Solar Disinfection Using Zero Valent Iron for Inactivation of Escherichia coli and Total Coliforms in Water Using a Raceway Reactor
Abstract
:1. Introduction
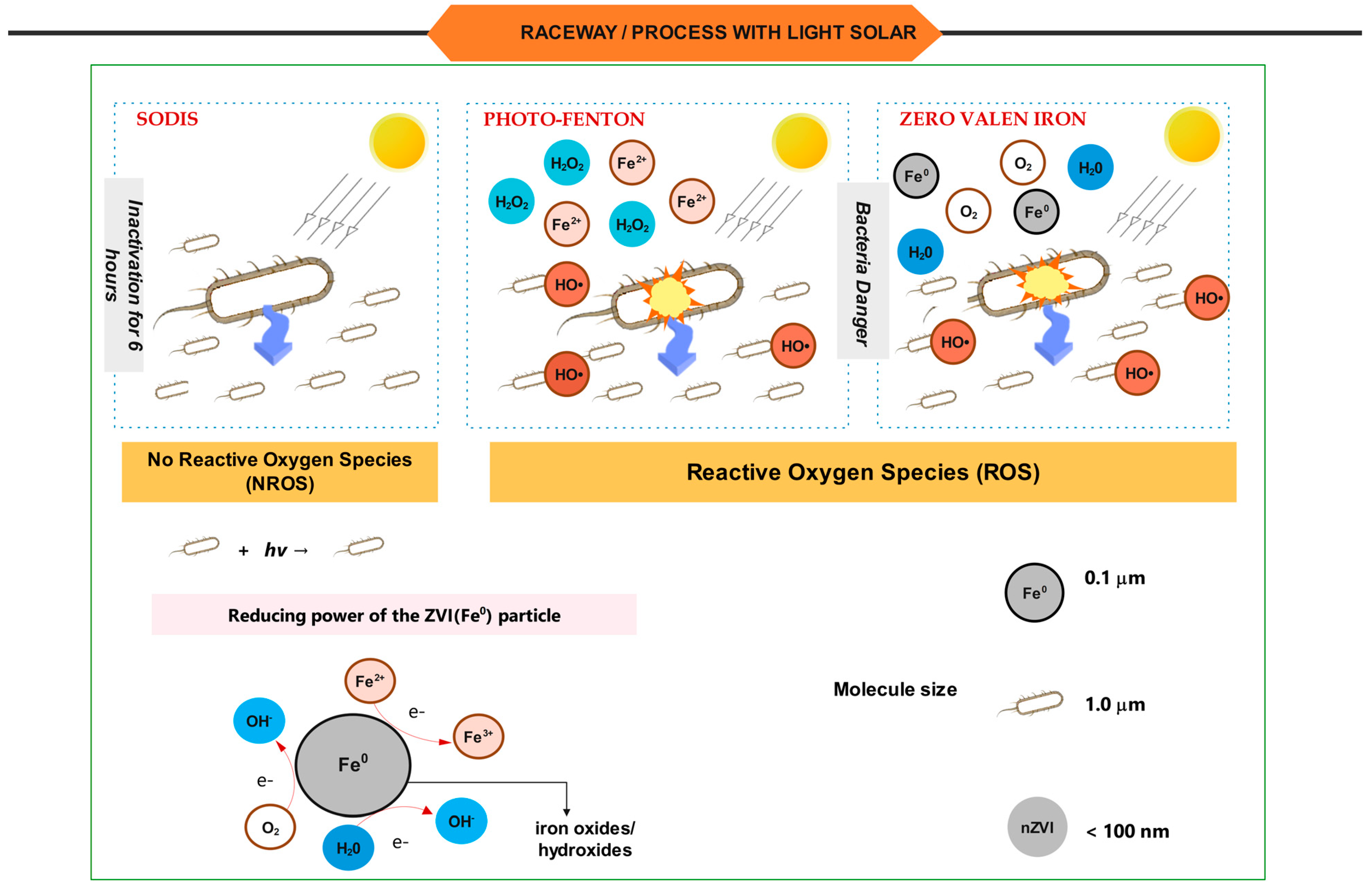
- − SODIS: The process (based on PET container reactors) is an established method for disinfecting domestic water. In 1984, this method was first used to economically disinfect water used to treat diarrhea and dehydration. In communities without access to potable or safe water, it is an inexpensive process for disinfecting natural water using solar radiation. Nevertheless, according to some authors, there are disadvantages associated with the volume of treated water and the possible reappearance of bacteria during consecutive storage periods in darkness [8,9].
- − AOP’s: Are presently regarded as one of the most prevalent and promising wastewater treatment technologies. Among the vast array of available technologies, the most prevalent are the combination of UV and peroxide (UV/H2O2), the Fenton reagent (Fe2+/H2O2), and two of its variants, photo-Fenton and zero-valent iron (Fe0/H2O2). This is a combination of photo-Fenton and zero valent iron (Fe0) or photocatalysis (UV/TiO2).
- − ZVI: Metallic iron (Fe0), also known as zero valent iron (ZVI), is introduced as an economical alternative to iron ions used in Fenton processes. Several studies discuss how Fe0 can be converted to Fe2+. This is referred to as a pseudo-catalytic iron (Fe0)/Fe2+ system. The produced Fe3+ can also be recycled [16,17]. Due to its non-toxic nature, abundance, environmental tolerance, and high surface area and high reactivity, this element has been studied extensively over the past 25 years [18]. ZVI readily corrodes in water. Iron supports spontaneous oxidative dissolution when submerged in water (H+ or H2O) because the redox potential of water is greater than that of iron (Equation (1)). This reaction is predominantly electrochemical [19,20,21].
2. Methodology Conditions
2.1. Materials and Methods
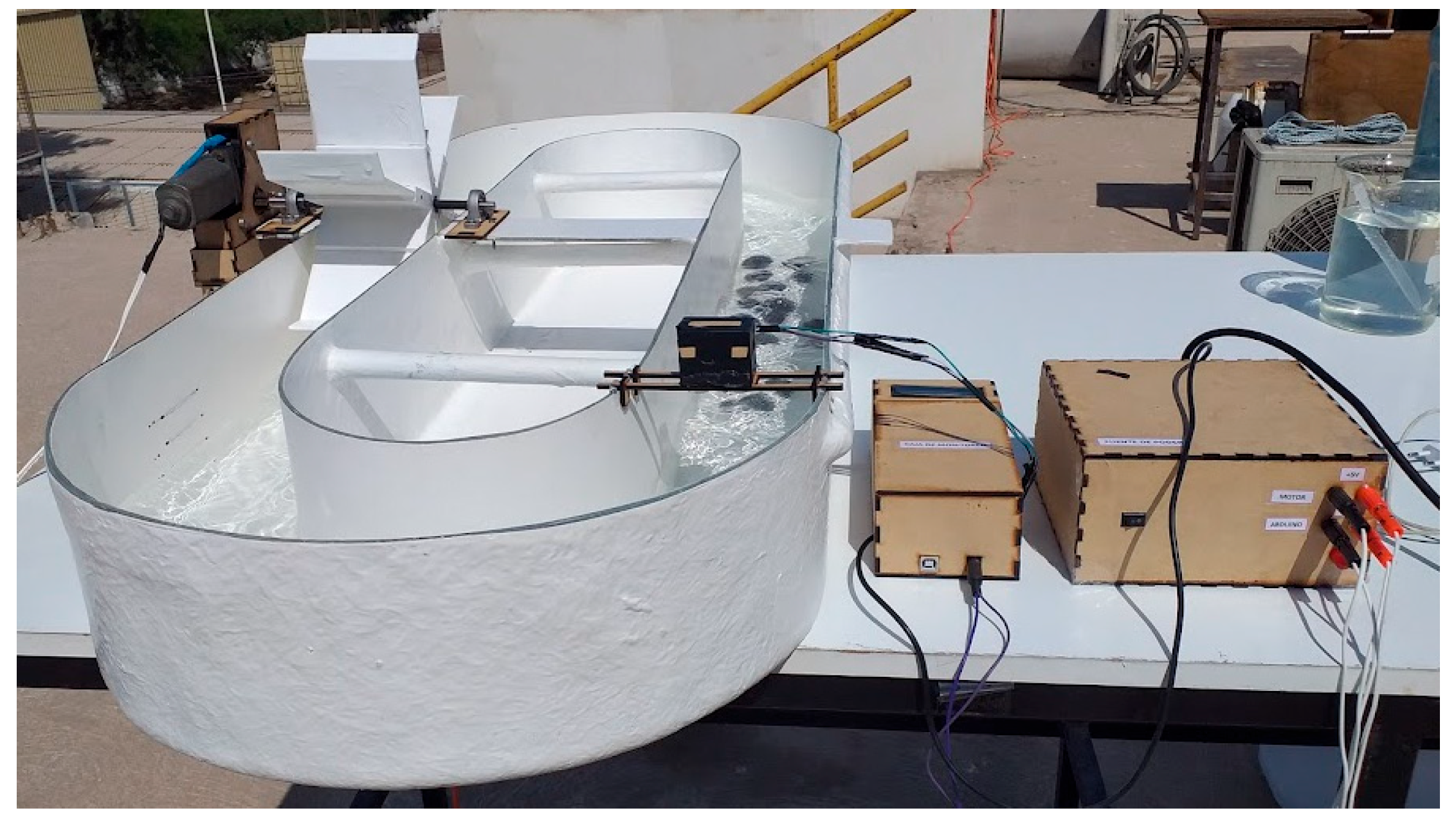
2.1.1. Construction and Sizing of a Raceway Reactor
2.1.2. Laboratory Validation of the Method
Steel Wool (Fe0)
Inoculated Synthetic Drinking Water Matrix (SDW)
Determination of Physicochemical Parameters
2.1.3. Cumulative Energy Absorbed at Raceway Surface (QUV)
2.1.4. Solar Disinfection by Raceway Reactor
2.1.5. Experimental Design and Statistical Analysis
3. Results
3.1. Initial Drinking Water Characterization
3.2. Inoculation
3.3. Solar Disinfection of the SDW on the Raceway
3.4. Data Processing and Statistical Analysis
3.5. Analysis of Radiation Dose in Inactivation Processes
3.6. Analysis of pH, Electrical Conductivity, Temperature, Turbidity, and Dissolved Iron
4. Discussion and Final Comments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ONU. Is Wastewater the New Black Gold? Africa Renewal. 2023. Available online: https://www.un.org/africarenewal/news/wastewater-new-black-gold (accessed on 20 July 2023).
- United Nation. UN World Water Development Report 2017. 2017. Available online: https://www.unwater.org/publications/un-world-water-development-report-2017 (accessed on 20 January 2023).
- Ma, J.; Shi, Y.; An, D.; Chen, Y.; Guo, J.; Qian, Y.; Wang, S.; Lu, J. Inactivation mechanism of E. coli in water by enhanced photocatalysis under visible light irradiation. Sci. Total Environ. 2023, 866, 161450. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/bitstream/handle/10665/254637/9789241549950-eng.pdf (accessed on 22 January 2023).
- Bain, R.; Johnston, R.; Khan, S.; Hancioglu, A.; Slaymaker, T. 2021. Monitoring Drinking Water Quality in Nationally Representative Household Surveys in Low-and Middle-Income Countries: Cross-Sectional Analysis of 27 Multiple Indicator Cluster Surveys 2014–2020. Environ. Health Perspect. 2021, 129, 097010. [Google Scholar] [CrossRef] [PubMed]
- NCh 409/1.Of2005; Agua Potable—Parte 1, Requisitos. INN: Instituto Nacional de Normalización: Santiago, Chile, 2006.
- De la Obra Jiménez, I.; Giannakis, S.; Grandjean, D.; Breider, F.; Grunauer, G.; Casas López, J.L.; Sánchez Pérez, J.A.; Pulgarin, C. Unfolding the action mode of light and homogeneous vs. Heterogeneous photo-Fenton in bacteria disinfection and concurrent elimination of micropollutants in urban wastewater, mediated by iron oxides in Raceway Pond Reactors. Appl. Catal. B 2020, 263, 118158. [Google Scholar] [CrossRef]
- Alvear-Daza, J.J.; García-Barco, A.; Osorio-Vargas, P.; Gutiérrez-Zapata, H.M.; Sanabria, J.; Rengifo-Herrera, J.A. Resistance and induction of viable but non culturable states (VBNC) during inactivation of E. coli and Klebsiella pneumoniae by addition of H2O2 to natural well water under simulated solar irradiation. Water Res. 2021, 188, 116499. [Google Scholar] [CrossRef]
- Chaúque, B.J.M.; Rott, M.B. Solar disinfection (SODIS) technologies as alternative for large-scale public drinking water supply: Advances and challenges. Chemosphere 2021, 281, 130754. [Google Scholar] [CrossRef]
- Nelson, K.L.; Boehm, A.B.; Davies-Colley, R.J.; Dodd, M.C.; Kohn, T.; Linden, K.G.; Liu, Y.; Maraccini, P.A.; McNeill, K.; Mitch, W.A.; et al. Sunlight-mediated inactivation of health-relevant microorganisms in water: A review of mechanisms and modeling approaches. Environ. Sci. Process Impacts 2018, 20, 1089–1122. [Google Scholar] [CrossRef]
- Cabrera-Reina, A.; Miralles-Cuevas, S.; Sánchez Pérez, J.A.; Salazar, R. Application of solar photo-Fenton in raceway pond reactors: A review. Sci. Total Environ. 2021, 800, 149653. [Google Scholar] [CrossRef]
- Faggiano, A.; Ricciardi, M.; Fiorentino, A.; Cucciniello, R.; Motta, O.; Rizzo, L.; Proto, A. Combination of foam fractionation and photo-Fenton like processes for greywater treatment. Sep. Purif. Technol. 2002, 293, 121114. [Google Scholar] [CrossRef]
- Soriano-Molina, P.; Miralles-Cuevas, S.; Esteban García, B.; Plaza-Bolaños, P.; Sánchez Pérez, J.A. Two strategies of solar photo-Fenton at neutral pH for the simultaneous disinfection and removal of contaminants of emerging concern. Comparative assessment in raceway pond reactors. Catal. Today 2021, 361, 17–23. [Google Scholar] [CrossRef]
- Rodrigues-Silva, F.; Masceno, G.P.; Panicio, P.P.; Imoski, R.; Prola, L.D.T.; Vidal, C.B.; Xavier, C.R.; Ramsdorf, W.A.; Passig, F.H.; Liz, M.V.d. Removal of micropollutants by UASB reactor and post-treatment by Fenton and photo-Fenton: Matrix effect and toxicity responses. Environ. Res. 2022, 212, 113396. [Google Scholar] [CrossRef]
- Prabhu, Y.T.; Rao, K.V.; Kumari, B.S.; Kumar, V.S.S.; Pavani, T. Synthesis of Fe3O4 nanoparticles and its antibacterial application. Int. Nano Lett. 2015, 5, 85–92. [Google Scholar] [CrossRef]
- Prousek, J.; Palackova, E.; Priesolova, S.A.; Markova, L.; Alevova, A. Fenton- and fenton-like AOPs for wastewater treat-ment: From Laboratory-to-plant-scale application. Sep. Sci. Technol. 2007, 42, 1505–1520. [Google Scholar] [CrossRef]
- Barndõk, H.; Blanco, L.; Hermosilla, D.; Blanco, A. Heterogeneous photo-Fenton processes using zero valent iron microspheres for the treatment of wastewaters contaminated with 1,4-dioxane. J. Chem. Eng. 2016, 284, 112–121. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.; Liu, H. Review: The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Gerasimov, Y.; Dreving, V.; Eremin, E.; Kiselev, A.; Lebedev, V.; Panchenkov, G.; Shlygin, A. Significance of Oxide-Film in Discussing the Mechanism of Contaminant Removal by Elemental Iron MaterialsPhysical Chemistry; MIR: Moscow, Russia, 1985; Volume 2. [Google Scholar]
- Noubactep, C.; Müller, A.; Martin, S. Significance of oxide-film in discussing the mechanism of contaminant removal by elemental iron materials. In Photo-Electrochemistry & Photo-Biology for the Sustainability; Kaneco, S., Viswanathan, B., Katsumata, H., Eds.; Chapter 2; Union Press: London, UK, 2012; pp. 97–122. [Google Scholar]
- Noubactep, C. Review: Metallic iron for environmental remediation: A review of reviews. Water Res. 2015, 85, 114–123. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. Review Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef]
- Triszcz, J.M.; Porta, A.; García Einschlag, F.S. Effect of operating conditions on iron corrosion rates in zero-valent iron systems for arsenic removal. J. Chem. Eng. 2009, 150, 431–439. [Google Scholar] [CrossRef]
- Santos-Juanes, L.; García-Ballesteros, S.; Vercher, R.F.; Amat, A.M.; Arques, A. Commercial steel wool used for Zero Valent Iron and as a source of dissolved iron in a combined red-ox process for pentachlorophenol degradation in tap water. Catal. Today 2019, 328, 252–258. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kim, J.W.; Watkins, J.; Wilkin, R. Formation of ferrihydrite and associated iron corrosion products in permeable reactive barriers of zero-valent iron. Environ. Sci. Technol. 2002, 36, 5469–5475. [Google Scholar] [CrossRef]
- Zhang, T.C.; Huang, Y.H. Profiling iron corrosion coating on iron grains in a zero valent iron system under the influence of dissolved oxygen. Water. Res. 2006, 40, 2311–2320. [Google Scholar] [CrossRef]
- Pham, A.N.; Rose, A.L.; Fetiz, A.J.; Waite, T.D. Kinetics of Fe(III) precipitation in aqueous solution at pH 6.0–9.5 and 25 °C. Geochim. Cosmochim. Acta 2006, 70, 640–650. [Google Scholar] [CrossRef]
- El Azher, N.; Gourich, B.; Vial, C.; Belhaj Soulami, M.; Ziyad, M. Study of ferrous iron oxidation in Morocco drinking water in an airlift reactor. Chem. Eng. Process 2008, 47, 1877–1886. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Corona, T.; Ray, K.; Nam, W. Heme and Nonheme High-Valent Iron and Manganese Oxo Cores in Biological and Abiological Oxidation Reactions. ACS Central Sci. 2019, 5, 13–28. [Google Scholar] [CrossRef]
- Zhu, F.; Lu, G.P.; Wang, F.; Ren, E.; Yu, Y.; Lin, Y. Iron catalyzed organic reactions in water: A “nature-like” synthesis. Curr. Opin. Green Sustain. Chem. 2023, 40, 100754. [Google Scholar] [CrossRef]
- Crichton, R. Chapter 13—Iron: Essential for Almost All Life. In Biological Inorganic Chemistry: A New Introduction to Molecular Structure and Function, 2nd ed.; Crichton, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 247–277. [Google Scholar]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Lee, C. Review Oxidation of organic contaminants in water by iron-induced oxygen activation: A short review. Environ. Eng. Res. 2015, 20, 205–211. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.Y.; Lee, W.I.; Nelson, K.L.; Yoon, J.; Sedlak, D.L. Bactericidal Effect of Zero-Valent Iron Nanoparticles on Escherichia coli. Environ. Sci. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, P.; Wei, Q.; Andrews, S.C.; Vinckx, T. Iron homeostasis and management of oxidative stress response in bacteria. Metallomics 2011, 3, 540–549. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.; Love, D.C.; Sedlak, D.L.; Yoon, J.; Nelson, K.L. Inactivation of MS2 coliphage by ferrous ion and zero-valent iron nanoparticles. Environ. Sci. Technol. 2011, 45, 6978–6984. [Google Scholar] [CrossRef]
- Imlay, J.A. Where in the world do bacteria experience oxidative stress? Environ. Microbiol. 2019, 21, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.L.; Santiago, F.A.; da Silva, G.A.; De Lima, L.B.; Amaral, L.P.; Nascimento, R.S.L. Inactivation of Escherichia coli in photobioreactors with microalgae and illuminated by light emitting diodes. Int. J. Environ. Sci. Technol. 2023, 20, 63–74. [Google Scholar] [CrossRef]
- Ike, I.A.; Orbell, J.D.; Duke, M. Steel wool and carbonyl iron powder activation of persulphate for the degradation of pollutants. J. Water Process. Eng. 2018, 25, 58–69. [Google Scholar] [CrossRef]
- Roozbeh Jamei, M.; Reza Khosravi, M.; Anvaripour, B. A novel ultrasound assisted method in synthesis of NZVI particles. Ultrason. Sonochem. 2014, 21, 226–233. [Google Scholar] [CrossRef]
- Zhao, P.; Geng, T.; Guo, Y.; Meng, Y.; Zhang, H.; Zhao, W. Transport of E. coli colloids and surrogate microspheres in the filtration process: Effects of flow rate, media size, and media species. Colloids Surf. B. 2022, 220, 112883. [Google Scholar] [CrossRef] [PubMed]
- Thakur, I.; Verma, A.; Örmeci, B. Mathematical modeling of E. coli inactivation in water using Fe-TiO2 composite in a fixed bed reactor. Sep. Purif. Technol. 2021, 260, 118242. [Google Scholar] [CrossRef]
- Sánchez Pérez, J.A.; Arzate, S.; Soriano-Molina, P.; García Sánchez, J.L.; Casas López, J.L.; Plaza-Bolaños, P. Neutral or acidic pH for the removal of contaminants of emerging concern in wastewater by solar photo-Fenton? A techno-economic assessment of continuous raceway pond reactors. Sci. Total Environ. 2020, 736, 139681. [Google Scholar] [CrossRef]
- Salazar, R.; Campos, S.; Martínez, J.; Luna, F.; Thiam, A.; Aranda, M.; Calzadilla, W.; Miralles-Cuevas, S.; Cabrera-Reina, A. New development of a solar electrochemical raceway pond reactor for industrial wastewater treatment. Environ. Res. 2022, 212, 113553. [Google Scholar] [CrossRef]
- Chisti, Y. Raceways-based Production of Algal Crude Oil. Green 2013, 3, 195–216. [Google Scholar] [CrossRef]
- Sompech, K.; Chisti, Y.; Srinophakun, T. Design of raceway ponds for producing microalgae. Biofuels 2012, 3, 387–397. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Elmore, S.; Van Gerven, T.; Stankiewicz, A. Hydrodynamic evaluations in high rate algae pond (HRAP) design. J. Chem. Eng. 2013, 217, 231–239. [Google Scholar] [CrossRef]
- Cornejo, L.; Lienqueo, H.; Arenas, M.; Acarapi, J.; Contreras, D.; Yañez, J.; Mansilla, H. In field arsenic removal from natural water by zero-valent iron assisted by solar radiatio. Environ. Pollut. 2008, 156, 827–831. [Google Scholar] [CrossRef] [PubMed]
- ISO 9308-2, 2012; Water Quality—Enumeration of Escherichia coli and Coliform Bacteria—Part 2: Most Probable Number Method, Second Edition. International Organization of Standard: Geneva, Switzerland, 2012.
- Cornejo, L.; Lienqueo, H.; Vilca, P. Hydro-chemical characteristics, water quality assessment and water relationship (HCA) of the Amuyo Lagoons, Andean Altiplano, Chile. Desalination Water Treat. 2019, 153, 36–45. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E.; Franson, M.A.H. (Eds.) Standard Methods for the Examination of Water & Wastewater, Centennial Edition; Amer Public Health Assn: New York, NY, USA, 2005. [Google Scholar]
- ISO 6332:1988; Water quality—Determination of Iron—Spectrometric Method Using 1,10-Phenanthroline. 2nd ed. International Organization of Standard: Geneva, Switzerland, 1988.
- Giannakis, S.; López, M.I.P.; Spuhler, D.; Pérez, J.A.S.; Ibáñez, P.F.; Pulgarin, C. Solar disinfection is an augmentable, in situ-generated photo-Fenton reaction—Part 2: A review of the applications for drinking water and wastewater disinfection. Appl. Catal. B 2016, 198, 431–446. [Google Scholar] [CrossRef]
- Cabrera-Reina, A.; Miralles-Cuevas, S.; Rivas, G.; Sánchez Pérez, J.A. Comparison of different detoxification pilot plants for the treatment of industrial wastewater by solar photo-Fenton: Are raceway pond reactors a feasible option? Sci. Total Environ. 2019, 648, 601–608. [Google Scholar] [CrossRef]
- Moraga-Contreras, C.; Cornejo-Ponce, L.; Vilca-Salinas, P.; Estupiñan, E.; Zuñiga, A.; Palma-Behnke, R.; Tapia-Caroca, H. Evolution of Solar Energy in Chile: Residential Opportunities in Arica and Parinacota. Energies 2022, 15, 551. [Google Scholar] [CrossRef]
- Cornejo, L.; Lienqueo, H.; Arenas, M.; Acarapi, J.; Mansilla, H.; Yañez, J. Removal of arsenic from natural waters of the Camarones River and their use for human consumption by the modified SORAS—Zerovalent iron technology. In Final Results of the OAS/AE/141 Project: Research, Development, Validation and Application of Solar Technologies for Water Potabilization in Isolated Rural Zones of Latin America and the Caribbean; Litter, M.I., Ed.; OEA: Buenos Aires, Argentina, 2006; pp. 135–157. [Google Scholar]
- Noubactep, C.; Schöner, A.; Woafo, P. Review Metallic Iron Filters for Universal Access to Safe Drinking Water. Clean 2009, 37, 930–937. [Google Scholar]
- Sun, H.; Wang, J.; Jiang, Y.; Shen, W.; Jia, F.; Wang, S.; Liao, X.; Zhang, L. Rapid Aerobic Inactivation and Facile Removal of Escherichia coli with Amorphous Zero-Valent Iron Microspheres: Indispensable Roles of Reactive Oxygen Species and Iron Corrosion Products. Environ. Sci. Technol. 2019, 53, 3707–3717. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, L.; Dong, H.; Li, R.; Tian, R.; Chen, J. Influence of several crucial groundwater components on the toxicity of nanoscale zero-valent iron towards Escherichia coli under aerobic and anaerobic conditions. Chemosphere 2021, 285, 131453. [Google Scholar] [CrossRef]
- Oprčkal, P.; Mladenovič, A.; Vidmar, J.; Mauko Pranjić, A.; Milačič, R.; Ščančar, J. Critical evaluation of the use of different nanoscale zero-valent iron particles for the treatment of effluent water from a small biological wastewater treatment plant. Chem. Eng. J. 2017, 321, 20–30. [Google Scholar] [CrossRef]
- Hsueh, Y.-H.; Tsai, P.-H.; Lin, K.-S.; Ke, W.-J.; Chiang, C.-L. Antimicrobial effects of zero-valent iron nanoparticles on gram-positive Bacillus strains and gram-negative Escherichia coli strains. J. Nanobiotechnol. 2017, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Ševců, A.; El-Temsah, Y.S.; Joner, E.J.; Černík, M. Oxidative Stress Induced in Microorganisms by Zero-valent Iron Nanoparticles. Microbes Environ. 2011, 26, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Achouak, W.; Rose, J.; Roncato, M.-A.; Chanéac, C.; Waite, D.T.; Masion, A.; Woicik, J.C.; Wiesner, M.R.; Bottero, J.-Y. Relation between the redox state of iron-based nanoparticles and their cytotoxicity toward Escherichia coli. Environ. Sci. Technol. 2008, 42, 6730–6735. [Google Scholar] [CrossRef] [PubMed]
- Mejri, A.; Soriano-Molina, P.; Miralles-Cuevas, S.; Sánchez Pérez, J.A. Fe3+-NTA as iron source for solar photo-Fenton at neutral pH in raceway pond reactors. Sci. Total Environ. 2020, 736, 139617. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Pulido, H.; De la Vara, R. Análisis y Diseño de Experimentos, 2nd ed.; McGraw-Hill Interamericana: Mexico City, Mexico, 2008; Volume 3, p. 564. [Google Scholar]
- Englehardt, J.; Meeroof, D.; Echegoyen, L.; Deng, Y.; Raymo, F.; Shibata, T. Oxidation of aqueous EDTA and associated or-ganics and coprecipitation of inorganics by ambient iron-mediated aeration. Environ. Sci. Technol. 2007, 41, 270–276. [Google Scholar] [CrossRef]
- Kallel, M.; Belaid, C.; Mechichi, T.; Ksibi, M.; Elleuch, B. Removal of organic load and phenolic compounds from olive mill wastewater by Fenton oxidation with zero-valent iron. Chem. Eng. J. 2009, 150, 391–395. [Google Scholar] [CrossRef]
- Bergendahl, J.A.; Thies, T.P. Fenton’s oxidation of MTBE with zero-valent iron. Water Res. 2004, 38, 327–334. [Google Scholar] [CrossRef]
- Solsona, F.; Méndez, J.P. Desinfección del Agua; Centro Panamerícano de Ingeniería Sanitaria y Ciencias del Ambiente: Lima, Peru, 2002; pp. 59–158. [Google Scholar]
- United State Environmental Protection Agency, US EPA. Secondary Drinking Water Standards: Guidance for Nuisance Chemicals. 2017. Available online: https://www.epa.gov/sdwa/secondary-drinking-water-standards-guidance-nuisance-chemicals#table (accessed on 20 March 2023).
- You, Y.; Han, J.; Chiu, P.C.; Jin, Y. Removal and Inactivation of Waterborne Viruses Using Zerovalent Iron. Environ. Sci. Technol. 2005, 39, 9263–9269. [Google Scholar] [CrossRef]





| Parameter | Unit | |
|---|---|---|
| pH | - | 6.97 |
| Electrical Conductivity | µScm−1 | 2067 |
| Turbidity | NTU | 0.17 |
| Total Hardness | CaCO3 mgL−1 | 755 |
| Alkalinity | CaCO3 mgL−1 | 138 |
| Free chlorine | mgL−1 | 0.00 |
| Total Coliforms | MPN 100 mL−1 | <1 |
| E. coli | MPN 100 mL−1 | <1 |
| h (cm) | S × Ac × h × 1000 | F (mL) |
|---|---|---|
| 5 | 15.5 | 6.2 |
| 7 | 21.6 | 8.7 |
| 9 | 27.8 | 11.1 |
| N° Exp. | Fe0 (gL−1) | Level (cm) | Time (h) | Initial (MPN 100 mL−1) | Final (MPN 100 mL−1) | Log-Red Colif. Tot. (%) | Initial (MPN 100 mL−1) | Final (MPN 100 mL−1) | E. coli (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.6 | 5 | 4 | >2419.6 | 4.1 | 99.83 | >2419.6 | 4.1 | 99.83 |
| 2 | 1.8 | 5 | 4 | >2419.6 | 14.6 | 99.40 | >2419.6 | 2.0 | 99.92 |
| 3 | 0.6 | 9 | 4 | >2419.6 | 14.6 | 99.40 | >2419.6 | 6.3 | 99.74 |
| 4 | 1.8 | 9 | 4 | 1732.9 | <1.0 | 99.94 | 1553.1 | <1.0 | 99.94 |
| 5 | 0.6 | 5 | 6 | >2419.6 | 25.4 | 98.95 | >2419.6 | <1.0 | 99.96 |
| 6 | 1.8 | 5 | 6 | >2419.6 | 2.0 | 99.92 | >2419.6 | <1.0 | 99.96 |
| 7 | 0.6 | 9 | 6 | >2419.6 | 11.0 | 99.55 | >2419.6 | <1.0 | 99.96 |
| 8 | 1.8 | 9 | 6 | >2419.6 | <1.0 | 99.96 | 115.3 | <1.0 | 99.13 |
| 9 | 1.2 | 7 | 5 | >2419.6 | 3.0 | 99.88 | >2419.6 | 3.0 | 99.88 |
| 10 | 1.2 | 7 | 5 | 727.0 | <1.0 | 99.86 | 686.7 | <1.0 | 99.85 |
| 11 | 1.2 | 7 | 5 | >2419.6 | 3.1 | 99.87 | >2419.6 | 3.0 | 99.88 |
| 12 | 1.2 | 7 | 5 | >2419.6 | <1.0 | 99.96 | >2419.6 | <1.0 | 99.96 |
| Source | Sum of Squares | Df | Mean Square | Ratio-F | p-Value |
|---|---|---|---|---|---|
| A: Dose Fe0 | 0.2775 | 1 | 0.2775 | 2.78 | 0.1562 |
| B: Liquid level | 0.0703 | 1 | 0.0703 | 0.70 | 0.4394 |
| C: Time | 0.0045 | 1 | 0.0045 | 0.05 | 0.8400 |
| AB | 0.0210 | 1 | 0.0210 | 0.21 | 0.6655 |
| AC | 0.2016 | 1 | 0.2016 | 2.02 | 0.2144 |
| BC | 0.0351 | 1 | 0.0351 | 0.35 | 0.5788 |
| Total error | 0.4987 | 5 | 0.0997 | ||
| Total (corr.) | 1.1088 | 11 |
| Source | Sum of Squares | Df | Mean Square | Ratio-F | p-Value |
|---|---|---|---|---|---|
| A: Dose Fe0 | 0.2775 | 1 | 0.2775 | 132.68 | 0.0014 |
| B: Liquid level | 0.0703 | 1 | 0.0703 | 33.62 | 0.0102 |
| C: Time | 0.0045 | 1 | 0.0045 | 2.16 | 0.2382 |
| AB | 0.0210 | 1 | 0.0210 | 10.05 | 0.0505 |
| AC | 0.2016 | 1 | 0.2016 | 96.39 | 0.0022 |
| BC | 0.0351 | 1 | 0.0351 | 16.79 | 0.0263 |
| Lack-of-fit | 0.4924 | 2 | 0.2462 | 117.72 | 0.0014 |
| Pure error | 0.0062 | 3 | 0.0021 | ||
| Total (corr.) | 1.1088 | 11 |
| Source | Sum of Squares | Df | Mean Square | Ratio-F | p-Value |
|---|---|---|---|---|---|
| A: Doses Fe0 | 0.0364 | 1 | 0.0364 | 1.33 | 0.3017 |
| B: Liquid level | 0.1012 | 1 | 0.1012 | 3.68 | 0.1131 |
| C:Time | 0.0221 | 1 | 0.0221 | 0.80 | 0.4116 |
| AB | 0.0648 | 1 | 0.0648 | 2.36 | 0.1854 |
| AC | 0.1568 | 1 | 0.1568 | 5.70 | 0.0626 |
| BC | 0.0722 | 1 | 0.0722 | 2.62 | 0.1661 |
| Total error | 0.1375 | 5 | 0.0275 | ||
| Total (corr.) | 0.5911 | 11 |
| Source | Sum of Squares | Df | Mean Square | Ratio-F | p-Value |
|---|---|---|---|---|---|
| A: Dose Fe0 | 0.0364 | 1 | 0.0364 | 16.38 | 0.0272 |
| B: Liquid level | 0.1012 | 1 | 0.1012 | 45.51 | 0.0067 |
| C: Time | 0.0221 | 1 | 0.0220 | 9.91 | 0.0513 |
| AB | 0.0648 | 1 | 0.0648 | 29.12 | 0.0125 |
| AC | 0.1568 | 1 | 0.1568 | 70.47 | 0.0035 |
| BC | 0.0722 | 1 | 0.0722 | 32.45 | 0.0107 |
| Lack-of-fit | 0.1308 | 2 | 0.0654 | 29.41 | 0.0107 |
| Pure error | 0.0066 | 3 | 0.0022 | ||
| Total (corr.) | 0.5911 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lienqueo-Aburto, H.; Cornejo-Ponce, L.; Baca-Delgado, L.; Vilca-Salinas, P.; Arenas-Herrera, M.J. Solar Disinfection Using Zero Valent Iron for Inactivation of Escherichia coli and Total Coliforms in Water Using a Raceway Reactor. Water 2023, 15, 3211. https://doi.org/10.3390/w15183211
Lienqueo-Aburto H, Cornejo-Ponce L, Baca-Delgado L, Vilca-Salinas P, Arenas-Herrera MJ. Solar Disinfection Using Zero Valent Iron for Inactivation of Escherichia coli and Total Coliforms in Water Using a Raceway Reactor. Water. 2023; 15(18):3211. https://doi.org/10.3390/w15183211
Chicago/Turabian StyleLienqueo-Aburto, Hugo, Lorena Cornejo-Ponce, Laura Baca-Delgado, Patricia Vilca-Salinas, and María Janet Arenas-Herrera. 2023. "Solar Disinfection Using Zero Valent Iron for Inactivation of Escherichia coli and Total Coliforms in Water Using a Raceway Reactor" Water 15, no. 18: 3211. https://doi.org/10.3390/w15183211
APA StyleLienqueo-Aburto, H., Cornejo-Ponce, L., Baca-Delgado, L., Vilca-Salinas, P., & Arenas-Herrera, M. J. (2023). Solar Disinfection Using Zero Valent Iron for Inactivation of Escherichia coli and Total Coliforms in Water Using a Raceway Reactor. Water, 15(18), 3211. https://doi.org/10.3390/w15183211








