Influence of Anthropogenic Sulfuric Acid on Different Lithological Carbonate Weathering and the Related Carbon Sink Budget: Examples from Southwest China
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area and Sampling Sites
2.2. Sampling and Analysis
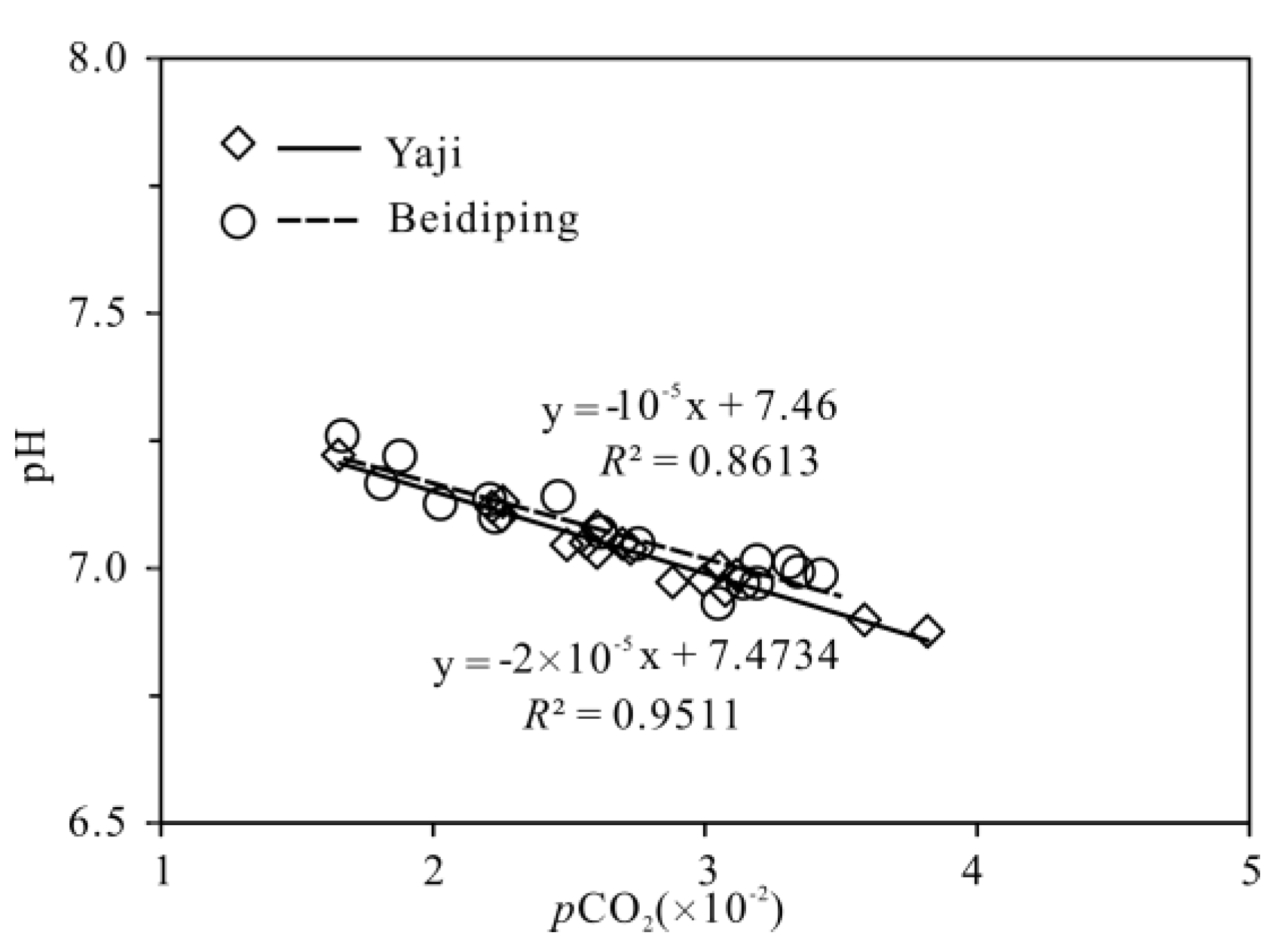
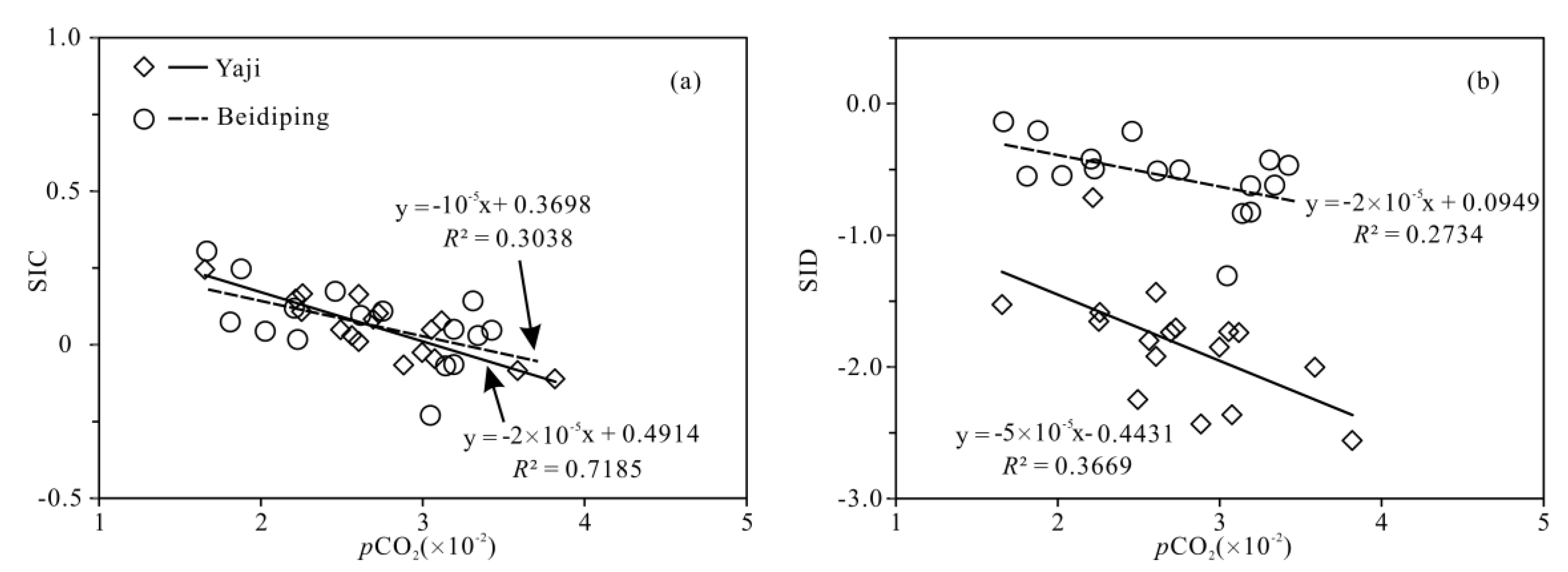
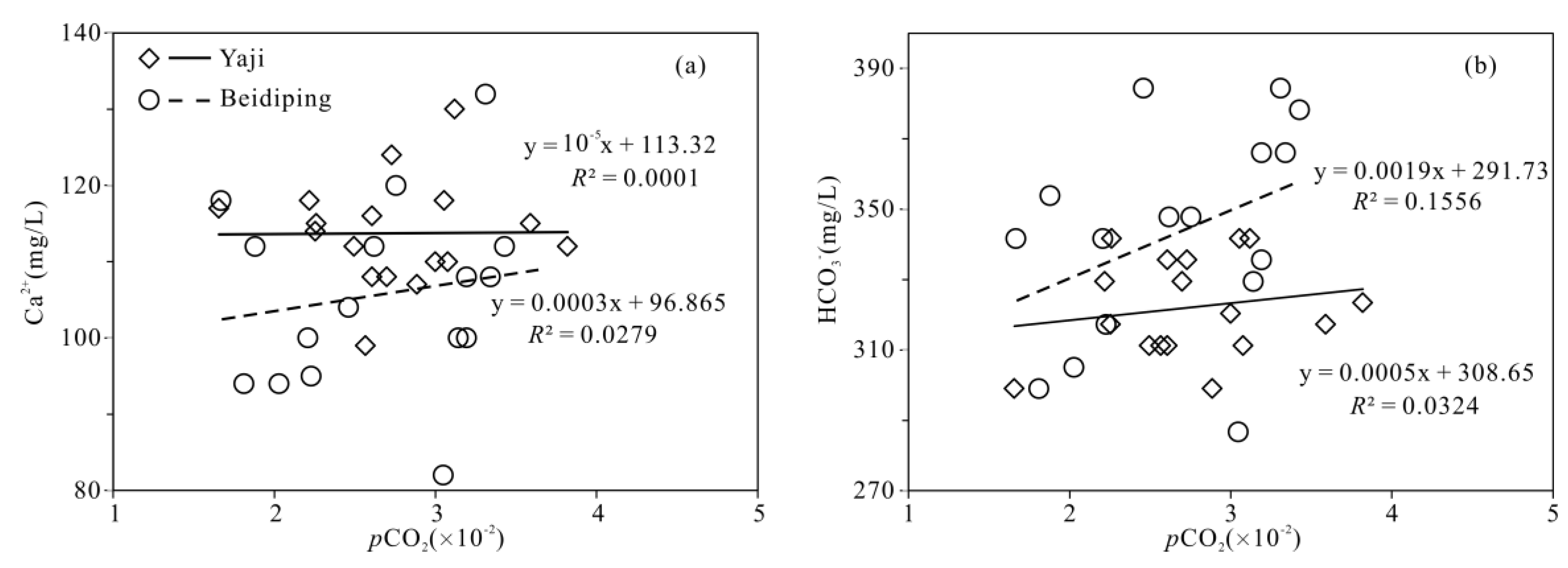
2.3. pCO2 and Calcite and Dolomite Saturation Indexes
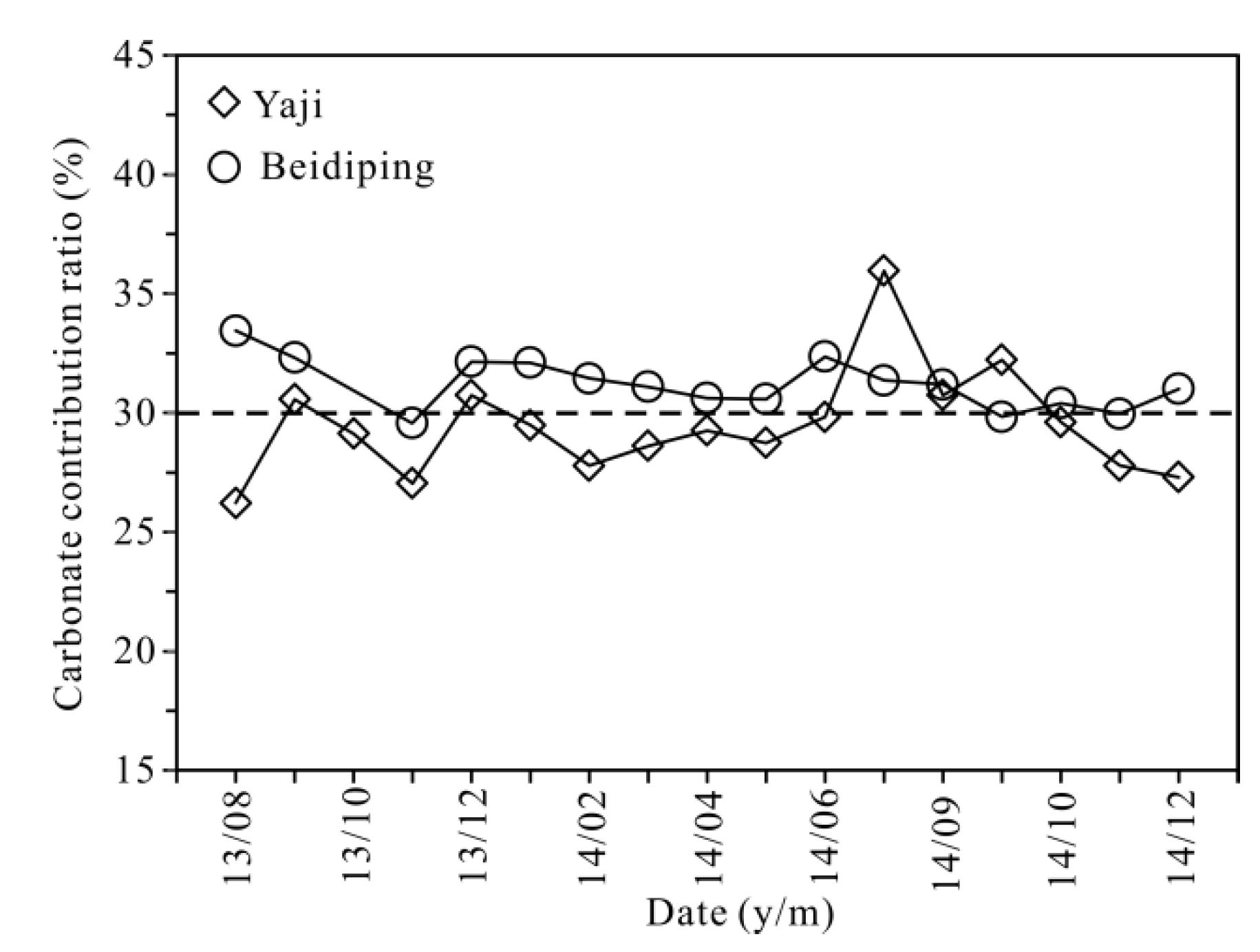
2.4. Calculation of the Effect of Anthropogenic H2SO4 on Carbonate Weathering and Carbon Sink
3. Results
4. Discussion
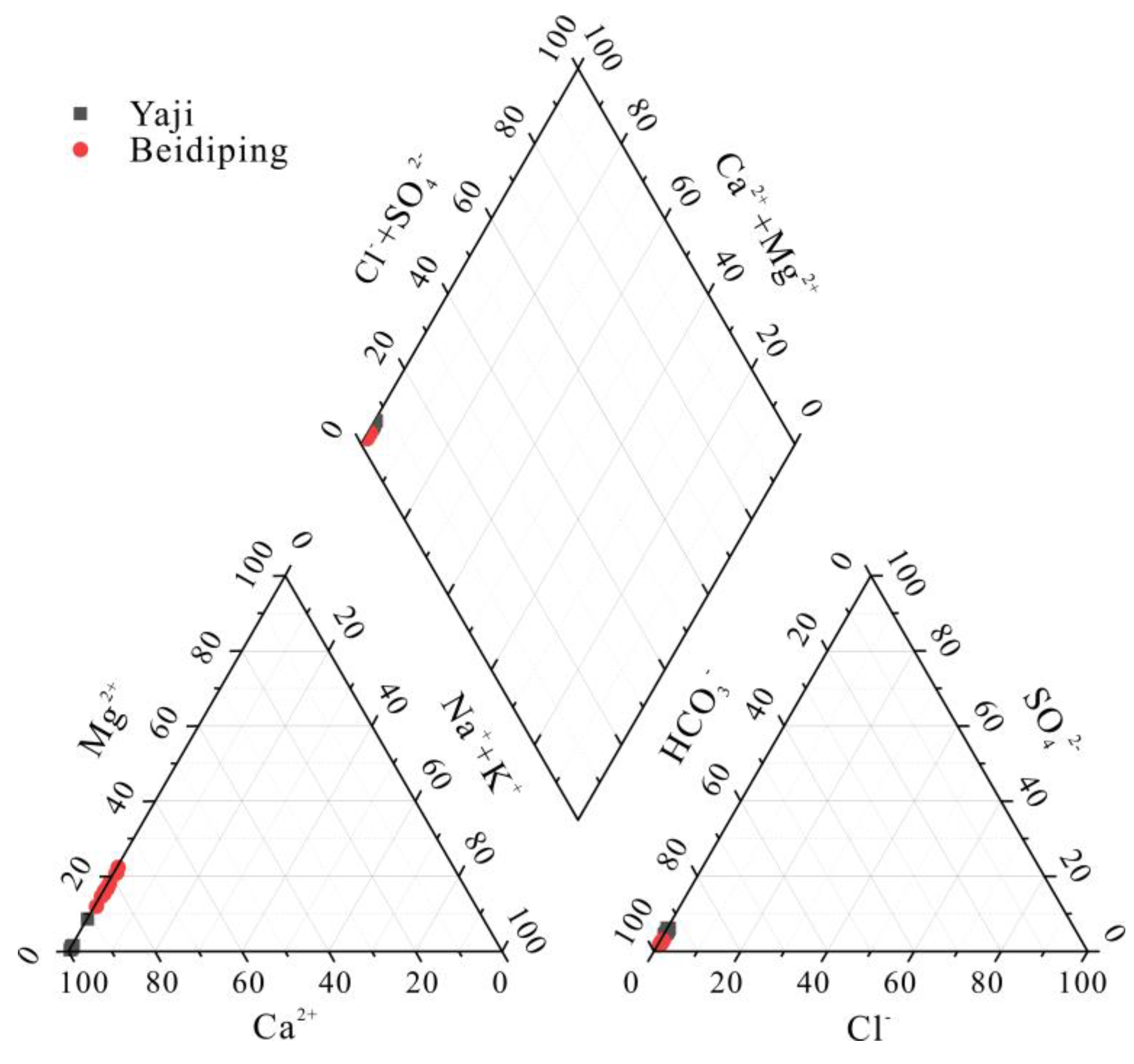
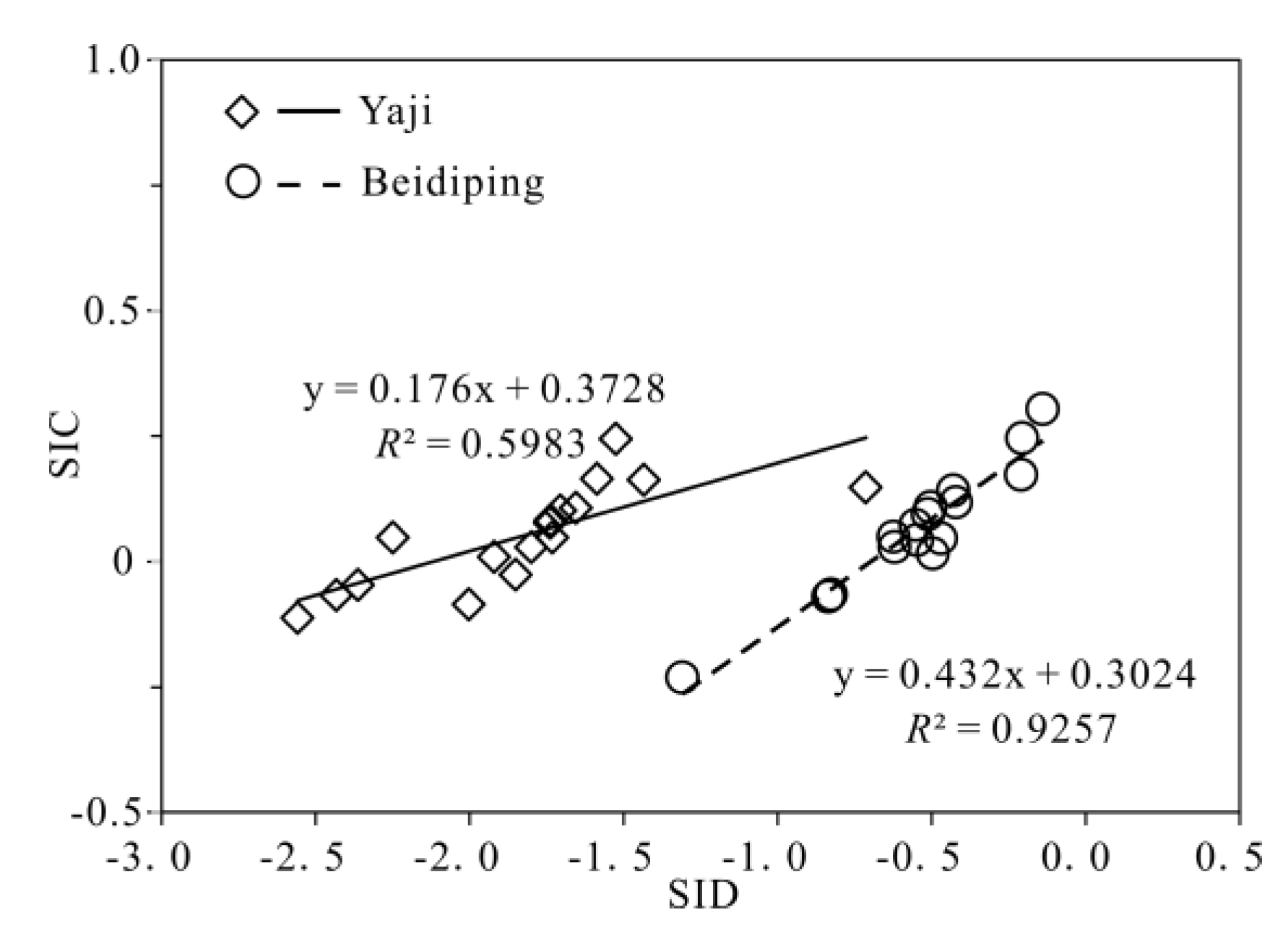
4.1. Sources of Dissolved Load
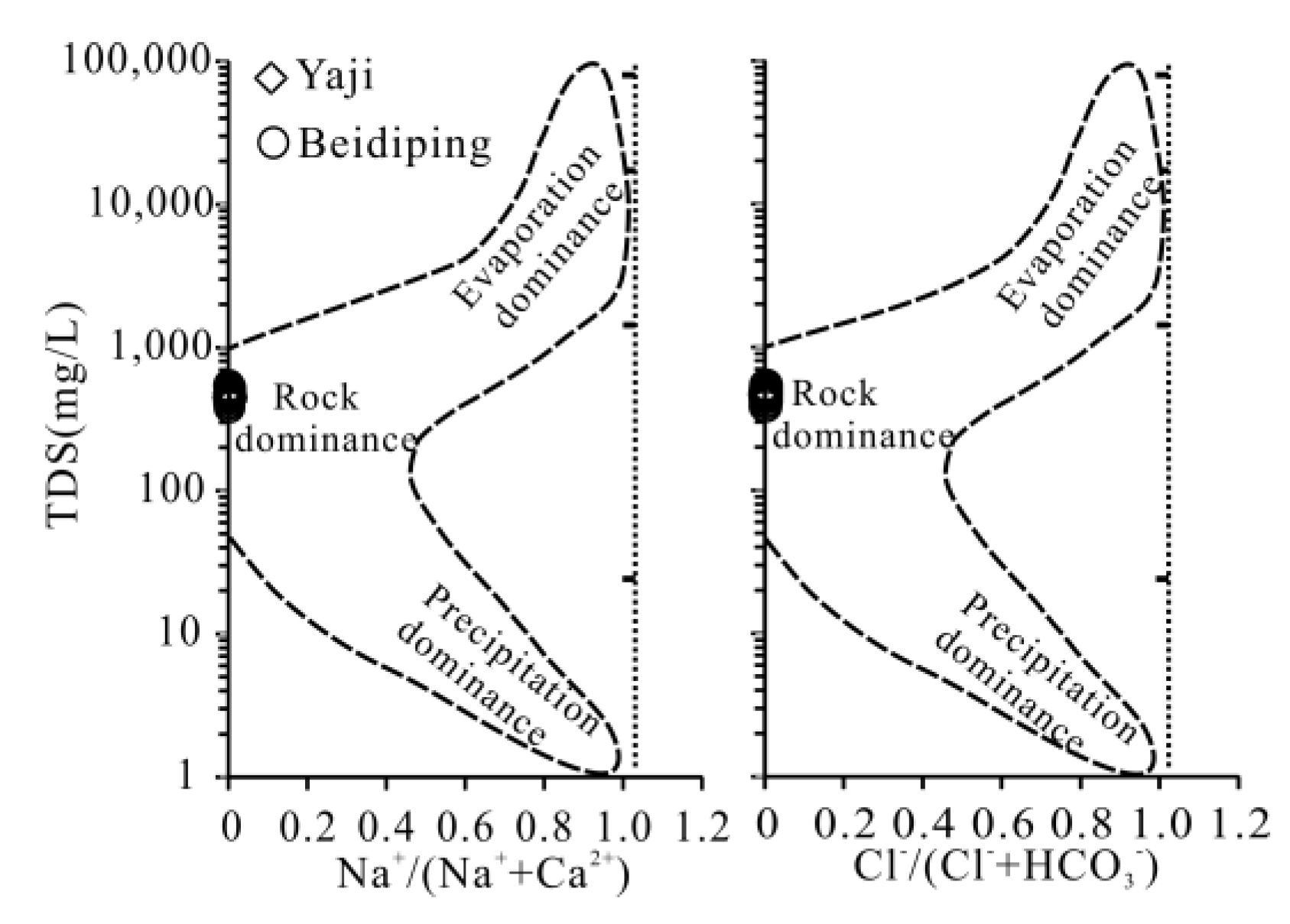
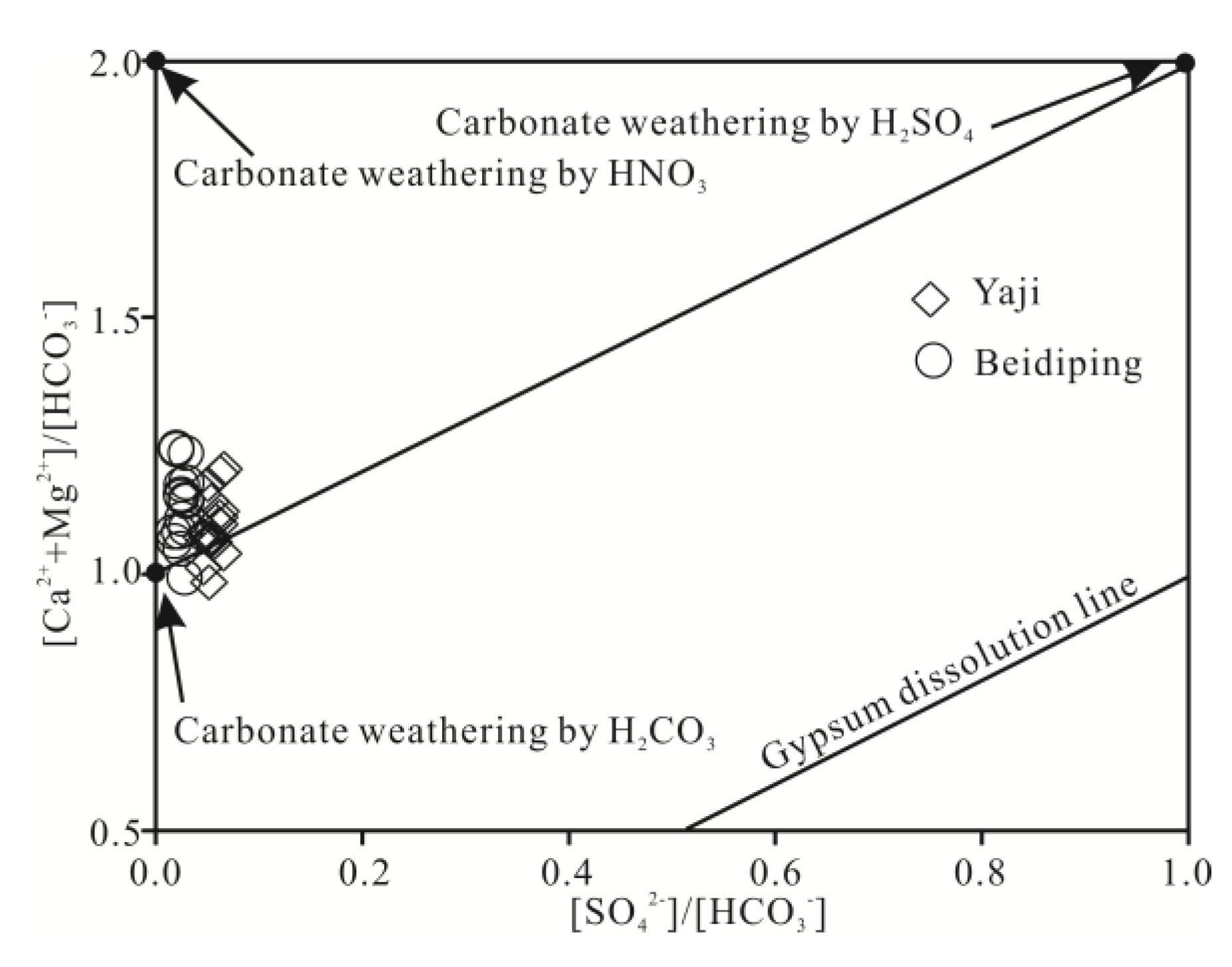
4.2. Anthropogenic Source of H2SO4
4.3. Hydrochemical Evidence of Sulfuric Acid Participation in Carbonate Weathering
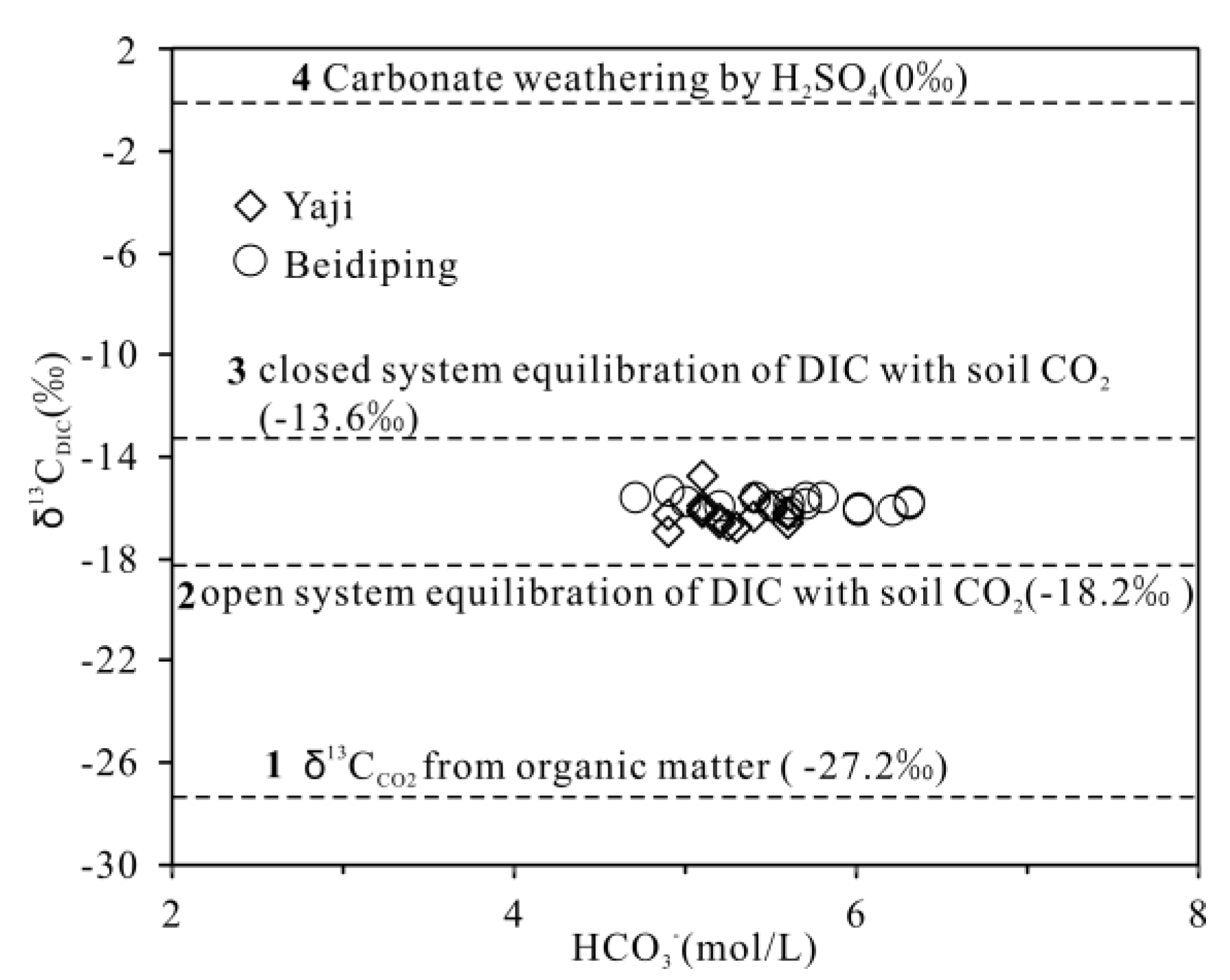
4.4. Carbon Isotopic (13CDIC) Evidence of Sulfuric Acid Weathering of Carbonate Rocks
4.5. Effect of Anthropogenic H2SO4 on Carbonate Weathering and Carbon Sink
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dreybrodt, W.; Wang, H. A new direction in effective accounting for the atmospheric CO2 budget: Considering the combined action of carbonate dissolution, the global water cycle and photosynthetic uptake of DIC by aquatic organisms. Earth-Sci. Rev. 2010, 99, 162–172. [Google Scholar] [CrossRef]
- Liu, Z.H.; Macphersonb, G.L.; Groves, C.; Martin, J.B.; Yuan, D.X.; Zeng, S.B. Large and active CO2 uptake by coupled carbonate weathering. Earth-Sci. Rev. 2018, 182, 42–49. [Google Scholar] [CrossRef]
- Berner, R.A. A model for atmospheric CO2 over Phanerozoic time. Am. J. Sci. 1991, 291, 339–376. [Google Scholar] [CrossRef]
- IPCC. Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Dreybrodt, W. Processes in Karst Systems (Series in Physical Environment); Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1988; p. 140. [Google Scholar]
- Morse, J.W.; Arvidson, R.S. The dissolution kinetics of major sedimentary carbonate minerals. Earth Sci. Rev. 2002, 58, 51–84. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dreybrodt, W.; Liu, H. Atmospheric CO2 sink: Silicate weathering or carbonate weathering. Appl. Geochem. 2011, 26, S292–S294. [Google Scholar] [CrossRef]
- Apollaro, C.; Fuoco, I.; Gennaro, E.; Giuliani, L.; Iezzi, G.; Marini, L.; Radica, F.; Di Luccio, F.; Ventura, G.; Vespasiano, G. Advanced argillic alteration at Cave di Caolino, Lipari, Aeolian Islands (Italy): Implications for the mitigation of volcanic risks and the exploitation of geothermal resources. Sci. Total Environ. 2023, 889, 164333. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.X. Contribution of IGCP 379 “Karst Processes and Carbon Cycle” to global change. Episodes 1998, 21, 198. [Google Scholar]
- Liu, Z.H.; Dreybrodt, W. Significance of the carbon sink produced by H2O-carbonate-CO2-aquatic phototroph interaction on land. Sci. Bull. 2015, 60, 182–191. [Google Scholar] [CrossRef]
- Detwiler, R.P.; Charles, A.S.H. Tropical forests and the global carbon cycle. Science 1988, 239, 42–47. [Google Scholar] [CrossRef]
- Tans, P.P.; Fung, I.; Takahashi, T. Observational constraints on the global atmospheric CO2 budget. Science 1990, 247, 1431–1438. [Google Scholar] [CrossRef]
- Berner, E.K.; Berner, R.A. The Global Water Cycle: Geochemistry and Environment; Prentice-Hall: New York, NY, USA, 1987; p. 394. [Google Scholar]
- Huang, T.M.; Fan, Y.F.; Long, Y.; Pang, Z.H. Quantitative calculation for the contribution of acid rain to carbonate weathering. J. Hydrol. 2019, 568, 360–371. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.S.; Liang, P.; Zhou, Y.; Chai, F.H.; Mo, Z.Y.; Chen, Z.M.; Li, H. Pollution characteristics and control countermeasures of acid rain in Guilin. Res. Environ. Sci. 2020, 33, 1393–1401. [Google Scholar]
- Zhang, G.Z.; Liu, D.Y.; He, X.H.; Yu, D.Y.; Pu, M.J. Acid rain in Jiangsu province, Eastern China: Tempo-spatial variations features and analysis. Atmos. Pollut. Res. 2017, 8, 1031–1043. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.L.; Wu, Q.X.; Tang, Y. Effects of agricultural alkaline substances on reducing the rainwater acidification: Insight from chemical compositions and calcium isotopes in a karst forests area. Agric. Ecosyst. Environ. 2020, 290, 106782. [Google Scholar] [CrossRef]
- Xie, S.Y.; Wang, S.J.; Yu, Y.; Liu, L.; Zhang, F.Y. Analysis on acid rain status and its change trends in China from 2003 to 2018. Environ. Monit. China 2020, 36, 80–88. [Google Scholar]
- Zhou, X.D.; Xu, Z.F.; Liu, W.J.; Wu, Y.; Zhao, T.; Jiang, H. Progress in the studies of precipitation chemistry in acid rain areas of Southwest China. Environ. Sci. 2017, 38, 4438–4446. [Google Scholar]
- Zhu, H.Y. Analysis on Cause and Source of Acid Precipitation in Guilin; Northwest Normal University: Lanzhou, China, 2020; pp. 1–62. [Google Scholar]
- Li, S.L.; Calmels, D.; Han, G.L.; Gaillardet, J.; Liu, C.Q. Sulfuric acid as an agent of carbonate weathering constrained by δ13CDIC: Examples from Southwest China. Earth Planet. Sci. Lett. 2008, 270, 180–199. [Google Scholar] [CrossRef]
- Ding, H.; Lang, Y.C.; Liu, C.Q.; Liu, T.Z. Chemical characteristics and δ34S-SO42− of acid rain: Anthropogenic sulfate deposition and its impacts on CO2 consumption in the rural karst area of southwest China. Geochem. J. 2013, 47, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.Y.; Wu, L.J.; Xin, C.L.; Yu, S.; Guo, Y.S.; Wang, J.J. Impact of anthropogenic sulfate deposition via precipitation of carbonate weathering in a typical industrial city in a karst basin of southwest China: A case study in Liuzhou. Appl. Geochem. 2019, 110, 104417. [Google Scholar] [CrossRef]
- Xie, Y.C.; Huang, F.; Yang, H.; Yu, S. Role of anthropogenic sulfuric and nitric acids in carbonate weathering and associated carbon sink budget in a karst catchment (Guohua), southwestern China. J. Hydrol. 2021, 599, 126287. [Google Scholar] [CrossRef]
- Li, D.T.; Luo, Y. Measurement of carbonate rocks distribution area in China. Carsologica Sin. 1983, 2, 147–150. [Google Scholar]
- Li, H.W.; Wang, S.J.; Bai, X.Y.; Cao, Y.; Wu, L.H. Spatiotemporal evolution of carbon sequestration of limestone weathering in China. Sci. China. Earth. Sci. 2019, 62, 974–991. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dreybrodt, W.; Li, H.J. Comparison of dissolution rate-determining mechanisms between limestone and dolomite. Earth Sci.-J. China Univ. Geosci. 2006, 31, 411–416. [Google Scholar]
- Blasco, M.; Auqué, L.F.; Gimeno, M.J.; Acero, P.; Asta, M.P. Geochemistry, geothermometry and influence of the concentration of mobile elements in the chemical characteristics of carbonate-evaporitic thermal systems. The case of the Tiermas geothermal system (Spain). Chem. Geol. 2017, 466, 696–709. [Google Scholar] [CrossRef] [Green Version]
- Kanduč, T.; Geršl, M.; Geršlová, E.; McIntosh, J. Temporal and seasonal variations of silicate Svratka River and sediment characterization, Czech Republic: Geochemical and isotopic approach. Aquat. Geochem. 2023, 29, 145–171. [Google Scholar] [CrossRef]
- Han, X.K.; Yan, Z.L.; Lang, Y.C.; Hu, D.; Guo, Q.J.; Li, S.L. Enhanced sulfide oxidation by monsoon rainfall in a small typical karstic catchment of Southwest China. J. Hydrol. 2022, 615, 128682. [Google Scholar] [CrossRef]
- Huang, Q.B.; Wu, H.Y.; Chen, R.R.; Li, T.F.; Luo, F.; Zhao, G.S.; Li, X.P. Buffering effect of lime soil on acid rain and its influence on the evaluation of the karst carbon sink effect. Acta Geosci. Sin. 2022, 43, 461–471. [Google Scholar]
- Wigley, T.M.L. WATSPEC: A computer program for determining the equilibrium speciation of aqueous solutions. Br. Geo Morphol. Res. Group Tech. Bull. 1977, 20, 1–46. [Google Scholar]
- Cao, J.H.; Zhou, L.; Yang, H.; Lu, Q.; Kang, Z.Q. Comparison of carbon transfer between forest soils in karst and clasolite areas and the karst carbon sink effect in Maocun village of Guilin. Quat. Sci. 2011, 31, 431–437. [Google Scholar]
- Meybeck, M. Pathways of major elements from land to oceans through rivers. In River Inputs to Ocean Systems; Martin, J.M., Burton, J.D., Eisma, D., Eds.; United Nations Press: New York, NY, USA, 1981; pp. 18–30. [Google Scholar]
- Zhao, M.; Liu, Z.H.; Li, H.C.; Zeng, C.; Yang, R.; Chen, B.; Yan, H. Response of dissolved inorganic carbon (DIC) and δ13CDic to changes in climate and land cover in SW China karst catchments. Geochim. Cosmochim. Acta 2015, 165, 123–136. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, G.H.; Pei, J.G.; Zhang, C. Assessment on the water qualities of major subterranean rivers in Guangxi and their changing trend. Carsologica Sin. 2002, 21, 195–201. [Google Scholar]
- Fuoco, I.; Figoli, A.; Criscuoli, A.; Brozzo, G.; De Rosa, R.; Gabriele, B.; Apollaro, C. Geochemical modeling of chromium release in natural waters and treatment by RO/NF membrane processes. Chemosphere 2020, 254, 126696. [Google Scholar] [CrossRef]
- Xie, Y.C.; Yu, S.; Miao, X.Y.; Li, J.; He, S.Y.; Sun, P.A. Chemical weathering and CO2 consumption flux in Tibetan Plateau: A case of Lhasa River Basin. Earth Sci. Front. 2023, 37, 688. [Google Scholar] [CrossRef]
- Roy, S.; Gaillardet, J.; Allègre, C.J. Geochemistry of dissolved and suspended load of the Seine river, France: Anthropogenic impact, carbonate and silicate weathering. Geochim. Cosmochim. Acta 1999, 63, 1277–1292. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Jiang, Y.J.; Yuan, D.X.; Cui, J.; Li, Y.; Yang, J.; Cao, M. Source and flux of anthropogenically enhanced dissolved inorganic carbon: A comparative study of urban and forest karst catchments in Southwest China. Sci. Total Environ. 2020, 725, 138255. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Kuo, Y.M.; Du, W.; He, S.Y.; Sun, P.A.; Yuan, Y.Q.; Li, R.; Li, Y.S. The hydrochemistry properties of precipitation in karst tourism city (Guilin), Southwest China. Environ. Earth Sci. 2015, 74, 1061–1069. [Google Scholar] [CrossRef]
- Galy, A.; France-Lanord, C. Weathering processes in the Ganges-Brahmaputra basin and the riverine alkalinity budget. Chem. Geol. 1999, 159, 31–60. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mehanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Dai, J.F.; Rong, F.Y.; Du, J.; Han, P.L.; Guan, B.L. Study on temporal and spatial variability of pan evaporation in Guangxi province. China Rural. Water Hydropower 2013, 1, 11–14. [Google Scholar]
- Grosbois, C.; Négrel, P.H.; Fouillac, C.; Grimaud, D. Chemical and isotopic characterization of the dissolved load of the Loire River. Chem. Geol. 2000, 170, 179–201. [Google Scholar] [CrossRef]
- Moquet, J.S.; Crave, A.; Viers, J.; Seyler, P.; Guyot, J.L. Chemical weathering and atmospheric/soil CO2 uptake in the Andean and Foreland Amazon basins. Chem. Geol. 2011, 287, 1–26. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, C.L.; Xie, Y.C.; Li, L.; Cao, J.H. Inorganic carbon flux and its source in the karst catchment of Maocun, Guilin, China. Environ. Earth Sci. 2015, 74, 1079–1089. [Google Scholar] [CrossRef]
- Liu, Z.H.; He, S.Y.; Yuan, D.X.; Zhao, J.B. CO2 in the soil and its function on karsts process. Hydrol. Geol. Eng. Geol. 1998, 4, 42–45. [Google Scholar]
- He, S.Y.; Pan, G.X.; Cao, J.H.; Tao, Y.X.; Teng, Y.Z. Research on characteristics of carbon cycle in epikarst ecological system. Quat. Sci. 2000, 20, 383–390. [Google Scholar]
- Yuan, D.X. Scientific innovation in karst resources and environment research field of China. Carsologica Sin. 2015, 34, 98–100. [Google Scholar]
- Yu, Z.L.; Yan, N.; Wu, G.J.; Xu, T.L.; Li, F. Chemical weathering in the upstream and midstream reaches of the Yarlung Tsangpo basin, southern Tibetan Plateau. Chem. Geol. 2021, 559, 119906. [Google Scholar] [CrossRef]
- Liu, Z.H.; Li, Q.; Sun, H.L.; Wang, J.L. Seasonal, diurnal and storm-scale hydrochemical variations of typical epikarst springs in subtropical karst areas of SW China: Soil CO2 and dilution effects. J. Hydrol. 2007, 337, 207–223. [Google Scholar] [CrossRef]
- Li, S.L.; Liu, C.Q.; Li, J.; Lang, Y.C.; Hu, D.; Li, L.B. Geochemistry of dissolved inorganic carbon and carbonate weathering in a small typical karstic catchment of Southwest China: Isotopic and chemical constraints. Chem. Geol. 2010, 277, 301–309. [Google Scholar] [CrossRef]
- Perrin, A.S.; Probst, A.; Probst, J.L. Impact of nitrogenous fertilizers on carbonate dissolution in small agricultural catchments: Implications for weathering CO2 uptake at regional and global scales. Geochim. Cosmochim. Acta 2008, 72, 3105–3123. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.J. The contribution of human activities to dissolved inorganic carbon fluxes in a karst underground river system: Evidence from major elements and δ13CDIC in Nandong, Southwest China. J. Contam. Hydrol. 2013, 152C, 1–11. [Google Scholar] [CrossRef]
- Smith, B.N. General characteristics of terrestrial plants (agronomic and forests)-C3, C4 and crustacean acid metabolism. In Basic Principles; Zaborsky, R., Ed.; CRC Handbook of Biosolar Resources: Boca Raton, FL, USA, 1982; pp. 99–118. [Google Scholar]
- Zheng, L.P. The stable carbon isotopic composition of soil CO2 in the karst, the middle parts of Guizhou province. Sci. China Ser. D Earth Sci. 1999, 29, 514–520. [Google Scholar]
- Kanduč, T.; Mori, N.; Kocman, D.; Stibilj, V.; Grassa, F. Hydrogeochemistry of Alpine springs from North Slovenia: Insights from stable isotopes. Chem. Geol. 2012, 300–301, 40–54. [Google Scholar] [CrossRef]
- Clark, I.; Fritz, P. Environmental Isotopes in Hydrogeology; Lewis Press: Boca Raton, FL, USA, 1997; pp. 111–169. [Google Scholar]
- Karim, A.; Veizer, J. Weathering processes in the Indus River Basin: Implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chem. Geol. 2000, 170, 153–177. [Google Scholar] [CrossRef]
- Deines, P. Carbon isotope effects in carbonate systems. Geochim. Cosmochim. Acta 2004, 68, 2659–2679. [Google Scholar] [CrossRef]
- Zhang, J.; Quay, P.D.; Wilbur, D.O. Carbon isotope fractionation during gas-water exchange and dissolution of CO2. Geochim. Cosmochim. Acta 1995, 59, 107–114. [Google Scholar] [CrossRef]
- Liu, J.; Han, G. Effects of chemical weathering and CO2 outgassing on δ13CDIC signals in a karst watershed. J. Hydrol. 2020, 589, 125192. [Google Scholar] [CrossRef]
- Xie, Y.C.; Zhu, T.B.; Yang, H.; Huang, F.; Zhang, C.L.; Luo, W.Q.; Huang, Q.B. Chemical weathering of carbonate rocks by sulfur acid in typical karst catchment and its implication for carbon cycle: A case study in Pingguo, Guangxi province, southwest China. Quat. Sci. 2017, 37, 1271–1282. [Google Scholar]
- Qin, C.; Li, S.L.; Yue, F.J.; Xu, S.; Ding, H. Spatiotemporal variations of dissolved inorganic carbon and controlling factors in a small karstic catchment, Southwestern China. Earth Surf. Proc. Land. 2019, 44, 2423–2436. [Google Scholar] [CrossRef]
- Wang, Z.J.; Yin, J.J.; Pu, J.B.; Wang, P.; Liang, X.; Yang, P.H.; He, Q.F.; Gou, P.F.; Yuan, D. Integrated understanding of the critical zone processes in a subtropical karst watershed (Qingmuguan, Southwestern China): Hydrochemical and isotopic constraints. Sci. Total Environ. 2020, 749, 141257. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, Z.; Zhao, M.; Yang, R. Hydrologically-driven variations in the karstrelated carbon sink fluxes: Insights from high-resolution monitoring of three karst catchments in Southwest China. J. Hydrol. 2016, 533, 74–90. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, Z.; Chen, B.; Hu, Y.; Zeng, S.; Zeng, C.; Yang, R.; He, H.; Zhu, H.; Cai, X.; et al. Carbonate weathering-related carbon sink fluxes under different land uses: A case study from the Shawan Simulation Test Site, Puding, Southwest China. Chem. Geol. 2017, 474, 58–71. [Google Scholar] [CrossRef]
- Williams, E.L.; Szramek, K.J.; Jin, L.X.; Timothy, C.W.K.; Lynn, M. The carbonate system geochemistry of shallow groundwater surface water systems in temperate glaciated watersheds (Michigan, USA): Significance of open system dolomite weathering. Geol. Soc. Am. Bull. 2007, 119, 515–528. [Google Scholar] [CrossRef]
- Szramek, K.; Walter, L.M.; Kandu, T.; Ogrinc, N. Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soca Rivers, Slovenia). Aquat. Geochem. 2011, 17, 357–396. [Google Scholar] [CrossRef]
- Lerman, A.; Wu, L.; Mackenzie, F.T. CO2 and H2SO4 consumption in weathering and material transport to the ocean, and their role in the global carbon balance. Mar. Chem. 2007, 106, 326–350. [Google Scholar] [CrossRef]
- Martin, J.B. Carbonate minerals in the global carbon cycle. Chem. Geol. 2017, 449, 58–72. [Google Scholar] [CrossRef]
- Calmels, D.; Gaillardet, J.; Brenot, A.; France-Lanord, C. Sustained sulfide oxidation by physical erosion processes in the Mackenzie River basin: Climatic perspectives. Geology 2007, 35, 1003–1006. [Google Scholar] [CrossRef]
- Ali, H.N.; Atekwana, E.A. The effect of sulfuric acid neutralization on carbonate and stable carbon isotope evolution of shallow groundwater. Chem. Geol. 2011, 284, 217–228. [Google Scholar] [CrossRef]
- Goldscheider, N.; Chen, Z.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Hartmann, J.; Jiang, G.H.; Moosdorf, N.; Stevanovic, Z.; et al. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 2020, 28, 1661–1677. [Google Scholar] [CrossRef] [Green Version]



| Location on the Map | Location | Latitude Deg N | Longitude Deg E | Date | T | pH | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | SIC | SID | TZ+ | TZ− | δ13CDIC | pCO2 | NICB | S1 | S2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day Month Year | °C | mg/L | meq/L | ‰ | ppmv | % | |||||||||||||||||
| 1 | Yaji | 25°10.685′ | 110°33.336′ | 31 August 2013 | 21.9 | 7.22 | 0.21 | 0.31 | 117 | 0.62 | 0.96 | 15.5 | 299 | 3.27 | 0.24 | −1.53 | 5.92 | 5.30 | −17.0 | 16,558 | 5.50 | 30.0 | 17.6 |
| 27 September 2013 | 20.0 | 7.05 | 0.04 | 0.32 | 112 | 0.29 | 0.89 | 14.3 | 311 | 2.67 | 0.05 | −2.25 | 5.64 | 5.47 | −16.0 | 24,946 | 1.55 | 14.4 | 7.77 | ||||
| 30 October 2013 | 19.8 | 6.97 | 0.06 | 0.32 | 107 | 0.31 | 0.96 | 14.6 | 299 | 2.65 | −0.07 | −2.43 | 5.39 | 5.27 | −16.3 | 28,840 | 1.09 | 12.9 | 6.88 | ||||
| 29 November 2013 | 19.6 | 6.88 | 0.07 | 0.30 | 112 | 0.30 | 0.86 | 14.3 | 323 | 2.49 | −0.11 | −2.56 | 5.64 | 5.66 | −16.8 | 38,194 | −0.21 | 7.32 | 3.80 | ||||
| 31 December 2013 | 19.7 | 7.04 | 0.40 | 0.31 | 124 | 0.89 | 1.15 | 14.1 | 336 | 2.63 | 0.10 | −1.71 | 6.30 | 5.87 | −15.9 | 27,290 | 3.53 | 20.0 | 11.1 | ||||
| 26 January 2014 | 19.5 | 7.00 | 0.08 | 0.24 | 118 | 1.02 | 0.91 | 14.1 | 342 | 2.45 | 0.05 | −1.73 | 6.00 | 5.96 | −16.2 | 30,549 | 0.33 | 8.70 | 4.55 | ||||
| 26 February 2014 | 19.7 | 6.99 | 0.07 | 0.23 | 130 | 0.96 | 0.87 | 13.6 | 342 | 2.20 | 0.08 | −1.74 | 6.59 | 5.94 | −16.6 | 31,189 | 5.17 | 26.5 | 15.3 | ||||
| 27 March 2014 | 19.8 | 7.10 | 0.08 | 0.19 | 114 | 0.89 | 1.03 | 15.4 | 317 | 2.16 | 0.11 | −1.65 | 5.78 | 5.58 | −16.4 | 22,542 | 1.76 | 15.2 | 8.20 | ||||
| 30 April 2014 | 19.4 | 7.13 | 0.10 | 0.20 | 115 | 0.81 | u.d. | 17.7 | 342 | 1.38 | 0.17 | −1.59 | 5.83 | 5.99 | −16.3 | 22,594 | −1.37 | 7.49 | 3.89 | ||||
| 30 May 2014 | 19.6 | 7.12 | 0.07 | 0.20 | 118 | 6.68 | 0.77 | 16.2 | 329 | 1.50 | 0.15 | −0.71 | 6.47 | 5.78 | −16.4 | 22,182 | 5.57 | 29.8 | 17.5 | ||||
| 27 June 2014 | 19.7 | 7.03 | 0.24 | 0.27 | 108 | 0.72 | 0.71 | 13.5 | 311 | 2.07 | 0.01 | −1.92 | 5.48 | 5.44 | −16.1 | 26,062 | 0.39 | 9.61 | 5.05 | ||||
| 29 July 2014 | 21.8 | 7.05 | 0.34 | 0.36 | 99 | 0.76 | 0.57 | 12.6 | 311 | 1.10 | 0.03 | −1.80 | 5.04 | 5.40 | −14.7 | 25,645 | −3.44 | 0 | 0 | ||||
| 3 September 2014 | 22.9 | 7.08 | 0.20 | 0.43 | 116 | 1.08 | 0.45 | 11.8 | 336 | 1.72 | 0.16 | −1.43 | 5.91 | 5.79 | −15.9 | 26,062 | 1.10 | 11.2 | 5.92 | ||||
| 28 September 2014 | 21.7 | 7.05 | 0.21 | 0.48 | 108 | 0.75 | 0.53 | 12.0 | 329 | 1.74 | 0.08 | −1.74 | 5.49 | 5.69 | −15.6 | 26,977 | −1.82 | 0 | 0 | ||||
| 25 October 2014 | 20.0 | 6.96 | 0.13 | 0.33 | 110 | 0.34 | 0.55 | 11.9 | 311 | 1.75 | −0.05 | −2.36 | 5.55 | 5.39 | −16.2 | 30,761 | 1.41 | 12.8 | 6.84 | ||||
| 27 November 2014 | 19.9 | 6.90 | 0.09 | 0.33 | 115 | 0.97 | 1.03 | 15.8 | 317 | 3.44 | −0.09 | −2.00 | 5.85 | 5.61 | −16.6 | 35,892 | 2.03 | 17.0 | 9.28 | ||||
| 25 December 2014 | 19.4 | 6.98 | 0.12 | 0.42 | 110 | 1.02 | 0.99 | 13.4 | 320 | 4.59 | −0.03 | −1.85 | 5.61 | 5.63 | −16.7 | 29,992 | −0.22 | 7.08 | 3.67 | ||||
| 2 | Beidiping | 25°10.963′ | 110°33.594′ | 28 August 2013 | 19.6 | 7.17 | 0.36 | 0.26 | 94 | 10.9 | 1.10 | 6.83 | 299 | 2.43 | 0.07 | −0.55 | 5.63 | 5.11 | −15.3 | 18,113 | 4.82 | 20.3 | 11.3 |
| 28 September 2013 | 19.5 | 6.93 | 0.29 | 0.30 | 82 | 6.75 | 0.82 | 6.32 | 287 | 1.70 | −0.23 | −1.31 | 4.68 | 4.88 | −15.6 | 30,479 | −2.08 | 0 | 0 | ||||
| 28 November 2013 | 19.5 | 7.14 | 0.45 | 0.46 | 100 | 12.7 | 1.20 | 6.17 | 342 | 3.37 | 0.12 | −0.42 | 6.09 | 5.82 | −16.2 | 22,080 | 2.29 | 9.69 | 5.09 | ||||
| 29 December 2013 | 19.3 | 7.07 | 0.54 | 0.32 | 112 | 13.1 | 1.18 | 6.82 | 348 | 2.32 | 0.09 | −0.51 | 6.72 | 5.91 | −15.6 | 26,182 | 6.35 | 25.1 | 14.3 | ||||
| 24 January 2014 | 19.0 | 7.22 | 0.70 | 0.32 | 112 | 13.2 | 1.13 | 6.78 | 354 | 2.18 | 0.25 | −0.21 | 6.73 | 6.01 | −15.6 | 18,793 | 5.68 | 22.5 | 12.7 | ||||
| 26 February 2014 | 19.8 | 7.01 | 0.54 | 0.35 | 132 | 14.7 | 1.28 | 6.08 | 384 | 3.69 | 0.14 | −0.43 | 7.86 | 6.52 | −15.8 | 33,113 | 9.28 | 35.2 | 21.3 | ||||
| 26 March 2014 | 19.7 | 7.26 | 0.45 | 0.22 | 118 | 12.2 | 1.13 | 7.79 | 342 | 2.00 | 0.30 | −0.14 | 6.94 | 5.83 | −15.9 | 16,672 | 8.70 | 34.1 | 20.6 | ||||
| 27 April 2014 | 19.0 | 6.97 | 0.46 | 0.22 | 100 | 11.9 | 1.00 | 7.74 | 336 | 1.90 | −0.06 | −0.83 | 6.01 | 5.72 | −16.0 | 31,915 | 2.48 | 11.8 | 6.28 | ||||
| 28 May 2014 | 19.0 | 7.10 | 0.35 | 0.20 | 95 | 16.5 | 0.74 | 7.40 | 317 | 1.58 | 0.02 | −0.50 | 6.14 | 5.40 | −16.0 | 22,284 | 6.43 | 27.2 | 15.7 | ||||
| 25 June 2014 | 19.3 | 6.97 | 0.37 | 0.21 | 100 | 11.7 | 0.68 | 6.70 | 329 | 1.68 | −0.07 | −0.83 | 6.00 | 5.59 | −15.6 | 31,405 | 3.54 | 16.3 | 8.88 | ||||
| 29 July 2014 | 19.8 | 7.13 | 0.38 | 0.38 | 94 | 12.6 | 0.83 | 6.09 | 305 | 2.05 | 0.04 | −0.55 | 5.78 | 5.18 | −15.8 | 20,277 | 5.42 | 22.4 | 12.6 | ||||
| 2 September 2014 | 19.8 | 7.05 | 0.41 | 0.46 | 120 | 13.2 | 0.72 | 5.50 | 348 | 2.24 | 0.11 | −0.50 | 7.13 | 5.87 | −15.8 | 27,542 | 9.67 | 37.0 | 22.7 | ||||
| 25 September 2014 | 19.7 | 6.99 | 0.64 | 0.60 | 108 | 13.1 | 0.94 | 4.88 | 366 | 2.83 | 0.03 | −0.62 | 6.53 | 6.17 | −16.1 | 33,420 | 2.82 | 11.1 | 5.89 | ||||
| 23 October 2014 | 19.8 | 7.01 | 0.60 | 0.45 | 108 | 11.8 | 0.83 | 5.18 | 366 | 2.57 | 0.05 | −0.62 | 6.42 | 6.17 | −16.0 | 31,915 | 1.93 | 8.35 | 4.36 | ||||
| 26 November 2014 | 19.6 | 6.99 | 0.70 | 0.60 | 112 | 17.7 | 1.54 | 8.88 | 378 | 6.65 | 0.05 | −0.47 | 7.12 | 6.54 | −16.1 | 34,277 | 4.29 | 19.1 | 10.6 | ||||
| 24 December 2014 | 19.4 | 7.14 | 0.66 | 0.61 | 104 | 16.8 | 1.53 | 7.28 | 384 | 4.53 | 0.17 | −0.21 | 6.65 | 6.57 | −15.9 | 24,604 | 0.60 | 2.61 | 1.32 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Hao, Y.; Li, J.; Guo, Y.; Xiao, Q.; Huang, F. Influence of Anthropogenic Sulfuric Acid on Different Lithological Carbonate Weathering and the Related Carbon Sink Budget: Examples from Southwest China. Water 2023, 15, 2933. https://doi.org/10.3390/w15162933
Xie Y, Hao Y, Li J, Guo Y, Xiao Q, Huang F. Influence of Anthropogenic Sulfuric Acid on Different Lithological Carbonate Weathering and the Related Carbon Sink Budget: Examples from Southwest China. Water. 2023; 15(16):2933. https://doi.org/10.3390/w15162933
Chicago/Turabian StyleXie, Yincai, Yupei Hao, Jun Li, Yongli Guo, Qiong Xiao, and Fen Huang. 2023. "Influence of Anthropogenic Sulfuric Acid on Different Lithological Carbonate Weathering and the Related Carbon Sink Budget: Examples from Southwest China" Water 15, no. 16: 2933. https://doi.org/10.3390/w15162933





