Processing of Carbon-Based Nanomaterials for the Removal of Pollutants from Water/Wastewater Application
Abstract
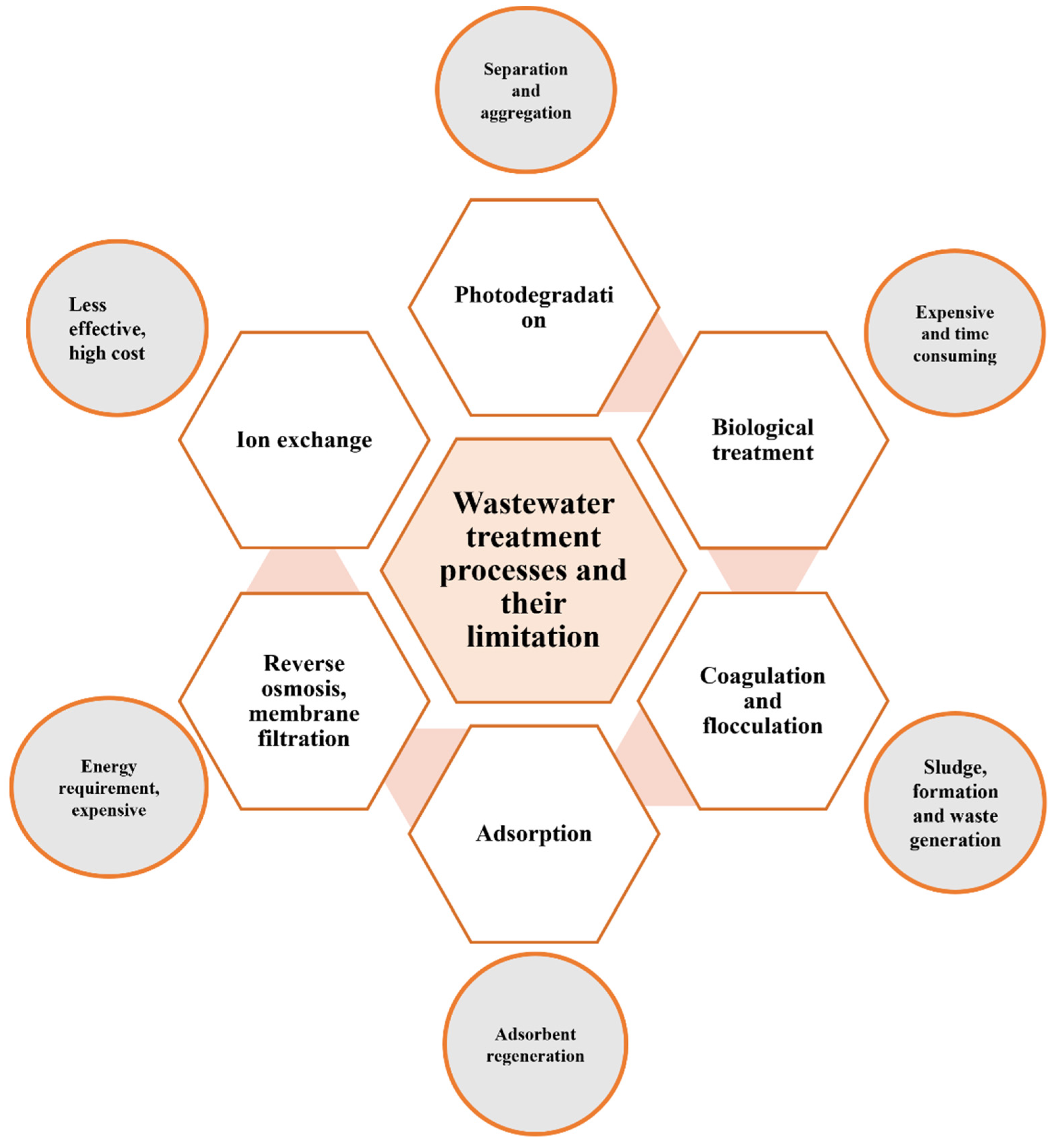
:1. Introduction
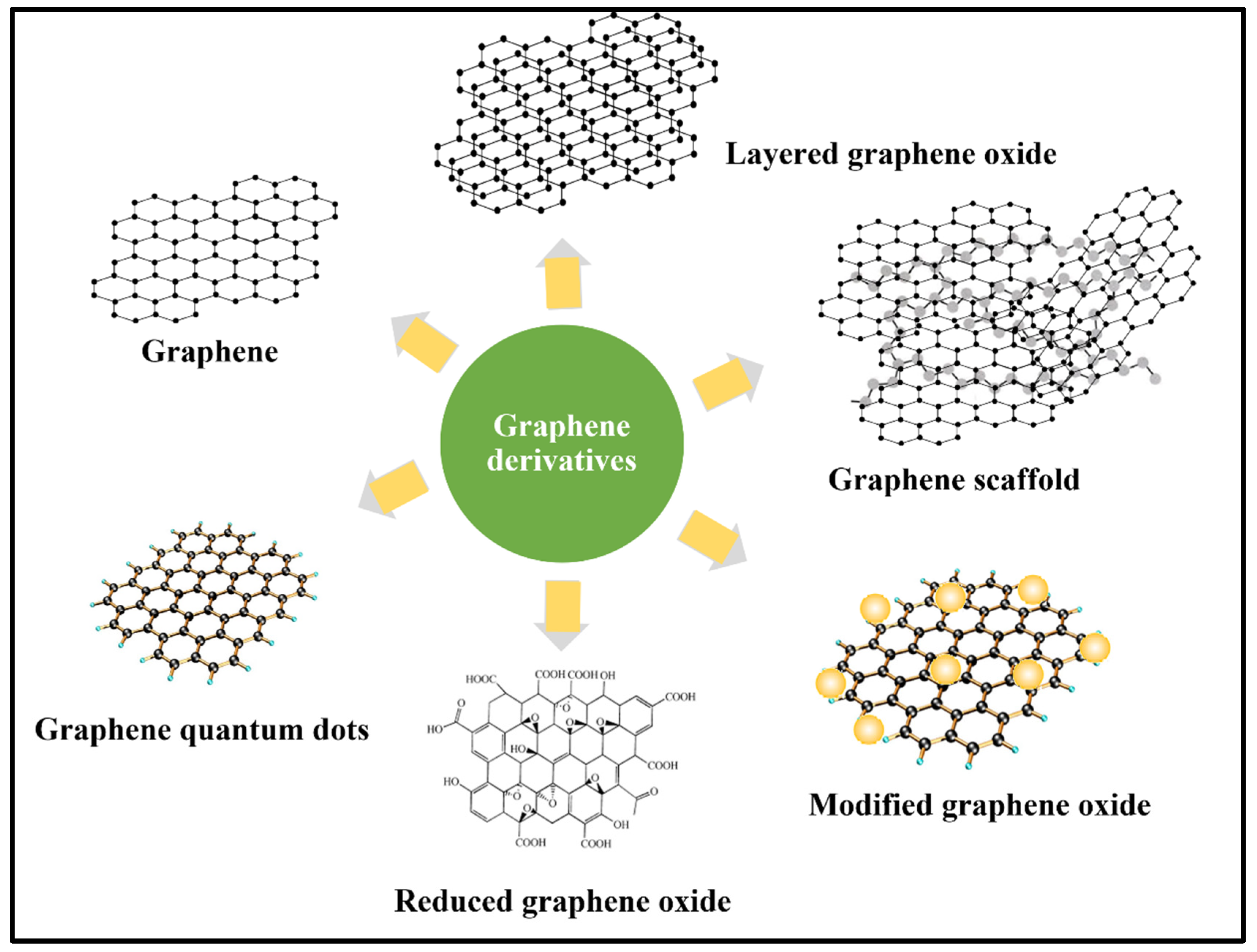
2. Application of Graphene Materials in Antibiotic Removal
3. Graphene-Based Adsorbents for the Sorption of Organic and Heavy Metal Pollutants
4. Applications of Graphene Based Membranes in Water Purification
5. Application of Graphene-Based Materials in EMs
6. Conclusions
Funding
Conflicts of Interest
References
- Minale, M.; Gu, Z.; Guadie, A.; Manaye, D.; Li, Y. Application of graphene-based materials for removal of tetracyclines using adsorption and photocatalytic-degradation: A review. J. Environ. Manag. 2020, 276, 111310. [Google Scholar] [CrossRef]
- Saleh, A.; Fadillah, G. Trends in Analytical Chemistry Recent trends in the design of chemical sensors based on graphene e metal oxide nanocomposites for the analysis of toxic species and biomolecules. TrAC Trends Anal. Chem. 2019, 120, 115660. [Google Scholar] [CrossRef]
- Ersan, G.; Apul, O.G.; Perreault, F.; Karan, T. Adsorption of organic contaminants by graphene nanosheets: A review. Water Res. 2017, 126, 385–398. [Google Scholar] [CrossRef]
- Yu, B.; Bai, Y.; Ming, Z.; Yang, H.; Chen, L.; Hu, X.; Feng, S.; Yang, S. Adsorption behaviors of tetracycline on magnetic graphene oxide sponge. Mater. Chem. Phys. 2017, 198, 283–290. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, L.; Cheng, J.; Jing, C. Mechanistic Insights into TiO2 Thickness in Fe3O4 @ TiO2 -GO Composites for Enrofloxacin Photodegradation. Chem. Eng. J. 2017, 325, 647–654. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Q.; Owens, G.; Chen, Z. Fenton-oxidation of rifampicin via a green synthesized rGO@nFe/Pd nanocomposite. J. Hazard. Mater. 2021, 402, 123544. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, J.; Xu, X.; He, G.; Chen, H. IFES sciences for life CdS–Bi2MoO6/RGO nanocomposites for efficient degradation of ciprofloxacin under visible light. J. Mater. Sci. 2020, 55, 6065–6077. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, X.; Zhang, C.; Zeng, G.; Peng, Z. Chemosphere Sorptive removal of ionizable antibiotic sulfamethazine from aqueous solution by graphene oxide-coated biochar nanocomposites: Influencing factors and mechanism. Chemosphere 2017, 186, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, J.; Meng, Y.; Su, J.; Wang, X. An investigation into the rapid removal of tetracycline using multilayered graphene-phase biochar derived from waste chicken feather. Sci. Total Environ. 2017, 603, 39–48. [Google Scholar] [CrossRef]
- Mariana, M.; HPS, A.K.; Yahya, E.B.; Olaiya, N.G.; Alfatah, T.; Suriani, A.B.; Mohamed, A. Recent trends and future prospects of nanostructured aerogels in water treatment applications. JWPE 2022, 45, 102481. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, R.; Zheng, Y.; Bai, H.; Shi, J.; Zhang, J.; Zhou, X.; Cai, M.; Fan, S.; Li, C. Graphene Oxide-Fe3O4 Nanocomposite Used as Aniline Adsorbent with a Wide PH Range. Colloid Polym. Sci. 2022, 300, 83–93. [Google Scholar] [CrossRef]
- Samuel, M.S.; Bhattacharya, J.; Raj, S. Efficient removal of Chromium(VI) from aqueous solution using chitosan grafted graphene oxide (CS-GO) nanocomposite. Int. J. Biol. Macromol. 2019, 121, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Subramaniyan, V.; Bhattacharya, J. A GO-CS@MOF [Zn(BDC)(DMF)] material for the adsorption of chromium(VI) ions from aqueous solution. Compos. Part B Eng. 2018, 152, 116–125. [Google Scholar] [CrossRef]
- Samuel, M.S.; Selvarajan, E.; Subramaniam, K. Synthesized β-cyclodextrin modified graphene oxide (β-CD-GO) composite for adsorption of cadmium and their toxicity profile in cervical cancer (HeLa) cell lines. Process Biochem. 2020, 93, 28–35. [Google Scholar] [CrossRef]
- Samuel, M.S.; Shah, S.S.; Bhattacharya, J. Adsorption of Pb(II) from aqueous solution using a magnetic chitosan/graphene oxide composite and its toxicity studies. Int. J. Biol. Macromol. 2018, 115, 1142–1150. [Google Scholar] [CrossRef]
- Shen, H.; Wang, J.; Jiang, J.; Luo, B.; Mao, B.; Shi, W. All-Solid-State Z-Scheme System of RGO-Cu2O/Bi2O3 for Tetracycline Degradation under Visible-Light Irradiation. Chem. Eng. J. 2017, 313, 508–517. [Google Scholar] [CrossRef]
- Selvamani, P.S.; Vijaya, J.J.; Kennedy, L.J.; Saravanakumar, B.; Bououdina, M. High-Performance Supercapacitor Based on Cu2O/MoS2/RGO Nanocomposite. Mater. Lett. 2020, 275, 128095. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Ruksana Alhabarah, A.N.; Alshehri, S.M. N/S doped highly porous magnetic carbon aerogel derived from sugarcane bagasse cellulose for the removal of bisphenol-A. Int. J. Biol. Macromol. 2019, 132, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Al-Kahtani, A.A.; Alshehri, S.M.; Naushad, M.; Ruksana Ahamad, T. Fabrication of highly porous N/S doped carbon embedded with ZnS as highly efficient photocatalyst for degradation of bisphenol. Int. J. Biol. Macromol. 2019, 121, 415–423. [Google Scholar] [CrossRef]
- Lawal, I.A.; Lawal, M.M.; Akpotu, S.O.; Okoro, H.K.; Klink, M.; Ndungu, P. Noncovalent graphene oxide functionalized with ionic liquid: Theoretical, isotherm, kinetics, and regeneration studies on the adsorption of pharmaceuticals. Ind. Eng. Chem. Res. 2020, 59, 4945–4957. [Google Scholar] [CrossRef]
- Delhiraja, K.; Vellingiri, K.; Boukhvalov, D.W.; Philip, L. Development of highly water stable graphene oxide-based composites for the removal of pharmaceuticals and personal care products. Ind. Eng. Chem. Res. 2019, 58, 2899–2913. [Google Scholar] [CrossRef]
- Rout, D.R.; Jena, H.M.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Graphene-Based Materials for Effective Adsorption of Organic and Inorganic Pollutants: A Critical and Comprehensive Review. Sci. Total Environ. 2023, 863, 160871. [Google Scholar] [CrossRef]
- Moradi, O.; Alizadeh, H.; Sedaghat, S. Removal of pharmaceuticals (diclofenac and amoxicillin) by maltodextrin/reduced graphene and maltodextrin/reduced graphene/copper oxide nanocomposites. Chemosphere 2022, 299, 134435. [Google Scholar] [CrossRef]
- Xiong, S.; Wu, Z.; Li, Z. Facile fabrication of robust, versatile, and recyclable biochar graphene oxide composite monoliths for efficient removal of different contaminants in water. Chemosphere 2022, 287, 132418. [Google Scholar] [CrossRef] [PubMed]
- Januário, E.F.D.; Fachina, Y.J.; Wernke, G.; Demiti, G.M.M.; Beltran, L.B.; Bergamasco, R.; Vieira, A.M.S. Application of activated carbon functionalized with graphene oxide for efficient removal of COVID-19 treatment-related pharmaceuticals from water. Chemosphere 2022, 289, 133213. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo Neves, T.; Camparotto, N.G.; Rodrigues, E.A.; Mastelaro, V.R.; Dantas, R.F.; Prediger, P. New Graphene Oxide-Safranin Modified@ Polyacrylonitrile Membranes for Removal of Emerging Contaminants: The Role of Chemical and Morphological Features. Chem. Eng. J. 2022, 446, 137176. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Q.; Zang, Z.; Han, R. Adsorptive removal of sulfosalicylic acid from aqueous medium by iron (III)-loaded magnetic chitosan/graphene oxide. J. Colloid. Interface Sci. 2022, 606, 1249–1260. [Google Scholar] [CrossRef]
- Feng, X.; Qiu, B.; Sun, D. Enhanced Naproxen Adsorption by a Novel β-Cyclodextrin Immobilized the Three-Dimensional Macrostructure of Reduced Graphene Oxide and Multiwall Carbon Nanotubes. Sep. Purif. Technol. 2022, 290, 120837. [Google Scholar] [CrossRef]
- Tian, D.; Geng, D.; Tyler Mehler, W.; Goss, G.; Wang, T.; Yang, S.; Niu, Y.; Zheng, Y.; Zhang, Y. Removal of perfluorooctanoic acid (PFOA) from aqueous solution by amino-functionalized graphene oxide (AGO) aerogels: Influencing factors, kinetics, isotherms, and thermodynamic studies. Sci. Total Environ. 2021, 783, 147041. [Google Scholar] [CrossRef] [PubMed]
- Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Gorring, M.; Kasner, M.L.; Hou, S. Adsorption behavior of EDTA-graphene oxide for pb (II) removal. ACS Appl. Mater. Interfaces 2012, 4, 1186–1193. [Google Scholar] [CrossRef]
- Verma, M.; Kumar, A.; Singh, K.P.; Kumar, R.; Kumar, V.; Srivastava, C.M.; Rawat, V.; Rao, G.; Kumari, S.; Sharma, P. Graphene Oxide-Manganese Ferrite (GO-MnFe2O4) Nanocomposite: One-Pot Hydrothermal Synthesis and Its Use for Adsorptive Removal of Pb2+ Ions from Aqueous Medium. J. Mol. Liq. 2020, 315, 113769. [Google Scholar] [CrossRef]
- Kong, Q.; Shi, X.; Ma, W.; Zhang, F.; Yu, T.; Zhao, F.; Zhao, D.; Wei, C. Strategies to improve the adsorption properties of graphene-based adsorbent towards heavy metal ions and their compound pollutants: A review. J. Hazard. Mater. 2021, 415, 125690. [Google Scholar] [CrossRef]
- Anuma, S.; Mishra, P.; Bhat, B.R. Polypyrrole functionalized cobalt oxide graphene (COPYGO) nanocomposite for the efficient removal of dyes and heavy metal pollutants from aqueous effluents. J. Hazard. Mater. 2021, 416, 125929. [Google Scholar] [CrossRef]
- Attia, N.F.; Diab, M.A.; Attia, A.S.; El-Shahat, M.F. Greener approach for fabrication of antibacterial graphene-polypyrrole nanoparticle adsorbent for removal of Mn2+ from aqueous solution. Synth. Met. 2021, 282, 116951. [Google Scholar] [CrossRef]
- Ranjan Rout, D.; Mohan Jena, H. Synthesis of novel reduced graphene oxide decorated β-cyclodextrin epichlorohydrin composite and its application for Cr(VI) removal: Batch and fixed-bed studies. Sep. Purif. Technol. 2022, 278, 119630. [Google Scholar] [CrossRef]
- Perlova, O.V.; Dzyazko, Y.S.; Palchik, A.V. Composites based on zirconium dioxide and zirconium hydrophosphate containing graphene-like additions for removal of U(VI) compounds from water. Appl. Nanosci. 2020, 10, 4591–4602. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Y. Covalently crosslinked graphene oxide membranes by esterification reactions for ions separation. J. Mater. Chem. 2015, 3, 4405–4412. [Google Scholar] [CrossRef]
- Liu, M.-L.; Wang, J.; Guo, J.-L.; Lu, T.-D.; Cao, X.-L.; Sun, S.-P. Graphene Oxide/Cross-Linked Polyimide (GO/CLPI) Composite Membranes for Organic Solvent Nanofiltration. Chem. Eng. Res. Des. 2019, 146, 182–189. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Song, X.; Su, B.; Mandal, B.; Prasad, B.; Gao, X.; Gao, C. Graphene Quantum Dots-Doped Thin Film Nanocomposite Polyimide Membranes with Enhanced Solvent Resistance for Solvent-Resistant Nanofiltration. ACS Appl. Mater. Interfaces 2019, 11, 6527–6540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.; An, J.; Fu, H.; Wu, X.; Pang, C.; Gao, H. Construction of an antibacterial membrane based on dopamine and polyethylenimine cross-linked graphene oxide. ACS Biomater. Sci. Eng. 2019, 5, 2732–2739. [Google Scholar] [CrossRef]
- Abounahia, N.; Qiblawey, H.; Zaidi, S.J. Progress for Co-Incorporation of Polydopamine and Nanoparticles for Improving Membranes Performance. Membranes 2022, 12, 675. [Google Scholar] [CrossRef]
- Pandey, R.P.; Kallem, P.; Hegab, H.M.; Rasheed, P.A.; Banat, F.; Hasan, S.W. Cross-Linked Laminar Graphene Oxide Membranes for Wastewater Treatment and Desalination: A Review. J. Environ. Manage. 2022, 317, 115367. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Yin, M.-J.; Zhao, Q.; Jin, C.-G.; Wang, N.; Ji, S.; Ritt, C.L.; Elimelech, M.; An, Q.-F. Graphene oxide membranes with stable porous structure for ultrafast water transport. Nat. Nanotechnol. 2021, 16, 337–343. [Google Scholar] [CrossRef]
- Lecaros, R.L.G.; Mendoza, G.E.J.; Hung, W.-S.; An, Q.-F.; Caparanga, A.R.; Tsai, H.-A.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Tunable interlayer spacing of composite graphene oxide- framework membrane for acetic acid dehydration. Carbon 2017, 123, 660–667. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, Y.; Zhao, W.; Yu, J.; Jiang, F.; Wu, Y.; Ma, L. Designing reduced graphene oxide/zinc rich epoxy composite coatings for improving the anticorrosion performance of carbon steel substrate. Mater. Des. 2019, 169, 107694. [Google Scholar] [CrossRef]
- Zheng, S.; Tu, Q.; Urban, J.J.; Li, S.; Mi, B. Swelling of graphene oxide membranes in aqueous solution: Characterization of interlayer spacing and insight into water transport mechanisms. ACS Nano 2017, 11, 6440–6450. [Google Scholar] [CrossRef]
- Gan, G.; Li, X.; Fan, S.; Wang, L.; Qin, M.; Yin, Z.; Chen, G. Carbon Aerogels for Environmental Clean-Up. Eur. J. Inorg. Chem. 2019, 2019, 3126–3141. [Google Scholar] [CrossRef]
- Sagadevan, S.; Lett, J.A.; Weldegebrieal, G.K.; Garg, S.; Oh, W.-C.; Hamizi, N.A.; Johan, M.R. Enhanced Photocatalytic Activity of rGO-CuO Nanocomposites for the Degradation of Organic Pollutants. Catalysts 2021, 11, 1008. [Google Scholar] [CrossRef]
- Samuel, M.S.; Subramaniyan, V.; Bhattacharya, J. Ultrasonic-assisted synthesis of graphene oxide–fungal hyphae: An efficient and reclaimable adsorbent for chromium(VI) removal from aqueous solution. Ultrason. Sonochem. 2018, 48, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Veena, R.; Samuel, M.S.; Selvarajan, E. Immobilization of laccases and applications for the detection and remediation of pollutants: A review. Environ. Chem. Lett. 2021, 19, 521–538. [Google Scholar] [CrossRef]
- Samuel, M.S.; Selvarajan, E.; Chidambaram, R. Clean approach for chromium removal in aqueous environments and role of nanomaterials in bioremediation: Present research and future perspective. Chemosphere 2021, 284, 131368. [Google Scholar] [CrossRef]
- Rajnish, K.N.; Samuel, M.S. Immobilization of cellulase enzymes on nano and micro-materials for breakdown of cellulose for biofuel production-a narrative review. Int. J. Biol. Macromol. 2021, 182, 603203. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Selvarajan, E.; Mathimani, T. Green synthesis of cobalt-oxide nanoparticle using jumbo Muscadine (Vitis rotundifolia): Characterization and photo-catalytic activity of acid Blue-74. J. Photochem. Photobiol. B Biol. 2020, 211, 112011. [Google Scholar] [CrossRef]
- Sikder, A.K.; Sikder, N. A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J. Hazard. Mater. 2004, 112, 1–15. [Google Scholar] [CrossRef]
- Talawar, M.B.; Sivabalan, R.; Anniyappan, M.; Gore, G.M.; Asthana, S.N.; Gandhe, B.R. Emerging trends in advanced high energy materials. Combust. Explos. Shock. 2007, 43, 62. [Google Scholar] [CrossRef]
- Yan, Q.L.; Gozin, M.; Zhao, F.Q.; Cohen, A.; Pang, S.P. Highly energetic compositionsbased on functionalized carbon nanomaterials. Nanoscale 2016, 8, 4799. [Google Scholar] [CrossRef]
- Ni, J.; Luo, X.F.; Zhan, Y.; Lin, J.X. Application and progress of the novel activated carbon in the field of catalysis. J. Mol. Catal. 2016, 30, 282. [Google Scholar]
- Qin, N.; Yao, J.Y. Review on development of polymer application in solid propellant. Chem. Adhesion 2003, 25–28, 0255. [Google Scholar]
- Zhang, X.; Zheng, J.; Fang, H.M.; Zhang, Y.F.; Bai, S.L.; He, G.S. High dimensional stability and low viscous response solid propellant binder based on graphene oxide nanosheets and dual cross-linked polyurethane. Compos. Sci. Technol. 2018, 161, 124. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, J.; Fang, H.M.; Zhang, Y.F.; Bai, S.L.; He, G.S. Al2O3/graphene reinforced bio-inspired interlocking polyurethane composites with superior mechanical and thermal properties for solid propulsion fuel. Compos. Sci. Technol. 2018, 167, 42. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342. [Google Scholar] [CrossRef]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; McWilliams, J.L.; Hamon, M.A.; Haddon, R.C. Solution properties of graphite and graphene. J. Am. Chem. Soc. 2006, 128, 7720. [Google Scholar] [CrossRef]
- Ting, A.; He, W.; Chen, S.W.; Zuo, B.L.; Qi, X.F.; Zhao, F.; Luo, Y.; Yan, Q.L. Thermal behavior and thermolysis mechanisms of AP under the effects of GO-doped complexes of triaminoguanidine. J. Phys. Chem. C 2018, 122, 26956. [Google Scholar]
- Zhang, L.P.; Xia, Z.H. Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells. J. Phys. Chem. C 2011, 115, 11170. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, Y.; Li, J.C.; Guan, Y.L.; Guo, Z.Q.; Ma, H.X. High catalytic activity of nitrogen-doped graphene on the thermal decomposition of CL-20. Propellants. Explos. Pyrotech. 2018, 43, 1263. [Google Scholar] [CrossRef]
- Zhuo, H.Y.; Zhang, X.; Liang, J.X.; Yu, Q.; Xiao, H.; Li, J. Theoretical understandings of graphene-based metal single-atom catalysts: Stability and catalytic performance. Chem. Rev. 2020, 120, 12315. [Google Scholar] [CrossRef]
- Rad, A.S. First principles study of Al-doped graphene as nanostructure adsorbent for NO2 and N2O: DFT calculations. Appl. Surf. Sci. 2015, 357, 1217. [Google Scholar] [CrossRef]


| S.No | Type of Graphene-Based Nanomaterial | Antibiotic/Heavy Metal | Mechanism | References |
|---|---|---|---|---|
| 1 | Magnetic graphene oxide sponge | tetracycline | Adsorption | [4] |
| 2 | Fe3O4@TiO2-GO | Enrofloxacin | Adsorption | [5] |
| 3 | GO@Fe3O4/ZnO/SnO2 | Azithromycin | Photocatalytic degradation | [6] |
| 4 | rGO@nFe/Pd | Rifampicin | Fenton-oxidation | [6] |
| 5 | CdS–Bi2MoO6/RGO | Ciprofloxacin | Photocatalytic degradation | [7] |
| 6 | Graphene-phase biochar | Tetracycline | Adsorption | [10] |
| 7 | Graphene–chitosan (GO-CS) | Chromium (VI) | Adsorption | [13] |
| 8 | GO-CS@MOF [Zn(BDC)(DMF)] | Chromium (VI) | Adsorption | [14] |
| 9 | β-cyclodextrin-modified graphene oxide (β-CD-GO) | Cadmium | Adsorption | [15] |
| 10 | chitosan/graphene oxide composite | Chromium (VI) | Adsorption | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Samuel, M.S.; Ravikumar, M.; Ethiraj, S.; Kirankumar, V.S.; Kumar, M.; Arulvel, R.; Suresh, S. Processing of Carbon-Based Nanomaterials for the Removal of Pollutants from Water/Wastewater Application. Water 2023, 15, 3003. https://doi.org/10.3390/w15163003
Singh R, Samuel MS, Ravikumar M, Ethiraj S, Kirankumar VS, Kumar M, Arulvel R, Suresh S. Processing of Carbon-Based Nanomaterials for the Removal of Pollutants from Water/Wastewater Application. Water. 2023; 15(16):3003. https://doi.org/10.3390/w15163003
Chicago/Turabian StyleSingh, Rashmi, Melvin S. Samuel, Madhumita Ravikumar, Selvarajan Ethiraj, Venkatesan Savunthari Kirankumar, Mohanraj Kumar, R. Arulvel, and Sagadevan Suresh. 2023. "Processing of Carbon-Based Nanomaterials for the Removal of Pollutants from Water/Wastewater Application" Water 15, no. 16: 3003. https://doi.org/10.3390/w15163003







