Glass Eel Restocking Experiments in Typologically Different Upland Rivers: How Much Have We Learned about the Importance of Recipient Habitats?
Abstract
:1. Introduction
2. Materials and Methods
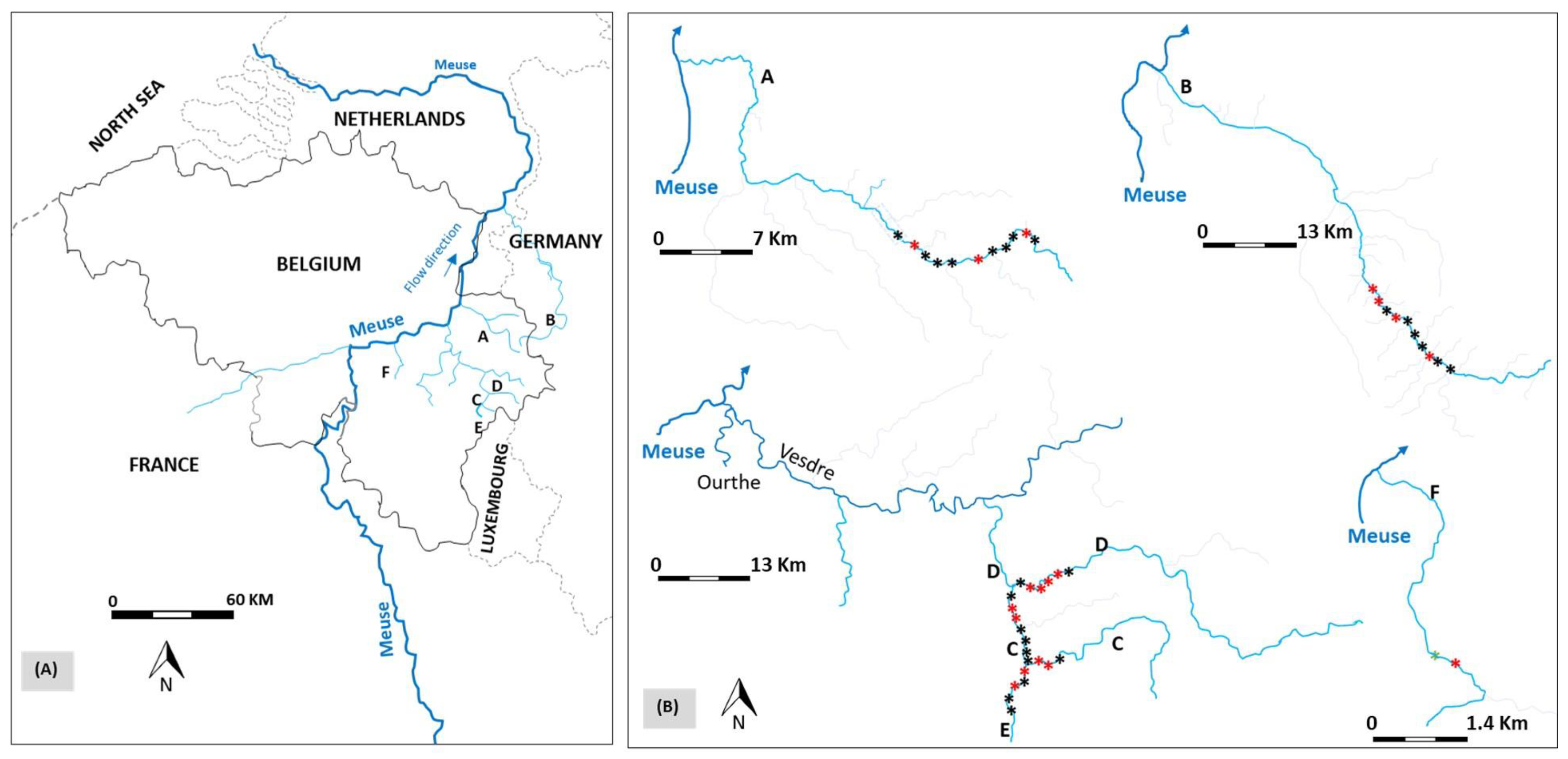
2.1. Study Area
2.2. Restocking Using Glass Eels
2.3. Eel Collection and Tagging
2.4. Demographic Metrics in Eels
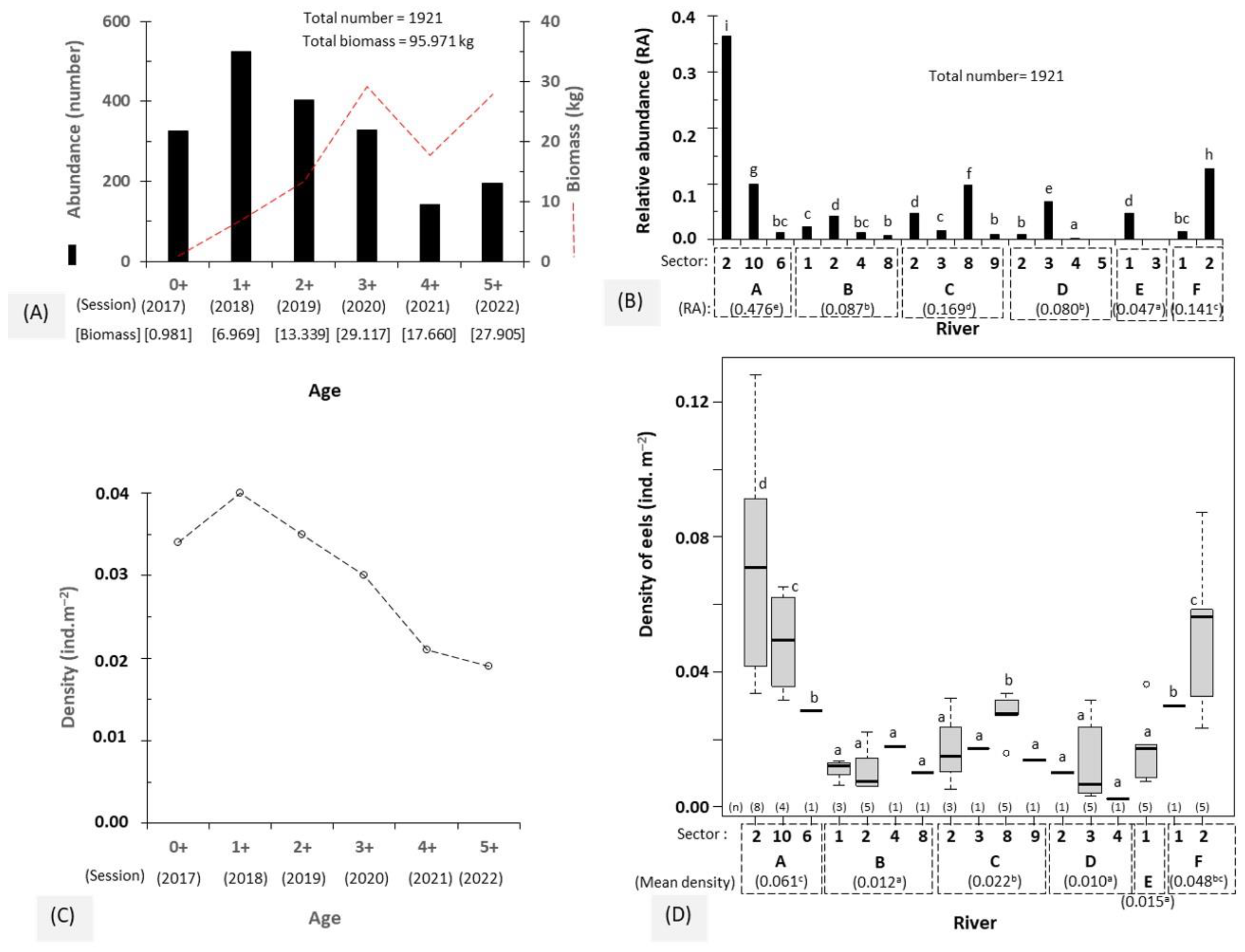
- The observed abundance is determined by counting the number of individuals caught at each age, and the biomass is the sum of the weights of all eels caught at each age. Age is expressed as the number of years after the glass eel restocking in the rivers. A value of 0+ means that the eels are in their first-year river life.
- The relative abundance is defined as the total number of eels captured in a sector/river divided by the sum of all eels caught in all sectors/rivers.
- The eels’ density is represented by the ratio between the number of eels caught and the total area electro-fished at each eel age.
- The Durif Silvering Index was assessed to ascertain the eel developmental phase. This index is predicated upon the variables of TL, pectoral fin length (±1 mm) and mean eye diameter (±1 mm) [58,59]. Given that eels restocked in rivers are in the growth phase during their first two years (from 0+ in 2017 to 1+ in 2018), we evaluated this index in these individuals from their third year (2+ in 2019) of river life.
- The length (TL) and weight (W) relationship at each age was calculated using the equation W = a × TL b that was logarithmically transformed into a linear relation as log10 (W) = b × log10 (TL) + log10 (a), where W is the weight (g), TL is total length (cm) and a and b are the coefficients. a is the intercept or coefficient referring to body shape, and b indicates the slope or growth coefficient to identify the type of growth with b = 3 meaning isometric growth, b < 3 negative allometric growth and b > 3 positive allometric growth [62].
- The mean annual TL increment (G in mm.year−1) was assessed in eels 5+ using the following formula: G = (TL − TL0) × (T)−1, where TL is the TL (mm) at their capture, TL0 is the TL (mm) of glass eels at release and T is the age after restocking [63]. It was also evaluated between two successive ages using the following equation: G = (TLi + 1 − TLi) × (T)−1, where TLi + 1 and TLi were the TL of eels at ages i + 1 and i, respectively.
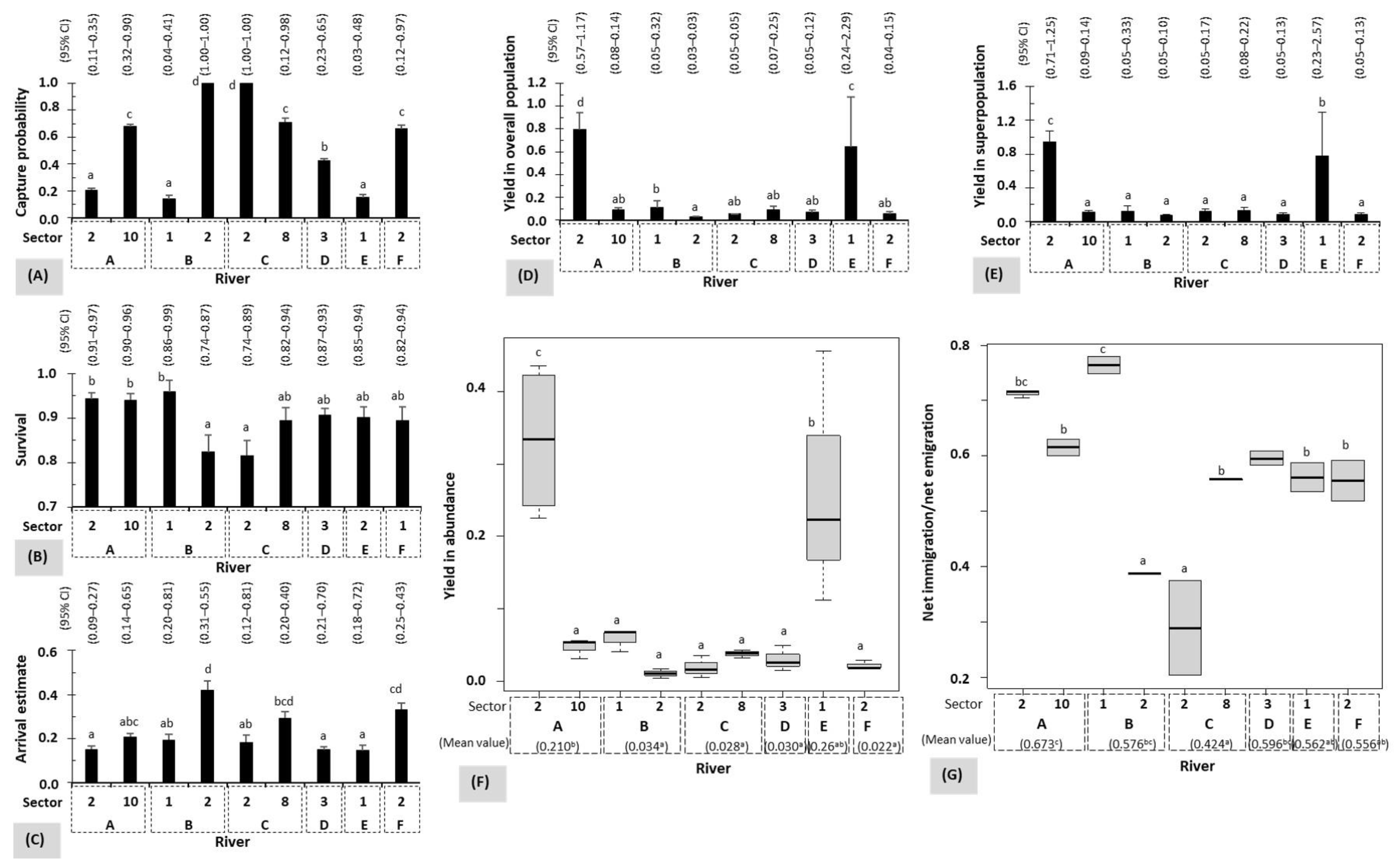
- The demographic parameters of eels 0+ (2017) to 3+ (2020) were estimated using the Jolly–Seber method by means of the Program MARK 8.0 POPAN module [64,65,66,67]. The strategy involved conducting multiple capture–mark–recapture sessions on the same site at different time intervals. We selected only data collected in autumn from 2017 to 2020. Data from 2021 (eels 4+) to 2022 (eels 5+) were not used in this demographic evaluation due to changes observed in the sites/sectors after the severe floods of July 2021, which completely changed the availability in cryptic habitats [47]. The model used was {p(.}, ϕ{.}, pent{t}, N(.)}, where p(.}, ϕ{.} and N(.) are constant over time and represent the capture probability, survival and overall population, respectively; pent{t} is the arrival probability varying with time or age [23,28,67]. Overall population was all individuals who inhabited the site throughout the study duration. This model also determined the superpopulation (N*-hat) that is constant over time, and the estimated abundance (N-hat i), net immigration (B-hat i) and net emigration (B*-hat i) which vary with time or session i. Superpopulation included eels that occasionally frequented the site and disappeared prior to the counting operation. It was selected based on Akaike’s Quasi-Probability Information Criterion (QAICc), species biology and study design as the same unaltered sampling site/sector was fished over a three (2018–2020) to four (2017–2020) year period. To allow objective comparisons between sites/rivers, demographic parameters were standardized as the yields in estimated abundance, overall population and superpopulation and the ratio between net immigration and net emigration. Yields were the quotient between the value of each estimated parameter and the number of glass eels released.
2.5. Statistical Analyses
3. Results
3.1. Abundance and Density of Eels
3.2. Growth Performance of Eels
3.3. Silvering Stage of Eels
3.4. Influence of Rivers in Recruitment Yields
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tzeng, W.N.; Wang, C.H.; Wickström, H.; Reizenstein, M. Occurrence of the semi-catadromous European eel Anguilla anguilla in the Baltic Sea. Mar. Biol. 2000, 137, 93–98. [Google Scholar] [CrossRef]
- Als, T.D.; Hansen, M.M.; Maes, G.E.; Castonguay, M.; Riemann, L.; Aarestrup, K.; Munk, P.; Sparholt, H.; Hanel, R.; Bernatchez, L. All roads lead to home: Panmixia of European eel in the Sargasso Sea. Mol. Ecol. 2011, 20, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Dannewitz, J.; Maes, G.E.; Johansson, L.; Wickström, H.; Volckaert, F.A.M.; Järvi, T. Panmixia in the European eel: A matter of time. Proc. R. Soc. B Biol. Sci. 2005, 272, 1129–1137. [Google Scholar] [CrossRef]
- Marohn, L.; Jakob, E.; Hanel, R. Implications of facultative catadromy in Anguilla anguilla. Does individual migratory behaviour influence eel spawner quality? J. Sea Res. 2013, 77, 100–106. [Google Scholar] [CrossRef]
- Moriarty, C. The Yellow Eel. In Eel Biology; Aida, K., Tsukamoto, K., Yamauchi, K., Eds.; Springer: Tokyo, Japan, 2003; pp. 89–105. [Google Scholar]
- Tesch, F.W.; Thorpe, J.E. The Eel, 3rd ed.; Blackwell Science: Oxford, UK, 2003. [Google Scholar]
- Daverat, F.; Limburg, K.; Thibault, I.; Shiao, J.; Dodson, J.; Caron, F.; Tzeng, W.; Iizuka, Y.; Wickström, H. Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A. japonica and A. rostrata. Mar. Ecol. Prog. Ser. 2006, 308, 231–241. [Google Scholar] [CrossRef]
- Denis, J.; Rabhi, K.; Le Loc’h, F.; Lasram, F.B.R.; Boutin, K.; Kazour, M.; Diop, M.; Gruselle, M.-C.; Amara, R. Role of estuarine habitats for the feeding ecology of the European eel (Anguilla anguilla L.). PLoS ONE 2022, 17, e0270348. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Arai, T. Facultative catadromy of the eel Anguilla japonica between freshwater and seawater habitats. Mar. Ecol. Prog. Ser. 2001, 220, 265–276. [Google Scholar] [CrossRef]
- Edeline, E. Adaptive phenotypic plasticity of eel diadromy. Mar. Ecol. Prog. Ser. 2007, 341, 229–232. [Google Scholar] [CrossRef]
- Harrod, C.; Grey, J.; McCarthy, T.K.; Morrissey, M. Stable isotope analyses provide new insights into ecological plasticity in a mixohaline population of European eel. Oecologia 2005, 144, 673–683. [Google Scholar] [CrossRef]
- Gross, M.R.; Coleman, R.M.; McDowall, R.M. Aquatic Productivity and the Evolution of Diadromous Fish Migration. Science 1988, 239, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Chino, N. Diverse migration strategy between freshwater and seawater habitats in the freshwater eel genus Anguilla. J. Fish Biol. 2012, 8, 442–455. [Google Scholar] [CrossRef]
- Arai, T. Ecology and evolution of migration in the freshwater eels of the genus Anguilla Schrank, 1798. Heliyon 2020, 6, e05176. [Google Scholar] [CrossRef] [PubMed]
- Daverat, F.; Tomás, J. Tactics and demographic attributes in the European eel Anguilla anguilla in the Gironde watershed, SW France. Mar. Ecol. Prog. Ser. 2006, 307, 247–257. [Google Scholar] [CrossRef]
- Daverat, F.; Beaulaton, L.; Poole, R.; Lambert, P.; Wickström, H.; Andersson, J.; Aprahamian, M.; Hizem, B.; Elie, P.; Yalçin-özdilek, S.; et al. One century of eel growth: Changes and implications: One century of eel growth. Ecol. Freshw. Fish 2012, 21, 325–336. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Partial migration: Niche shift versus sexual maturation in fishes. Rev. Fish Biol. Fish. 1993, 3, 348–365. [Google Scholar] [CrossRef]
- Van den Thillart, G.V.; Van Ginneken, F.; Korner, R.; Heijmans, R.; Van der Linden, R.; Gluvers, A. European eel endurance swimming. J. Fish Biol. 2004, 65, 312–318. [Google Scholar] [CrossRef]
- Imbert, H.; Labonne, J.; Rigaud, C.; Lambert, P. Resident and migratory tactics in freshwater European eels are size dependent. Freshw. Biol. 2010, 55, 1483–1493. [Google Scholar] [CrossRef]
- Delrez, N.; Zhang, H.; Lieffrig, F.; Mélard, C.; Farnir, F.; Boutier, M.; Donohoe, O.; Vanderplasschen, A. European eel restocking programs based on wild-caught glass eels: Feasibility of quarantine stage compatible with implementation of prophylactic measures prior to scheduled reintroduction to the wild. J. Nat. Conserv. 2021, 59, 125933. [Google Scholar] [CrossRef]
- Pedersen, M.I. Long-term survival and growth of stocked eel, Anguilla anguilla (L.), in a small eutrophic lake. Dana 2000, 12, 71–76. [Google Scholar]
- Nzau Matondo, B.; Séleck, E.; Dierckx, A.; Benitez, J.-P.; Rollin, X.; Ovidio, M. What happens to glass eels after restocking in upland rivers? A long-term study on their dispersal and behavioural traits. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 374–388. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Benitez, J.P.; Dierckx, A.; Rollin, X.; Ovidio, M. An evaluation of restocking practice and demographic stock assessment methods for cryptic juvenile European eel in upland rivers. Sustainability 2020, 12, 1124. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Benitez, J.P.; Dierckx, A.; Renardy, S.; Rollin, X.; Colson, D.; Baltus, L.; Romain, V.R.M.; Ovidio, M. What are the best upland river characteristics for glass eel restocking practice? Sci. Total Environ. 2021, 784, 147042. [Google Scholar] [CrossRef] [PubMed]
- Belpaire, C.; Geeraerts, C.; Evans, D.; Ciccotti, E.; Poole, R. The European eel quality database: Towards a pan-European monitoring of eel quality. Environ. Monit. Assess. 2011, 183, 273–284. [Google Scholar] [CrossRef]
- Miller, M.J.; Feunteun, E.; Tsukamoto, K. Did a “perfect storm” of oceanic changes and continental anthropogenic impacts cause northern hemisphere anguillid recruitment reductions? ICES J. Mar. Sci. 2016, 73, 43–56. [Google Scholar] [CrossRef]
- Bonnineau, C.; Scaion, D.; Lemaire, B.; Belpaire, C.; Thomé, J.-P.; Thonon, M.; Leermaker, M.; Gao, Y.; Debier, C.; Silvestre, F.; et al. Accumulation of neurotoxic organochlorines and trace elements in brain of female European eel (Anguilla anguilla). Environ. Toxicol. Pharmacol. 2016, 45, 346–355. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Delrez, N.; Bardonnet, A.; Vanderplasschen, A.; Joaquim-Justo, C.; Rives, J.; Benitez, J.P.; Dierckx, A.; Séleck, E.; Rollin, X.; et al. A complete check-up of European eel after eight years of restocking in an upland river: Trends in growth, lipid content, sex ratio and health status. Sci. Total Environ. 2022, 807, 151020. [Google Scholar] [CrossRef]
- Pike, C.; Crook, V.; Gollock, M. Anguilla anguilla. The IUCN Red List of Threatened Species 2020: e.T60344A152845178. 2020. Available online: https://www.fishsec.org/app/uploads/2022/11/200709-Assessment-10.2305_IUCN.UK_.2020-2.RLTS_.T60344A152845178.en_.pdf (accessed on 9 May 2023).
- Feunteun, E. Management and restoration of European eel population (Anguilla anguilla): An impossible bargain. Ecol. Eng. 2002, 18, 575–591. [Google Scholar] [CrossRef]
- Robinet, T.; Feunteun, E. Sublethal effects of exposure to chemical compounds: A cause for the decline in Atlantic eels? Ecotoxicology 2002, 11, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Haenen, O.L.M.; Mladineo, I.; Konecny, R.; Yoshimizu, M.; Groman, D.; Munoz, P. Diseases of eels in an international perspective: Workshop on eel diseases at the 15th international conference on the diseases of fish and shellfish, Split, Croatia, 2011. Bull. Eur. Assoc. Fish Pathol. 2012, 32, 109–115. [Google Scholar]
- Muñoz, P.; Barcala, E.; Peñalver, J.; Romero, D. Can inorganic elements affect herpesvirus infections in European eels? Environ. Sci. Pollut. Res. 2019, 26, 35266–35269. [Google Scholar] [CrossRef]
- Ibbotson, A.; Smith, J.; Scarlett, P.; Aprahamian, M.W. Colonisation of freshwater habitats by the European eel Anguilla anguilla. Freshw. Biol. 2002, 47, 1696–1706. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Ovidio, M. Decreased stock entering the Belgian Meuse is associated with the loss of colonisation behaviour in yellow-phase European eels. Aquat. Living Resour. 2018, 31, 7. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Ovidio, M. Dynamics of upstream movements of the European eel Anguilla Anguilla in an inland area of the river Meuse over the last 20 years. Environ. Biol. Fish 2016, 99, 223–235. [Google Scholar] [CrossRef]
- ICES. ICES Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL); ICES Scientific Reports: Copenhagen, Denmark, 2020; 223p. [Google Scholar]
- Pedersen, M.I.; Rasmussen, C.H. Yield per recruit from stocking two different sizes of eel (Anguilla anguilla) in the brackish Roskilde Fjord. ICES J. Mar. Sci. 2016, 73, 158–164. [Google Scholar] [CrossRef]
- Prigge, E.; Marohn, L.; Hanel, R. Tracking the migratory success of stocked European eels Anguilla anguilla in the Baltic Sea. J. Fish Biol. 2013, 82, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Dörner, H.; Scott, R.D.; Schreckenbach, K.; Knösche, R. Comparison of growth and condition of European eels stocked as glass and farm sourced eels in lakes in the first four years after stocking. J. Appl. Ichthyol. 2013, 29, 323–330. [Google Scholar] [CrossRef]
- Ovidio, M.; Seredynski, A.; Philippart, J.C.; Nzau Matondo, B. A bit of quiet between the migrations: The resting life of the European eel during their freshwater growth phase in a small stream. Aquat. Ecol. 2013, 47, 291–301. [Google Scholar] [CrossRef]
- Desprez, M.; Crivelli, A.J.; Lebel, I.; Massez, G.; Gimenez, O. Demographic assessment of a stocking experiment in European eels. Ecol. Freshw. Fish 2013, 22, 412–420. [Google Scholar] [CrossRef]
- Kullmann, B.; Thiel, R. Bigger is better in eel stocking measures? Comparison of growth performance, body condition, and benefit-cost ratio of simultaneously stocked glass and farmed eels in a brackish fjord. Fish. Res. 2018, 205, 132–140. [Google Scholar] [CrossRef]
- Félix, P.M.; Costa, J.L.; Monteiro, R.; Castro, N.; Quintella, B.R.; Almeida, P.R.; Domingos, I. Can a restocking event with European (glass) eels cause early changes in local biological communities and its ecological status? Glob. Ecol. Conserv. 2020, 21, e00884. [Google Scholar] [CrossRef]
- Félix, P.M.; Costa, J.L.; Quintella, B.R.; Almeida, P.R.; Monteiro, R.; Santos, J.; Portela, T.; Domingos, I. Early settlement and growth of stocked European glass eels in a fragmented watercourse. Fish. Manag. Ecol. 2020, 28, 91–100. [Google Scholar] [CrossRef]
- Ovidio, M.; Tarrago-Bès, F.; Nzau Matondo, B. Short-term responses of glass eels transported from UK to small Belgian streams. Ann. Limnol. Int. J. Limnol. 2015, 51, 219–226. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Backory, L.; Dupuy, G.; Amoussou, G.; Oumarou, A.A.; Gelder, J.; Renardy, S.; Benitez, J.-P.; Dierckx, A.; Dumonceau, F.; et al. Space and Time Use of European Eel Restocked in Upland Continental Freshwaters, a Long-Term Telemetry Study. Fishes 2023, 8, 137. [Google Scholar] [CrossRef]
- Sadler, K. Effects of temperature on the growth and survival of the European eel, Anguilla anguilla L. J. Fish Biol. 1979, 15, 499–507. [Google Scholar] [CrossRef]
- Morin, A.; Lamoureux, W.; Busnarda, J. Empirical models predicting primary productivity from chlorophyll and water temperature for stream periphyton and lake and ocean phytoplankton. J. N. Am. Benthol. Soc. 1999, 18, 299–307. [Google Scholar] [CrossRef]
- Baras, E.; Philippart, J.C.; Salmon, B. Estimation of migrant yellow eels stock in large rivers through the survey of fish passes: A preliminary in the River Meuse (Belgium). In Stock Assessment in Inland Fisheries; Cowx, I.G., Ed.; Oxford Fishing News Books (Blackwell): London, UK, 1996; pp. 314–325. [Google Scholar]
- Philippart, J.C. L’érosion de la Biodiversité: Les Poissons; Université de Liège: Liège, Belgique, 2006; 320p. [Google Scholar]
- Philippart, J.C.; Ovidio, M.; Rimbaud, G.; Dierckx, A.; Poncin, P. Bilan des Observations Sur Les Populations de L’anguille Dans Les Sous-Bassins Hydrographiques Meuse Aval, Ourthe, Amblève et Vesdre; Commission Provinciale de Liège du Fonds Piscicole du Service Public de Wallonie: Liège, Belgique, 2010; 162p. [Google Scholar]
- Huet, M. Aperçu de la relation entre la pente et les populations piscicoles des eaux courantes. Schweiz. Z. Hydrol. 1949, 11, 332–351. [Google Scholar] [CrossRef]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Elie, P.; Lecomte-Finiger, R.; Cantrelle, I.; Charlon, N. Définition des limites des différents stades pigmentaires durant la phase civelle d’Anguilla anguilla (L.). Vie Milieu 1982, 32, 149–157. [Google Scholar]
- Baras, E.; Jeandrain, D.; Serouge, B.; Philippart, J.C. Seasonal variations in time and space utilization by radio-tagged yellow eels Anguilla anguilla (L.) in A Small Stream. Hydrobiologia 1998, 371/372, 187–198. [Google Scholar] [CrossRef]
- Jepsen, N.; Koed, A.; Thorstad, E.B.; Baras, E. Surgical implantation of telemetry transmitters in fish: How much have we learned? Hydrobiologia 2002, 483, 239–248. [Google Scholar] [CrossRef]
- Durif, C.; Dufour, S.; Elie, P. The silvering process of Anguilla Anguilla: A new classification from the yellow resident to the silver migrating stage. J. Fish Biol. 2005, 66, 1025–1043. [Google Scholar] [CrossRef]
- Durif, C.; Guibert, A.; Elie, P. Morphological discrimination of the silvering stages of the European eel. In Eels at the Edge Science, Status, and Conservation Concerns; American Fisheries Society: Bethesda, MD, USA, 2009; pp. 103–111. [Google Scholar]
- Fulton, T.W. The rate of growth of fishes. Fish. Board. Scotl. Ann. Rep. 1904, 22, 141–241. [Google Scholar]
- Simon, J. Age, growth, and condition of European eel (Anguilla anguilla) from six lakes in the river havel system (Germany). ICES J. Mar. Sci. 2007, 64, 1414–1422. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Kumai, Y.; Tsukamoto, K.; Kuroki, M. Growth and habitat use of two anguillid eels, Anguilla marmorata and A. Japonica, on Yakushima Island Japan. Ichthyol. Res. 2020, 67, 375–384. [Google Scholar] [CrossRef]
- Schwarz, C.J.; Arnason, A.N. A general methodology for the analysis of capture-recapture experiments in open populations. Biometrics 1996, 52, 860–873. [Google Scholar] [CrossRef]
- White, G.C.; Burnham, K.P. Program MARK: Survival estimation from populations of marked animals. Bird Study 1999, 46, 120–139. [Google Scholar] [CrossRef]
- Pledger, S.; Pollock, K.H.; Norris, J.L. Open Capture–Recapture Models with Heterogeneity: II. Jolly–Seber Model. Biometrics 2010, 66, 883–890. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer-Verlag Science and Business Média: New York, NY, USA, 2002; 454p. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 2 March 2023).
- Fox, J. Using the R Commander: A Point-and-Click Interface for R; Chapman and CRC Press: Boca Raton, FL, USA, 2017; Available online: http://socserv.mcmaster.ca/jfox/Books/RCommander/ (accessed on 2 March 2023).
- Harrell, F.E., Jr. Hmisc: Harrell Miscellaneous, Version 4.1-1; Cran: Washington DC, USA, 2018; Available online: http://CRAN.Rproject.org/package=Hmisc (accessed on 2 March 2023).
- Dorow, M.; Kullmann, L.; Buck, M.; Frankowski, J. Yellow eel (Anguilla anguilla) density trends along the German part of the southern Baltic Sea between 2009 and 2020. Fish. Res. 2023, 257, 106497. [Google Scholar] [CrossRef]
- Reynolds, C. The Effect of Acidification on the Survival of American Eel. Master’s Thesis, Dalhousie University, Halifax, NS, Canada, 2011; 78p. [Google Scholar]
- Larsen Bjørn, M.; Hesthagen, T.; Thorstad, E.B.; Diserud, O.H. Increased abundance of European eel (Anguilla anguilla) in acidified Norwegian rivers after liming. Ecol. Freshw. Fish 2015, 24, 575–583. [Google Scholar] [CrossRef]
- Bisgaard, J.; Pedersen, M.I. Mortality and growth of wild and introduced cultured eels Anguilla anguilla (L.) in a Danish stream, with special reference to a new tagging technique. Dana 1991, 9, 57–69. [Google Scholar]
- Mazel, V.; Charrier, F.; Legault, A.; Laffaille, P. Long-term effects of passive integrated transponder tagging (PIT tags) on the growth of the yelloweel (Anguilla anguilla (Linnaeus, 1758)). J. Appl. Ichthyol. 2013, 29, 906–908. [Google Scholar] [CrossRef]
- Silm, M.; Bernotas, P.; Haldna, M.; Järvalt, A.; Nõges, T. Age and growth of European eel, Anguilla anguilla (Linnaeus, 1758), in Estonian lakes. J. Appl. Ichthyol. 2017, 33, 236–241. [Google Scholar] [CrossRef]
- Pedersen, M.I. Does Stocking of Danish lowland streams with Elvers increase European Eel Populations? Am. Fish. Soc. Symp. 2009, 58, 149–156. [Google Scholar]
- van Ginneken, V.; Durif, C.; Balm, S.P.; Boot, R.; Verstegen, M.W.A.; Antonissen, E.; van den Thillart, G. Silvering of European eel (Anguilla anguilla L.): Seasonal changes of morphological and metabolic parameters. Anim. Biol. 2007, 57, 63–77. [Google Scholar] [CrossRef]
- Moura, A.; Dias, E.; López, R.; Antunes, C. Regional population structure of the European eel at the southern limit of its distribution revealed by otolith shape signature. Fishes 2022, 7, 135. [Google Scholar] [CrossRef]
- Cattrijsse, A.; Hampel, H. Life History and Habitat Use Tables. In Final Report. Subproject 1—‘Nursery Function Westerschelde’; University of Gent, Department of Biology, Marine Biology Section: Gent, Belgium, 2000; 30p. [Google Scholar]
- Jellyman, D.J. An enigma: How can freshwater eels (Anguilla spp.) be such a successful genus yet be universally threatened? Rev. Fish Biol. Fish. 2022, 32, 701–718. [Google Scholar] [CrossRef]
- Shiao, J.C.; Lozys, L.; Iizuka, Y.; Tzeng, W.N. Migratory patterns and contribution of stocking to the population of European eel in Lithuanian waters as indicated by otolith Sr:Ca ratios. J. Fish Biol. 2006, 69, 749–769. [Google Scholar] [CrossRef]
- Sjöberg, N.B.; Wickström, H.; Asp, A.; Petersson, E. Migration of eels tagged in the Baltic Sea and Lake Mälaren in the context of the stocking question. Ecol. Freshw. Fish 2017, 26, 517–532. [Google Scholar] [CrossRef]
- Cucherousset, J.; Britton, J.R.; Beaumont, W.R.C.; Nyqvist, M.; Sievers, K.; Gozlan, R.E. Determining the effects of species environmental conditions and tracking method on the detection efficiency of portable PIT telemetry. J. Fish Biol. 2010, 7, 1039–1045. [Google Scholar] [CrossRef]
- Keithan, E.D.; Lowe, R.L. Primary productivity and spatial structure of phytolithic growth in streams in the Great Smoky Mountains National Park, Tennessee. Hydrobiologia 1985, 123, 59–67. [Google Scholar] [CrossRef]
- Boulenger, C.; Crivelli, A.J.; Charrier, F.; Roussel, J.M.; Feunteun, E.; Acou, A. Difference in factors explaining growth rate variability in European eel subpopulations: The possible role of habitat carrying capacity. Ecol. Freshw. Fish 2014, 25, 281–294. [Google Scholar] [CrossRef]
- Verbiest, H.; Breukelaar, A.; Ovidio, M.; Philippart, J.-C.; Belpaire, C. Escapement success and patterns of downstream migration of female silver eel Anguilla anguilla in the River Meuse. Ecol. Freshw. Fish 2012, 21, 395–403. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Benitez, J.P.; Dierckx, A.; Philippart, J.C.; Ovidio, M. Assessment of the entering stock, migration dynamics and fish pass fidelity of European eel in the Belgian Meuse River. River Res. Appl. 2017, 33, 292–301. [Google Scholar] [CrossRef]


| Year | Age | n | b | a | F−Statistic | Adjusted R² | K, Mean ± SE |
|---|---|---|---|---|---|---|---|
| 2017 | 0+ | 327 | 3.285 | −3.103 | 2927 | 0.901 *** | 0.162 ± 0.027 a |
| 2018 | 1+ | 524 | 3.019 | −2.81 | 9659 | 0.950 *** | 0.166 ± 0.043 a |
| 2019 | 2+ | 403 | 3.129 | −2.95 | 9746 | 0.963 *** | 0.171 ± 0.034 b |
| 2020 | 3+ | 329 | 3.156 | −2.998 | 5277 | 0.945 *** | 0.174 ± 0.037 c |
| 2021 | 4+ | 143 | 3.269 | −3.137 | 2301 | 0.946 *** | 0.194 ± 0.040 e |
| 2022 | 5+ | 195 | 3.102 | −2.92 | 7858 | 0.944 *** | 0.181 ± 0.050 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nzau Matondo, B.; Fontaine, F.; Detrait, O.; Poncelet, C.; Vandresse, S.; Orban, P.; Gelder, J.; Renardy, S.; Benitez, J.P.; Dierckx, A.; et al. Glass Eel Restocking Experiments in Typologically Different Upland Rivers: How Much Have We Learned about the Importance of Recipient Habitats? Water 2023, 15, 3133. https://doi.org/10.3390/w15173133
Nzau Matondo B, Fontaine F, Detrait O, Poncelet C, Vandresse S, Orban P, Gelder J, Renardy S, Benitez JP, Dierckx A, et al. Glass Eel Restocking Experiments in Typologically Different Upland Rivers: How Much Have We Learned about the Importance of Recipient Habitats? Water. 2023; 15(17):3133. https://doi.org/10.3390/w15173133
Chicago/Turabian StyleNzau Matondo, Billy, François Fontaine, Olivier Detrait, Cathérine Poncelet, Stéphanie Vandresse, Patrice Orban, Justine Gelder, Séverine Renardy, Jean Philippe Benitez, Arnaud Dierckx, and et al. 2023. "Glass Eel Restocking Experiments in Typologically Different Upland Rivers: How Much Have We Learned about the Importance of Recipient Habitats?" Water 15, no. 17: 3133. https://doi.org/10.3390/w15173133





