Unravelling Relationships between In Vivo Effects on Plants and Detected Pesticide Mixtures in Freshwaters of a South-European Agro-Ecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterisation of the Study Area
2.2. Crop Occupation in the Study Area
2.3. Selection of the Sampling Surface Sites
2.4. Surface Water Sampling
2.5. Analytical Methods
2.5.1. Individual Parameters for Water Quality Intended for Irrigation Use
2.5.2. Pesticides
2.5.3. Phytotoxkit Liquid Samples
2.6. Environmental Risk Assessment
3. Results
3.1. Individual Water Quality Parameters
3.2. Detected Pesticide Compounds
3.3. Environmental Risk Assessment
3.4. Phytotestkit
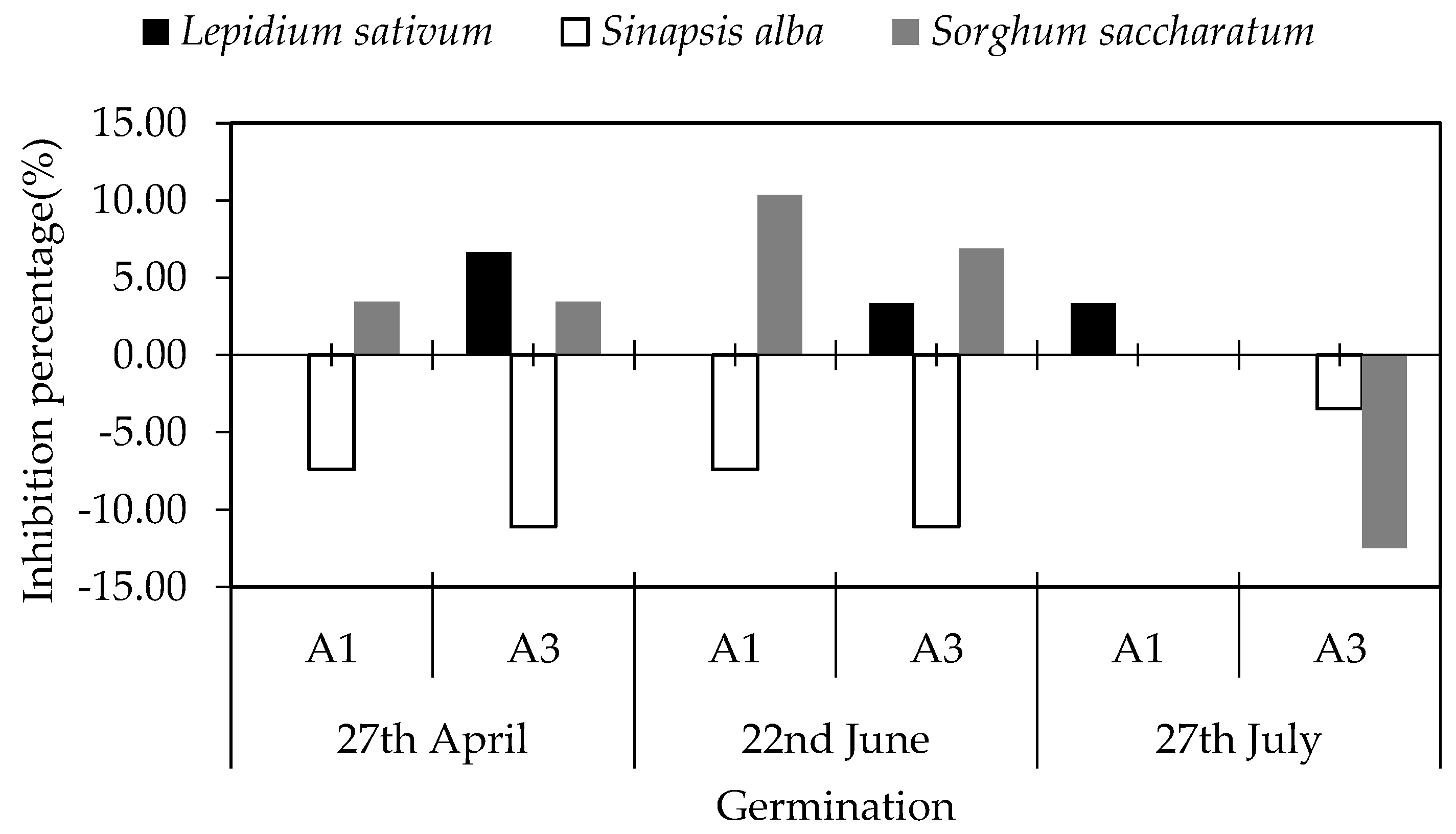
3.4.1. Germination
3.4.2. Growth of Roots and Shoot
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Azcón-Bieto, J.; Talón, M. Fundamentos de Fisiología Vegetal, 2nd ed.; McGraw-Hill—Interamericana de España, S.L.: Madrid, Spain, 2008. [Google Scholar]
- TOPPS—Water Protection. Surface Water Protection. 2018. Available online: www.toppslife.org/ (accessed on 6 July 2023).
- Pereira, A.S.; Dâmaso-Rodrigues, M.L.; Amorim, A.; Daam, M.A.; Cerejeira, M.J. Aquatic community structure in Mediterranean edge-of-field waterbodies as explained by environmental factors and the presence of pesticide mixtures. Ecotoxicology 2018, 27, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Knillmann, S.; Orlinskiy, P.; Kaske, O.; Foit, O.; Liess, M. Indication of pesticide effects and recolonization in streams. Sci. Total Environ. 2018, 630, 1619–1627. [Google Scholar] [CrossRef]
- Christou, A.; Michael, C.; Fatta-Kassinos, D.; Fotopoulos, V. Can the pharmaceutically active compounds released in agroecosystems be considered as emerging plant stressors? Environ. Int. 2018, 114, 360–364. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R.; Brix, H. A review of plant–pharmaceutical interactions: From uptake and effects in crop plants to phytoremediation in constructed wetlands. Environ. Sci. Pollut. Res. 2014, 21, 11729–11763. [Google Scholar] [CrossRef] [PubMed]
- Margenat, A.; Matamoros, V.; Díez, S.; Cañameras, N.; Comas, J.; Bayona, J.M. Occurrence of chemical contaminants in peri-urban agricultural irrigation waters and assessment of their phytotoxicity and crop productivity. Sci. Total Environ. 2017, 599, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Marsoni, M.; De Mattia, F.; Labra, M.; Bruno, A.; Bracale, M.; Vannini, C. Uptake and effects of a mixture of widely used therapeutic drugs in Eruca sativa L. and Zea mays L. Plants. Ecotoxicol. Environ. Saf. 2014, 108, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Decreto 33210, de 11 de Novembro. Diário do Govêrno n.º 245/1943, Série I de 1943-11-11, 743-744. Available online: https://diariodarepublica.pt/dr/detalhe/decreto/33210-1943-257447 (accessed on 6 July 2023).
- Campos, J.; Madaleno, C. Edições Anteriores da Revista Agrotec. AGROTEC® 2020, 34, 22–24. Available online: http://www.agrotec.pt/noticias/seccao/edicoes-anteriores/ (accessed on 6 July 2023).
- Instituto Nacional de Estatística (INE). Recenseamento Agrícola—Análise Dos Principais Resultados—2019; Instituto Nacional de Estatística: Lisboa, Portugal, 2021. [Google Scholar]
- Narciso, G.A. Contribuição para o Estudo da Relação Entre a Qualidade da água de Superfície da área Agrícola Lezíria Grande de Vila Franca de Xira e os Efeitos Tóxicos Sobre três Espécies de Plantas Terrestres. Master’s Thesis, Unviversity of Lisbon, Lisbon, Portugal, 2021. [Google Scholar]
- Cerejeira, M.J.; Silva, E. Aquatic Risk Assessment of Pesticides. In Concepts and Applications in Portuguese River Basins; ISAPress: Lisboa, Portugal, 2019; 189p. [Google Scholar]
- Decreto-lei 236/98, de 1 de Agosto. Diário da República n.º 176/1998, Série I-A de 1998-08-01. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/236-1998-430457 (accessed on 6 July 2023).
- Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on minimum requirements for water reuse. Off. J. Eur. Union 2020, 177, 32–55. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0741 (accessed on 6 July 2023).
- MicroBioTests. Phytotoxicity Test with Phytotoxkit Liquid samples. Available online: https://www.microbiotests.com/toxkit/phytotoxicity-test-with-phytotoxkit-liquid-samples/ (accessed on 6 July 2023).
- ISO 18763:2016; Soil Quality—Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants. ISO: Geneva, Switzerland, 2016.
- European Commission. Technical Guidance for deriving Environmental Quality Standards. In Guidance Document No. 27—Updated Version 2018, Document Directors at Their Meeting in Sofia on 11–12 June 2018; European Commission: Sofia, Bulgaria, 2018. [Google Scholar]
- European Chemicals Agency (ECHA). Guidance on the BPR: Volume IV Environment, Assessment & Evaluation (Parts B+C), Version 2.0, Reference: ECHA-17-G-23-EN; European Chemicals Agency (ECHA): Helsinki, Finland, 2017; ISBN 978-92-9020-151-9.
- European Chemicals Agency (ECHA). Guidance on Information Requirements and Chemical Safety Assessment Part E: Risk Characterisation, Version 3.0, Reference: ECHA-2016-G-04-EN; European Chemicals Agency (ECHA): Helsinki, Finland, 2016; ISBN 978-92-9495-055-0. [Google Scholar]
- European Commission. Technical Guidance Document on Risk Assessment; Part 2 EUR 20418 EN/2; Joint Research Centre: Brussels Belgium; European Communities: Luxembourg, 2003. [Google Scholar]
- Oekotoxzentrum. ad hoc EQS List Swiss Ecotoxcentre. Available online: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.ecotoxcentre.ch%2Fmedia%2F195581%2Fquality-criteria-used-by-oz-for-aquatic-risk-assessment.xlsx&wdOrigin=BROWSELINK (accessed on 6 July 2023).
- Norman. Norman Database System. Available online: https://www.norman-network.com/nds/ (accessed on 6 July 2023).
- Monte, H.M.D.; Albuquerque, A. Reutilização de Águas Residuais; ERSAR: Lisboa, Portugal, 2010.
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G., Jr. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Carlesa, L.; Gardona, H.; Josepha, L.; Sanchísb, J.; Farréb, M.; Artigasa, J. Meta-analysis of glyphosate contamination in surface waters and dissipation by biofilms. Environ. Int. 2019, 124, 284–293. [Google Scholar] [CrossRef]
- Sistema de Gestão das Autorizações de Produtos Fitofarmacêuticos (SIFITO). Available online: www.sifito.dgav.pt (accessed on 6 July 2023).
- Pesticide Properties DataBase, PPDB. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en (accessed on 6 July 2023).
- Barbieri, M.V.; Peris, A.; Postigo, C.; Moya-Garcés, A.; Monllor-Alcaraz, L.S.; Rambla-Alegre, M.; Eljarrat, E.; Alda, M.L. Evaluation of the occurrence and fate of pesticides in a typical Mediterranean delta ecosystem (Ebro River Delta) and risk assessment for aquatic organisms☆. Environ. Pollut. 2021, 274, 115813. [Google Scholar] [CrossRef] [PubMed]
- Associação de Regantes e Beneficiários do Vale do Sorraia, ARBVS. Available online: www.arbvs.pt (accessed on 6 July 2023).
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union 2013, 226, 1–17.
- Agência Portuguesa do Ambiente (APA). Critérios Para a Classificação das Massas de Água—Ficha Técnica. Ficha Técnica, DRH/DEQA. Available online: https://www.apambiente.pt/sites/default/files/_Agua/DRH/ParticipacaoPublica/PGRH/2022-2027/3_Fase/PGRH_3_SistemasClassificacao.pdf (accessed on 6 July 2023).
- Commission Implementing Decision (EU). 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2015, 78, 40–42. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015D0495&rid=4 (accessed on 6 July 2023).
- Commission Implementing Decision (EU). 2018/840 of 5 June 2018 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing Commission Implementing Decision (EU) 2015/495. Off. J. Eur. Union 2018, 141, 9–12. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0840 (accessed on 6 July 2023).
- Commission Implementing Decision (EU). 2022/1307 of 22 July 2022 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2022, 197, 117–120. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022D1307 (accessed on 6 July 2023).
- Brito, I.P.F.S.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Hormetic effects of glyphosate on plants. Pest Manag. Sci. 2018, 74, 1064–1070. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA). Ecotox Knowledgebase. Available online: https://cfpub.epa.gov/ecotox/ (accessed on 6 July 2023).
- Cedergreen, N. Quantifying Synergy: A Systematic Review of Mixture Toxicity Studies within Environmental Toxicology. PLoS ONE 2014, 9, e96580. [Google Scholar] [CrossRef] [PubMed]
- Weisner, O.; Frische, T.; Liebmanna, L.; Reemtsma, T.; Roß-Nickoll, M.; Schäfer, R.B.; Schäffer, A.; Scholz-Starke, B.; Vormeier, P.; Knillmann, S.; et al. Risk from pesticide mixtures—The gap between risk assessment and reality. Sci. Total Environ. 2021, 796, 149017. [Google Scholar] [CrossRef]



| Pesticide | Concentration (μg L−1) | |||||
|---|---|---|---|---|---|---|
| 27 April | 22 June | 27 July | ||||
| A1 | A3 | A1 | A3 | A1 | A3 | |
| Fungicide | ||||||
| azoxystrobin | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | 0.04 (±0.02) |
| Herbicide | ||||||
| azimsulfuron | <0.05 | <0.05 | <0.05 | 0.2 (±0.1) | <0.05 | 0.12 (±0.06) |
| bentazone | <0.05 | 0.51 (±0.26) | <0.05 | 3.4 (±1.7) | 0.2 (±0.1) | 8.0 (±4.0) |
| clomazone | <0.05 | 0.1 (±0.1) | <0.05 | 0.16 (±0.08) | <0.05 | <0.05 |
| glyphosate | 0.058 (±0.029) | 0.13 (±0.07) | 0.19 (±0.10) | 0.24 (±0.12) | 0.061 (±0.031) | 0.091 (±0.046) |
| imazamox | <0.05 | <0.05 | <0.05 | 0.092 (±0.046) | <0.05 | 0.09 (±0.05) |
| MCPA | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.066 (±0.033) |
| oxadiazon | <0.03 | 0.031 (±0.016) | <0.03 | <0.03 | <0.03 | 0.16 (±0.08) |
| Herbicide metabolite | ||||||
| AMPA | 0.41 (±0.21) | 0.36 (±0.18) | 0.62 (±0.31) | 0.58 (±0.29) | 0.62 (±0.31) | 0.68 (±0.34) |
| Insecticide | ||||||
| flonicamid | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | 0.079 (±0.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, E.; Narciso, G.A.; Castro, J.C. Unravelling Relationships between In Vivo Effects on Plants and Detected Pesticide Mixtures in Freshwaters of a South-European Agro-Ecosystem. Water 2023, 15, 2936. https://doi.org/10.3390/w15162936
Silva E, Narciso GA, Castro JC. Unravelling Relationships between In Vivo Effects on Plants and Detected Pesticide Mixtures in Freshwaters of a South-European Agro-Ecosystem. Water. 2023; 15(16):2936. https://doi.org/10.3390/w15162936
Chicago/Turabian StyleSilva, Emília, Guilherme Anágua Narciso, and Joel Carvalho Castro. 2023. "Unravelling Relationships between In Vivo Effects on Plants and Detected Pesticide Mixtures in Freshwaters of a South-European Agro-Ecosystem" Water 15, no. 16: 2936. https://doi.org/10.3390/w15162936
APA StyleSilva, E., Narciso, G. A., & Castro, J. C. (2023). Unravelling Relationships between In Vivo Effects on Plants and Detected Pesticide Mixtures in Freshwaters of a South-European Agro-Ecosystem. Water, 15(16), 2936. https://doi.org/10.3390/w15162936







