A Review of the Harvesting Techniques of Microalgae
Abstract
:1. Introduction
2. Harvesting Methods
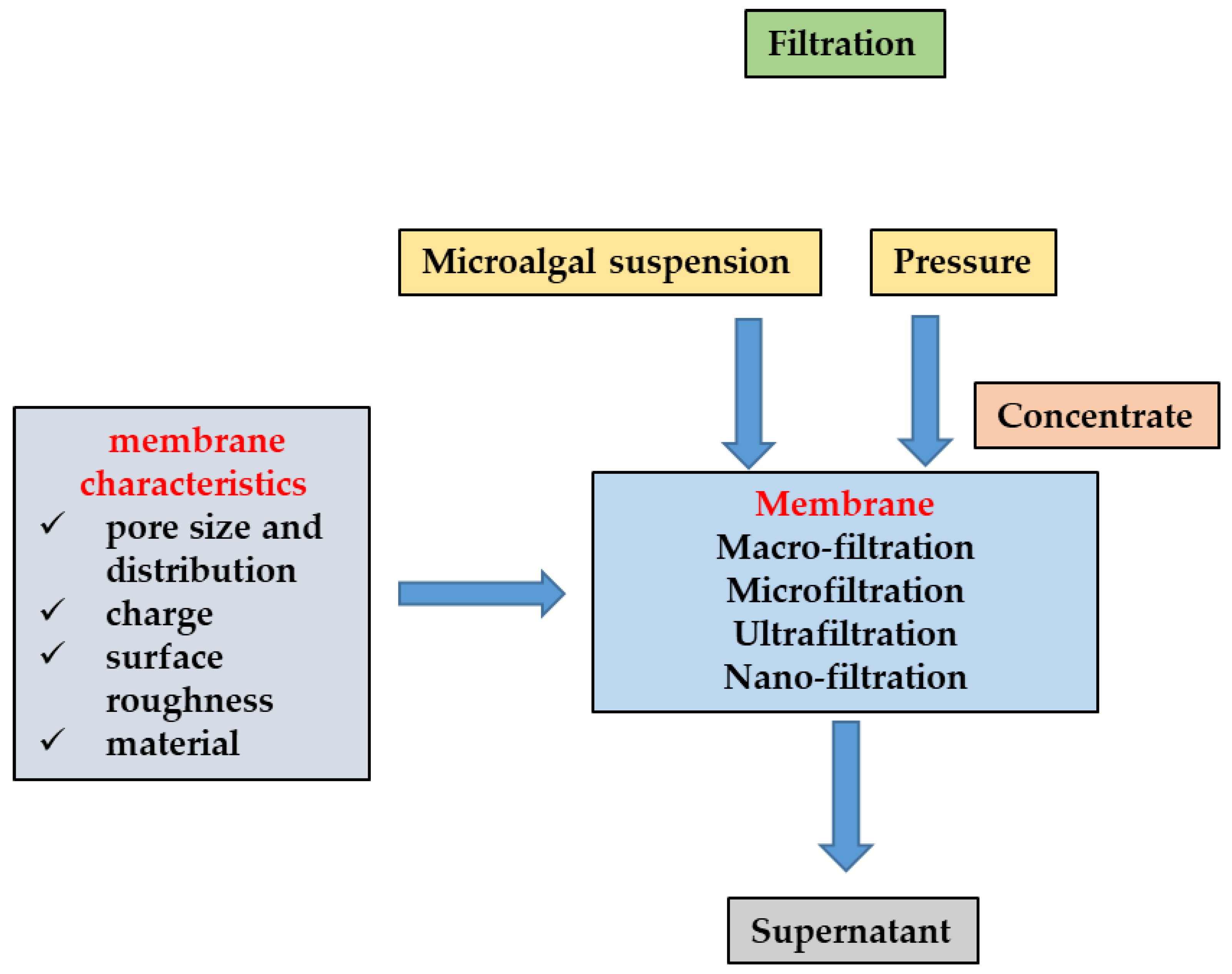
2.1. Filtration
2.2. Flotation
2.3. Flocculation
2.3.1. Chemical Flocculation
2.3.2. Electro-Flocculation
2.3.3. Bio-Flocculation
2.4. Electrochemical Harvesting
2.5. Other Harvesting Methods
3. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Ananthi, V.; Balaji, P.; Sindhu, R.; Kim, S.H.; Pugazhendhi, A.; Arun, A. A critical review on different harvesting techniques for algal based biodiesel production. Sci. Total Environ. 2021, 780, 146467. [Google Scholar] [CrossRef]
- Ndikubwimana, T.; Chang, J.; Xiao, Z.; Shao, W.; Zeng, X.; Ng, I.S.; Lu, Y. Flotation: A promising microalgae harvesting and dewatering technology for biofuels production. Biotechnol. J. 2016, 11, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Elcik, H.; Cakmakci, M. Harvesting microalgal biomass using crossflow membrane filtration: Critical flux, filtration performance, and fouling characterization. Environ. Technol. 2017, 38, 1585–1596. [Google Scholar] [CrossRef]
- Zamalloa, C.; Vulsteke, E.; Albrecht, J.; Verstraete, W. The techno-economic potential of renewable energy through the anaerobic digestion of microalgae. Bioresour. Technol. 2011, 102, 1149–1158. [Google Scholar] [CrossRef]
- Huang, W.-C.; Kim, J.-D. Cationic surfactant-based method for simultaneous harvesting and cell disruption of a microalgal biomass. Bioresour. Technol. 2013, 149, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottle neck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 012701–012715. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef] [PubMed]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sust. Energ. Rev. 2019, 115, 109361. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; García-Depraect, O. Membrane-Based Harvesting Processes for Microalgae and Their Valuable-Related Molecules: A Review. Membranes 2021, 11, 585. [Google Scholar] [CrossRef]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae—A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sust. Energ. Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic waste water and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Hwang, T.; Park, S.J.; Oh, Y.K.; Rashid, N.; Han, J.I. Harvesting of Chlorella sp. KR-1 using a cross-flow membrane filtration system equipped with an anti-fouling membrane. Bioresour. Technol. 2013, 139, 379–382. [Google Scholar] [CrossRef]
- Hwang, T.; Kotte, M.R.; Han, J.I.; Oh, Y.K.; Diallo, M.S. Microalgae recovery by ultrafiltration using novel fouling-resistant PVDF membranes with in situ PEGylated polyethyleneimine particles. Water Res. 2015, 73, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Discart, V.; Bilad, M.R.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F.J. Role of transparent exopolymeric particles in membrane fouling: Chlorella vulgaris broth filtration. Bioresour. Technol. 2013, 129, 18–25. [Google Scholar] [CrossRef]
- Osman, W.N.A.W.; Nawi, N.I.M.; Samsuri, S.; Bilad, M.R.; Khan, A.L.; Hunaepi, H.; Jaafar, J.; Lam, M.K. Ultra low-pressure filtration system for energy efficient microalgae filtration. Heliyon 2021, 7, e07367. [Google Scholar] [CrossRef]
- Lee, D.J.; Liao, G.Y.; Chang, Y.R.; Chang, J.S. Coagulation-membrane filtration of Chlorella vulgaris. Bioresour. Technol. 2012, 108, 184–189. [Google Scholar] [CrossRef]
- Bilad, M.R.; Azizo, A.S.; Wirzal, M.D.H.; Jia, L.J.; Putra, Z.A.; Nordin, N.A.H.; Mavukkandy, M.O.; Jasni, M.J.F.; Yusoff, A.R.M. Tackling membrane fouling in microalgae filtration using nylon 6, 6 nanofiber membrane. J. Environ. Manag. 2018, 223, 23–28. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Halim, N.S.A.; Lee, L.C.; Wirzal, M.D.H.; Bilad, M.R.; Nordin, N.A.H.; Putra, Z.A. Improved nylon 6,6 nanofiber membrane in a tilted panel filtration system for fouling control in microalgae harvesting. Polymers 2020, 12, 252. [Google Scholar] [CrossRef]
- Bilad, M.R.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F.J. Harvesting microalgal biomass using submerged microfiltration membranes. Bioresour. Technol. 2012, 111, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Chu, H.; Zhou, X.; Dong, B. Dewatering of Chlorella pyrenoidosa using diatomite dynamic membrane: Filtration performance, membrane fouling and cake behaviour. Colloids Surf. B Biointerfaces 2014, 113, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Nurra, C.; Clavero, E.; Salvado, J.; Torras, C. Vibrating membrane filtration as improved technology for microalgae dewatering. Bioresour. Technol. 2014, 157, 247–253. [Google Scholar] [CrossRef]
- Zhao, Z.; Muylaert, K.; Vankelecom, I.F.J. Combining patterned membrane filtration and flocculation for economical microalgae harvesting. Water Res. 2021, 198, 117181. [Google Scholar] [CrossRef]
- Kim, K.; Shin, H.; Moon, M.; Ryu, B.G.; Han, J.I.; Yang, J.W.; Chang, Y.K. Evaluation of various harvesting methods for high-density microalgae, Aurantiochytrium sp. KRS101. Bioresour. Technol. 2015, 198, 828–835. [Google Scholar] [CrossRef]
- Chen, W.; Wang, T.; Dou, Z.; Xie, X. Microalgae harvesting by self-driven 3D microfiltration with rationally designed porous superabsorbent polymer (PSAP) beads. Environ. Sci. Technol. 2021, 55, 15446–15455. [Google Scholar] [CrossRef]
- Zhao, Z.; Ilyas, A.; Muylaert, K.; Vankelecom, I.F.J. Optimization of patterned polysulfone membranes for microalgae harvesting. Bioresour. Technol. 2020, 309, 123367. [Google Scholar] [CrossRef]
- Kurniawati, H.A.; Ismadji, S.; Liu, J.C. Microalgae harvesting by flotation using natural saponin and chitosan. Bioresour. Technol. 2014, 166, 429–434. [Google Scholar] [CrossRef]
- Koley, S.; Prasad, S.; Bagchi, S.K.; Mallick, N. Development of a harvesting technique for large-scale microalgal harvesting for biodiesel production. RSC Adv. 2017, 7, 7227–7237. [Google Scholar] [CrossRef]
- Shen, Z.; Li, Y.; Wen, H.; Ren, X.; Liu, J.; Yang, L. Investigation on the role of surfactants in bubble-algae interaction in flotation harvesting of Chlorella vulgaris. Sci. Rep. 2018, 8, 3303. [Google Scholar] [CrossRef]
- Xu, K.; Zou, X.; Wen, H.; Xue, Y.; Zhao, S.; Li, Y. Buoy-bead flotation harvesting of the microalgae Chlorella vulgaris using surface-layered polymeric microspheres: A novel approach. Bioresour. Technol. 2018, 267, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Xu, K.; Wen, H.; Xue, Y.; Qu, Y.; Li, Y. Efficient microalgae harvesting using a thermal flotation method with response surface methodology. Water Sci. Technol. 2019, 80, 426–436. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, C.; Liu, Z.; Luo, W.; Zhong, H.; He, G.; Liang, C.; Li, L.; Deng, L.; Fu, W. Gemini surfactant: A novel flotation collector for harvesting of microalgae by froth flotation. Bioresour. Technol. 2019, 275, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zou, X.; Xu, K.; Shen, Z.; Ren, X.; Li, Y. Buoy-bead flotation application for the harvesting of microalgae and mechanistic analysis of significant factors. Bioprocess Biosyst. Eng. 2019, 42, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Corpuz, A.G.; Hasan, S.W.; Sillanpaa, M.; Banat, F. Microalgae harvesting using colloidal gas aphrons generated from single and mixed surfactants. Chemosphere 2021, 273, 128568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wen, H.; Yin, H.; Qin, W.; Liu, X.; Wang, Y.; Liu, Y. A novel approach for harvesting the microalgae Chlorella vulgaris with sodium alginate microspheres using buoy-bead flotation method. Sci. Total Environ. 2022, 851, 158418. [Google Scholar] [CrossRef]
- Al-Humairi, S.T.; Lee, J.G.M.; Harvey, A.P.; Salman, A.D.; Juzsakova, T.; Van, B.; Le, P.C.; La, D.D.; Mungray, A.K.; Show, P.L.; et al. A foam column system harvesting freshwater algae for biodiesel production: An experiment and process model evaluations. Sci. Total Environ. 2023, 862, 160702. [Google Scholar] [CrossRef]
- Zhang, X.; Amendola, P.; Hewson, J.C.; Sommerfeld, M.; Hu, Q. Influence of growth phase on harvesting of Chlorella zofingiensis by dissolved air flotation. Bioresour. Technol. 2012, 116, 477–484. [Google Scholar] [CrossRef]
- Xia, L.; Li, Y.; Huang, R.; Song, S. Effective harvesting of microalgae by coagulation-flotation. R. Soc. Open Sci. 2017, 4, 170867. [Google Scholar] [CrossRef]
- Leite, L.D.S.; Santos, P.R.D.; Daniel, L.A. Microalgae harvesting from wastewater by pH modulation and flotation: Assessing and optimizing operational parameters. J. Environ. Manag. 2020, 254, 109825. [Google Scholar] [CrossRef]
- Hosseini, M.; Starvaggi, H.; Ju, L.K. Additive-free harvesting of oleaginous phagotrophic microalga by oil and air flotation. Bioprocess Biosyst. Eng. 2016, 39, 1181–1190. [Google Scholar] [CrossRef]
- Besson, A.; Formosa-Dague, C.; Guiraud, P. Flocculation-flotation harvesting mechanism of Dunaliella salina: From nanoscale interpretation to industrial optimization. Water Res. 2019, 155, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Shaleh, S.R.M. Flotation removal of the microalga Nannochloropsis sp. using Moringa protein-oil emulsion: A novel green approach. Bioresour. Technol. 2018, 247, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Fuad, N.; Omar, R.; Kumarudin, S.; Harun, R.; Idris, A.; Azlina, W.A.K.G.W. Harvesting marine microalgae Nannochloropsis sp. using dissolved air flotation (DAF) technique. Sains Malays. 2021, 50, 73–83. [Google Scholar] [CrossRef]
- Lal, A.; Das, D. Biomass production and identification of suitable harvesting technique for Chlorella sp. MJ 11/11 and Synechocystis PCC 6803. 3 Biotech 2016, 6, 41. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, K.; Lee, J.; Lee, Y.H.; Han, J.I.; Park, J.Y.; Oh, Y.K. Acidified-flocculation process for harvesting of microalgae: Coagulant reutilization and metal-free-microalgae recovery. Bioresour. Technol. 2017, 239, 190–196. [Google Scholar] [CrossRef]
- Lin, Z.; Li, C.; Liu, J.; Yang, Z.; Zhang, H. An effective process of harvesting Chlorella sp. biomass for bioresource by rapid flocculation in a helical tube. Desalin. Water Treat. 2021, 221, 440–445. [Google Scholar] [CrossRef]
- Kim, D.H.; Oh, Y.K.; Lee, K. Harvesting of Oleaginous Microalgae Chlorella sp. by CaCO3 Mineralization. Korean J. Mater. Res. 2021, 31, 386–391. [Google Scholar] [CrossRef]
- Farooq, W.; Moon, M.; Ryu, B.G.; Suh, W.I.; Shrivastav, A.; Park, M.S.; Mishra, S.K.; Yang, J.W. Effect of harvesting methods on the reusability of water for cultivation of Chlorella vulgaris, its lipid productivity and biodiesel quality. Algal Res. 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Gorin, K.; Sergeeva, Y.E.; Butylin, V.V.; Komova, A.V.; Pojidaev, V.M.; Badranova, G.U.; Shapovalova, A.A.; Konova, I.A.; Gotovtsev, P.M. Methods coagulation/flocculation and flocculation with ballast agent for effective harvesting of microalgae. Bioresour. Technol. 2015, 193, 178–184. [Google Scholar] [CrossRef]
- Lee, S.M.; Choi, H.J. Harvesting of microalgae species using Mg-sericite flocculant. Bioprocess Biosyst. Eng. 2015, 38, 2323–2330. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, H.; Zhou, W.; Liu, Y.; Chen, P.; Ruan, R. Enhanced harvesting of Chlorella vulgaris using combined flocculants. Appl. Biochem. Biotechnol. 2016, 180, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Gerchman, Y.; Vasker, B.; Tavasi, M.; Mishael, Y.; Kinel-Tahan, Y.; Yehoshua, Y. Effective harvesting of microalgae: Comparison of different polymeric flocculants. Bioresour. Technol. 2017, 228, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Potocar, T.; Leite, L.D.S.; Daniel, L.A.; Pivokonsky, M.; Matoulkova, D.; Branyik, T. Cooking oil-surfactant emulsion in water for harvesting Chlorella vulgaris by sedimentation or flotation. Bioresour. Technol. 2020, 311, 123508. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cui, Y.; Cheng, P.; Huo, S.; Ma, X.; Chen, Q.; Cobb, K.; Chen, P.; Ma, J.; Gao, X.; et al. Microwave assisted flocculation for harvesting of Chlorella vulgaris. Bioresour. Technol. 2020, 314, 123770. [Google Scholar] [CrossRef]
- Mohseni, F.; Zenooz, A.M. Flocculation of Chlorella vulgaris with alum and pH adjustment. Biotechnol. Appl. Biochem. 2022, 69, 1112–1120. [Google Scholar] [CrossRef]
- Ma, W.; Feng, C.; Guan, F.; Ma, D.; Cai, J. Effective Chlorella vulgaris biomass harvesting through sulfate and chloride flocculants. J. Mar. Sci. Eng. 2023, 11, 47. [Google Scholar] [CrossRef]
- Kuzhiumparambil, U.; Labeeuw, L.; Commault, A.; Vu, H.P.; Nguyen, L.N.; Ralph, P.J.; Nghiem, L.D. Effects of harvesting on morphological and biochemical characteristics of microalgal biomass harvested by polyacrylamide addition, pH-induced flocculation, and centrifugation. Bioresour. Technol. 2022, 359, 127433. [Google Scholar] [CrossRef]
- Machado, C.A.; Esteves, A.F.; Pires, J.C.M. Optimization of microalgal harvesting with inorganic and organic flocculants using factorial design of experiments. Processes 2022, 10, 1124. [Google Scholar] [CrossRef]
- Escapa, C.; Coimbra, R.N.; Paniagua, S.; Garcia, A.I.; Otero, M. Nutrients and pharmaceuticals removal from wastewater by culture and harvesting of Chlorella sorokiniana. Bioresour. Technol. 2015, 185, 276–284. [Google Scholar] [CrossRef]
- Vandamme, D.; Pohl, P.I.; Beuckels, A.; Foubert, I.; Brady, P.V.; Hewson, J.C.; Muylaert, K. Alkaline flocculation of Phaeodactylum tricornutum induced by brucite and calcite. Bioresour Technol. 2015, 196, 656–661. [Google Scholar] [CrossRef]
- Das, P.; Thaher, M.I.; Hakim, M.A.Q.M.A.; Al-Jabri, H.M.S.J.; Alghasal, G.S.H.S. Microalgae harvesting by pH adjusted coagulation-flocculation, recycling of the coagulant and the growth media. Bioresour. Technol. 2016, 216, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Khadim, S.R.; Singh, P.; Singh, A.K.; Tiwari, A.; Mohanta, A.; Asthana, R.K. Mass cultivation of Dunaliella salina in a flat plate photobioreactor and its effective harvesting. Bioresour. Technol. 2018, 270, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Caetano, N.S.; Martins, A.A.; Gorgich, M.; Gutierrez, D.M.; Ribeiro, T.J.; Mata, T.M. Flocculation of Arthrospira maxima for improved harvesting. Energy Rep. 2020, 6, 423–428. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Cheng, S.; Zhang, W.; Zhang, X. Enhanced microalgal harvesting using microalgae-derived extracellular polymeric substance as flocculation aid. ACS Sustain. Chem. Eng. 2020, 8, 4069–4075. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, L.; Chen, Q.; Lu, J.; Pan, G.; Hu, L.; Yi, Q. Synergy of flocculation and flotation for microalgae harvesting using aluminium electrolysis. Bioresour. Technol. 2017, 233, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Griffith, R.; Li, W.; Peng, P.; Cheng, Y.; Chen, P.; Addy, M.M.; Liu, Y.; Ruan, R. A continuous flocculants-free electrolytic flotation system for microalgae harvesting. Bioresour. Technol. 2017, 238, 439–449. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, C.; Liu, Z.; Han, T.; Hao, N.; Guo, Z.; Wang, W.; Chen, S.; Zhao, L.; Safavi, M.; et al. A novel salt-bridge electroflocculation technology for harvesting microalgae. Front. Bioeng. Biotechnol. 2022, 10, 902524. [Google Scholar] [CrossRef]
- Zenouzi, A.; Ghobadian, B.; Hejazi, M.A.; Rahnemoon, P. Harvesting of microalgae Dunaliella salina using electroflocculation. J. Agric. Sci. Technol. 2013, 15, 879–887. [Google Scholar]
- Xiong, Q.; Pang, Q.; Pan, X.; Chika, A.O.; Wang, L.; Shi, J.; Jia, L.; Chen, C.; Gao, Y. Facile sand enhanced electro-flocculation for cost-efficient harvesting of Dunaliella salina. Bioresour. Technol. 2015, 187, 326–330. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Lv, T.; Chen, H.; Chika, A.O.; Xiang, C.; Guo, M.; Wu, M.; Li, J.; Jia, L. Energy-producing electro-flocculation for harvest of Dunaliella salina. Bioresour. Technol. 2017, 241, 1022–1026. [Google Scholar] [CrossRef]
- Cassini, S.T.; Francisco, S.A.; Antunes, P.W.P.; Oss, R.N.; Keller, R. Harvesting microalgal biomass grown in anaerobic sewage treatment effluent by the coagulation-flocculation method: Effect of pH. Braz. Arch. Biol. Technol. 2017, 60, e160174. [Google Scholar] [CrossRef]
- Chen, J.; Leng, L.; Ye, C.; Lu, Q.; Addy, M.; Wang, J.; Liu, J.; Chen, P.; Ruan, R.; Zhou, W. A comparative study between fungal pellet- and spore-assisted microalgae harvesting methods for algae bioflocculation. Bioresour. Technol. 2018, 259, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.M.; Yunos, F.H.M.; Jusoh, H.H.W.; Mohammad, A.; Lam, S.S.; Jusoh, A. Subtopic: Advances in water and wastewater treatment harvesting of Chlorella sp. microalgae using Aspergillus niger as bio-flocculant for aquaculture wastewater treatment. J. Environ. Manag. 2019, 249, 109373. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wu, X.; Jiang, H.; Yu, M.; Liu, Y.; Min, A.; Li, W.; Ruan, R. Edible fungi-assisted harvesting system for efficient microalgae bio-flocculation. Bioresour. Technol. 2019, 282, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Gao, Z.; Yin, J.; Tang, X.; Ji, X.; Huang, H. Harvesting of microalgae by flocculation with poly (γ-glutamic acid). Bioresour. Technol. 2012, 112, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Gultom, S.O.; Zamalloa, C.; Hu, B. Microalgae harvest through fungal pelletization-Co-culture of Chlorella vulgaris and Aspergillus niger. Energies 2014, 7, 4417–4429. [Google Scholar] [CrossRef]
- Prochazkova, G.; Kastanek, P.; Branyik, T. Harvesting freshwater Chlorella vulgaris with flocculant derived from spent brewer’s yeast. Bioresour. Technol. 2015, 177, 28–33. [Google Scholar] [CrossRef]
- Razack, S.A.; Duraiarasan, S.; Shellomith, A.S.S.; Muralikrishnan, K. Statistical optimization of harvesting Chlorella vulgaris using a novel bio-source, Strychnos potatorum. Biotechnol. Rep. 2015, 7, 150–156. [Google Scholar] [CrossRef]
- Tork, M.B.; Khalilzadeh, R.; Kouchakzadeh, H. Efficient harvesting of marine Chlorella vulgaris microalgae utilizing cationic starch nanoparticles by response surface methodology. Bioresour. Technol. 2017, 243, 583–588. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Hiltunen, E. Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: Effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol. Biofuels. 2018, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zou, X.; Mouradov, A.; Spangenberg, G.; Chang, W.; Li, Y. Efficient bioflocculation of Chlorella vulgaris with a chitosan and walnut protein extract. Biology 2021, 10, 352. [Google Scholar] [CrossRef]
- Chu, R.; Li, S.; Yin, Z.; Hu, D.; Zhang, L.; Xiang, M.; Zhu, L. A fungal immobilization technique for efficient harvesting of oleaginous microalgae: Key parameter optimization, mechanism exploration and spent medium recycling. Sci. Total Environ. 2021, 790, 148174. [Google Scholar] [CrossRef]
- Niemi, C.; Gentili, F.G. The use of natural organic flocculants for harvesting microalgae grown in municipal wastewater at different culture densities. Physiol. Plant. 2021, 173, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Hou, J.; Miao, L. Harvesting freshwater microalgae with natural polymer flocculants. Algal Res. 2021, 57, 102358. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Feng, C.; Li, J.; Wang, N.; Cai, J. High-quality Chlorella vulgaris biomass harvesting through chitosan and polyacrylamide. Environ. Sci. Pollut. Res. 2022, 29, 34651–34658. [Google Scholar] [CrossRef]
- Dai, Y.R.; Wang, D.; Zhu, Y.R.; Yang, K.X.; Jiao, N.; Sun, Z.L.; Wang, S.K. Thermal-tolerant potential of ordinary Chlorella pyrenoidosa and the promotion of cell harvesting by heterotrophic cultivation at high temperature. Front. Bioeng. Biotechnol. 2022, 10, 1072942. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Machado, E.L.; Knoll, R.S.; Osbel, N.D.; Colares, G.S.; Rodrigues, L.R. Combined system for wastewater treatment: Ozonization and coagulation via tannin-based agent for harvesting microalgae by dissolved air flotation. Environ. Technol. 2022, 43, 1370–1380. [Google Scholar] [CrossRef]
- Teixeira, M.S.; Speranza, L.G.; Silva, I.C.D.; Moruzzi, R.B.; Silva, G.H.R. Tannin-based coagulant for harvesting microalgae cultivated in wastewater: Efficiency, floc morphology and products characterization. Sci. Total Environ. 2022, 807, 150776. [Google Scholar] [CrossRef] [PubMed]
- Letelier-Gordo, C.O.; Holdr, S.L.; Francisci, D.D.; Karakashev, D.B.; Angelidaki, I. Effective harvesting of the microalgae Chlorella protothecoides via bioflocculation with cationic starch. Bioresour. Technol. 2014, 167, 214–218. [Google Scholar] [CrossRef]
- Xu, Y.; Purton, S.; Baganz, F. Chitosan flocculation to aid the harvesting of the microalga Chlorella sorokiniana. Bioresour. Technol. 2013, 129, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Toscano, L.P.; Ogden, K.L.; Brown, J.K.; Ogden, G.; Cervantes, L.D.; Steichen, S.A.; Samaniego, B.G. Harvesting the microalga Chlorella sorokiniana by fungal-assisted Pelletization. J. Biobased Mater. Bioenergy 2018, 12, 493–505. [Google Scholar] [CrossRef]
- Lopez-Exposito, P.; Campano, C.; Ven, T.G.M.V.D.; Negro, C.; Blanco, A. Microalgae harvesting with the novel flocculant hairy cationic nanocrystalline cellulose. Colloids Surf. B Biointerfaces. 2019, 178, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Noh, W.; Kim, J.; Lee, S.J.; Ryu, B.G.; Kang, C.M. Harvesting and contamination control of microalgae Chlorella ellipsoidea using the bio-polymeric flocculant α-poly-l-lysine. Bioresour. Technol. 2018, 249, 206–211. [Google Scholar] [CrossRef]
- Verfaillie, A.; Blockx, J.; Praveenkumar, R.; Thielemans, W.; Muylaert, K. Harvesting of marine microalgae using cationic cellulose nanocrystals. Carbohydr. Polym. 2020, 240, 116165. [Google Scholar] [CrossRef]
- Ndikubwimana, T.; Zeng, X.; Murwanashyaka, T.; Manirafasha, E.; He, N.; Shao, W.; Lu, Y. Harvesting of freshwater microalgae with microbial bioflocculant: A pilot-scale study. Biotechnol. Biofuels. 2016, 9, 47. [Google Scholar] [CrossRef]
- Srinuanpan, S.; Chawpraknoi, A.; Chantarit, S.; Cheirsilp, B.; Prasertsan, P. A rapid method for harvesting and immobilization of oleaginous microalgae using pellet-forming filamentous fungi and the application in phytoremediation of secondary effluent. Int. J. Phytoremediation 2018, 20, 1017–1024. [Google Scholar] [CrossRef]
- Fayad, N.; Yehya, T.; Audonnet, F.; Vial, C. Harvesting of microalgae Chlorella vulgaris using electro-coagulation-flocculation in the batch mode. Algal Res. 2017, 25, 1–11. [Google Scholar] [CrossRef]
- Pishgar, Z.; Samimi, A.; Mohebbi-Kalhori, D.; Shokrollahzadeh, S. Comparative study on the harvesting of marine Chlorella vulgaris microalgae from a dilute slurry using autoflocculation-sedimentation and electrocoagulation-flotation methods. Int. J. Environ. Res. 2020, 14, 615–628. [Google Scholar] [CrossRef]
- Lucakova, S.; Branyikova, I.; Kovacikova, S.; Masojidek, J.; Ranglova, K.; Branyik, T.; Ruzicka, M.C. Continuous electrocoagulation of Chlorella vulgaris in a novel channel-flow reactor: A pilot-scale harvesting study. Bioresour. Technol. 2022, 351, 126996. [Google Scholar] [CrossRef]
- Mehrgan, M.S.; Shekarabi, S.P.H. Electrochemical harvesting of the marine microalgae, Nannochloropsis oculata: Effect on approximate composition, fatty acid profile, and metals biosorption. Iran. J. Fish. Sci. 2022, 21, 33–47. [Google Scholar]
- Misra, R.; Guldhe, A.; Singh, P.; Rawat, I.; Stenstrom, T.A.; Bux, F. Evaluation of operating conditions for sustainable harvesting of microalgal biomass applying electrochemical method using non sacrificial electrodes. Bioresour. Technol. 2015, 176, 1–7. [Google Scholar] [CrossRef]
- Sossella, F.D.S.; Rempel, A.; Nunes, J.M.A.; Biolchi, G.; Migliavaca, R.; Antunes, A.C.F.; Costa, J.A.V.; Hemkemeier, M.; Colla, L.M. Effects of harvesting Spirulina platensis biomass using coagulants and electrocoagulation-flotation on enzymatic hydrolysis. Bioresour. Technol. 2020, 311, 123526. [Google Scholar] [CrossRef]
- Qi, S.; Chen, J.; Hu, Y.; Hu, Z.; Zhan, X.; Stengel, D.B. Low energy harvesting of hydrophobic microalgae (Tribonema sp.) by electro-flotation without coagulation. Sci. Total Environ. 2022, 838, 155866. [Google Scholar] [CrossRef]
- Hawari, A.H.; Alkhatib, A.M.; Das, P.; Thaher, M.; Benamor, A. Effect of the induced dielectrophoretic force on harvesting of marine microalgae (Tetraselmis sp.) in electrocoagulation. J. Environ. Manag. 2020, 260, 110106. [Google Scholar] [CrossRef] [PubMed]
- Khatib, W.A.; Ayari, A.; Yasir, A.T.; Talhami, M.; Das, P.; Quadir, M.A.; Hawari, A.H. Enhancing the electrocoagulation process for harvesting marine microalgae (Tetraselmis sp.) using interdigitated electrodes. J. Environ. Manag. 2021, 292, 112761. [Google Scholar] [CrossRef]
- Kim, D.Y.; Oh, Y.K.; Park, J.Y.; Kim, B.; Choi, S.A.; Han, J.I. An integrated process for microalgae harvesting and cell disruption by the use of ferric ions. Bioresour. Technol. 2015, 191, 469–474. [Google Scholar] [CrossRef]
- Seo, Y.H.; Park, D.; Oh, Y.K.; Yoon, S.; Han, J.I. Harvesting of microalgae cell using oxidized dye wastewater. Bioresour. Technol. 2015, 192, 802–806. [Google Scholar] [CrossRef]
- Tiron, O.; Bumbac, C.; Manea, E.; Stefanescu, M.; Lazar, M.N. Overcoming microalgae harvesting barrier by activated algae granules. Sci. Rep. 2017, 7, 4646. [Google Scholar] [CrossRef]
- Behera, B.; Balasubramanian, P. Natural plant extracts as an economical and ecofriendly alternative for harvesting microalgae. Bioresour. Technol. 2019, 283, 45–52. [Google Scholar] [CrossRef]
- Prochazkova, G.; Podolova, N.; Safarik, I.; Zachleder, V.; Branyik, T. Physicochemical approach to freshwater microalgae harvesting with magnetic particles. Colloids Surf. B Biointerfaces. 2013, 112, 213–218. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, Z.; Tan, D.; Liu, C.; Kuang, Y.; Li, Z. A novel method to harvest Chlorella sp. by co-flocculation/air flotation. Biotechnol. Lett. 2017, 39, 79–84. [Google Scholar] [CrossRef]
- Leite, L.D.S.; Daniel, L.A. Optimization of microalgae harvesting by sedimentation induced by high pH. Water Sci. Technol. 2020, 82, 1227–1236. [Google Scholar] [CrossRef]
- Savvidou, M.G.; Dardavila, M.M.; Georgiopoulou, I.; Louli, V.; Stamatis, H.; Kekos, D.; Voutsas, E. Optimization of microalga Chlorella vulgaris magnetic harvesting. Nanomaterials 2021, 11, 1614. [Google Scholar] [CrossRef]
- Dassey, A.J.; Theegala, C.S. Harvesting economics and strategies using centrifugation for cost effective separation of microalgae cells for biodiesel applications. Bioresour. Technol. 2013, 128, 241–245. [Google Scholar] [CrossRef]
- Hu, Y.R.; Wang, F.; Wang, S.K.; Liu, C.Z.; Guo, C. Efficient harvesting of marine microalgae Nannochloropsis maritima using magnetic nanoparticles. Bioresour. Technol. 2013, 138, 387–390. [Google Scholar] [CrossRef]
- Markeb, A.A.; Llimos-Turet, J.; Ferrer, I.; Blanquez, P.; Alonso, A.; Sanchez, A.; Moral-Vico, J.; Font, X. The use of magnetic iron oxide based nanoparticles to improve microalgae harvesting in real wastewater. Water Res. 2019, 159, 490–500. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, J.; Lin, Y.; Cui, S.; Luo, S. The selection of a surfactant for freshwater microalgae harvesting and separation by the foam separation method. Bioprocess Biosyst. Eng. 2019, 42, 1721–1730. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Fermentative hydrogen production using pretreated microalgal biomass as feedstock. Microb. Cell Fact. 2018, 17, 22. [Google Scholar] [CrossRef]
- Liber, J.A.; Bryson, A.B.; Bonito, G.; Du, Z.-Y. Harvesting Microalgae for Food and Energy Products. Small Methods 2020, 2020, 2000349. [Google Scholar] [CrossRef]
- Matter, I.A.; Bui, V.K.H.; Jung, M.; Seo, J.Y.; Kim, Y.-E.; Lee, Y.-C.; Oh, Y.-K. Flocculation Harvesting Techniques for Microalgae: A Review. Appl. Sci. 2019, 9, 3069. [Google Scholar] [CrossRef]
- Branyikova, I.; Prochazkova, G.; Potocar, T.; Jezkova, Z.; Branyik, T. Harvesting of Microalgae by Flocculation. Fermentation 2018, 4, 93. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, C.; Zhang, S.; Zhang, M.; Li, D.; Wang, X.; Mao, B. Comprehensive analysis of the combined flocculation and filtration process for microalgae harvesting at various operating parameters. Sci. Total Environ. 2023, 857, 159658. [Google Scholar] [CrossRef] [PubMed]

| Microalgae | Place | Filtration | Recovery (%) | References |
|---|---|---|---|---|
| Chlorella sp. | South Korea | Crossflow membrane filtration—hydrophilic polyvinyl alcohol polymer | 100% | [14] |
| South Korea | Ultrafiltration, fouling-resistant PVDF membranes | 94% and 100% | [15] | |
| Chlorella vulgaris | Germany | Transparent exopolymeric particles—polycarbonate filter | 97% | [16] |
| Istanbul, Turkey | Crossflow membrane filtration, UH050 membrane—hydrophilic polyethersulfone | 100% | [5] | |
| Crossflow filtration, ultra-low-pressure filtration system | 76% | [17] | ||
| Southern Taiwan | Coagulation—polyaluminum chloride and polytetrafluoroethylene membrane | 31% lipid, 28% protein, and 8% carbohydrate | [18] | |
| Malaysia | Nylon 6,6 nanofiber membrane, polyvinylidene fluoride phase-inverted membrane | Enhanced its competitiveness | [19] | |
| Perak, Malaysia | Nylon 6,6 nanofiber membrane | - | [20] | |
| Belgium | Combining the submerged membrane bioreactor microfiltration with centrifugation | - | [21] | |
| Chlorella pyrenoidosa (Syn: Chlorella vulgaris) | China | Diatomite dynamic membrane | - | [22] |
| Nannochloropsis gaditana (Syn: Microchloropsis gaditana) | Tarragona, Spain | Dynamic filtration, polyethersulfone membrane | - | [23] |
| Dictyosphaerium sp. | Belgium | Combination of patterned membrane filtration and flocculation at standardized chitosan dosage, crossflow filtration, polyethylene glycol | Highest stable membrane permeance | [24] |
| Phaeodactylum tricornutum | Belgium | Combining the submerged membrane bioreactor microfiltration with centrifugation | - | [21] |
| Tarragona, Spain | Dynamic filtration, polyethersulfone membrane | - | [23] | |
| Aurantiochytrium sp. | South Korea | Dynamic filtration module, an FMX B-class | 100% | [25] |
| Microalgae | UK | Microfiltration, porous superabsorbent polymer beads | 90% | [26] |
| Desmodesmus sp. | Belgium | Polysulfone and polyethylene glycol | 100% | [27] |
| Microalgae | Place | Flotation | Recovery (%) | References |
|---|---|---|---|---|
| Chlorella vulgaris | Taiwan | Dispersed air flotation | 93% | [28] |
| India | Dissolved air flotation | 90% | [29] | |
| China | Surfactant, hexadecyltrimethyl-ammonium bromide and tea saponin. | 89.23% | [30] | |
| China | Buoy-bead flotation, surface-layered polymeric microspheres | 98.43% | [31] | |
| China | Thermal flotation | 91.96% | [32] | |
| China | N,N′-bis(cetyl dimethyl)-1,4-butane diammonium dibromide | 99.2% | [33] | |
| China | Buoy-bead flotation | 89.9% | [34] | |
| Abu Dhabi | Colloidal gas aphrons technology, surfactants—cationic hexadecyl trimethyl ammonium bromide, anionic sodium dodecylbenzene sulfonate, sodium dodecyl sulfate, and combinations of these surfactants | 95% | [35] | |
| China | Buoy-bead flotation, sodium alginate microspheres | 93.78% | [36] | |
| UK | Continuous foam flotation, cationic trimethyl-ammonium bromide | 96% | [37] | |
| Chromochloris zofingiensis | US | Dissolved air flotation, dissolved organic matter, increasing Al3+ concentration | 95.2% | [38] |
| Chlorella sp. | Mexico | Al3+ and cetyltrimethylammonium bromide | 98.73% | [39] |
| Chlorella sorokiniana | Brotas, Brazil | Dissolved air flotation, pH modulation | 96.5–97.9% | [40] |
| Scenedesmus obliquus (Syn: Tetradesmus obliquus) | Taiwan | Dispersed air flotation | 93% microalgae | [28] |
| India | Dissolved air flotation | 90% | [29] | |
| China | Thermal flotation | 91.96% | [32] | |
| Ochromonas danica | USA | Oil and air flotation | 98% | [41] |
| Dunaliella salina | France | Flocculation/flotation | 80% | [42] |
| Arthrospira platensis | Abu Dhabi | surfactants—cationic hexadecyltrimethylammonium bromide, anionic sodium dodecylbenzene sulfonate, sodium dodecyl sulfate, and combinations of these surfactants | 95% | [35] |
| Nannochloropsis sp. | Malaysia | Flotation, Moringa protein–oil emulsion | 86% | [43] |
| Malaysia | Dissolved air flotation, tannin-based biopolymer flocculant, AFlok-BP1 | - | [44] | |
| Nannochloropsis oculata | Abu Dhabi | surfactants—cationic hexadecyl trimethyl ammonium bromide, anionic sodium dodecylbenzene sulfonate, sodium dodecyl sulfate, and combinations of these surfactants | 95% | [35] |
| Microalgae | Place | Flocculation | Recovery (%) | References |
|---|---|---|---|---|
| Chlorella sp. | IARI, India, and Uppsala University, Sweden | Ferric chloride, potassium aluminum sulfate, chitosan solution | 82% | [45] |
| Republic of Korea | Acidified flocculation, coagulant—Fe2(SO4)3 and H2SO4 | 98% | [46] | |
| China | FeCl3 and polyacrylamide | 90.5% | [47] | |
| Republic of Korea | Ca2+ and CO32−, amorphous nano-flakes, rhombohedral calcites, and spherical vaterites | 90–99% | [48] | |
| Chlorella vulgaris | Texas, USA | Centrifugation or flocculation with FeCl3 | 90% | [49] |
| Russia | Mixture of coagulant—FeCl3 and flocculant—PEO-based Sibfloc-718 | 90% | [50] | |
| Republic of Korea | Mg-sericite flocculant | 99% | [51] | |
| China | Mixture of flocculants, poly-γ-glutamic acid, and calcium oxide | 95% | [52] | |
| India | Alum and ferric chloride | 90% | [29] | |
| Israel | Polydiallyldimethylammonium chloride | 90% | [53] | |
| Trebon, Czech Republic | Cooking oil (rapeseed oil) in an aqueous solution of cetyl-trimethylammonium bromide (2.7 mg/L) | 90% | [54] | |
| Austin, USA | Fe3+ (FeCl3), chitosan, and Ca2+ (CaCl2) | 43.2%, 49.5% and 39.6% | [55] | |
| Tehran, Iran | Alum and pH adjustment | 90% | [56] | |
| Wuhan, China | Sulfate (Al2(SO4)3 and Fe2(SO4)3) and chloride flocculants (AlCl3 and FeCl3) | 93.5–98.8% | [57] | |
| Australia | Polyacrylamide addition, alkaline addition, and centrifugation | - | [58] | |
| Oban, UK | Aluminum sulfate, ferric sulfate, and ferric chloride | 98.8% | [59] | |
| Chlorella sorokiniana | Spain | AlCl3 | 95.23% | [60] |
| Porphyridium purpureum | Australia | Polyacrylamide addition, alkaline addition, and centrifugation | - | [58] |
| Phaeodactylum tricornutum | Belgium | Brucite and calcite | 90% | [61] |
| Australia | Polyacrylamide addition, alkaline addition, and centrifugation | - | [58] | |
| Synechocystis sp. | IARI, India and Uppsala University, Sweden, | Ferric chloride, potassium aluminum sulfate, chitosan solution | 82% | [45] |
| Scenedesmus sp. | Doha, Qatar | Coagulation flocculation (ferric chloride (72–96 mg/L) | 90% | [62] |
| Scenedesmus obliquus (Syn: (Tetradesmus obliquus) | India | Alum and ferric chloride | 90% | [29] |
| Dunaliella salina | India | Potash alum or FeCl3·6H2O | 99% | [63] |
| Arthrospira maxima (Syn: Limnospira maxima) | Porto, Portugal | NaOH or CaCl2 | 90% | [64] |
| Scenedesmus acuminatus (Syn: (Tetradesmus lagerheimii) | China | Alum coagulation with extracellular polymeric substances | - | [65] |
| Microalgae | Place | Electro-Flocculation | Recovery (%) | References |
|---|---|---|---|---|
| Chlorella sp. | IARI, India, and Uppsala University, Sweden | Different DC voltages (6, 9, and 12 V) | 98% | [45] |
| Chlorella vulgaris | China | Aluminium electrolysis | 98% | [66] |
| China | Flocculant-free electrolytic flotation | 90% | [67] | |
| Synechocystis sp. | IARI, New Delhi, India, and Uppsala University, Sweden | Different DC voltages (6, 9, and 12 V) | 98% | [45] |
| Nannochloropsis oculata | UK | Salt bridge electro-flocculation (300 mA in 45 min.) | 90.4% | [68] |
| Dunaliella salina | Iran | Aluminum electrodes | 97.44% | [69] |
| China | Electro-flocculation | 95.13% to 98.09% | [70] | |
| China | Precipitation of aluminum hydroxide hydrates | 97% | [71] |
| Microalgae | Place | Bioflocculation | Recovery (%) | References |
|---|---|---|---|---|
| Chlorella sp. | IARI, India, and Uppsala University, Sweden | Chitosan | 98% | [45] |
| Brazil | Tanfloc, seed powder of Moringa oleifera, gum from Hibiscus esculentus, and cationic starch | 80.3 to 92% | [72] | |
| USA | Fungi-assisted harvesting, Penicillium sp. | 99.26% | [73] | |
| Malaysia | Aspergillus niger | 90% | [74] | |
| China | Edible fungi-assisted harvesting—Pleurotus ostreatus | 64.86% | [75] | |
| Chlorella vulgaris | China | Microbial flocculant poly (γ-glutamic acid) | 90% | [76] |
| USA | Fungal pelletization—Aspergillus niger | 90% | [77] | |
| USA | Yeast modified with 2-chloro-N,N-diethylethylamine hydrochloride | - | [78] | |
| India | Strychnos potatorum | 99.68% | [79] | |
| India | Chitosan | 90% | [29] | |
| Tehran, Iran | Cationic starch nanoparticles | 90% | [80] | |
| Finland | Chitosan | 90% | [81] | |
| Wuhan, China | Chitosan (10 mg/L), neutral pH | 89% | [82] | |
| Wuhan, China | Walnut protein extract | 40% | [82] | |
| Wuhan, China | Chitosan (6 mg/L) and walnut protein extract | 98% | [82] | |
| China | Aspergillus oryzae | 99.23% | [83] | |
| Vakin, Umeå, Sweden | Cationic starch, chitosan, and acacia tannin S5T | 93% | [84] | |
| Wuhan, China | Chitosan, Tanfloc, cationic starch, and Moringa oleifera | >90% | [85] | |
| China | Chitosan and polyacrylamide | 98.10% and 94.57% | [86] | |
| Oban, UK | Zetag 8185, chitosan, Tanfloc SG | 97.9% | [59] | |
| Chlorella pyrenoidosa (Syn: Auxenochlorella pyrenoidosa) | China | Chitosan | 96.83% | [87] |
| Microalgae | Brazil | Tannin-based coagulant | 84% | [88] |
| Microalgae | Brazil | Tannin-based coagulant | 90% | [89] |
| Chlorella protothecoides (Syn: Auxenochlorella protothecoides) | China | Microbial flocculant poly (γ-glutamic acid) | 90% | [76] |
| UK | Cationic starch—coagulation flocculation | 80% | [90] | |
| Chlorella sorokiniana | Texas, USA | Chitosan | 99% | [91] |
| Mexico | Aspergillus flavus-assisted pelletization | 96% | [92] | |
| Canada | Flocculant hairy cationic nanocrystalline cellulose | 82% | [93] | |
| Chlorella ellipsoidea (Syn: Chloroidium ellipsoideum) | Republic of Korea | Bio-polymeric flocculant α-poly-l-lysine | 98% | [94] |
| Nannochloropsis oculata | Belgium | Cationic cellulose nanocrystals | 95% | [95] |
| Desmodesmus brasiliensis | γ-PGA obtained from Bacillus licheniformis | 98% | [96] | |
| Synechocystis sp. | Brazil | Tanfloc, seed powder of Moringa oleifera, gum from Hibiscus esculentus, and cationic starch | 80.3 to 92% | [72] |
| Scenedesmus sp. | Thailand | Aspergillus niger, Trichoderma reesei, and Aspergillus oryzae—pellet formation | 94% | [97] |
| Scenedesmus obliquus (Syn: (Tetradesmus obliquus) | Vakin, Umeå, Sweden | Cationic starch, chitosan, and acacia tannin S5T | 93% | [84] |
| Wuhan, China | Chitosan, Tanfloc, cationic starch, and Moringa oleifera | >90% | [85] |
| Microalgae | Place | Electrochemical | Recovery (%) | References |
|---|---|---|---|---|
| Chlorella vulgaris | France | Aluminum and iron electrodes, metal hydroxide | 36.6% | [98] |
| Iran | Aluminium electrodes—carbon cloth (anode) and stainless-steel (cathode) | 98.00% | [99] | |
| India | Electroflotation | 99% | [29] | |
| Czech Republic | Electrocoagulation, electrolysis with iron electrodes | 85% | [100] | |
| Nannochloropsis oculata | Iran | Aluminum, iron, and graphite electrodes | 89.68% | [101] |
| Scenedesmus obliquus (Syn: (Tetradesmus obliquus) | South Africa | Metallic electrodes—NaCl | 83% | [102] |
| India | Electroflotation | 99% | [29] | |
| Arthrospira platensis | Brazil | Electrocoagulation flotation, aluminum and carbon electrode | 98–99% | [103] |
| Tribonema sp. | China | Electroflotation | 96.3% | [104] |
| Tetraselmis sp. | Doha, Qatar | Electrocoagulation (asymmetrical aluminum electrodes) | 90.9% | [105] |
| Qatar | Electrocoagulation, interdigitated electrodes | 96.18% | [106] |
| Microalgae | Place | Methods | Recovery (%) | References |
|---|---|---|---|---|
| Chlorella sp. | Republic of Korea | Coagulation (FeCl3 and Fe2(SO4)3) | 99% | [107] |
| Republic of Korea | 1 mM of FeCl3 and 0.5% of H2O2 | 90% | [108] | |
| Romania | Activated algae granules | 99% | [109] | |
| India | Coagulation, M. oleifera | 95.76% | [110] | |
| Prague, Czech Republic | Magnetic particles (diethylaminoethyl and polyethylenimine) | 90% | [111] | |
| Co-flocculation/air flotation (helix tube flocculation reactor) | 94% | [112] | ||
| Brazil | Sedimentation | 97.8% | [113] | |
| Iran | sedimentation | 66.00% | [99] | |
| Greece | Magnetic harvesting (microwave-synthesized naked magnetite (Fe3O4) particles) | 99% | [114] | |
| Aurantiochytrium sp. | Republic of Korea | Centrifugation | 90% | [107] |
| Nannochloris sp. | USA | Centrifugation with high flow rate | 90% | [115] |
| Nannochloropsis maritima | China | Magnetic nanoparticles, Fe3O4 nanoparticles | 95% | [116] |
| Scenedesmus sp. | Spain | Adsorbents of magnetite-based nanoparticles (Fe3O4 NPs) | 95% | [117] |
| India | Coagulation, M. oleifera | 95.76% | [110] | |
| Desmodesmus brasiliensis | China | Foam separation (natural surfactant cocamidopropyl betaine) | 93.6% | [118] |
| Synechocystis sp. | India | Coagulation, M. oleifera | 95.76% | [110] |
| Spirulina sp. | India | Coagulation, M. oleifera | 95.76% | [110] |
| Harvesting Methods | Advantages | Disadvantages | References |
|---|---|---|---|
| Filtration |
|
| [14,15,17,19,22,26] |
| Flotation |
|
| [33,39,40,42] |
| Chemical flocculation |
|
| [57,58,59] |
| Electro-flocculation |
|
| [45,66,67] |
| Bio-flocculation |
|
| [79,84,85,90] |
| Electrochemical techniques |
|
| [98,99,103] |
| Coagulation |
|
| [25,108,109] |
| Sedimentation |
|
| [99,113] |
| Centrifugation |
|
| [25,115] |
| Magnetic harvesting |
|
| [116,117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deepa, P.; Sowndhararajan, K.; Kim, S. A Review of the Harvesting Techniques of Microalgae. Water 2023, 15, 3074. https://doi.org/10.3390/w15173074
Deepa P, Sowndhararajan K, Kim S. A Review of the Harvesting Techniques of Microalgae. Water. 2023; 15(17):3074. https://doi.org/10.3390/w15173074
Chicago/Turabian StyleDeepa, Ponnuvel, Kandhasamy Sowndhararajan, and Songmun Kim. 2023. "A Review of the Harvesting Techniques of Microalgae" Water 15, no. 17: 3074. https://doi.org/10.3390/w15173074







