Genotoxic Effects on Daphnia magna Fed with Aquatic Green Algae Exposed to Silver Nanoclusters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
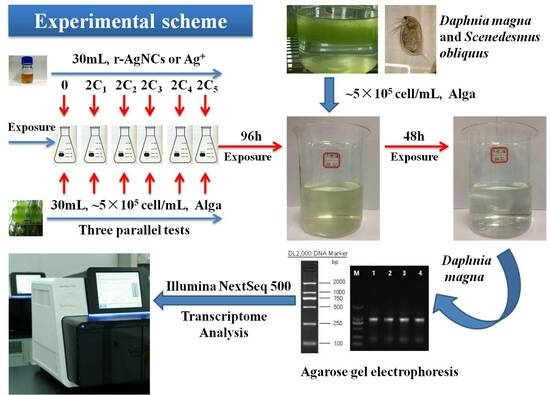
2.2. Algae Culture and Nanosilver Exposure Conditions
2.3. Daphnia Magna Culture and Ag Exposure
2.4. RNA Sequencing
2.5. Transcriptomic Analysis
3. Results and Discussion
3.1. Sequencing and De Novo Transcriptome Assembly
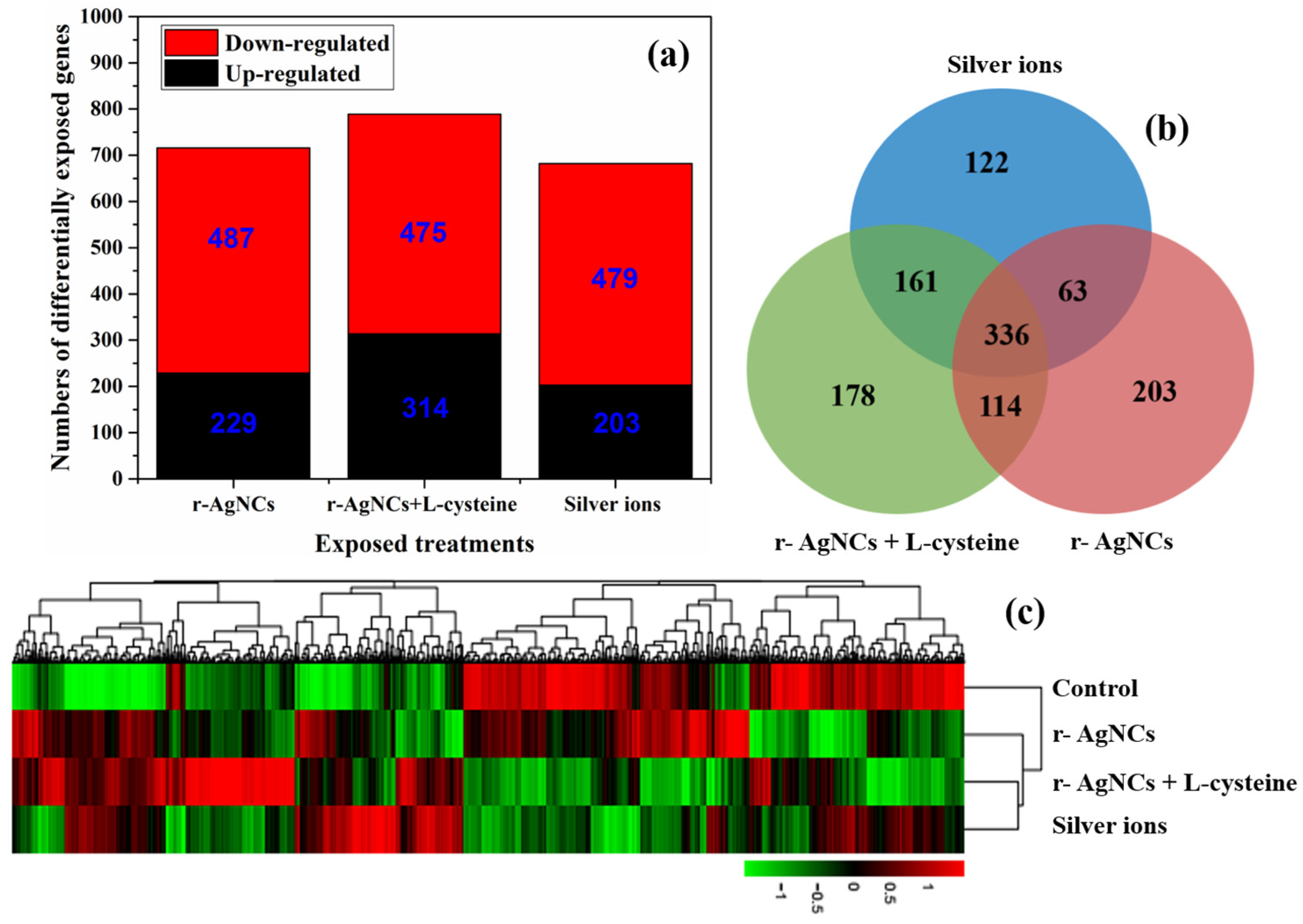
3.2. Differential Expression Gene Analysis
3.3. Enrichment Analysis of the Differential Expression Genes
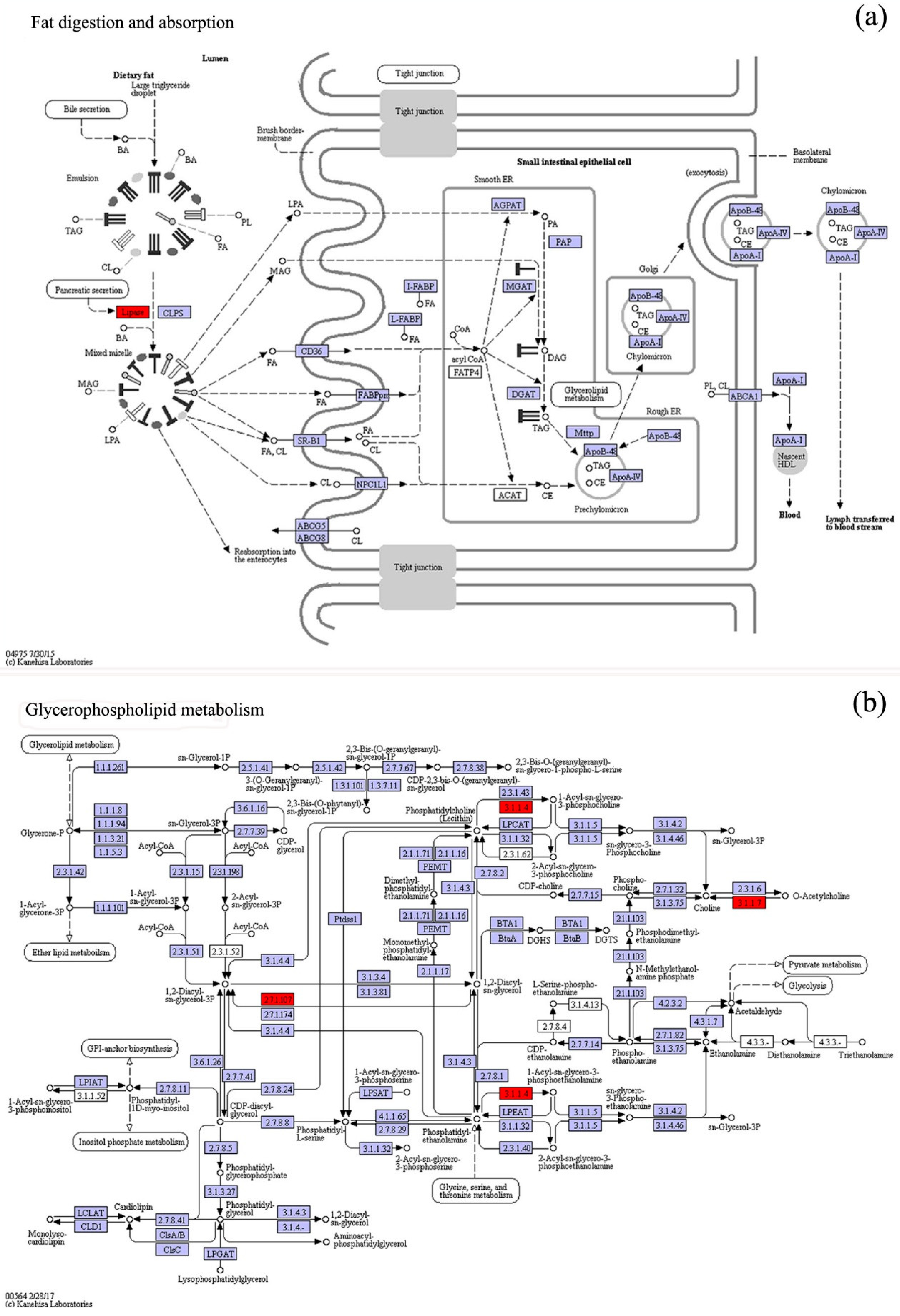
3.4. KO Metabolic Pathway Analysis of the Differential Expression Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.Z.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623. [Google Scholar] [CrossRef] [PubMed]
- Diez, I.; Ras, R.H. Fluorescent silver nanoclusters. Nanoscale 2011, 3, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Dong, S.J.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Xu, H.X.; Suslick, K.S. Water-soluble fluorescent silver nanoclusters. Adv. Mater. 2010, 22, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Guidez, E.B.; Aikens, C.M. Theoretical analysis of the optical excitation spectra of silver and gold nanowires. Nanoscale 2012, 4, 4190–4198. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yeow, T.J.; Zhang, Q.; Lee, J.Y.; Xie, J.P. Highly luminescent Ag+ nanoclusters for Hg2+ ion detection. Nanoscale 2012, 4, 1968–1971. [Google Scholar] [CrossRef]
- Zheng, K.; Yuan, X.; Kuah, K.; Luo, Z.; Yao, Q.; Zhang, Q.; Xie, J.P. Boiling water synthesis of ultrastable thiolated silver nanoclusters with aggregation-induced emission. Chem. Commun. 2015, 51, 15165–15168. [Google Scholar] [CrossRef]
- Murray, R.W. Nanoelectrochemistry: Metal Nanoparticles, Nanoelectrodes, and Nanopores. Chem. Rev. 2008, 108, 2688–2720. [Google Scholar] [CrossRef]
- Yuan, X.; Tay, Y.; Dou, X.; Luo, Z.; Leong, D.T.; Xie, J.P. Glutathione-protected silver nanoclusters as cysteine-selective fluorometric and colorimetric probe. Anal. Chem. 2013, 85, 1913–1919. [Google Scholar] [CrossRef]
- Guo, W.W.; Yuan, J.P.; Dong, Q.Z.; Wang, E. Highly sequence-dependent formation of fluorescent silver nanoclusters in hybridized DNA duplexes for single nucleotide mutation identification. J. Am. Chem. Soc. 2010, 132, 932–934. [Google Scholar] [CrossRef]
- Yeh, H.C.; Sharma, J.; Han, J.J.; Martinez, J.S.; Werner, J.H. A DNA–silver nanocluster probe that fluoresces upon hybridization. Nano Lett. 2010, 10, 3106–3110. [Google Scholar] [CrossRef]
- Zheng, C.R.; Li, S.; Ye, C.; Li, X.; Zhang, C.; Yu, X. Particulate respirators functionalized with silver nanoparticles showed excellent real-time antimicrobial effects against pathogens. Environ. Sci. Technol. 2016, 50, 7144–7151. [Google Scholar] [CrossRef]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Leclerc, S.; Wilkinson, K.J. Bioaccumulation of nanosilver by Chlamydomonas reinhardtii-nanoparticle or the free ion? Environ. Sci. Technol. 2014, 48, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Kaveh, R.; Li, Y.S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Van Aken, B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013, 47, 10637–10644. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.S.; Guagliardo, P.; Jiang, H.B.; Wang, W.X. Intra- and intercellular silver nanoparticle translocation and transformation in Oyster gill filaments: Coupling nanoscale secondary ion mass spectrometry and dual stable isotope tracing study. Environ. Sci. Technol. 2021, 55, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.P.; Coleman, J.G.; Bednar, A.J.; Kennedy, A.J.; Ranville, J.F.; Higgins, C.P. 2013. Extraction and analysis of silver and gold nanoparticles from biological tissues using single particle inductively coupled plasma mass spectrometry. Environ. Sci. Technol. 2013, 47, 14315–14323. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Liu, L.H.; Liu, X.L.; Yin, Y.G.; Qu, G.B.; Shi, J.B.; Song, M.Y.; He, B.; Hu, L.G.; et al. Administration of silver nasal spray leads to nanoparticle accumulation in rat brain tissues. Environ. Sci. Technol. 2022, 56, 403–413. [Google Scholar] [CrossRef]
- Wang, M.Y.; Wang, W.X. Nanoscale whole-body expansion microscopy revealed the early skeletal developmental malformation induced by silver nanoparticles. Environ. Sci. Technol. Lett. 2023, 10, 471–477. [Google Scholar] [CrossRef]
- Farre, M.; Gajda-Schrantz, K.; Kantiani, L.; Barcelo, D. Ecotoxicity and analysis of nanomaterials in the aquatic environment. Anal. Bioanal. Chem. 2009, 393, 81–95. [Google Scholar] [CrossRef]
- Gupta, G.S.; Kumar, A.; Senapati, V.A.; Pandey, A.K.; Shanker, R.; Dhawan, A. Laboratory scale microbial food chain to study bioaccumulation, biomagnification, and ecotoxicity of cadmium telluride quantum dots. Environ. Sci. Technol. 2017, 51, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- McTeer, J.; Dean, A.P.; White, K.N.; Pittman, J.K. Bioaccumulation of silver nanoparticles into Daphnia magna from a freshwater algal diet and the impact of phosphate availability. Nanotoxicology 2014, 8, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Quigg, A.; Chin, W.-C.; Chen, C.-S.; Zhang, S.; Jiang, Y.; Miao, A.J.; Schwehr, K.A.; Xu, C.; Santschi, P.H. Direct and Indirect Toxic Effects of Engineered Nanoparticles on Algae: Role of Natural Organic Matter. ACS Sustain. Chem. Eng. 2013, 1, 686–702. [Google Scholar] [CrossRef]
- Chisholm, S.W. Stirring times in the Southern Ocean. Nature 2000, 407, 685–687. [Google Scholar] [CrossRef]
- Yan, N.; Tang, B.Z.; Wang, W.X. In vivo bioimaging of silver nanoparticle dissolution in the gut environment of zooplankton. ACS Nano 2018, 12, 12212–12223. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E., Jr. Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of nanomaterials in the environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef]
- Yu, S.J.; Yin, Y.G.; Liu, J.F. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts 2013, 15, 78–92. [Google Scholar] [CrossRef]
- Gil-Allue, C.; Schirmer, K.; Tlili, A.; Gessner, M.O.; Behra, R. Silver nanoparticle effects on stream periphyton during short-term exposures. Environ. Sci. Technol. 2015, 49, 1165–1172. [Google Scholar] [CrossRef]
- Ankley, G.T.; Daston, G.P.; Degitz, S.J. Toxicogenomics in Regulatory Ecotoxicology. Environ. Sci. Technol. 2006, 40, 4055–4065. [Google Scholar] [CrossRef]
- Chen, C.; Unrine, J.M.; Judy, J.D.; Lewis, R.W.; Guo, J.; McNear, D.H.; Tsyusko, O.V. Toxicogenomic responses of the model legume medicago truncatula to aged biosolids containing a mixture of nanomaterials (TiO2, Ag, and ZnO) from a pilot wastewater treatment plant. Environ. Sci. Technol. 2015, 49, 8759–8768. [Google Scholar] [CrossRef] [PubMed]
- Poynton, H.C.; Lazorchak, J.M.; Impellitteri, C.A.; Blalock, B.J.; Rogers, K.; Allen, H.J.; Loguinov, A.; Heckman, J.L.; Govindasmawy, S. Toxicogenomic responses of nanotoxicity in Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Environ. Sci. Technol. 2012, 46, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Setyawati, M.I.; Tan, A.S.; Ong, C.N.; Leong, D.T.; Xie, J.P. Highly luminescent silver nanoclusters with tunable emissions: Cyclic reduction–decomposition synthesis and antimicrobial properties. NPG Asia Mater. 2013, 5, e39. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.L.; Goswami, N.; Xie, J.P.; Zhang, B.; Tao, X.J. Uptake and effect of highly fluorescent silver nanoclusters on Scenedesmus obliquus. Chemosphere 2016, 153, 322–331. [Google Scholar] [CrossRef]
- Zhang, L.; Goswami, N.; Xie, J.P.; Zhang, B.; He, Y.L. Unraveling the molecular mechanism of photosynthetic toxicity of highly fuorescent silver nanoclusters to Scenedesmus obliquus. Sci. Rep. 2017, 7, 16432. [Google Scholar] [CrossRef]
- Yuan, X.; Setyawati, M.I.; Leong, D.T.; Xie, J.P. Ultrasmall Ag+-rich nanoclusters as highly efficient nanoreservoirs for bacterial killing. Nano Res. 2013, 7, 301–307. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Li, P.; Huang, W.; Tang, J.; Wang, P.; Liu, J.; Yuan, Q.; Bai, R.; Li, B.; et al. Use of Synchrotron radiation-analytical techniques to reveal chemical origin of silver-nanoparticle cytotoxicity. ACS Nano 2015, 9, 6532–6547. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Tan, H. Genotoxic Effects on Daphnia magna Fed with Aquatic Green Algae Exposed to Silver Nanoclusters. Water 2023, 15, 3172. https://doi.org/10.3390/w15183172
Zhang L, Tan H. Genotoxic Effects on Daphnia magna Fed with Aquatic Green Algae Exposed to Silver Nanoclusters. Water. 2023; 15(18):3172. https://doi.org/10.3390/w15183172
Chicago/Turabian StyleZhang, Li, and Haoqiang Tan. 2023. "Genotoxic Effects on Daphnia magna Fed with Aquatic Green Algae Exposed to Silver Nanoclusters" Water 15, no. 18: 3172. https://doi.org/10.3390/w15183172
APA StyleZhang, L., & Tan, H. (2023). Genotoxic Effects on Daphnia magna Fed with Aquatic Green Algae Exposed to Silver Nanoclusters. Water, 15(18), 3172. https://doi.org/10.3390/w15183172