Enhancing Fishery Management in Tanghe Reservoir, China: Insights from Food Web Structure and Ecosystem Analysis
Abstract
1. Introduction
2. Materials and Methods
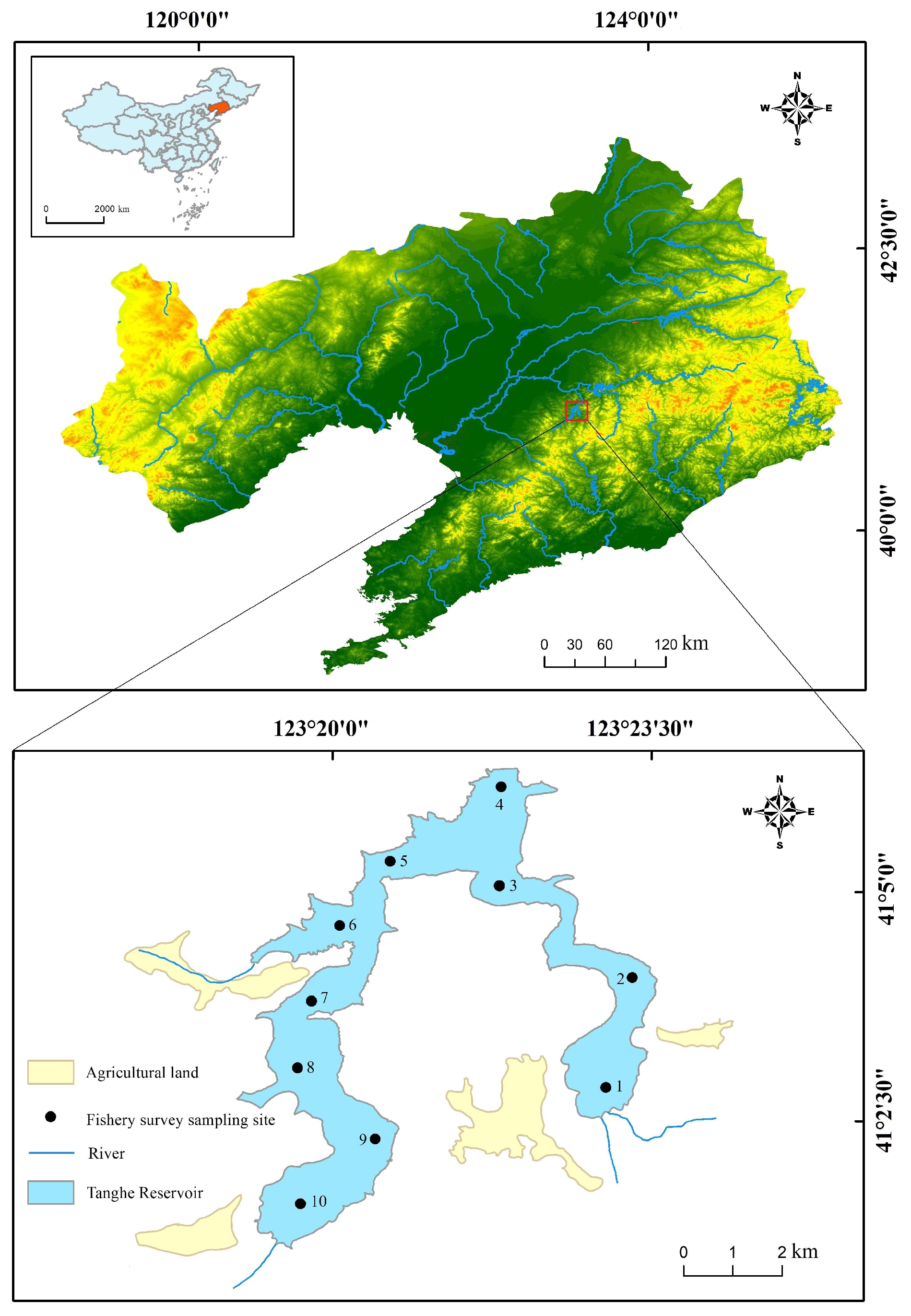
2.1. Study Area
2.2. Trophic Modeling Method
2.3. Functional Group and Input Data Collection
2.3.1. Functional Group Division
2.3.2. Fish
2.3.3. Plankton, Shrimp, and Zoobenthos
2.3.4. Macrophytes and Detritus
2.3.5. Diet Composition
2.3.6. Model Balance and Analysis
3. Results
3.1. Basic Input and Estimates
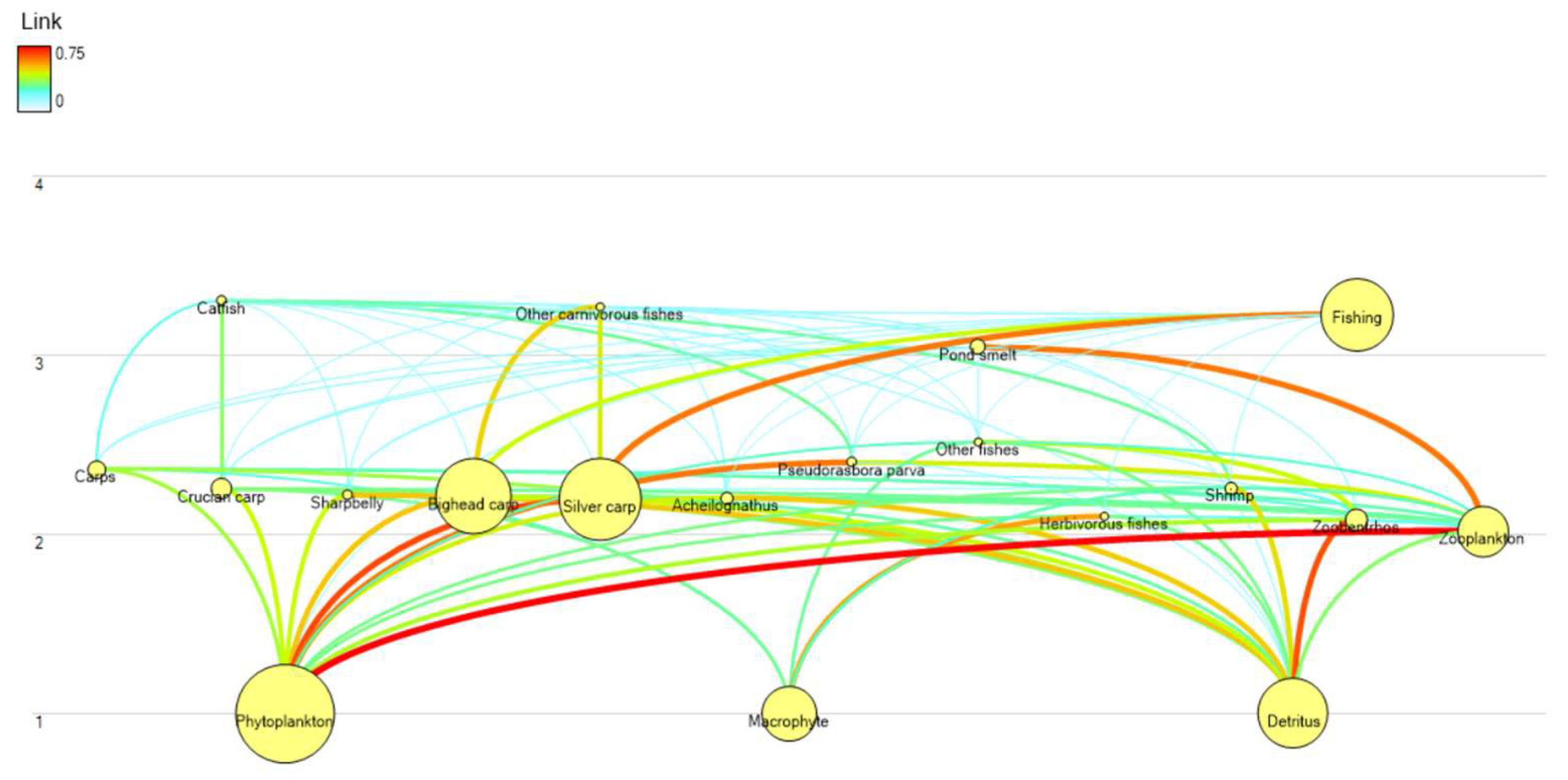
3.2. Food Web Structure and Trophic Analysis
3.2.1. Trophic Structure
3.2.2. Transfer Efficiencies
3.2.3. Mixed Trophic Impacts (MTI)
3.3. Ecosystem Properties and Indicators
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Funge-Smith, S.; Bennett, A. A fresh look at inland fisheries and their role in food security and livelihoods. Fish Fish. 2019, 20, 1176–1195. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. China Statistical Yearbook in 2021; China Statistics Press: Beijing, China, 2022. [Google Scholar]
- Chen, J.G.; Wang, J.F.; Guo, J.Y.; Yu, J.; Zeng, Y.; Yang, H.Q.; Zhang, R.Y. Eco-environment of reservoirs in China: Characteristics and research prospects. Prog. Phys. Geogr. 2018, 42, 185–201. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C.C.; Ridoutt, B.G.; Wang, X.C.; Ren, P.A. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Lin, S.S.; Shen, S.L.; Zhou, A.; Lyu, H.M. Assessment andmanagement of lake eutrophication: A case study in Lake Erhai, China. Sci. Total Environ. 2021, 751, 141618. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.L.; Huang, J.C. Development orientation and emphases on the high efficient eco-agriculture in the Three Gorges Area. J. Nat. Resour. 2002, 17, 444–450. [Google Scholar]
- Liu, B.Y. The structure and growth characteristics of the main economic fish species in the Tanghe Reservoir in recent years. Chin. J. Fish. 2010, 23, 26–30. [Google Scholar]
- Chen, D.Q.; Li, S.J.; Wang, K. Enhancement and conservation of inland fisheries resources in China. Environ. Biol. Fishes 2012, 93, 531–545. [Google Scholar] [CrossRef]
- Su, S.; Tang, Y.; Chang, B.W.; Zhu, W.B.; Chen, Y. Evolution of marine fisheries management in China from 1949 to 2019: How did China get here and where does China go next? Fish Fish. 2020, 21, 435–452. [Google Scholar] [CrossRef]
- Costello, C.; Ovando, D.; Clavelle, T.; Strauss, C.K.; Hilborn, R.; Melnychuk, M.C.; Branch, T.A.; Gaines, S.D.; Szuwalski, C.S.; Cabral, R.B.; et al. Global fishery prospects under contrasting management regimes. Proc. Natl. Acad. Sci. USA 2016, 113, 5125–5129. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J. Ecopath with Ecosim: Methods, capabilities and limitations. Eco. Model. 2004, 172, 109–139. [Google Scholar] [CrossRef]
- Guo, C.B.; Chen, Y.S.; Li, W.; Xie, S.G.; Lek, S.; Li, Z.J. Food web structure and ecosystem properties of the largest impounded lake along the eastern route of China’s South-to-North Water Diversion Project. Ecol. Inform. 2018, 43, 174–184. [Google Scholar] [CrossRef]
- Guo, C.B.; Ye, S.W.; Lek, S.; Liu, J.S.; Zhang, T.L.; Yuan, J.; Li, Z.J. The need for improved fishery management in a shallow macrophytic lake in the Yangtze River basin: Evidence from the food web structure and ecosystem analysis. Eco. Model. 2013, 267, 138–147. [Google Scholar] [CrossRef]
- Heymans, J.J.; Coll, M.; Link, J.S.; Mackinson, S.; Steenbeek, J.; Walters, C.; Christensen, V. Best practice in Ecopath with Ecosim food-web models for ecosystem-based management. Eco. Model. 2016, 331, 173–184. [Google Scholar] [CrossRef]
- Yin, C.J.; Gong, L.; Chen, Y.S.; Ni, L.Y.; Pitcher, T.J.; Kang, B.; Guo, L.G. Modeling ecosystem impacts of the invasive Japanese smelt Hypomesus nipponensis in Lake Erhai, southwestern China. Ecol. Inform. 2022, 67, 101488. [Google Scholar] [CrossRef]
- Li, B.; Xie, Y.J.; Fu, L.J. Fishery biological base of Tanghe Reservoir. Fish Sci. 1989, 2, 1–6. [Google Scholar] [CrossRef]
- Yan, H.Q.; Zhao, W.; Guo, K.; Li, W.K.; Xue, F.; Cai, Z.L. Evaluation and anlysis of eutrophication in six domestic water supply reservoirs in Liaoning Province. J. Dalian Ocean Univ. 2016, 31, 180–184. [Google Scholar] [CrossRef]
- Gao, D.P. Analysis of the causes of algal blooms in Tanghe Reservoir and countermeasures research. Tech. Superv. Water Resour. 2017, 25, 40–42+47. [Google Scholar]
- Zhang, X.; Li, C. Analysis of phytoplankton community characteristic and its influencing factors in Tanghe Reservoir. Environ. Sci. Technol. 2016, 39, 394–401. [Google Scholar]
- Liu, B.Y.; Zhao, W.; Guo, K.; Li, Y.Y. The community structure and spatio-temporal pattern of zooplankton in Tanghe Reservoir in 2007. J. Dalian Ocean Univ. 2011, 26, 526–531. [Google Scholar] [CrossRef]
- GB 11982; Water Quality—Determination of Permanganate Index. National Standardization Administration of the People’s Republic of China: Beijing, China, 1989.
- Patton, C.J.; Kryskalla, J.R. Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Evaluation of Alkaline Persulfate Digestion as an Alternative to Kjeldahl Digestion for Determination of Total and Dissolved Nitrogen and Phosphorus in Water; United States Geological Survey: Sunrise Valley Drive Reston, VA, USA, 2003.
- Allen Radway, K. Relation Between Production and Biomass. J. Fish Res. 1971, 28, 1573–1581. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J.; Pauly, D. Ecopath with Ecosim: A User’s Guide. 2005. Available online: http://www.reseachgate.net/publication/267193103 (accessed on 16 October 2023).
- Steenbeek, J.; Buszowski, J.; Christensen, V.; Akoglu, E.; Aydin, K.; Ellis, N.; Felinto, D.; Guitton, J.; Lucey, S.; Kearney, K.; et al. Ecopath with Ecosim as a model-building toolbox: Source code capabilities, extensions, and variations—ScienceDirect. Eco. Model. 2016, 319, 178–189. [Google Scholar] [CrossRef]
- Li, C.; Wang, Q.D.; Ye, S.W.; Huang, G.; Liu, J.S.; Li, Z.G. Modeling trophic structure and energy flows in a shallow lake, Yangtze River Basin, China: A case analysis for culture-based fishery practices. Aquac. Environ. Interact. 2018, 10, 213–226. [Google Scholar] [CrossRef]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Palomares, M.L.D.; Pauly, D. Predicting food consumption of fish populations as functions of mortality, food type, morphometrics, temperature and salinity. Mar. Freshw Res. 1998, 49, 447–453. [Google Scholar] [CrossRef]
- Liu, Q.G.; Chen, Y.; Li, J.L.; Chen, L.Q. The food web structure and ecosystem properties of a filter-feeding carps dominated deep reservoir ecosystem. Eco. Model. 2007, 203, 279–289. [Google Scholar] [CrossRef]
- Halfon, E.; Schito, N.; Ulanowicz, R.E. Energy flow through the Lake Ontario food web: Conceptual model and an attempt at mass balance. Eco. Model. 1996, 86, 1–36. [Google Scholar] [CrossRef]
- Yan, Y.J.; Liang, Y.L. Energy flow of macrozoobenthic community in a macrophytic lake, Baiyangdian Lake. Acta Ecolo. Sin. 2003, 03, 527–538. [Google Scholar]
- Park, R.A. Generalized model for simulating lake ecosystems. Simulation 1974, 23, 33–50. [Google Scholar] [CrossRef]
- Deng, Y.; Zheng, Y.C.; Chang, J.B. Evaluation of the effect of stocking silver carp and bighead carp on the ecosystem of Qiandao Lake using Ecopath model. Acta Ecol. Sin. 2022, 42, 6853–6862. [Google Scholar] [CrossRef]
- Li, H.; Shen, Y.H.; Li, S.J.; Liang, Y.Z.; Lu, C.Y.; Zhang, L.L. Effects of eutrophication on the benthic-pelagic couping food web in Baiyangdian Lake. Acta Ecol. Sin. 2018, 38, 2017–2030. [Google Scholar]
- Zhang, Y. The Study of Fishery Resources and Ecopath Model in Jinshahe Reservoir Ecosystem, Hubei Province. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar]
- Fan, Z.Y.; Bai, X.L.; Xu, J.C.; Wang, X.N.; Lv, Y.B.; Hou, J.; He, X.G. Analysis of ecological system characteristics and ecological capacity of Hypophthalmichthys molitrix and Aristichthys nobilis in the Weishui Reservoir based on Ecopath model. J. Fish. Sci. China 2021, 28, 773–784. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Wang, X.Q.; Liu, J.; Yang, S.W.; Zhao, D.S. The food habit of Opsariichthys bidens in Biliuhe Reservoir and fishery countermea sure. Fish. Sci. 1995, 03, 35–38. [Google Scholar] [CrossRef]
- Wen, H.S.; Wang, L.; Mao, Y.Z.; Zhang, X.D.; Yang, X.Z. Growth, food habits and utilization of population resources of the Western Liaohe Catfish (Silurus asotus). J. Hydroecol. 1999, 02, 35–37. [Google Scholar] [CrossRef]
- Xie, Y.H.; Pu, X.P. The biologucal aspects of pond smelt (Hypomesus olidus) in the Shuifeng Reservoir. Acta Hydrobio. Sin. 1984, 04, 457–468. [Google Scholar]
- Yu, J.; Liu, J.R.; Wang, L.; Wu, Z.X.; Yu, Z.M.; Liu, M.L.; Han, Y.C.; Xie, P. Analysis on the ecosystem structure and function of lake Qiandao based on Ecopath model. Acta Hydrobiol. Sin. 2021, 45, 308–317. [Google Scholar] [CrossRef]
- Funtowicz, S.O.; Ravetz, J.R. Uncertainty and Quality in Science for Policy; Kluwer Academic Publishers: Dortrecht, The Netherlands, 1990. [Google Scholar]
- Pauly, D.; Christensen, V.; Walters, C. Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES J. Mar. Sci. 2000, 57, 697–706. [Google Scholar] [CrossRef]
- Ulanowicz, R.E. Ecosystem Trophic Foundations: Lindeman Exonerata; Patten, B.C., Jørgensen, S.E., Eds.; Complex Ecology: The Part–Whole Relation in Ecosystems; Prentice Hall: Bergen, NJ, USA, 1995; pp. 549–560, Chapter 21. [Google Scholar]
- Banerjee, A.; Banerjee, M.; Mukherjee, J.; Rakshit, N.; Ray, S. Trophic relationships and ecosystem functioning of Bakreswar Reservoir, India. Ecol. Inform. 2016, 36, 50–60. [Google Scholar] [CrossRef]
- Philippsen, J.S.; Minte-Vera, C.V.; Coll, M.; Angelini, R. Assessing fishing impacts in a tropical reservoir through an ecosystem modeling approach. Rev. Fish Biol. Fish. 2019, 29, 125–146. [Google Scholar] [CrossRef]
- Khan, M.F.; Preetha, P.; Sharma, A.P. Modelling the food web for assessment of the impact of stock supplementation in a reservoir ecosystem in India. Fish. Manag. Ecol. 2015, 22, 359–370. [Google Scholar] [CrossRef]
- Thapanand, T.; Jutagatee, T.; Wongrat, P.; Lekcholayut, T.; Meksumpun, C.; Janekitkarn, S.; Rodloi, A.; Moreau, J.; Wongrat, L. Trophic relationships and ecosystem characteristics in a newly-impounded man-made lake in Thailand. Fish. Manag. Ecol. 2009, 16, 77–87. [Google Scholar] [CrossRef]
- Morissete, L.; Hammill, M.O.; Savenkoff, C. The trophic role of marine mammals in the northern Gulf of St. Lawrence. Mar. Mamm. Sci. 2006, 22, 74–103. [Google Scholar] [CrossRef]
- Jia, P.Q.; Hu, Z.J.; Wu, Z.; Liu, Q.G.; Wu, Z.X.; Kong, Y.J.; Zhu, Y. Quantitative analysis on the structure and function of the Gehu Lake ecosystem based on Ecopath modeling. Resour. Environ. Yangtze Basin 2013, 22, 189–197. [Google Scholar]
- Cremer, M.C.; Smitherman, R.O. Food-habits and growth of silver and bighead carp in cages and ponds. Aquaculture 1980, 20, 57–64. [Google Scholar] [CrossRef]
- Spataru, P.; Gophen, M. Feeding-behavior of silver carp (hypophthalmichthys molitrix) and its impact on the food web in lake Kinneret, Israel. Hydrobiologia 1985, 120, 53–61. [Google Scholar] [CrossRef]
- Christensen, V.; Pauly, D. Trophic Models of Aquatic Ecosystems; Working Papers; WorldFish: Penang, Malaysia, 1993; Volume 26, pp. 338–352. [Google Scholar]
- Hossain, M.M.; Matsuishi, T.; Arhonditsis, G. Elucidation of ecosystem attributes of an oligotrophic lake in Hokkaido, Japan, using Ecopath with Ecosim (EwE). Eco. Model. 2010, 221, 1717–1730. [Google Scholar] [CrossRef]
- Barausse, A.; Duci, A.; Mazzoldi, C.; Artioli, Y.; Palmeri, L. Trophic network model of the Northern Adriatic Sea: Analysis of an exploited and eutrophic ecosystem. Estuar. Coast Shelf Sci. 2009, 83, 577–590. [Google Scholar] [CrossRef]
- Odum, E.P. Strategy of ecosystem development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef]
- Finn, J.T. Measures of ecosystem structure and function derived from analysis of flows. J. Theor. Biol. 1976, 56, 363–380. [Google Scholar] [CrossRef]
- Odum, E.P. Fundamental of Ecology; Saunders: Philadelphia, PA, USA, 1971. [Google Scholar]
- Han, R.; Chen, Q.W.; Wang, L.; Tang, X.W. Preliminary investigation on the changes in trophic structure and energy flow in the Yangtze estuary and adjacent coastal ecosystem due to the Three Gorges Reservoir. Ecol. Inform. 2016, 36, 152–161. [Google Scholar] [CrossRef]
- Angellni, R.; Agostinho, A.A.; Gomes, L.C. Modeling energy flow in a large Neotropical Reservoir: A tool do evaluate fishing and stability. Neotrop. Ichthyol. 2018, 4, 253–260. [Google Scholar] [CrossRef]
- Dong, S.P.; Gao, Y.F.; Gao, Y.P.; He, M.D.; Liu, F.; Yan, F.J.; Wang, F. Evaluation of the trophic structure and energy flow of a rice-crayfish integrated farming ecosystem based on the Ecopath model. Aquaculture 2021, 539, 736626. [Google Scholar] [CrossRef]
- Outeiro, L.; Byron, C.; Angelini, R. Ecosystem maturity as a proxy of mussel aquaculture carrying capacity in Ria de Arousa (NW Spain): A food web modeling perspective. Aquaculture 2018, 496, 270–284. [Google Scholar] [CrossRef]
- Zhao, Q.S.; Huang, H.M.; Zhu, Y.G.; Cao, M.; Zhao, L.L.; Hong, X.G.; Chu, J.S. Analysing ecological carrying capacity of bivalve aquaculture within the Yellow River Estuary ecoregion through mass-balance modelling. Aquac. Environ. Interact. 2022, 14, 147–161. [Google Scholar] [CrossRef]
- Mace. A new role for MSY in single-species and ecosystem approaches to fisheries stock assessment and management. Fish Fish. 2001, 2, 2–32. [Google Scholar] [CrossRef]



| Parameters | April (n = 10) | August (n = 10) | October (n = 10) | December (n = 10) | One-Way ANOVA |
|---|---|---|---|---|---|
| Cond (μS/cm) | 312.70 ± 10.71 b | 368.96 ± 11.45 a | 293.14 ± 5.29 c | 268.72 ± 3.38 d | p = 0.000 |
| DO | 12.48 ± 0.76 a | 7.28 ± 0.64 d | 7.99 ± 0.60 c | 11.26 ± 0.55 b | p = 0.000 |
| ORP (mV) | 84.26 ± 14.12 a | 79.33 ± 4.26 a | 79.52 ± 4.87 a | 80.57 ± 13.78 a | p = 0.727 |
| pH | 8.96 ± 0.08 a | 8.60 ± 0.48 b | 8.64 ± 0.05 b | 8.50 ± 0.20 b | p = 0.005 |
| SD (m) | 1.67 ± 0.29 c | 2.20 ± 0.47 b | 1.69 ± 0.15 c | 2.84 ± 0.54 a | p = 0.000 |
| TDS (mg/L) | 274.37 ± 1.89 a | 238.81 ± 1.31 d | 243.56 ± 4.30 c | 267.22 ± 3.29 b | p = 0.000 |
| WT (°C) | 11.45 ± 1.22 c | 26.14 ± 0.32 a | 13.61 ± 0.09 b | 6.74 ± 0.43 d | p = 0.000 |
| CODMn (mg/L) | 1.79 ± 0.35 c | 1.40 ± 0.18 bc | 2.17 ± 1.06 ab | 2.60 ± 0.73 a | p = 0.004 |
| TN (mg/L) | 1.87 ± 0.57 b | 0.86 ± 0.30 c | 2.74 ± 0.15 a | 0.79 ± 0.21 c | p = 0.000 |
| TP (mg/L) | 0.02 ± 0.01 b | 0.04 ± 0.00 a | 0.03 ± 0.01 ab | 0.03 ± 0.02 b | p = 0.007 |
| NO. | Functional Group | Dominant Species Composition |
|---|---|---|
| 1 | Catfish | Silurus asotus |
| 2 | Other carnivorous fishes | Cultrichthys erythropterus |
| Opsariichthys bidens | ||
| Channa argus | ||
| 3 | Carp | Cyprinus carpio |
| 4 | Crucian carp | Carassius auratus |
| 5 | Pond smelt | Hypomesus olidus |
| 6 | Sharpbelly | Hemiculter leucisculus |
| 7 | Bighead carp | Aristichthys nobilis |
| 8 | Silver carp | Hypophthalmichthys molitrix |
| 9 | Acheilognathus | Acheilognathus chankaensis |
| Rhodeus lighti | ||
| 10 | Pseudorasbora parva | Pseudorasbora parva |
| 11 | Other fishes | Abbottina rivularis |
| Zacco sinensis | ||
| Hemibarbus labeo | ||
| Misgurnus anguillicaudatus | ||
| Pelteobagrus fulvidraco | ||
| 12 | Herbivorous fishes | Ctenopharyngodon idella |
| Megalobrama amblycephala | ||
| 13 | Shrimp | Shrimp |
| 14 | Zoobenthos | Oligochaeta |
| Chironomidae larvae | ||
| 15 | Zooplankton | Protozoan |
| Rotifer | ||
| Cladocera | ||
| Copepoda | ||
| 16 | Phytoplankton | Cyanophyta |
| Chlorophyta | ||
| Bacillariophyta | ||
| Euglenophyta | ||
| Pyrrophyta | ||
| Cryptophyta | ||
| 17 | Macrophyte | Acorus calamus |
| Vallisneria natans | ||
| 18 | Detritus | Organic ditritus |
| Group Number | Group | Trophic Level | Biomass (t/km2) | P/B (/year) | Q/B (/year) | EE | P/Q |
|---|---|---|---|---|---|---|---|
| 1 | Catfish | 3.284 | 0.23 | 0.97 | 6.566 | 0.188 | 0.148 |
| 2 | Other carnivorous fishes | 3.357 | 0.14 | 1.95 | 12.61 | 0.937 | 0.155 |
| 3 | Carp | 2.366 | 1.90 | 0.92 | 4.59 | 0.970 | 0.200 |
| 4 | Crucian carp | 2.253 | 2.39 | 1.035 | 7.34 | 0.974 | 0.141 |
| 5 | Pond smelt | 3.047 | 1.32 | 1.38 | 14.34 | 0.615 | 0.096 |
| 6 | Sharpbelly | 2.219 | 0.33 | 2.63 | 12.6 | 0.976 | 0.209 |
| 7 | Bighead carp | 2.415 | 22.59 | 1.02 | 4.848 | 0.407 | 0.210 |
| 8 | Silver carp | 2.197 | 32.98 | 1.15 | 8.128 | 0.380 | 0.141 |
| 9 | Acheilognathus | 2.206 | 0.72 | 2.68 | 13.21 | 0.368 | 0.203 |
| 10 | Pseudorasbora parva | 2.402 | 0.31 | 2.83 | 14.46 | 0.998 | 0.196 |
| 11 | Other fishes | 2.516 | 0.17 | 2.55 | 12.65 | 0.987 | 0.202 |
| 12 | Herbivorous fishes | 2.102 | 0.11 | 0.71 | 9.388 | 0.685 | 0.076 |
| 13 | Shrimp | 2.261 | 0.89 | 1.83 | 24.4 | 0.950 | 0.075 |
| 14 | Zoobentrhos | 2.082 | 2.64 | 5.3 | 265 | 0.972 | 0.020 |
| 15 | Zooplankton | 2.020 | 11.45 | 24.68 | 493.6 | 0.995 | 0.050 |
| 16 | Phytoplankton | 1.000 | 52.30 | 140.2 | 0.637 | ||
| 17 | Macrophyte | 1.000 | 13.49 | 1.25 | 0.500 | ||
| 18 | Detritus | 1.000 | 22.02 | 0.256 |
| Trophic Level | Flow to Detritus (t km−2 year−1) | Throughput (t km−2 year−1) |
|---|---|---|
| IV | 0.896 | 2.992 |
| III | 98.04 | 180.4 |
| II | 4409 | 6516 |
| I | 2673 | 14,530 |
| Sum | 7181 | 21,230 |
| Attribute Parameter | Value | Units |
|---|---|---|
| Sum of all consumption (TC) | 6820.278 | t km−2 year−1 |
| Sum of all exports (TE) | 5364.935 | t km−2 year−1 |
| Sum of all respiratory flows (TR) | 1984.388 | t km−2 year−1 |
| Sum of all flows into detritus (TD) | 7180.641 | t km−2 year−1 |
| Total system throughput (TST) | 21,350.240 | t km−2 year−1 |
| Sum of all production (TP) | 7719.199 | t km−2 year−1 |
| Mean trophic level of the catch (TLc) | 2.303 | |
| Calculated total net primary production (TPP) | 7349.324 | t km−2 year−1 |
| Total primary production/total respiration (TPP/TR) | 3.704 | |
| Net system production (NSP) | 5364.936 | t km−2 year−1 |
| Total primary production/total biomass (TPP/TB) | 51.056 | |
| Total biomass (excluding detritus) (TB) | 143.948 | t km−2 |
| Total catch | 24.326 | t km−2 year−1 |
| Connectance index (CI) | 0.299 | |
| System omnivory index (SOI) | 0.145 | |
| Ecopath pedigree | 0.481 | |
| Measure of fit (t*) | 2.122 | |
| Shannon diversity index | 1.776 | |
| Ascendancy (A) | 0.3127 | |
| System overhead (O) | 0.6873 | |
| Finn’s cycling index (FCI) | 10.5 | % of total throughput |
| Finn’s mean path length (FML) | 2.905 |
| Parameters | Bao’an Lake [13] (2012–2013) | Gehu Lake [49] (2010–2011) | Jinshahe Reservoir [35] (2013–2014) | Qiandao Lake [40] (2016–2017) | Tanghe Reservoir (2021–2022) | Weishui Reservoir [36] (2020–2021) |
|---|---|---|---|---|---|---|
| Finn’s cycling index (FCI) | 9.25% | 7.99% | 6.73% | 5.15% | 10.50% | 11.35% |
| Connectance index (CI) | 0.205 | 0.219 | 0.277 | 0.263 | 0.299 | 0.351 |
| System omnivory index (SOI) | 0.058 | 0.189 | 0.087 | 0.132 | 0.145 | 0.099 |
| Total primary production/total respiration (TPP/TR) | 1.64 | 2.761 | 6.735 | 6.509 | 3.704 | 1.394 |
| Total system throughput (TST) | 37,418.04 | 12,131.76 | 27,247.68 | 24,698.27 | 21,350.24 | 44,254.86 |
| Total transfer efficiencies | 8.68% | 6.40% | 7.60% | 3.50% | 4.78% | 4.24% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Qiu, Y.; Peng, L.; Shen, J.; Li, G.; Li, J. Enhancing Fishery Management in Tanghe Reservoir, China: Insights from Food Web Structure and Ecosystem Analysis. Water 2024, 16, 200. https://doi.org/10.3390/w16020200
Qiu L, Qiu Y, Peng L, Shen J, Li G, Li J. Enhancing Fishery Management in Tanghe Reservoir, China: Insights from Food Web Structure and Ecosystem Analysis. Water. 2024; 16(2):200. https://doi.org/10.3390/w16020200
Chicago/Turabian StyleQiu, Longhui, Yuhui Qiu, Legen Peng, Jianzhong Shen, Guangyu Li, and Jiangwei Li. 2024. "Enhancing Fishery Management in Tanghe Reservoir, China: Insights from Food Web Structure and Ecosystem Analysis" Water 16, no. 2: 200. https://doi.org/10.3390/w16020200
APA StyleQiu, L., Qiu, Y., Peng, L., Shen, J., Li, G., & Li, J. (2024). Enhancing Fishery Management in Tanghe Reservoir, China: Insights from Food Web Structure and Ecosystem Analysis. Water, 16(2), 200. https://doi.org/10.3390/w16020200






