphate. Mater. Chem. Phys. 2003, 80, 269–277. [Google Scholar] [CrossRef]
- Meejoo, S.; Maneeprakorn, W.; Winotai, P. Phase and thermal stability of nanocrystalline hydroxyapatite prepared via microwave heating. Thermochim. Acta 2006, 447, 115–120. [Google Scholar] [CrossRef]
- Novais, S.V.; Zenero, M.D.O.; Tronto, J.; Conz, R.F.; Cerri, C.E.P. Poultry manure and sugarcane straw biochars modified with MgCl2 for phosphorus adsorption. J Environ. Manag. 2018, 214, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Hadiko, G.; Fuji, M.; Takahashi, M. Factors affecting the phase and morphology of CaCO3 prepared by a bubbling method. J. Eur. Ceram. Soc. 2006, 26, 843–847. [Google Scholar] [CrossRef]
- Torit, J.; Phihusut, D. Phosphorus removal from wastewater using eggshell ash. Environ. Sci. Pollut. Res. 2019, 26, 34101–34109. [Google Scholar] [CrossRef] [PubMed]
- Boro, J.; Konwar, L.J.; Deka, D. Transesterification of non-edible feedstock with lithium incorporated eggshell derived CaO for biodiesel production. Fuel Process. Technol. 2014, 122, 72–78. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Couture, L. Etude des spectres de vibration de monocristaux ioniques. Ann. Phys. 1947, 2, 5–94. [Google Scholar] [CrossRef]
- Murphy, A.E.; Jakubek, R.S.; Steele, A. Raman spectroscopy provides insight into carbonate rock fabric based on calcite and dolomite crystal orientation. J. Raman Spectrosc. 2021, 52, 1155–1166. [Google Scholar] [CrossRef]
- Wang, L.J.; Ruiz-Agudo, E.; Putnis, C.V.; Menneken, M.; Putnis, A. Kinetics of calcium phosphate nucleation and growth on calcite: Implications for predicting the fate of dissolved phosphate species in alkaline soils. Environ. Sci. Technol. 2012, 46, 834–842. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, S.; Ruiz-Agudo, E.; Putnis, C.V.; Putnis, A. Posner’s cluster revisited: Direct imaging of nucleation and growth of nanoscale calcium phosphate clusters at the calcite-water interface. CrystEngComm 2012, 14, 6252–6256. [Google Scholar] [CrossRef]
- Gomes, G.C.; Borghi, F.F.; Ospina, R.O. Nd: YAG (532 nm) pulsed laser deposition produces crystalline hydroxyapatite thin coatings at room temperature. Surf. Coat. Technol. 2017, 329, 174–183. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, W.; Xu, H.; Li, S.; Peng, Y. Adsorption removal and reuse of phosphate from wastewater using a novel adsorbent of lanthanum-modified Platanus biochar. Process Saf. Environ. Prot. 2020, 140, 221–232. [Google Scholar] [CrossRef]
- Li, S.; Zeng, W.; Xu, H.; Jia, Z.; Peng, Y. Performance investigation of struvite high-efficiency precipitation from wastewater using silicon-doped magnesium oxide. Environ. Sci. Pollut. Res. Int. 2020, 27, 15463–15474. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kong, M. Simultaneous removal of ammonium and phosphate from eutrophic waters using natural calcium-rich attapulgite-based versatile adsorbent. Desalination 2014, 351, 128–137. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Yan, F.Y. Bioinspired porous octacalcium phosphate/silk fibroin composite coating materials prepared by electrochemical deposition. ACS Appl. Mater. Interfaces 2015, 7, 5634–5642. [Google Scholar] [CrossRef]
- Moulder, W.F.S.J.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronic Inc.: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Wu, J.; Huang, X.; Chen, H. Fabrication of Cu-Al2O3/ceramic particles by using brick particles as supports for highly-efficient selenium adsorption. J. Environ. Chem. Eng. 2021, 9, 105008. [Google Scholar] [CrossRef]
- Hou, Y.; Peng, J.; Wang, M. Tribochemical behavior of pure magnesium during sliding friction. Metals 2019, 9, 311. [Google Scholar]
- Chubar, N. XPS determined the mechanism of selenite (HSeO3−) sorption in the absence/presence of sulfate (SO42−) on Mg-Al-CO3 Layered double hydroxides (LDHs): Solid phase speciation focus. J. Environ. Chem. Eng. 2023, 11, 109669. [Google Scholar] [CrossRef]
- Din, I.U.; Alotaibi, M.A.; Alharthi, A.I. Synthesis, characterization, and activity of CeO2-supported Cu-Mg bimetallic catalysts for CO2 to methanol. Chem. Eng. Res. Des. 2023, 192, 158–166. [Google Scholar] [CrossRef]
- Stipp, S.L.; Hochella, M.F., Jr. Structure and bonding environments at the calcite surface as observed with X-ray photoelectron spectroscopy (XPS) and low energy electron diffraction. Geochim. Cosmochim. Acta 1991, 55, 1723–1736. [Google Scholar] [CrossRef]
- Hu, X.; Xue, Y.; Liu, L.; Zeng, Y.; Long, L. Preparation and characterization of Na2S-modified biochar for nickel removal. Environ. Sci. Pollut. Res. Int. 2018, 25, 9887–9895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Miao, R.; Ning, P. From wastes to functions: A paper mill sludge-based calcium-containing porous biochar adsorbent for phosphorus removal. J. Colloid Interface Sci. 2021, 593, 434–446. [Google Scholar] [CrossRef]
- Yin, H.; Kong, M.; Fan, C. Batch investigations on P immobilization from wastewaters and sediment using natural calcium-rich sepiolite as a reactive material. Water Res. 2013, 47, 4247–4258. [Google Scholar] [CrossRef]
- Kõiv, M.; Liira, M.; Mander, Ü.; Mõtlep, R.; Vohla, C.; Kirsimäe, K. Phosphorus removal using Ca-rich hydrated oil shale ash as filter material—The effect of different phosphorus loadings and wastewater compositions. Water Res. 2010, 44, 5232–5239. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Xiao, R. Effect of pyrolysis temperature on phosphate adsorption characteristics and mechanisms of crawfish char. J. Colloid Interface Sci. 2018, 525, 143–151. [Google Scholar] [CrossRef]
- Falini, G.; Gazzano, M.; Ripamonti, A. Magnesium calcite crystallization from water-alcohol mixtures. Chem. Commun. 1996, 26, 1037–1038. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, S.; Zheng, Q. Removal and recovery of phosphorus from solution by bifunctional biochar. Inorg. Chem. Commun. 2022, 139, 109341. [Google Scholar] [CrossRef]
- Sparks, D.L.; Hartemink, A.E.; McBratney, A.B. Elucidating the fundamental chemistry of soils; past and recent achievements and future frontiers. Geoderma 2001, 100, 303–319. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Huo, S. Recent progress in metal-based composites toward adsorptive removal of phosphate: Mechanisms, behaviors, and prospects. Chem. Eng. J. 2022, 446, 137081. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and recovery of phosphate from water using sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, Z.Y.; Khoso, S.A. Selective adsorption of benzhydroxamic acid on fluorite rendering selective separation of fluorite/calcite. Appl. Surf. Sci. 2018, 435, 752–758. [Google Scholar] [CrossRef]
- Huang, X.; Huang, L.; Babu Arulmani, S.R. Research progress of metal organic frameworks and their derivatives for adsorption of anions in water: A review. Environ. Res. 2022, 204, 112–381. [Google Scholar] [CrossRef] [PubMed]



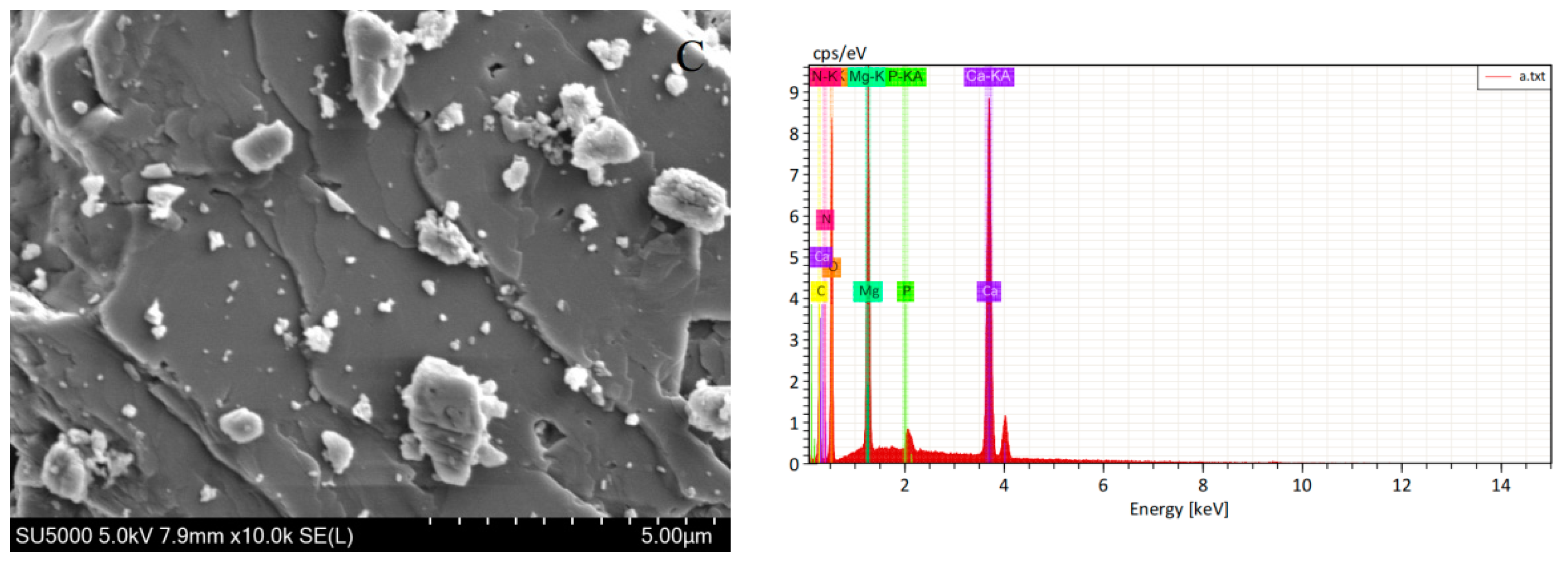
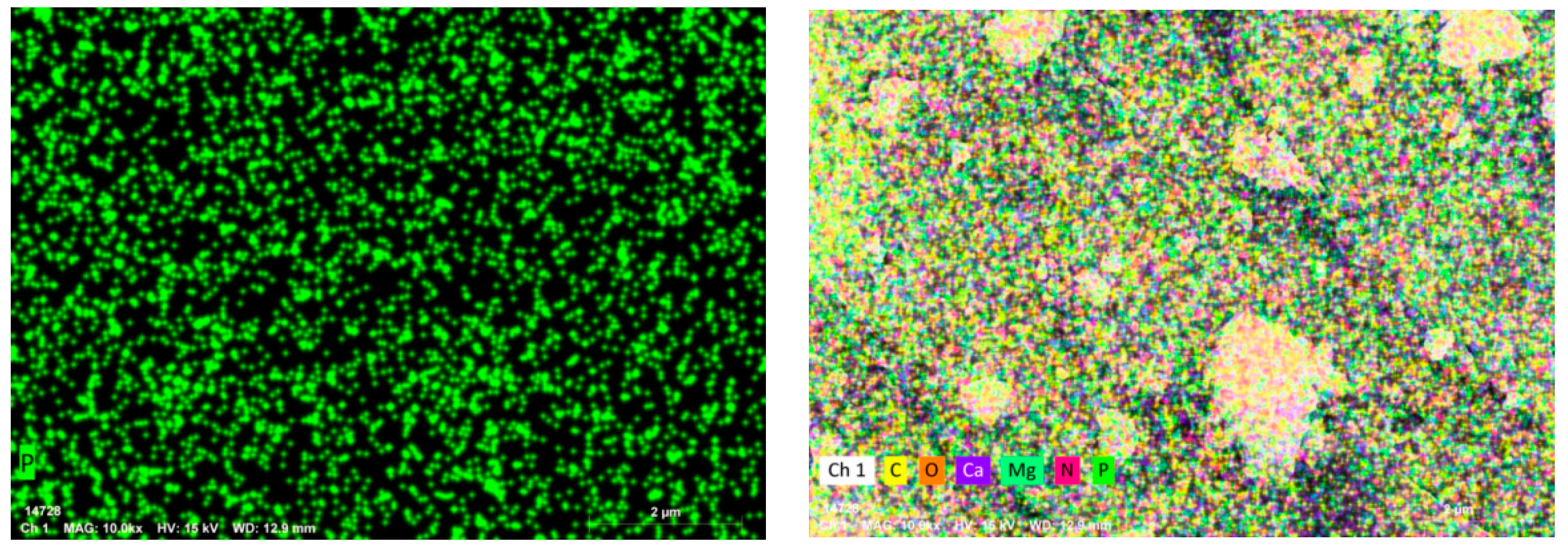

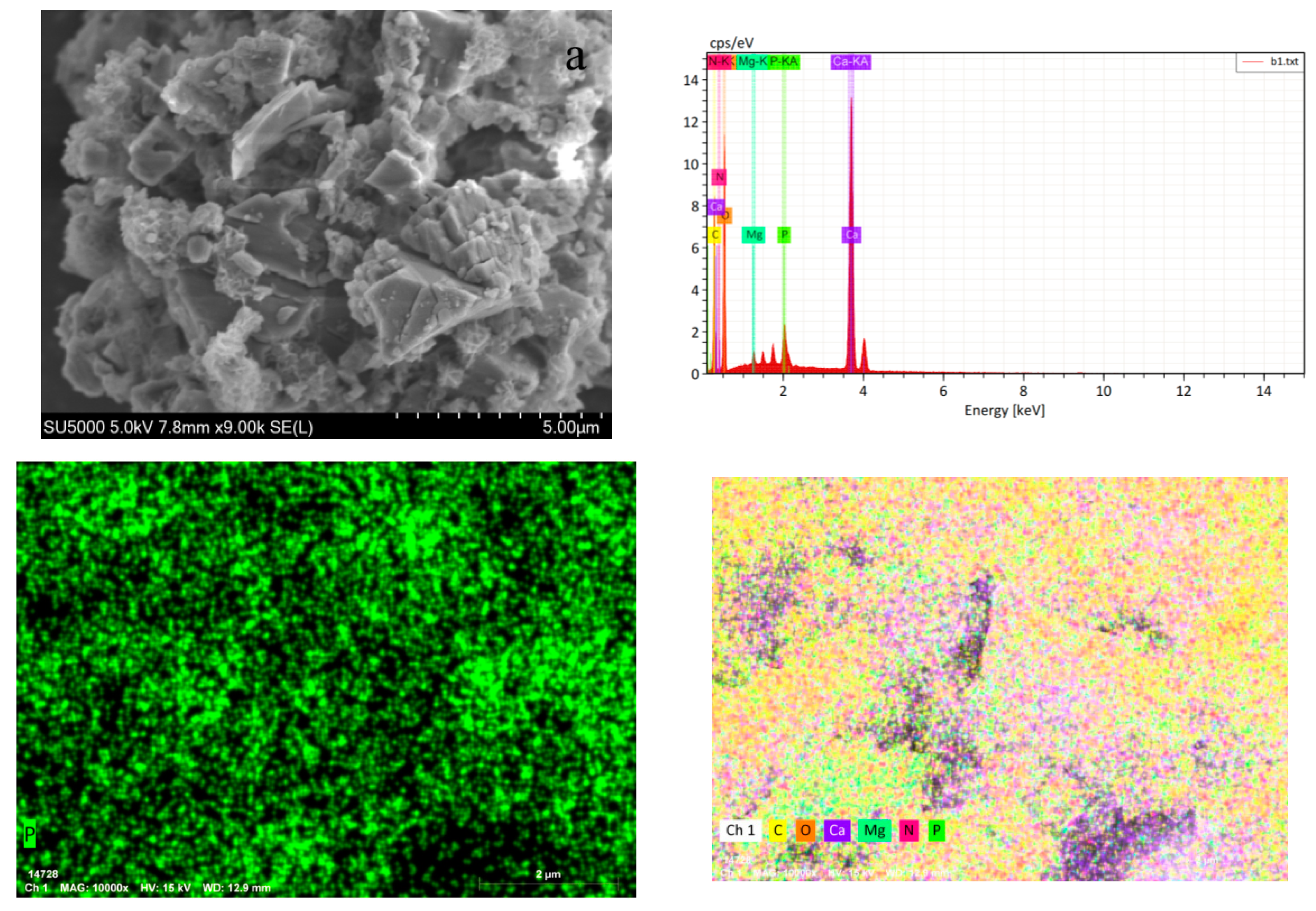
| Elemental | Line Type | Apparent Concentration | wt% | wt% Sigma |
|---|---|---|---|---|
| O | K | 23,335 | 44.78 | 4.37 |
| C | K | 7300 | 11.69 | 1.34 |
| Ca | K | 59,389 | 30.38 | 0.73 |
| Mg | K | 35,215 | 12.95 | 0.56 |
| P | K | 38 | 0.01 | 0.00 |
| Total: | 100.00 |
| Elemental | Line Type | Apparent Concentration | wt% | wt% Sigma |
|---|---|---|---|---|
| O | K | 40,178 | 45.3 | 5.4 |
| C | K | 34,647 | 21.77 | 2.64 |
| Ca | K | 119,194 | 32.19 | 0.98 |
| Mg | K | 1256 | 0.19 | 0.04 |
| P | K | 3647 | 0.54 | 0.05 |
| Total: | 100.00 |
| Elemental | Line Type | Apparent Concentration | wt% | wt% Sigma |
|---|---|---|---|---|
| O | K | 32,120 | 46.48 | 5.37 |
| C | K | 19,982 | 18.44 | 2.25 |
| Ca | K | 90,662 | 32.34 | 0.94 |
| Mg | K | 2373 | 0.58 | 0.06 |
| P | K | 10,001 | 2.16 | 0.1 |
| Total: | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wang, W.; Jiang, Z.; Luo, A. Phosphate in Aqueous Solution Adsorbs on Limestone Surfaces and Promotes Dissolution. Water 2023, 15, 3230. https://doi.org/10.3390/w15183230
Li L, Wang W, Jiang Z, Luo A. Phosphate in Aqueous Solution Adsorbs on Limestone Surfaces and Promotes Dissolution. Water. 2023; 15(18):3230. https://doi.org/10.3390/w15183230
Chicago/Turabian StyleLi, Liang, Wenhai Wang, Zhiwei Jiang, and Anzhong Luo. 2023. "Phosphate in Aqueous Solution Adsorbs on Limestone Surfaces and Promotes Dissolution" Water 15, no. 18: 3230. https://doi.org/10.3390/w15183230
APA StyleLi, L., Wang, W., Jiang, Z., & Luo, A. (2023). Phosphate in Aqueous Solution Adsorbs on Limestone Surfaces and Promotes Dissolution. Water, 15(18), 3230. https://doi.org/10.3390/w15183230







