Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles Loaded on Nanoclay
Abstract
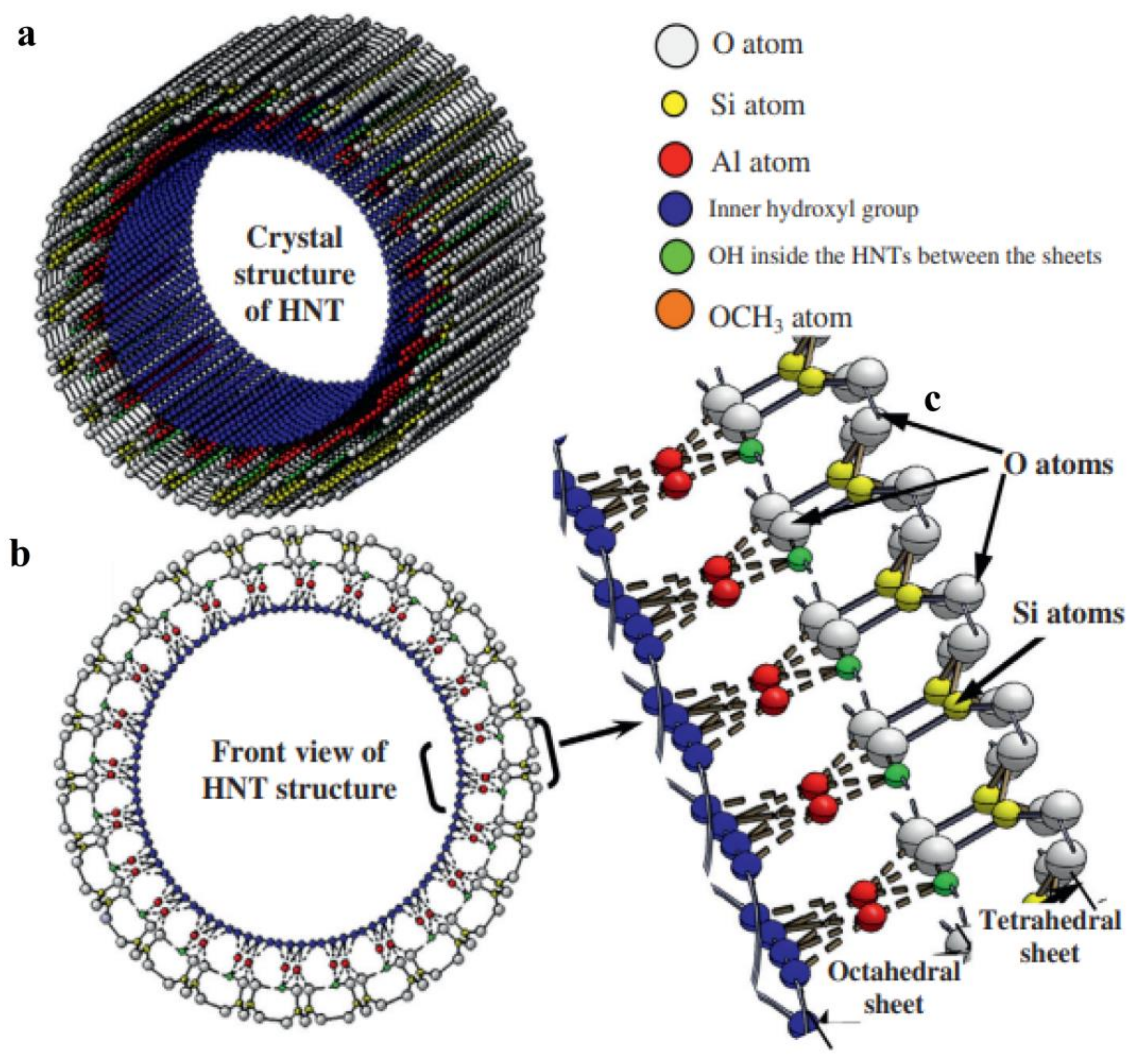
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Methods
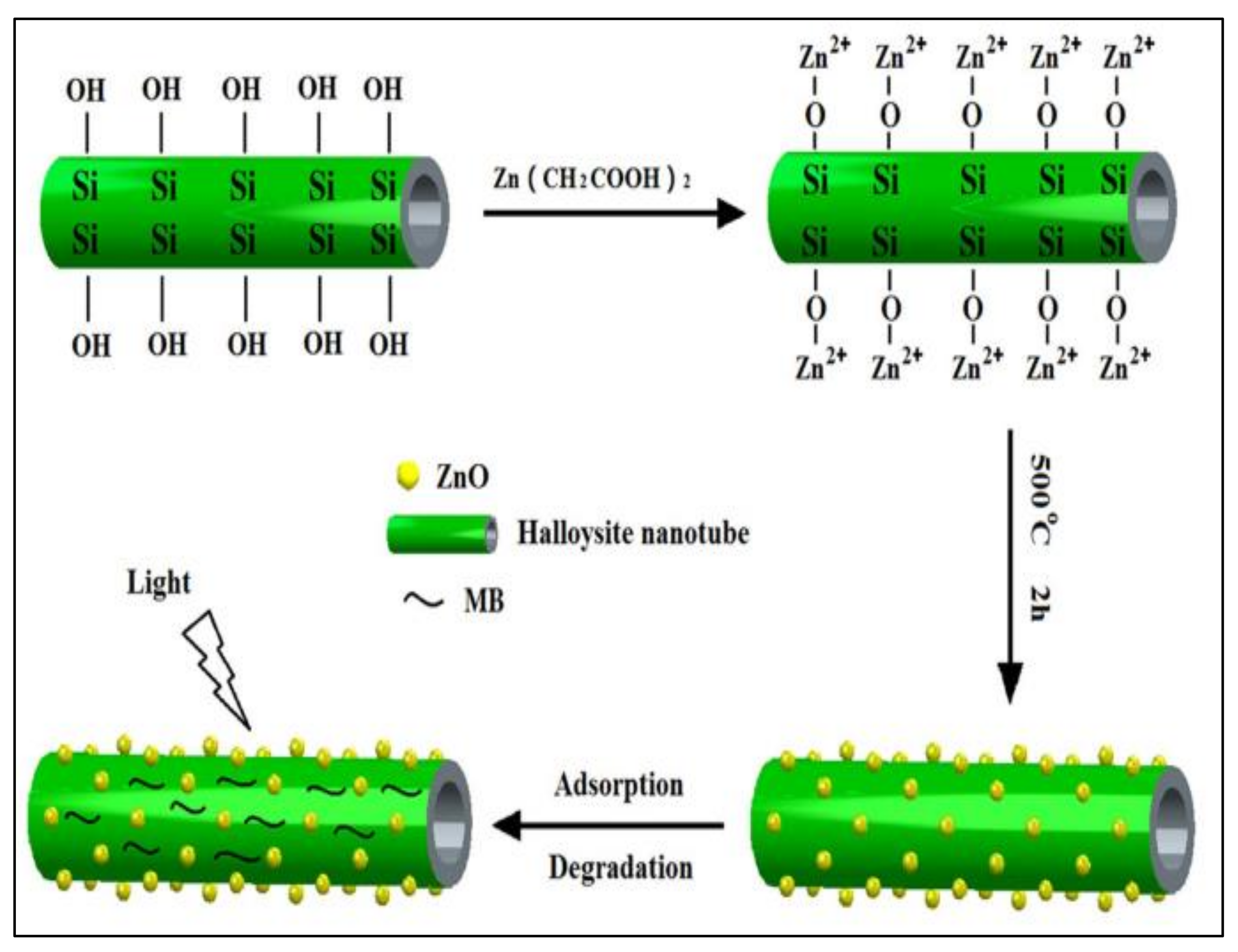
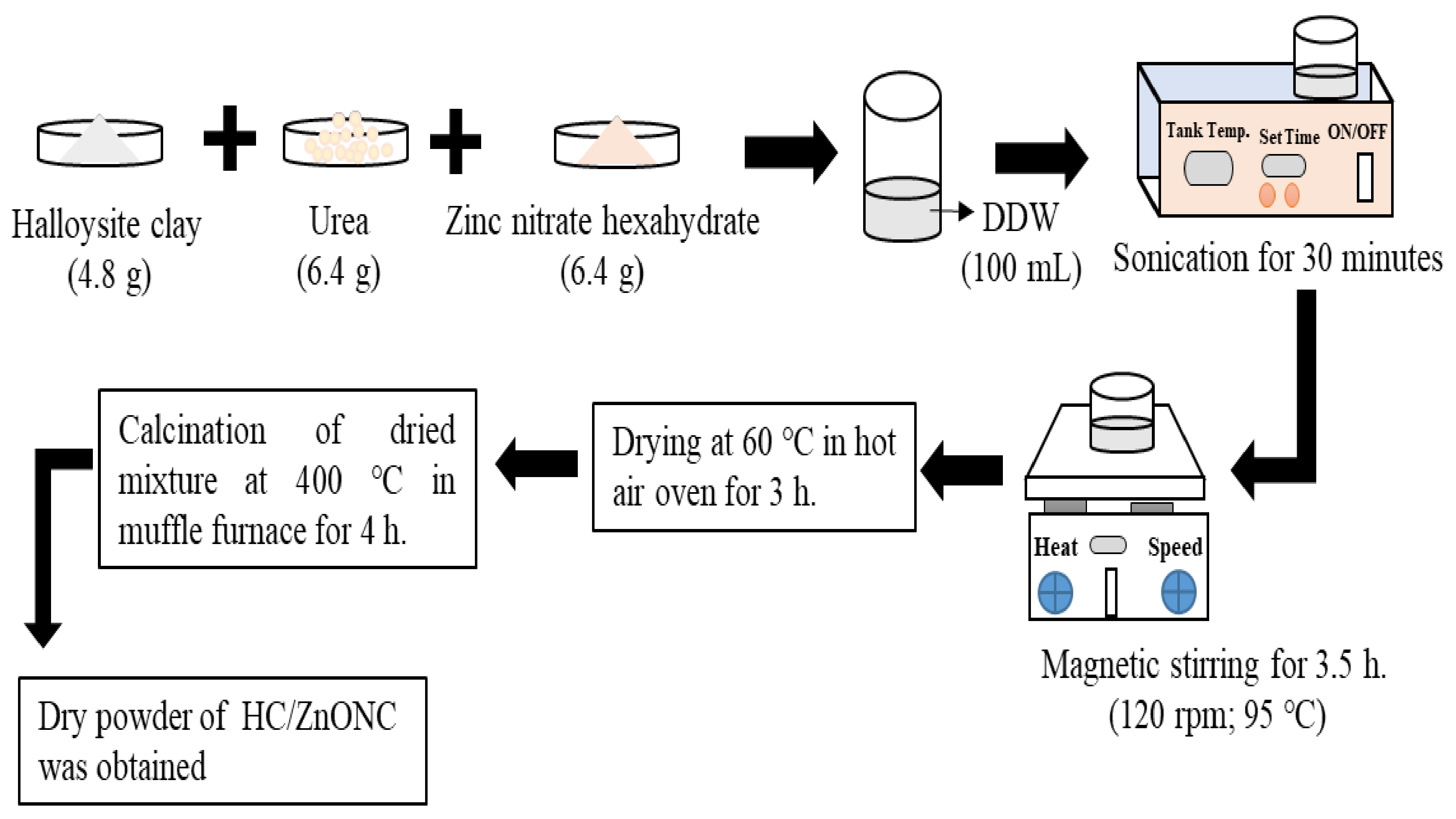
2.2.1. Synthesis Procedure for HC/ZnONC
2.2.2. Synthesis of Nanocomposite
2.2.3. Preparation of Dye Solution
2.2.4. Remediation of Dye
3. Characterization of Synthesized HC/ZnO Nanocomposite
4. Result and Discussions
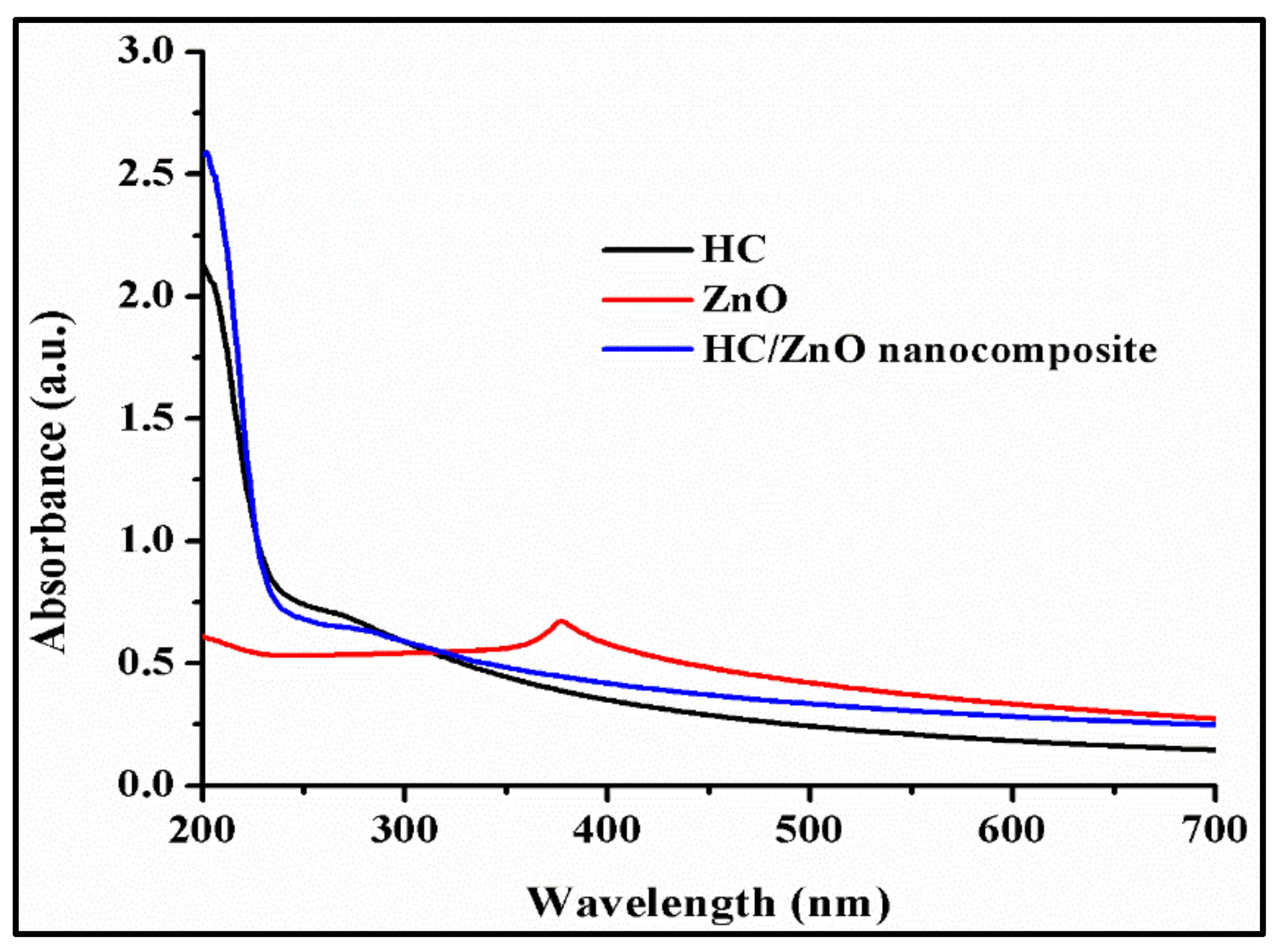
4.1. UV-Vis Study of ZnONPs, HC and HC/ZnONC
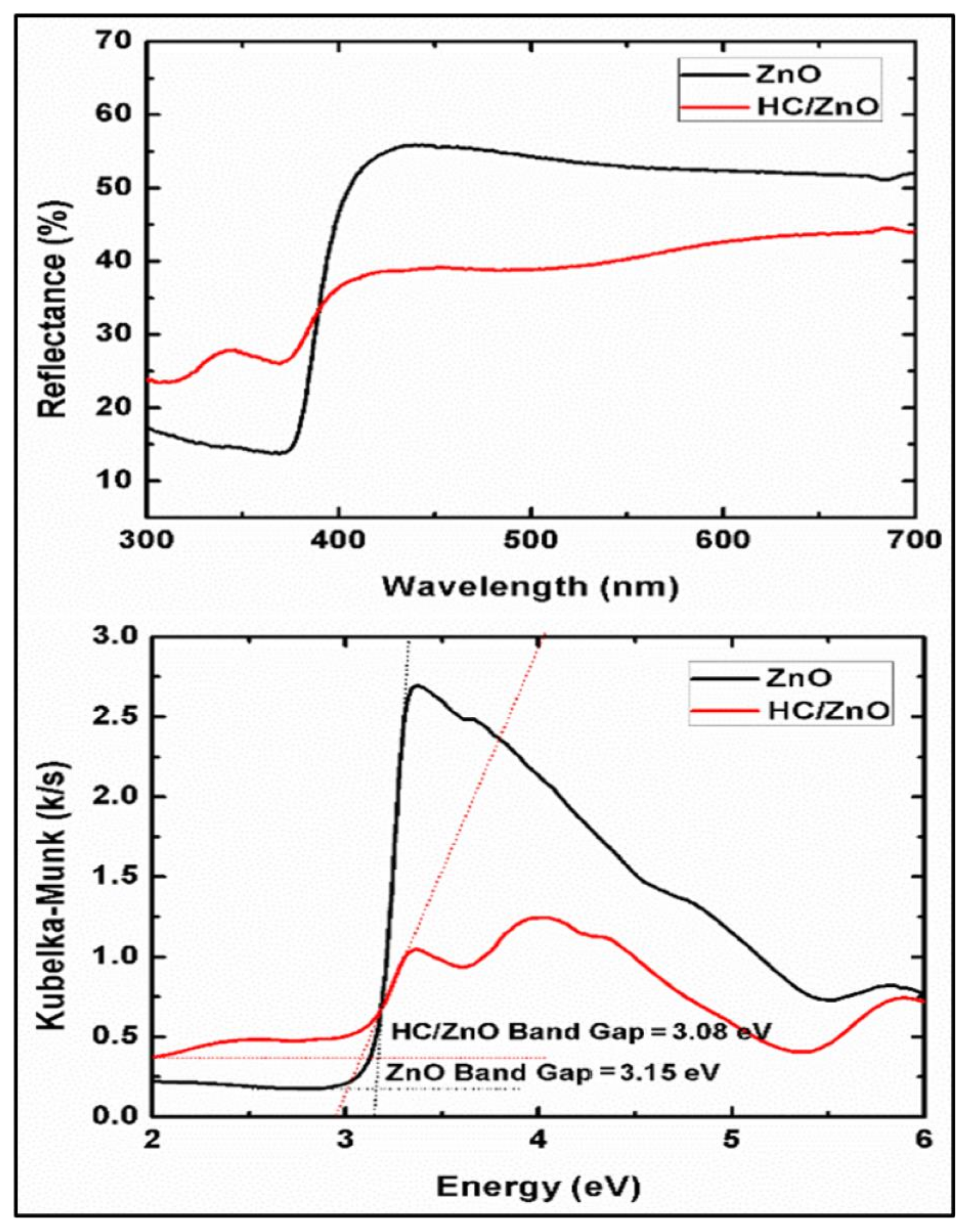
4.2. UV-Diffuse Reflectance Spectroscopic (DRS) Analysis
4.3. FTIR Analysis ZnONPs, HC and HC/ZnONC
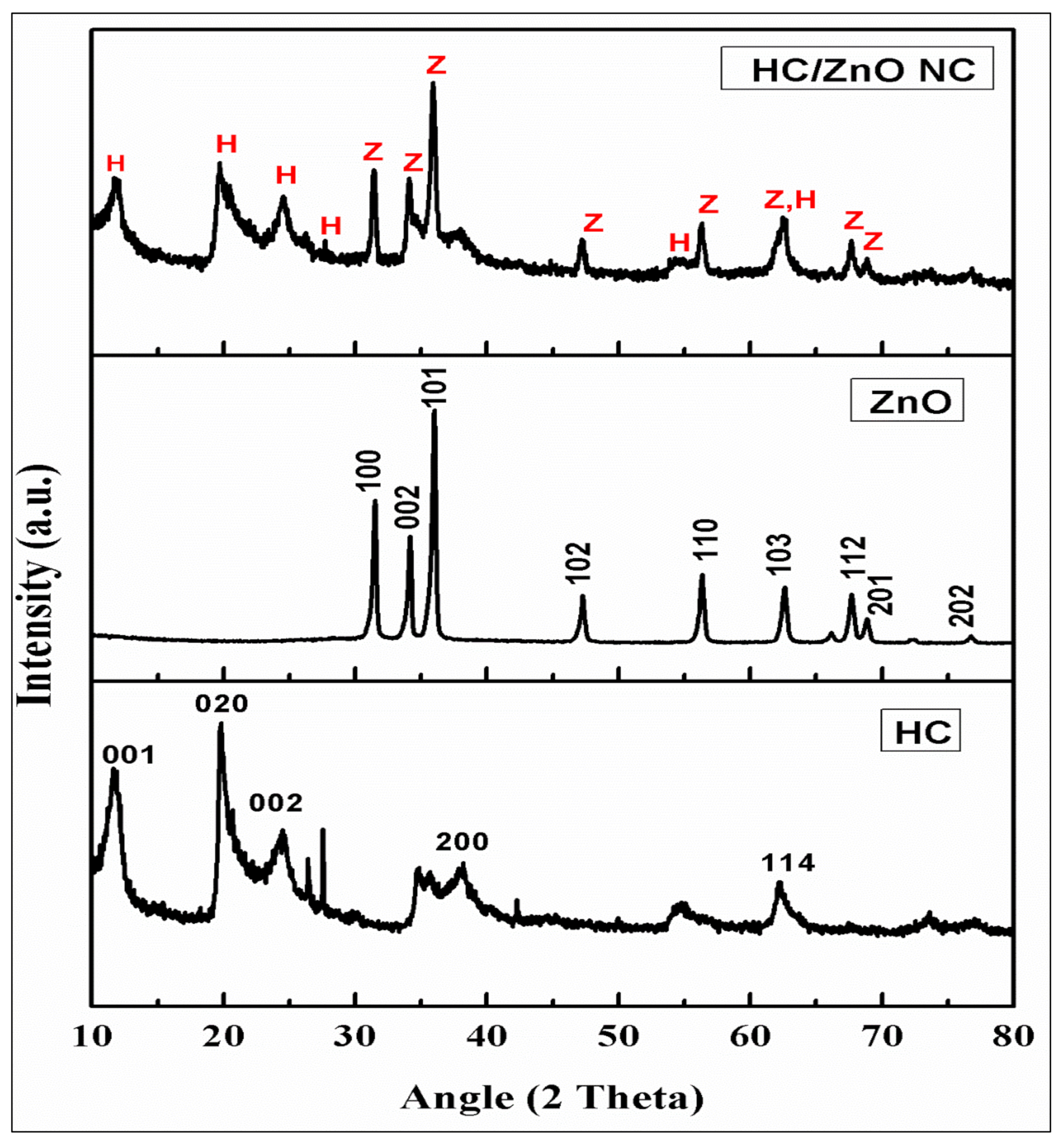
4.4. XRD Analysis of ZnONPs, HC and HC/ZnONC
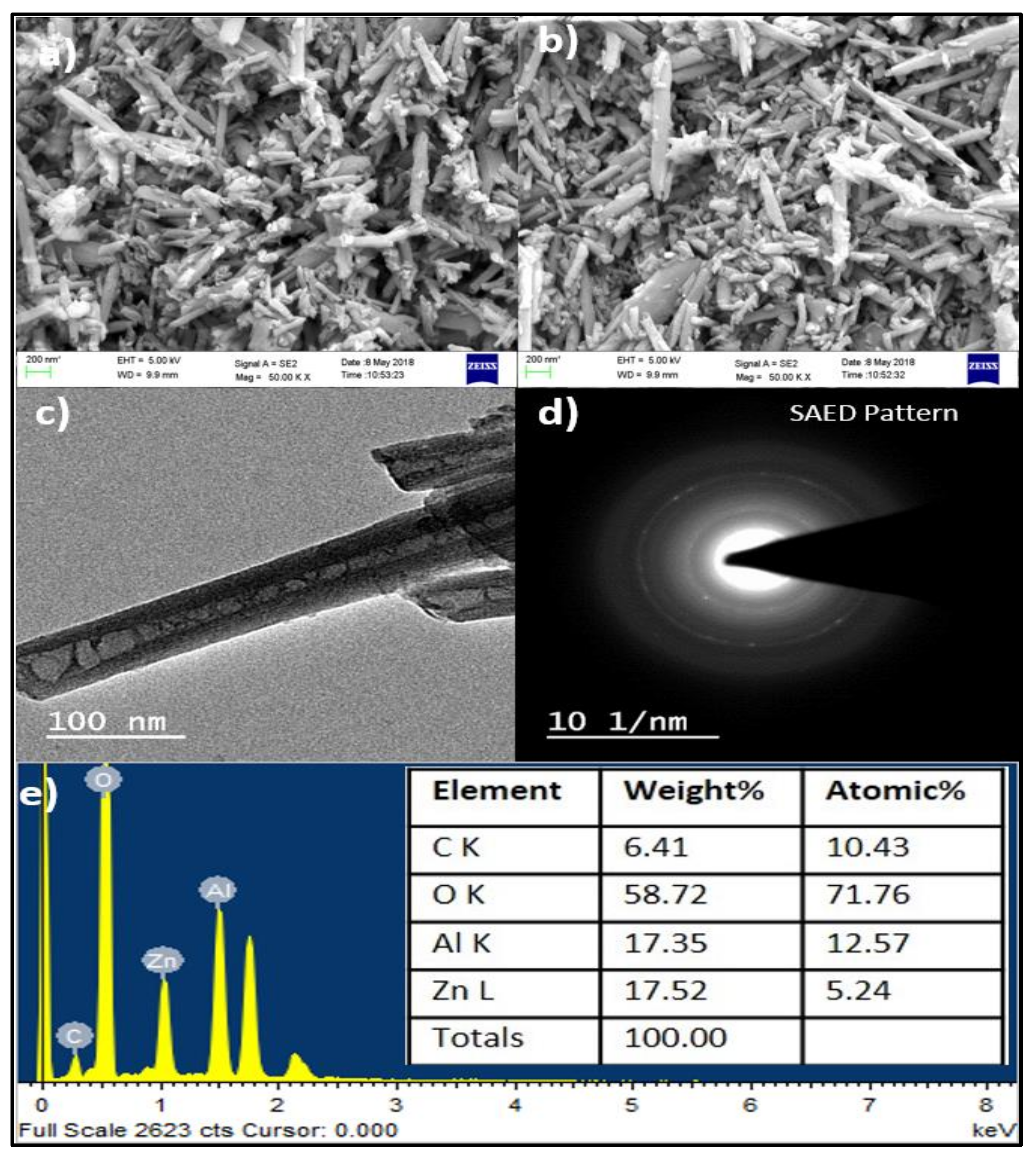
4.5. Morphological and Elemental Analysis
4.6. BET Surface Area Analysis
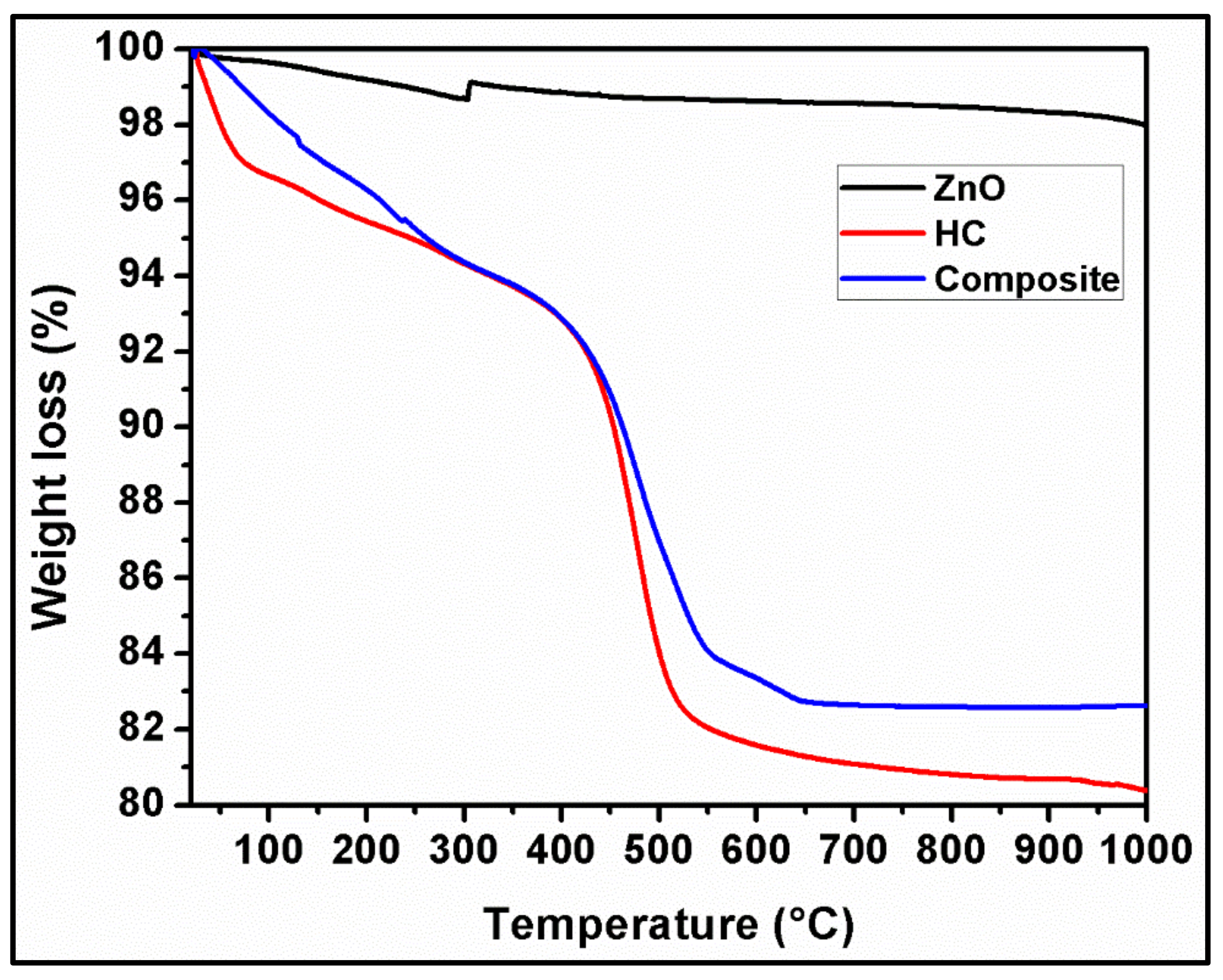
4.7. TGA Analysis of Halloysite Clay and HC/ZnONC
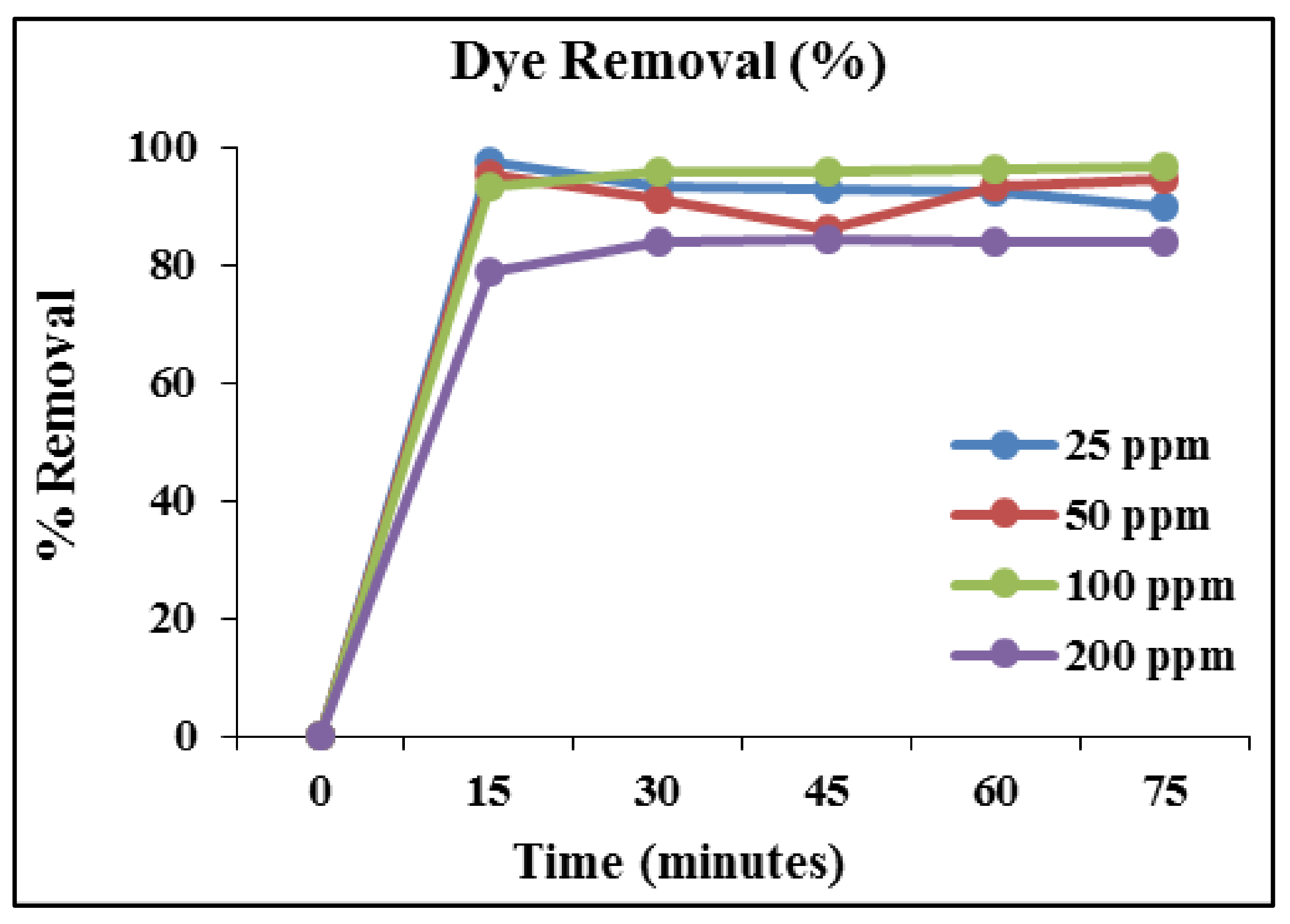
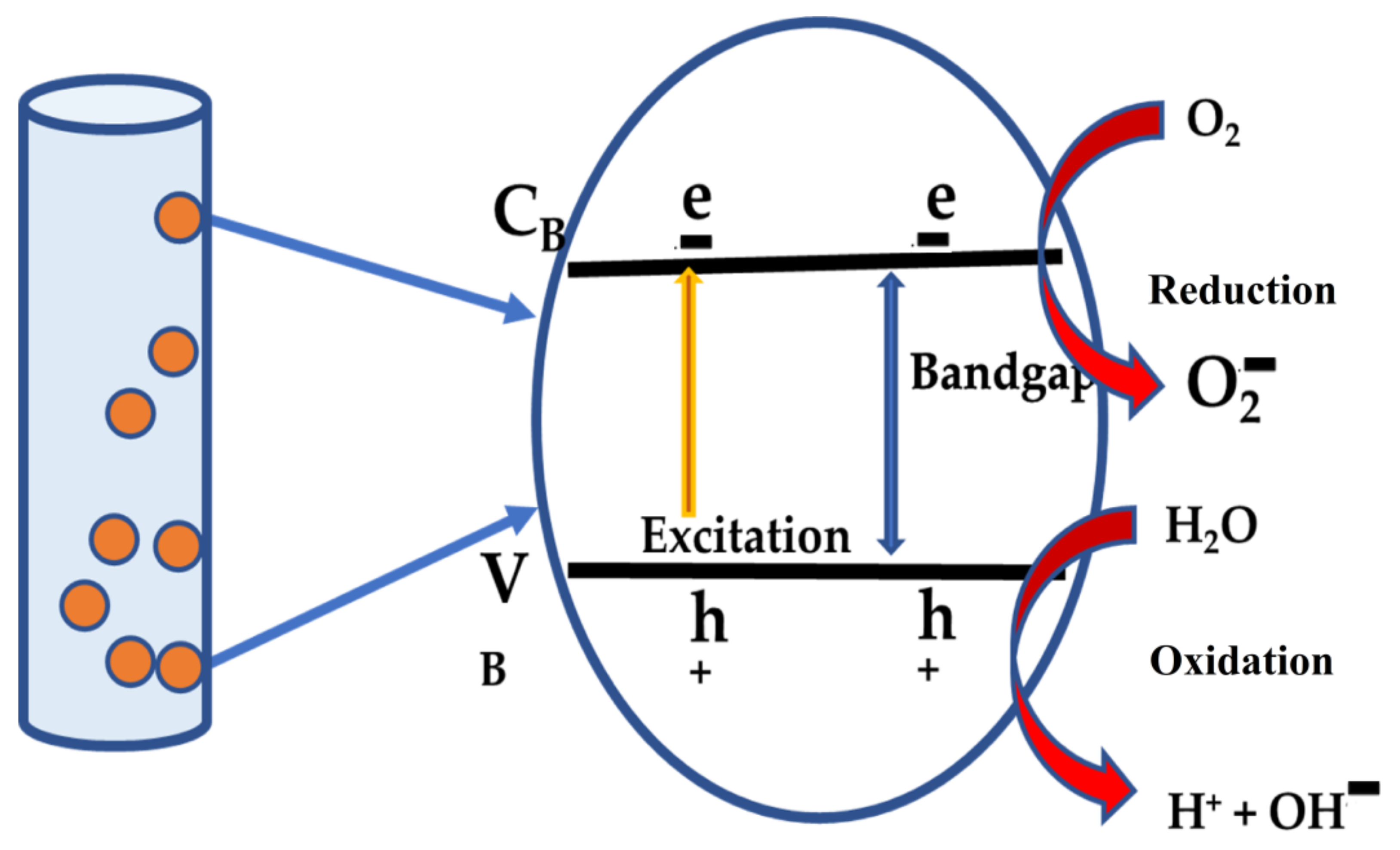
5. MB Dye Removal Study by HC/ZnO Nanocomposite
6. Conclusions
7. Future Prospective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, Y.; Chhaya, U.; Rudakiya, D.M.; Joshi, S. Biological Decolorization and Degradation of Synthetic Dyes: A Green Step Toward Sustainable Environment. In Microbial Rejuvenation of Polluted Environment; Panpatte, D.G., Jhala, Y.K., Eds.; Springer: Singapore, 2021; Volume 2, pp. 77–110. [Google Scholar] [CrossRef]
- Soleimani-Gorgani, A.; Taylor, J. Dyeing of nylon with reactive dyes. Part 1. The effect of changes in dye structure on the dyeing of nylon with reactive dyes. Dye. Pigment. 2006, 68, 109–117. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, U.; Singh, K.; Gupta, S.P.; Beg, M.S.J. Structure and Properties of Dyes and Pigments. In Dyes and Pigments; Dixit, U., Ed.; IntechOpen: Rijeka, Croatia, 2021; p. 8. [Google Scholar] [CrossRef]
- Sumathi, S.; Manju, B. Uptake of reactive textile dyes by Aspergillus foetidus. Enzym. Microb. Technol. 2000, 27, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Dwivedi, S.K.; Mishra, J. Role of Fungal Enzymes in the Removal of Azo Dyes. In Microbial Enzymes: Roles and Applications in Industries; Arora, N.K., Mishra, J., Mishra, V., Eds.; Springer: Singapore, 2020; pp. 231–257. [Google Scholar] [CrossRef]
- Bello, O.S.; Bello, I.A.; Adegoke, K.A. Adsorption of Dyes Using Different Types of Sand: A Review. S. Afr. J. Chem. 2013, 66, 117–129. [Google Scholar]
- Dapson, R. Benzidine-based dyes: Effects of industrial practices, regulations, and world trade on the biological stains market. Biotech. Histochem. 2009, 84, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Dellamatrice, P.M.; Silva-Stenico, M.E.; De Moraes, L.A.B.; Fiore, M.F.; Monteiro, R.T.R. Degradation of textile dyes by cyanobacteria. Braz. J. Microbiol. 2016, 48, 25–31. [Google Scholar] [CrossRef]
- Bijekar, S.; Padariya, H.D.; Yadav, V.K.; Gacem, A.; Hasan, M.A.; Awwad, N.S.; Yadav, K.K.; Islam, S.; Park, S.; Jeon, B.-H. The State of the Art and Emerging Trends in the Wastewater Treatment in Developing Nations. Water 2022, 14, 2537. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Karthikeyan, V.; Ali, D.; Kumar, G.; Yadav, V.K.; Narayanan, V. Global popularization of CuNiO2 and their rGO nanocomposite loveabled to the photocatalytic properties of methylene blue. Environ. Res. 2022, 204, 112338. [Google Scholar] [CrossRef]
- Kara, H.T.; Anshebo, S.T.; Sabir, F.K.; Workineh, G.A. Removal of Methylene Blue Dye from Wastewater Using Periodiated Modified Nanocellulose. Int. J. Chem. Eng. 2021, 2021, 9965452. [Google Scholar] [CrossRef]
- Modi, S.; Yadav, V.K.; Gacem, A.; Ali, I.H.; Dave, D.; Khan, S.H.; Yadav, K.K.; Rather, S.-U.; Ahn, Y.; Son, C.T.; et al. Recent and Emerging Trends in Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles. Water 2022, 14, 1749. [Google Scholar] [CrossRef]
- Yadav, V.K.; Choudhary, N.; Ali, D.; Kumar, G.; Gnanamoorthy, G.; Khan, A.U.; Kumar, P.; Kumar, S.H.; Tizazu, B.Z. Determination of Adsorption of Methylene Blue Dye by Incense Stick Ash Waste and Its Toxicity on RTG-2 Cells. Adsorpt. Sci. Technol. 2022, 2022, 8565151. [Google Scholar] [CrossRef]
- Zhambolova, A.; Vocaturo, A.; Tileuberdi, Y.; Ongarbayev, Y.; Caputo, P.; Aiello, I.; Rossi, C.O.; Godbert, N. Functionalization and Modification of Bitumen by Silica Nanoparticles. Appl. Sci. 2020, 10, 6065. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, J.; Islam, R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Bin Emran, T.; Cavalu, S. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Kumar, A.; Tripathi, R.; Kumar, A. Advancing of Zinc Oxide Nanoparticles for Cosmetic Applications. In Handbook of Consumer Nanoproducts; Mallakpour, S., Hussain, C.M., Eds.; Springer: Singapore, 2021; pp. 1–16. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B.; Fulekar, M.H. Synthesis, characterization and photocatalytic degradation of chlorpyrifos by novel Fe: ZnO nanocomposite material. Nanotechnol. Environ. Eng. 2018, 3, 13. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc oxide based photocatalytic degradation of persistent pesticides: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B.; Fulekar, M.H. A study on the influence of metal (Fe, Bi, and Ag) doping on structural, optical, and antimicrobial activity of ZnO nanostructures. Adv. Compos. Hybrid Mater. 2020, 3, 551–569. [Google Scholar] [CrossRef]
- Khan, S.H.; Yadav, V.K.; Ali, D.; Varghese, R. Influence of precursor ions on the structural morphological and optical properties of ZnO nanostructure and cytotoxicity on murine NIH 3T3 cells. Chem. Pap. 2022, 76, 477–489. [Google Scholar] [CrossRef]
- Singh, R.; Volli, V.; Lohani, L.; Purkait, M.K. Polymeric ultrafiltration membranes modified with fly ash based carbon nanotubes for thermal stability and protein separation. Case Stud. Chem. Environ. Eng. 2021, 4, 100155. [Google Scholar] [CrossRef]
- Cheng, Z.; An, J.; Li, F.; Lu, Y.; Li, S. Effect of fly ash cenospheres on properties of multi-walled carbon nanotubes and polyvinyl alcohol fibers reinforced geopolymer composites. Ceram. Int. 2022, 48, 18956–18971. [Google Scholar] [CrossRef]
- Purcar, V.; Şomoghi, R.; Niţu, S.G.; Nicolae, C.-A.; Alexandrescu, E.; Gîfu, I.C.; Gabor, A.R.; Stroescu, H.; Ianchiş, R.; Căprărescu, S.; et al. The Effect of Different Coupling Agents on Nano-ZnO Materials Obtained via the Sol–Gel Process. Nanomaterials 2017, 7, 439. [Google Scholar] [CrossRef]
- Mukai, H.; Hirose, A.; Motai, S.; Kikuchi, R.; Tanoi, K.; Nakanishi, T.M.; Yaita, T.; Kogure, T. Cesium adsorption/desorption behavior of clay minerals considering actual contamination conditions in Fukushima. Sci. Rep. 2016, 6, 21543. [Google Scholar] [CrossRef] [PubMed]
- Huggett, J. Clay Minerals☆. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Hillier, S. Clay Mineralogy. In Sedimentology; Springer: Berlin/Heidelberg, Germany, 1978; pp. 223–228. [Google Scholar] [CrossRef]
- Hanif, M.; Jabbar, F.; Sharif, S.; Abbas, G.; Farooq, A.; Aziz, M. Halloysite nanotubes as a new drug-delivery system: A review. Clay Miner. 2016, 51, 469–477. [Google Scholar] [CrossRef]
- Sarkar, B.; Rusmin, R.; Ugochukwu, U.C.; Mukhopadhyay, R.; Manjaiah, K.M. Chapter 5—Modified clay minerals for environmental applications. In Modified Clay and Zeolite Nanocomposite Materials; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier: Amsterdam, The Nethelands, 2019; pp. 113–127. [Google Scholar] [CrossRef]
- Lu, Z.; Sha, A.; Gao, J.; Jia, M.; Wang, W. An Improved Predictive Model for Determining the Permeability Coefficient of Artificial Clayey Soil Based on Double T2 Cut-Offs. Adv. Mater. Sci. Eng. 2020, 2020, 5323820. [Google Scholar] [CrossRef]
- Biswas, B.; Labille, J.; Prelot, B. Clays and modified clays in remediating environmental pollutants. Environ. Sci. Pollut. Res. 2020, 27, 38381–38383. [Google Scholar] [CrossRef]
- Amar, A.; Loulidi, I.; Kali, A.; Boukhlifi, F.; Hadey, C.; Jabri, M. Physicochemical Characterization of Regional Clay: Application to Phenol Adsorption. Appl. Environ. Soil Sci. 2021, 2021, 8826063. [Google Scholar] [CrossRef]
- Shubbar, A.A.; Sadique, M.; Kot, P.; Atherton, W. Future of clay-based construction materials—A review. Constr. Build. Mater. 2019, 210, 172–187. [Google Scholar] [CrossRef]
- Mintis, D.; Mavrantzas, V.G. Effect of pH and Molecular Length on the Structure and Dynamics of Short Poly(acrylic acid) in Dilute Solution: Detailed Molecular Dynamics Study. J. Phys. Chem. B 2019, 123, 4204–4219. [Google Scholar] [CrossRef]
- Aluvihara, S.; Kalpage, C.S.; Lemle, K.L. Elementary chemical analysis of different clay types. J. Phys. Conf. Ser. 2021, 1781, 012007. [Google Scholar] [CrossRef]
- Albdiry, M.; Yousif, B. Role of silanized halloysite nanotubes on structural, mechanical properties and fracture toughness of thermoset nanocomposites. Mater. Des. 2014, 57, 279–288. [Google Scholar] [CrossRef]
- Peng, H.; Liu, X.; Tang, W.; Ma, R. Facile synthesis and characterization of ZnO nanoparticles grown on halloysite nanotubes for enhanced photocatalytic properties. Sci. Rep. 2017, 7, 2250. [Google Scholar] [CrossRef]
- Kamble, R.; Ghag, M.; Gaikawad, S.; Kumar Panda, B. Halloysite nanotubes and applications: A review. J. Adv. Sci. Res. 2012, 3, 25–29. [Google Scholar]
- Borah, D.; Nath, H.; Saikia, H. Modification of bentonite clay & its applications: A review. Rev. Inorg. Chem. 2021, 42, 265–282. [Google Scholar] [CrossRef]
- Khan, I.; Bhat, A.H. Micro and Nano Calcium Carbonate Filled Natural Rubber Composites and Nanocomposites. In Natural Rubber Materials; Royal Society of Chemistry: London, UK, 2014; pp. 467–487. [Google Scholar] [CrossRef]
- Isawi, H. Using Zeolite/Polyvinyl alcohol/sodium alginate nanocomposite beads for removal of some heavy metals from wastewater. Arab. J. Chem. 2020, 13, 5691–5716. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Yadav, V.K.; Latha, D.; Karthikeyan, V.; Narayanan, V. Enhanced photocatalytic performance of ZnSnO3/rGO nanocomposite. Chem. Phys. Lett. 2020, 739, 137050. [Google Scholar] [CrossRef]
- Gadore, V.; Ahmaruzzaman, M. Fly ash–based nanocomposites: A potential material for effective photocatalytic degradation/elimination of emerging organic pollutants from aqueous stream. Environ. Sci. Pollut. Res. 2021, 28, 46910–46933. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Ali, D.; Yadav, V.K.; Dhinagaran, G.; Venkatachalam, K.; Narayanan, V. New construction of Fe3O4/rGO/ZnSnO3 nanocomposites enhanced photoelectro chemical properties. Opt. Mater. 2020, 109, 110353. [Google Scholar] [CrossRef]
- Ab Rahman, I.; Padavettan, V. Synthesis of Silica Nanoparticles by Sol-Gel: Size-Dependent Properties, Surface Modification, and Applications in Silica-Polymer Nanocomposites—A Review. J. Nanomater. 2012, 2012, 132424. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Karthikeyan, V.; Ali, D.; Kumar, G.; Jenifer, S.G.; Yadav, V.K.; Choudhary, N.; Narayanan, V. Realization of rGO/ZnCo2O4 nanocomposites enhanced for the antimicrobial, electrochemical and photocatalytic activities. Diam. Relat. Mater. 2021, 120, 108677. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Nicola, R.; Costişor, O.; Ciopec, M.; Negrea, A.; Lazău, R.; Ianăşi, C.; Picioruş, E.-M.; Len, A.; Almásy, L.; Szerb, E.I.; et al. Silica-Coated Magnetic Nanocomposites for Pb2+ Removal from Aqueous Solution. Appl. Sci. 2020, 10, 2726. [Google Scholar] [CrossRef]
- Rajendran, S.; Wanale, S.G.; Gacem, A.; Yadav, V.K.; Ahmed, I.A.; Algethami, J.S.; Kakodiya, S.D.; Modi, T.; Alsuhaibani, A.M.; Yadav, K.K.; et al. Nanostructured Iron Oxides: Structural, Optical, Magnetic, and Adsorption Characteristics for Cleaning Industrial Effluents. Crystals 2023, 13, 472. [Google Scholar] [CrossRef]
- Yadav, V.K.; Amari, A.; Gacem, A.; Elboughdiri, N.; Eltayeb, L.B.; Fulekar, M.H. Treatment of Fly-Ash-Contaminated Wastewater Loaded with Heavy Metals by Using Fly-Ash-Synthesized Iron Oxide Nanoparticles. Water 2023, 15, 908. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, Y.; Yang, Q.; Yang, H. Halloysite Nanotubes Supported Ag and ZnO Nanoparticles with Synergistically Enhanced Antibacterial Activity. Nanoscale Res. Lett. 2017, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, Y.; Selvakumar, M.; Bhat, D.K. Investigations on thermo-mechanical properties of organically modified polymer clay nanocomposites for packaging application. Polym. Polym. Compos. 2021, 29, 1191–1199. [Google Scholar] [CrossRef]
- Choudhary, N.; Yadav, V.K.; Yadav, K.K.; Almohana, A.I.; Almojil, S.F.; Gnanamoorthy, G.; Kim, D.-H.; Islam, S.; Kumar, P.; Jeon, B.-H. Application of Green Synthesized MMT/Ag Nanocomposite for Removal of Methylene Blue from Aqueous Solution. Water 2021, 13, 3206. [Google Scholar] [CrossRef]
- Zsirka, B.; Vágvölgyi, V.; Horváth, E.; Juzsakova, T.; Fónagy, O.; Szabó-Bárdos, E.; Kristóf, J. Halloysite-Zinc Oxide Nanocomposites as Potential Photocatalysts. Minerals 2022, 12, 476. [Google Scholar] [CrossRef]
- Caprarescu, S.; Miron, A.R.; Purcar, V.; Radu, A.-L.; Sarbu, A.; Ion-Ebrasu, D.; Atanase, L.I.; Ghiurea, M. Efficient removal of Indigo Carmine dye by a separation process. Water Sci. Technol. 2016, 74, 2462–2473. [Google Scholar] [CrossRef]
- Caprarescu, S.; Miron, A.R.; Purcar, V.; Radu, A.-L.; Sarbu, A.; Nicolae, C.A.; Pascu, M.; Ion-Ebrasu, D.; Raditoiu, V. Treatment of Crystal Violet from Synthetic Solution Using Membranes Doped with Natural Fruit Extract. CLEAN Soil Air Water 2018, 46, 1700413. [Google Scholar] [CrossRef]
- Caprarescu, S.; Miron, A.R.; Purcar, V.; Radu, A.-L.; Sarbu, A.; Ianchis, R.; Ebrasu, D.I. Commercial Gooseberry Buds Extract Containing Membrane for Removal of Methylene Blue Dye from Synthetic Wastewaters. Rev. Chim. 2017, 68, 1757–1762. [Google Scholar] [CrossRef]
- Modrogan, C.; Cǎprǎrescu, S.; Dǎncilǎ, A.M.; Orbuleț, O.D.; Grumezescu, A.M.; Purcar, V.; Radițoiu, V.; Fierascu, R.C. Modified Composite Based on Magnetite and Polyvinyl Alcohol: Synthesis, Characterization, and Degradation Studies of the Methyl Orange Dye from Synthetic Wastewater. Polymers 2021, 13, 3911. [Google Scholar] [CrossRef]
- Ringu, T.; Ghosh, S.; Das, A.; Pramanik, N. Zinc oxide nanoparticles: An excellent biomaterial for bioengineering applications. Emergent Mater. 2022, 5, 1629–1648. [Google Scholar] [CrossRef]
- Pradeeswari, K.; Venkatesan, A.; Pandi, P.; Karthik, K.; Krishna, K.V.H.; Kumar, R.M. Study on the electrochemical performance of ZnO nanoparticles synthesized via non-aqueous sol-gel route for supercapacitor applications. Mater. Res. Express 2019, 6, 105525. [Google Scholar] [CrossRef]
- Chitra, M.; Mangamma, G.; Uthayarani, K.; Neelakandeswari, N.; Girija, E. Band gap engineering in ZnO based nanocomposites. Phys. E Low Dimens. Syst. Nanostructures 2020, 119, 113969. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Yoo, I.-H.; Cho, I.-S.; Seo, H. Effect of oxygen vacancies on the band edge properties of WO3 producing enhanced photocurrents. Electrochim. Acta 2019, 296, 517–527. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Kadhum, A.A.H.; Al-Amiery, A.A.; Nassir, M.H.; Jaaz, A.H. The Impact of Halloysite on the Thermo-Mechanical Properties of Polymer Composites. Molecules 2017, 22, 838. [Google Scholar] [CrossRef] [PubMed]
- Alkatheeri, M.S.; Palasuk, J.; Eckert, G.J.; Platt, J.A.; Bottino, M.C. Halloysite nanotube incorporation into adhesive systems—Effect on bond strength to human dentin. Clin. Oral Investig. 2015, 19, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Bordeepong, S.; Bhongsuwan, D.; Pungrassami, T.; Bhongsuwan, T. Mineralogy, Chemical Composition and Ceramic Properties of Clay Deposits in Southern Thailand. Agric. Nat. Resour. 2012, 46, 485–500. [Google Scholar]
- Thangapandiyan, S.; Monika, S. Green Synthesized Zinc Oxide Nanoparticles as Feed Additives to Improve Growth, Biochemical, and Hematological Parameters in Freshwater Fish Labeo rohita. Biol. Trace Elem. Res. 2020, 195, 636–647. [Google Scholar] [CrossRef]
- Getie, S.; Belay, A.; Chandra Reddy, A.R.; Belay, Z. Synthesis and Characterizations of Zinc Oxide Nanoparticles for Antibacterial Applications. J. Nanomed. Nanotechnol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Biddeci, G.; Spinelli, G.; Colomba, P.; Di Blasi, F. Halloysite Nanotubes and Sepiolite for Health Applications. Int. J. Mol. Sci. 2023, 24, 4801. [Google Scholar] [CrossRef]
- Alekseeva, O.V.; Shibaeva, V.D.; Noskov, A.V.; Ivanov, V.K.; Agafonov, A.V. Enhancing the Thermal Stability of Ionogels: Synthesis and Properties of Triple Ionic Liquid/Halloysite/MCC Ionogels. Molecules 2021, 26, 6198. [Google Scholar] [CrossRef] [PubMed]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Jian, S.; Tian, Z.; Zhang, K.; Duan, G.; Yang, W.; Jiang, S. Hydrothermal Synthesis of Ce-doped ZnO Heterojunction Supported on Carbon Nanofibers with High Visible Light Photocatalytic Activity. Chem. Res. Chin. Univ. 2021, 37, 565–570. [Google Scholar] [CrossRef]
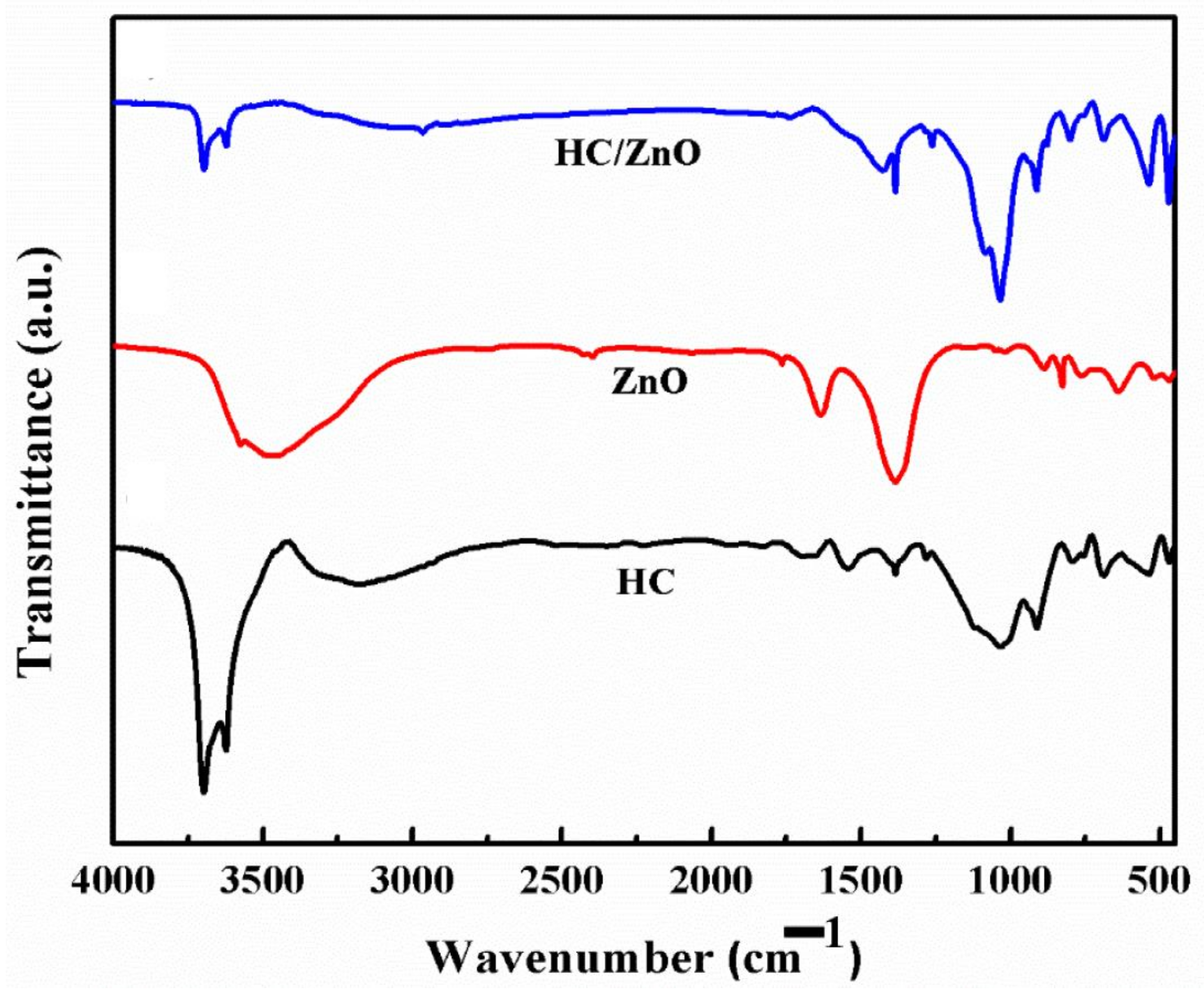




| Samples | Hydroxyl Group | Atmospheric Carbon | Si-O-Si | Al-O/Al-OH | Zn-O-Zn | |
|---|---|---|---|---|---|---|
| ZnONPs | 3576 & 3474 cm−1 | 2397 & 2427 cm−1 | 1634 & 1385 cm−1 | 630, 519 & 460 cm−1 | ||
| HC | 3698, 3622 &1680 cm−1 | 1120, 1034 & 790 cm−1 | 910 & 536 cm−1 | |||
| HC/ZnONC | 3800 cm−1 | 1400 cm−1 | 1132 | 900–400 cm−1 |
| Material | Specific Surface Area (m2/g) | Pore Volume (cc/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Halloysite clay (raw) | 54.751 | 0.269 | 3.813 |
| HC/ZnO nanocomposite | 60.258 | 0.149 | 3.707 |
| Breakdown Step | Halloysite Clay (Temp.) | HC/ZnONC (Temp.) | Due to |
|---|---|---|---|
| 1st step | 25–100 °C | 25–400 °C | Loss of water molecule |
| 2nd Step | 100–400 °C | - | dehydroxylation |
| 3rd step | 400–550 °C | 400–600 °C | dehydroxylation and volatilization |
| Parameters | ZnONPs | HC | HC/ZnONC | MB 25 ppm | MB 50 ppm | MB 100 ppm | MB 200 ppm |
|---|---|---|---|---|---|---|---|
| BET (SSA, m2/g) | 54.751 | 60.258 | |||||
| Morphology | Spherical shaped | Rod-shaped | Rod-shaped with spherical ZnONPs | ||||
| XRD | Crystalline | Crystalline | Crystalline | ||||
| Dye removal with HC/ZnONPs | 97.5% | 95.45% | 93% | 78.61% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhary, N.; Yadav, V.K.; Ali, H.; Ali, D.; Almutairi, B.O.; Cavalu, S.; Patel, A. Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles Loaded on Nanoclay. Water 2023, 15, 1427. https://doi.org/10.3390/w15071427
Choudhary N, Yadav VK, Ali H, Ali D, Almutairi BO, Cavalu S, Patel A. Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles Loaded on Nanoclay. Water. 2023; 15(7):1427. https://doi.org/10.3390/w15071427
Chicago/Turabian StyleChoudhary, Nisha, Virendra Kumar Yadav, Huma Ali, Daoud Ali, Bader O. Almutairi, Simona Cavalu, and Ashish Patel. 2023. "Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles Loaded on Nanoclay" Water 15, no. 7: 1427. https://doi.org/10.3390/w15071427
APA StyleChoudhary, N., Yadav, V. K., Ali, H., Ali, D., Almutairi, B. O., Cavalu, S., & Patel, A. (2023). Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles Loaded on Nanoclay. Water, 15(7), 1427. https://doi.org/10.3390/w15071427









