Organic Carbon in the Bottom Sediments of Lake Baikal: Geochemical Processes of Burial and Balance Values
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Pore Waters and Benthic Water Chemical Analysis
2.3. Redox Potential (Eh) of Sediments
2.4. Sediment: OC, Iron, and Manganese Extraction, Analysis, and Calculations
2.5. Calculations of Diffusion Fluxes of Dissolved Organic Carbon
3. Results
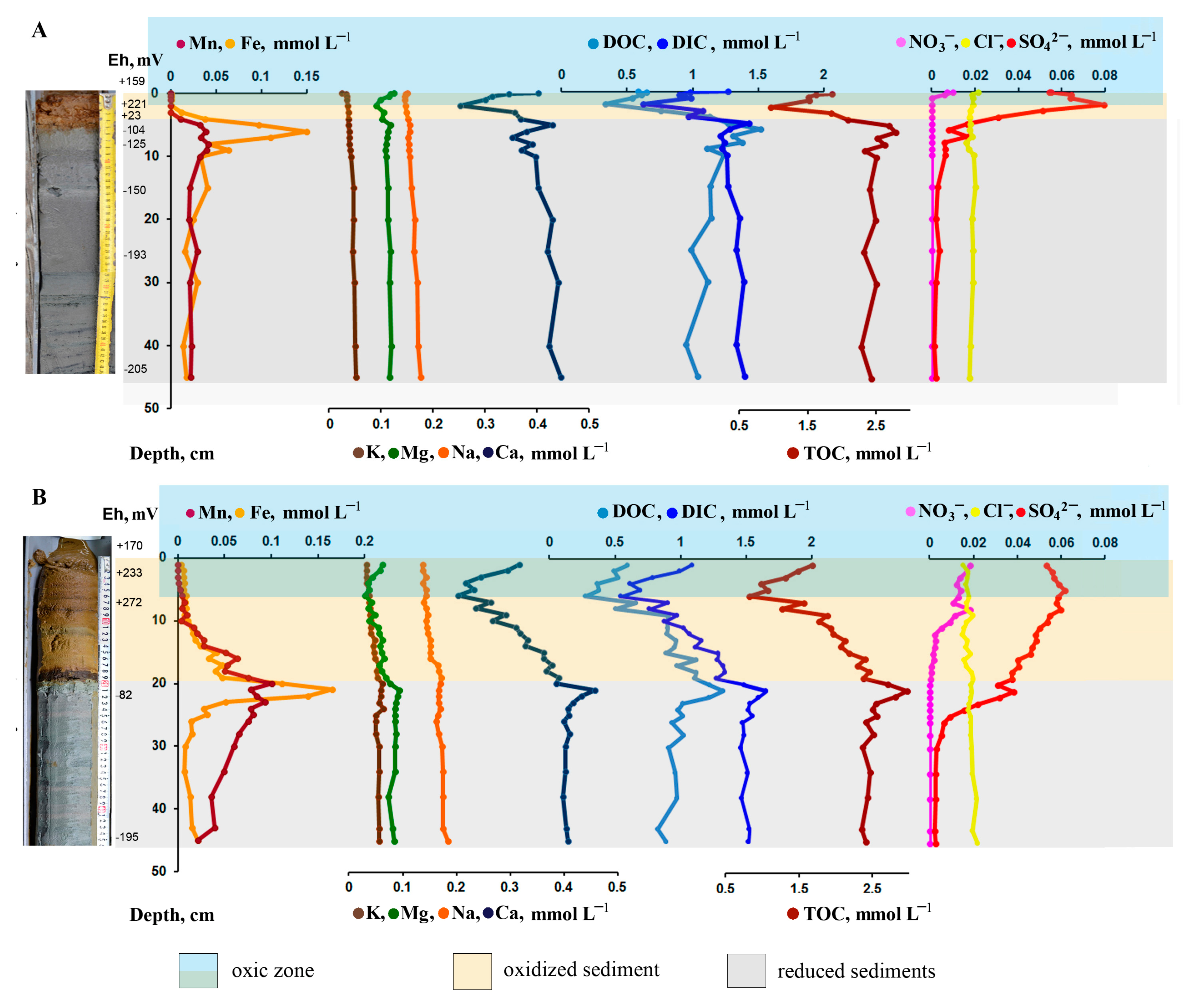
3.1. Lithology of Bottom Sediments
3.2. Oxygen
3.3. Redox Values
3.4. Pore Water Chemical Composition
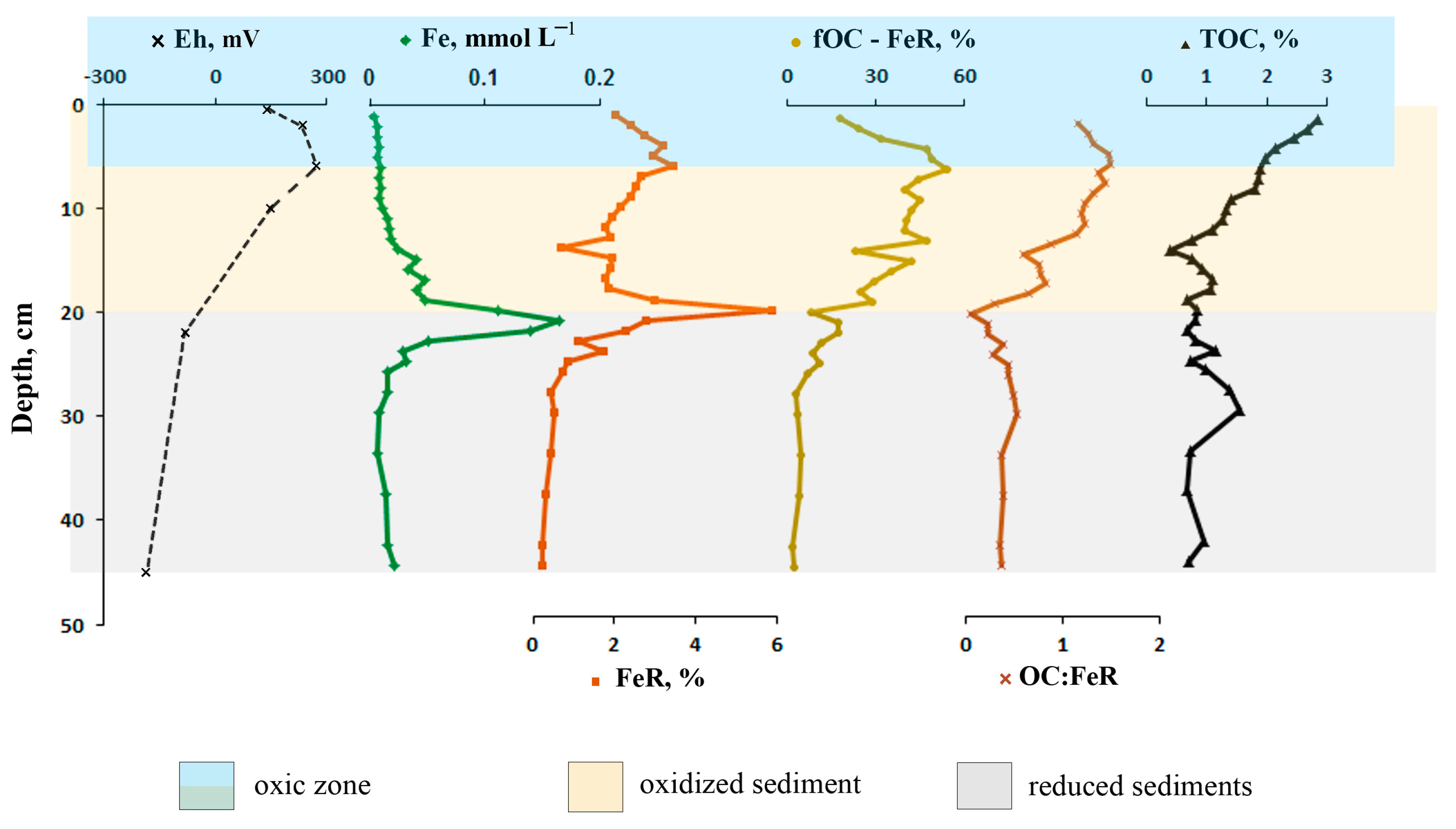
3.5. Reactive Iron and Organic Carbon Associated with Iron
3.6. Reactive Manganese and Organic Carbon Associated with Manganese
4. Discussion
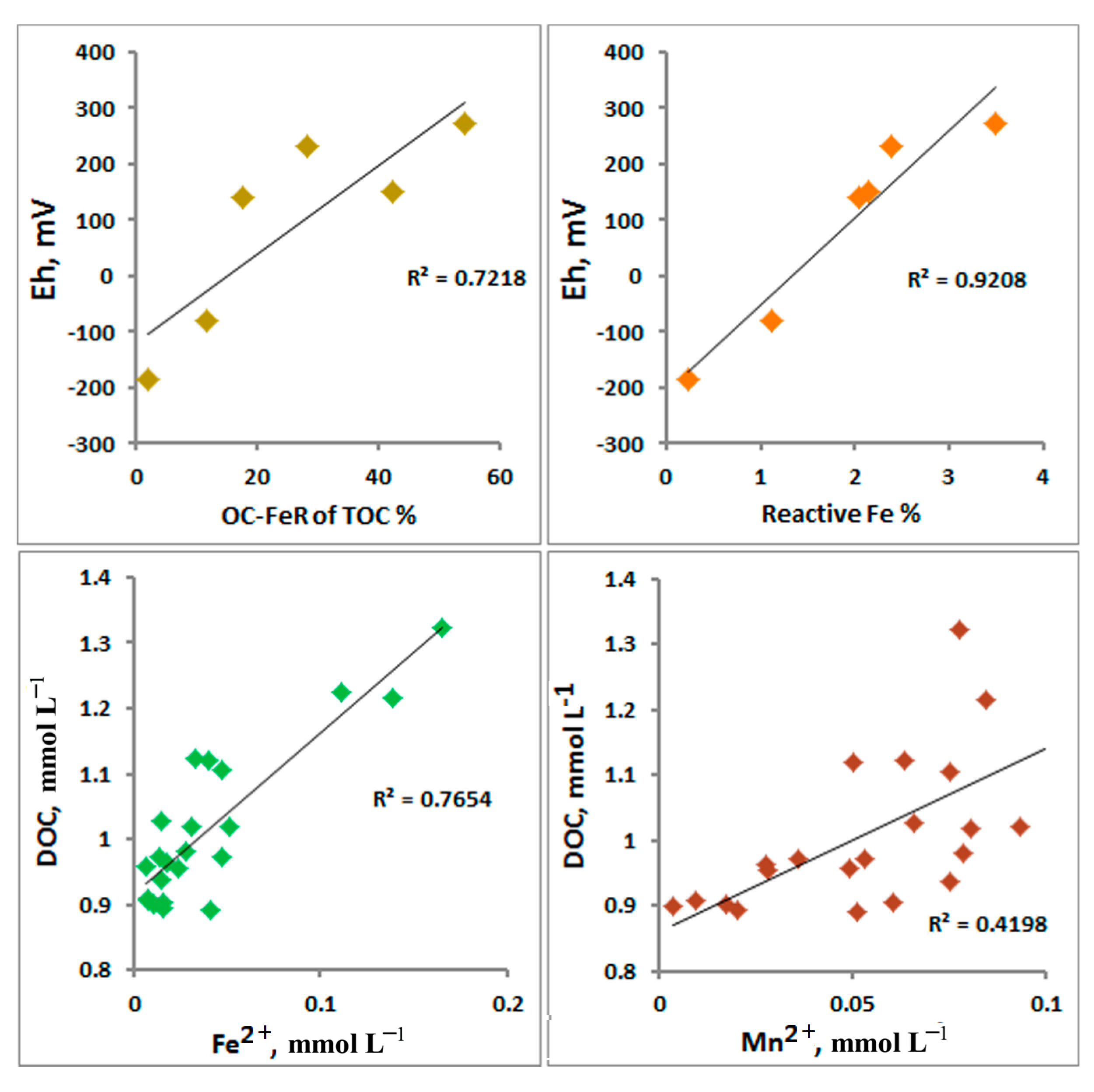
4.1. Peculiarities and Influence of Sorption Properties of Iron Hydroxides. Features of the Absorption of Dissolved Organic Matter, Cations, and Anions
4.2. Features of the Absorption of Dissolved Organic Matter by Fe (Oxyhydr)oxides. The Effect of Calcium on the Absorption of DOC
4.3. Influence of Redox Conditions on the Sediments
4.4. Factors Affecting the Stabilization of OC
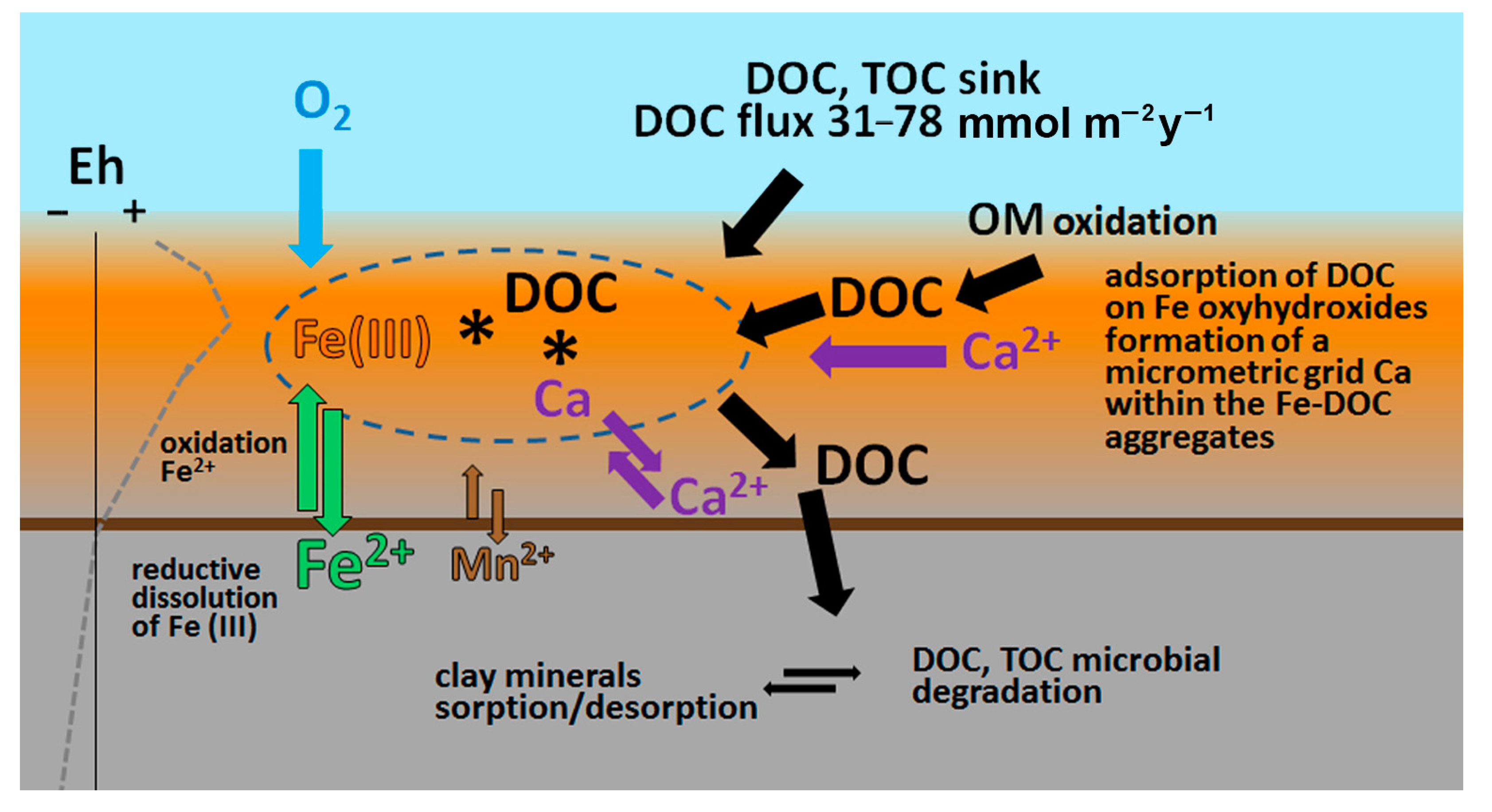
4.5. Conceptual Model of Organic Carbon Burial
4.6. DOC Fluxes and Adjustment of Balance Values
| Areas | Zones in Sediments | Depth, cm | OC 1 Flux mmol m−2 yr−1 | DOC 2 Flux mmol m−2 yr−1 |
|---|---|---|---|---|
| Southern basin | 220 | |||
| I | 0.1–2 | 78 | ||
| II | 2–6 | −102 | ||
| III | 6–45 | 5.9 | ||
| Northern basin | 125 | |||
| I | 0–6 | 31 | ||
| II | 6–21 | −33 | ||
| III | 21–45 | 8.6 |
| System | Oxygenation | Water Depth, m | DOC 1,2 Flux mmol m−2 d−1 | References |
|---|---|---|---|---|
| Freshwater | ||||
| Southern basin | oxic | 1476 | 0.21 | This study |
| anoxic | −0.27 | This study | ||
| Northern basin | oxic | 925 | 0.09 | This study |
| anoxic | −0.09 | This study | ||
| Southern basin | - | 1415 | −0.17 | [26] |
| Northern basin | - | 897 | −0.05 | [26] |
| Lake Erssjön | anoxic | 4.5 | −3.3 | [13] |
| Sweden | [13] | |||
| Hassel reservoir | oxic | 5 | 2.4 | [4] |
| Germany | anoxic | −1.8 | [4] | |
| Uiam Lake | oxic | 13 | 4.8 | [8] |
| South Korea | anoxic | −5.1 | [8] | |
| Marine | ||||
| Arabian Sea | anoxic | 3190–4420 | −0.06–−0.22 | [50] |
| NE Atlantic | anoxic | 4500–4800 | −0.05–−0.12 | [50] |
| Baltic Sea | oxic | 231 | 0.02 | [49] |
| anoxic | −0.44 | [49] | ||
| Long Island Sound | oxic | 15 | 1.5 | [3] |
| USA | anoxic | −5.2 | [3] |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Sobek, S.; Durisch-Kaiser, E.; Zurbrügg, R.; Wongfun, N.; Wessels, M.; Pasche, N.; Wehrli, B. Organic carbon burial efficiency in lake sediments controlled by oxygen exposure time and sediment source. Limnol. Oceanogr. 2009, 54, 2243–2254. [Google Scholar] [CrossRef] [Green Version]
- Skoog, A.C.; Arias-Esquivel, V.A. The effect of induced anoxia and reoxygenation on benthic fluxes of organic carbon, phosphate, iron, and manganese. Sci. Total Environ. 2009, 407, 6085–6092. [Google Scholar] [CrossRef] [PubMed]
- Dadi, T.; Friese, K.; Wendt-Potthoff, K. Benthic dissolved organic carbon fluxes in a drinking water reservoir. Limnol. Oceanogr. 2016, 61, 445–459. [Google Scholar] [CrossRef] [Green Version]
- Sachse, A.; Babenzien, D.; Ginzel, G.; Gelbrecht, J.; Steinberg, C.E.W. Characterization of dissolved organic carbon (DOC) in a dystrophic lake and an adjacent fen. Biogeochemistry 2001, 54, 279–296. [Google Scholar] [CrossRef]
- Fu, P.; Wu, F.; Liu, C.-Q. Spectroscopic characterization and molecular weight distribution of dissolved organic matter in sediment porewaters from Lake Erhai, Southwest China. Biogeochemistry 2006, 81, 179–189. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Choi, J.H.; Hur, J. Benthic flux of dissolved organic matter from lake sediment at different redox conditions and the possible effects of biogeochemical processes. Water Res. 2014, 61, 97–107. [Google Scholar] [CrossRef]
- Peter, S.; Agstam, O.; Sobek, S. Widespread release of dissolved organic carbon from anoxic boreal lake sediments. Inland Waters 2017, 7, 151–163. [Google Scholar] [CrossRef]
- Hemingway, J.D.; Rothman, D.H.; Grant, K.E.; Rosengard, S.Z.; Eglinton, T.I.; Derry, L.A.; Galy, V.V. Mineral protection regulates long-term global preservation of natural organic carbon. Nature 2019, 570, 228–231. [Google Scholar] [CrossRef]
- Faust, J.C.; Tessin, A.; Fisher, B.J.; Zindorf, M.; Papadaki, S.; Hendry, K.R.; Doyle, K.A.; März, C. Millennial scale persistence of organic carbon bound to iron in Arctic marine sediments. Nat. Commun. 2021, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.; Lalonde, K.; Mucci, A. The role of iron in the diagenesis of organic carbon and nitrogen in sediments: A long-term incubation experiment. Mar. Chem. 2014, 162, 1–9. [Google Scholar] [CrossRef]
- Peter, S.; Isidorova, A.; Sobek, S. Enhanced carbon loss from anoxic lake sediment through diffusion of dissolved organic carbon. J. Geophys. Res. Biogeosci. 2016, 121, 1959–1977. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, T.; Ueda, S.; Khodzher, T.; Bashenkhaeva, N.; Korovyakova, I.; Sorokovikova, L.; Gorbunova, L. Distribution of dissolved organic carbon in Lake Baikal and its watershed. Limnology 2002, 3, 159–168. [Google Scholar] [CrossRef]
- Hutchinson, D.R.; Golmshtok, A.S.; Zonenshain, L.P.; Moore, T.C.; Scholz, C.A.; Klitgord, K.D. Depositional and tectonic frame work of the rift basin of Lake Baikal from multichannel seismic data. Geology 1992, 20, 589–592. [Google Scholar] [CrossRef]
- Atlas of Baikal; RAN, Federal’naya Sluzhba Geodezii i Kartografii Rossii: Moscow, Russia, 1993. (In Russian)
- Shimaraev, M.N.; Grachev, M.A.; Imboden, D.I. International hydrophysical experiment at Lake Baikal. Dokl. Earth Sci. 1995, 343, 824–828. (In Russian) [Google Scholar]
- Killworth, P.D.; Carmack, E.C.; Weiss, R.F.; Matear, R. Modeling deep-water renewal in Lake Baikal. Limnol. Oceanogr. 1996, 41, 1521–1538. [Google Scholar] [CrossRef]
- Granina, L.; Müller, B.; Wehrli, B. Origin and dynamics of Fe and Mn sedimentary layers in Lake Baikal. Chem. Geol. 2004, 205, 55–72. [Google Scholar] [CrossRef]
- Och, L.M.; Müller, B.; Voegelin, A.; Ulrich, A.; Göttlicher, J.; Steiniger, R.; Mangold, S.; Vologina, E.G.; Sturm, M. New insights into the formation and burial of Fe/Mn accumulations in Lake Baikal sediments. Chem. Geol. 2012, 330–331, 244–259. [Google Scholar] [CrossRef]
- Vologina, E.G.; Sturm, M.S.; Vorob’eva, S.; Granina, L.Z.; Toshchakov, S.Y. Character of sedimentation in Lake Baikal in the Holocene. Russ. Geol. Geophys. 2003, 50, 722–727. (In Russian) [Google Scholar] [CrossRef]
- Maerki, M.; Müller, B.; Wehrli, B. Microscale mineralization pathways in surface sediments: A chemical sensor study in Lake Baikal. Limnol. Oceanogr. 2006, 51, 1342–1354. [Google Scholar] [CrossRef]
- Torres, N.T.; Och, L.M.; Hauser, P.C.; Furrer, G.; Brandl, H.; Vologina, E.; Sturm, M.; Bürgmanna, H.; Müller, B. Early diagenetic sediment processes generate iron and manganese oxide layers in the sediments of Lake Baikal, Siberia. Environ. Sci. Process. Impacts 2014, 16, 879–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Och, L.M.; Müller, B.; Wichser, A.; Ulrich, A.; Vologina, E.G.; Sturm, M. Rare earth elements in the sediments of Lake Baikal. Chem. Geol. 2014, 376, 61–75. [Google Scholar] [CrossRef]
- Och, L.M.; Müller, B.; März, C.; Wichser, A.; Vologina, E.G.; Sturm, M. Elevated uranium concentrations in Lake Baikal sediments: Burial and early diagenesis. Chem. Geol. 2016, 441, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Klump, J.V.; Edgington, D.N.; Granina, L. Estimates of the remineralization and burial of organic carbon in Lake Baikal sediments. J. Great Lakes Res. 2020, 46, 102–114. [Google Scholar] [CrossRef]
- Baram, G.I.; Vereshchagin, A.L.; Golobokova, L.P. Application of high-efficiency microcolumn liquid chromatography with UV detection for determining anions in environmental objects. J. Anal. Chem. 1999, 54, 962–965. [Google Scholar]
- Pogodaeva, T.V.; Zemskaya, T.I.; Golobokova, L.P.; Khlystov, O.M.; Minami, H.; Sakagami, H. Chemical composition of pore waters of bottom sediments in different Baikal basins. Russ. Geol. Geophys. 2007, 48, 886–900. [Google Scholar] [CrossRef]
- Salvadó, J.A.; Tesi, T.; Andersson, A.; Ingri, J.; Dudarev, O.V.; Semiletov, I.P.; Gustafsson, Ö. Organic carbon remobilized from thawing permafrost is resequestered by reactive iron on the Eurasian Arctic Shelf. Geophys. Res. Lett. 2015, 42, 8122–8130. [Google Scholar] [CrossRef] [Green Version]
- Berner, R.A. Early Diagenesis: A Theoretical Approach; Princeton University Press: Princeton, NJ, USA, 1980. [Google Scholar]
- Maerki, M.; Wehrli, B.; Dinkel, C.; Müller, B. The influence of tortuosity on molecular diffusion in freshwater sediments of high porosity. Geochim. Cosmochim. Acta 2004, 68, 1519–1528. [Google Scholar] [CrossRef]
- Li, Y.-H.; Gregory, S. Diffusion of ions in sea water and in deep-sea sediments. Geochim. Cosmochim. Acta 1974, 38, 703–714. [Google Scholar]
- O’Loughlin, E.J.; Chin, Y.P. Quantification and characterization of dissolved organic carbon and iron in sedimentary porewater from Green Bay, WI, USA. Biogeochemistry 2004, 71, 371–386. [Google Scholar] [CrossRef]
- Pogodaeva, T.V.; Lopatina, I.N.; Khlystov, O.M. Background composition of pore waters in Lake Baikal bottom sediments. J. Great Lakes Res. 2017, 43, 1030–1043. [Google Scholar] [CrossRef]
- Riedel, T.; Zak, D.; Biester, H. Iron traps terrestrially derived dissolved organic matter at redox interfaces. Proc. Natl. Acad. Sci. USA 2013, 110, 10101–10105. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, T.; Van Riemsdijk, W.H. A surface structural model for ferrihydrite I: Sites related to primary charge, molar mass, and mass density. Geochim. Cosmochim. Acta 2009, 73, 4423–4436. [Google Scholar] [CrossRef]
- Hiemstra, T.; Mendez, J.C.; Li, J. Evolution of the Reactive Surface Area of Ferrihydrite: Time, PH, and Temperature Dependency of Growth by Ostwald Ripening. Environ. Sci. Nano 2019, 6, 820–833. [Google Scholar] [CrossRef]
- Mendez, J.C.; Hiemstra, T. High and low affinity sites of ferrihydrite for metal ion adsorption: Data and modeling of the alkaline-earth ions Be, Mg, Ca, Sr, Ba, and Ra. Geochim. Cosmochim. Acta 2020, 286, 289–305. [Google Scholar] [CrossRef]
- Ding, Y.; Huang, X.; Zhang, H.; Ma, J.; Li, F.; Zeng, Q.; Hu, N.; Wang, Y.; Dai, Z.; Ding, D. Coupled variations of dissolved organic matter distribution and iron (oxyhydr)oxides transformation: Effects on the kinetics of uranium adsorption and desorption. J. Hazard. Mater. 2022, 436, 129298. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, S.Z.; Wang, S.S.; Luo, L.; Cao, D.; Christie, P. Molecular-scale investigation with ESI-FT-ICR-MS on fractionation of dissolved organic matter induced by adsorption on iron oxyhydroxides. Environ. Sci. Technol. 2016, 50, 2328–2336. [Google Scholar] [CrossRef]
- Linkhorst, A.; Dittmar, T.; Waska, H. Molecular fractionation of dissolved organic matter in a shallow subterranean estuary: The role of the iron curtain. Environ. Sci. Technol. 2017, 51, 1312–1320. [Google Scholar] [CrossRef]
- Li, Y.; Gong, X.; Sun, Y.; Shu, Y.; Niu, D.; Ye, H. High molecular weight fractions of dissolved organic matter (DOM) determined the adsorption and electron transfer capacity of DOM on iron minerals. Chem. Geol. 2022, 604, 120907. [Google Scholar] [CrossRef]
- Jelavić, S.; Mitchell, A.C.; Sand, K.K. Fate of organic compounds during transformation of ferrihydrite in iron formations. Geochem. Perspect. Lett. 2020, 15, 25–29. [Google Scholar] [CrossRef]
- Beauvois, A.; Vantelon, D.; Jestin, J.; Rivard, C.; Bouhnik-Le Coz, M.; Dupon, A.; Briois, V.; Bizien, T.; Sorrentino, A.; Wu, B.; et al. How does calcium drive the structural organization of iron–organic matter aggregates? A multiscale investigation. Environ. Sci. Nano 2020, 7, 2833–2849. [Google Scholar] [CrossRef]
- Ma, W.-W.; Zhu, M.-X.; Yang, G.-P.; Li, T. Iron geochemistry and organic carbon preservation by iron (oxyhydr)oxides in surface sediments of the East China Sea and the South Yellow Sea. J. Mar. Syst. 2018, 178, 62–74. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M. Mineral–organic associations: Formation, properties, and relevance in soil environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar] [CrossRef]
- Chen, C.; Dynes, J.J.; Wang, J.; Sparks, D.L. Properties of Fe-Organic Matter Associations via Coprecipitation versus Adsorption. Environ. Sci. Technol. 2014, 48, 13751–13759. [Google Scholar] [CrossRef]
- Li, H.; Santos, F.; Butler, K.; Herndon, E. A Critical Review on the Multiple Roles of Manganese in Stabilizing and Destabilizing Soil Organic Matter. Environ. Sci. Technol. 2021, 55, 12136–12152. [Google Scholar] [CrossRef]
- Lengier, M.; Szymczycha, B.; Brodecka-Goluch, A.; Kłostowska, Z.; Kuliński, K. Benthic diffusive fluxes of organic and inorganic carbon, ammonium and phosphates from deep water sediments of the Baltic Sea. Oceanologia 2021, 63, 370–384. [Google Scholar] [CrossRef]
- Lahajnar, N.; Rixen, T.; Gaye-Haake, B.; Schäfer, P.; Ittekkot, V. Dissolved organic carbon (DOC) fluxes of deep-sea sediments from the Arabian Sea and NE Atlantic. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 1947–1964. [Google Scholar] [CrossRef]
- Müller, B.; Maerki, M.; Schmid, M.; Vologina, E.G.; Wehrli, B.; Wüest, A.; Sturm, M. Internal carbon and nutrient cycling in Lake Baikal: Sedimentation, upwelling, and early diagenesis. Glob. Planet. Chang. 2005, 46, 101–124. [Google Scholar] [CrossRef]
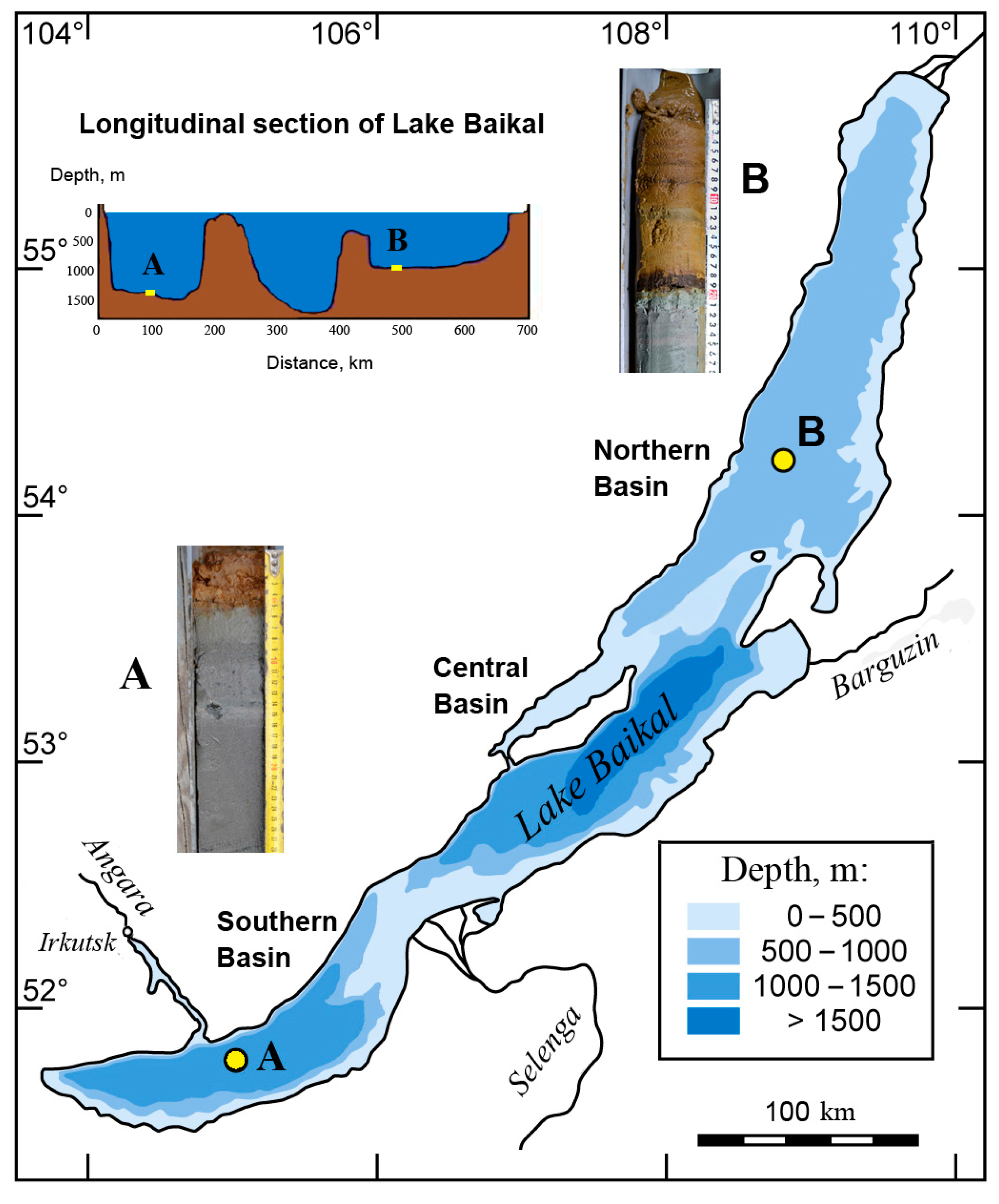
| Location | Station | Core | Date | Position | Lake Depth, m | Core Length, cm | Number of Samples |
|---|---|---|---|---|---|---|---|
| Southern basin | A | Ver 20 01 St13BGC1 | 24 June 2020 | 51°42′10.4″ N 104°58′47.39′′ E | 1476 | 55 | 19 * |
| Northern basin | B | Ver 20 01 St6BGC1 | 19 June 2020 | 54°14′16.99″ N 108°48′16.38′′ E | 925 | 50 | 33 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogodaeva, T.; Khodzher, T. Organic Carbon in the Bottom Sediments of Lake Baikal: Geochemical Processes of Burial and Balance Values. Water 2023, 15, 2941. https://doi.org/10.3390/w15162941
Pogodaeva T, Khodzher T. Organic Carbon in the Bottom Sediments of Lake Baikal: Geochemical Processes of Burial and Balance Values. Water. 2023; 15(16):2941. https://doi.org/10.3390/w15162941
Chicago/Turabian StylePogodaeva, Tatyana, and Tamara Khodzher. 2023. "Organic Carbon in the Bottom Sediments of Lake Baikal: Geochemical Processes of Burial and Balance Values" Water 15, no. 16: 2941. https://doi.org/10.3390/w15162941
APA StylePogodaeva, T., & Khodzher, T. (2023). Organic Carbon in the Bottom Sediments of Lake Baikal: Geochemical Processes of Burial and Balance Values. Water, 15(16), 2941. https://doi.org/10.3390/w15162941






