Abstract
Accurate estimate of carbonate weathering and the related carbon sink flux induced by anthropogenic H2SO4 is of great significance for improving understanding of the hydrogeochemical evolution and the global carbon cycle. Here, to quantitatively evaluate the influence of anthropogenic H2SO4 on different lithological carbonate weathering and the related carbon sink budget, karst spring water in the typical limestone and mixed limestone–dolomite catchments in Yaji and Beidiping affected by acid precipitation in southwest China were sampled monthly for the analysis of hydrochemical and δ13CDIC characteristics. Results show for the period of sampling (August 2013 to December 2014) that the average contribution rates of atmospheric inputs and carbonate weathering to total dissolved cations are 2.24% and 97.8%, and 3.09% and 96.9% in Yaji and Beidiping, respectively. The δ13CDIC values (−17.0% to −14.7‰) and the [Ca2+ + Mg2+]/[HCO3−] (0.98 to 1.25) and [Ca2+ + Mg2+]/[HCO3− + SO42−] (approximately 1) equivalent ratios of samples prove that H2CO3 and H2SO4 simultaneously participate in carbonate weathering. The contribution rates of H2SO4 to [Ca2+ + Mg2+] and [HCO3−] produced by carbonate weathering in Yaji and Beidiping are 0–30% and 0–18%, and 0–37% and 0–23%, with average values of 14% and 7%, and 19% and 11%, respectively, suggesting that the influence of H2SO4 on different lithological carbonate weathering is different. H2SO4 precipitation participating in carbonate weathering increases the weathering rate by 14–19%, whereas it decreases the flux of karst carbon sink by 7–11% in Southwest China. Therefore, anthropogenic acids have influenced the global carbon cycle and climate change by carbonate weathering due to the large karst areas in the world, and their influences on different lithological carbonate weathering should not be ignored in the regional and global carbon cycles in future studies.
1. Introduction
Rock weathering affects the atmospheric CO2 balance by consuming CO2 in the atmosphere or soil and plays an important role in the global carbon cycle and climate change [1,2,3]. However, the carbon sink from rock weathering was considered a long-term (104–106 years) geological process in previous global carbon cycle models, and it was believed that the carbon cycle driven by lithosphere-related carbonate weathering did not actively participate in the modern carbon cycle, making no or negligible contributions to current atmospheric CO2 sources and sinks [4,5]. With the deepening of research, the karst carbon sink effect has gradually been recognized. On a short time scale, due to the kinetics of the rapid dissolution [6,7] and high solubility [6,8,9] of carbonate, the carbon sink generated by carbonate weathering can reach up to about 0.6 Pg C/a [2,3,10,11], accounting for 18–50% of the total “residual land sink” (1.2–3.4 Pg C/a) [3,12,13]. In addition, new evidence shows that carbonate weathering based on the H2O–carbonate–CO2–aquatic phototroph interaction can form endogenous organic carbon burial through the utilization of DIC by photosynthetic organisms in the terrestrial aquatic ecosystem (biological carbon pumping), which may make the carbon sink generated by carbonate weathering important in the control of climate change at any time scale [2,3,8]. Therefore, the karst carbon sink by carbonate weathering is a key part of the global carbon budget at different time scales and thus plays an important role in climate change.
In general, soil CO2 dissolved in water can produce carbonic acid and thus dissolves carbonate rocks in the natural state following Equation (1):
CaxMg1−xCO3 + H2O + CO2 → xCa2+ + (1 − x) Mg2+ + 2HCO3− (0 ≤ x ≤ 1).
In this case, half of the HCO3− in water comes from carbonate rocks, and the other half is from soil CO2. This process saves soil CO2 in the form of HCO3− in water, leading to a reduction in the amount of CO2 released from the soil into the atmosphere, thereby realizing the carbon sink effect. However, in recent years, with the strengthening of human activities, in addition to carbonic acid, anthropogenic sulfuric acid has been widely involved in the geochemical cycle of carbonate rocks. The participation of sulfuric acid in carbonate weathering also increases HCO3− in water following Equation (2):
2CaxMg1−xCO3 + H2SO4 → 2 × Ca2+ + 2(1 − x)Mg2+ + 2HCO3− +SO42− (0 ≤ x ≤ 1).
But the HCO3− all comes from carbonate rocks. Considering that the residence time of SO42− (8.7 Ma) is two orders of magnitude higher than that of HCO3− (0.083 Ma) in seawater [14], sulfuric acid weathering of carbonate rocks is essentially a net release process of atmospheric CO2. Therefore, a systematic study on the role of sulfuric acid in carbonate weathering is crucial for an accurate estimate of the budgets of karst carbon sources and sinks [14].
China is one of the world’s three major acid precipitation areas after Europe and North America [15,16], and Southwest China is not only the world’s largest continuous karst area, but also one of the most important sulfuric acid precipitation areas due to the increase in energy demand, coal combustion and air pollution emissions [17,18]. In the past decade, the frequency of acid precipitation in Southern China has decreased [19], but the incidence of acid precipitation still reaches 58–66% [20,21]; thus, acid precipitation remains at a relatively severe level. In this context, Southwest China is an ideal area to study the influence of anthropogenic sulfuric acid on the weathering mechanism of carbonate rocks and the associated karst carbon sink and source effects. In recent years, more and more attention has been paid to the role of atmospheric acid precipitation (mainly sulfuric acid) in weathering of carbonate rocks. At the karst catchment scale, atmospheric acid precipitation has reduced the karst carbon sink by 4.5–19.3% [22,23,24,25], significantly changing the karst carbon cycle. Many studies contribute to clarifying the spatial distribution and importance of anthropogenic sulfuric acid in carbonate weathering. However, the decreased proportion of karst carbon sink caused by anthropogenic sulfuric acid participating in carbonate weathering still has great uncertainty, which seriously restricts the accurate assessment of the karst carbon sink effect. As we know, carbonate rocks are composed of limestone, dolomite and a series of transitional types of mixed limestone and dolomite, and the distribution of dolomite is as common as that of limestone [26,27]; thus, dolomite is an important component of carbonate rocks. Under natural conditions, the dissolution rate of dolomite is only 1/3–1/60 of that of limestone [28,29], but its solubility is usually 20–30% higher than that of limestone [6,29]. Nonetheless, the current research mainly focuses on the limestone catchment, while the related studies in other lithological carbonate catchments are relatively scarce [30]; thus, anthropogenic sulfuric acid weathering characteristics of different lithological carbonate rocks on different time scales are not yet clear. Therefore, simply using limestone or dolomite to completely replace carbonate rocks may lead to significant uncertainty in the assessment of the influence of anthropogenic sulfuric acid on the karst carbon sink effect at regional or global scales. Therefore, it is necessary to have a more comprehensive and in-depth understanding of the impact of anthropogenic sulfuric acid on the weathering mechanisms of carbonate rocks and the associated karst carbon sink effect from the perspective of carbonate lithology so as to provide theoretical reference for the study of the global carbon cycle and climate change. In addition, the current research data on the involvement of anthropogenic sulfuric acid in carbonate weathering is mostly based on water samples collected in the rainy and/or dry seasons, with only a few studies utilizing high-frequency sampling data (e.g., monthly sampling strategy) [23,31] to study the seasonal variation pattern of the contribution of anthropogenic sulfuric acid to carbonate weathering. Such studies can reduce the uncertainty of the assessment of karst carbon sink fluxes generated by the sulfuric acid weathering of carbonate rocks and are required for a better understanding of the biogeochemistry cycle of sulfur and carbon in karst systems and its feedback to modern global climate change.
In this study, spring water samples were collected monthly for one and a half years for analyses of the hydrochemical and carbon isotopic characteristics to compare the processes of carbonate weathering by carbonic acid and sulfuric acid generated by anthropogenic activities in two small different lithological karst catchments (limestone and mixed limestone–dolomite). The main aims of the study are to (1) compare the influence on CO2 consumption flux from carbonate weathering by anthropogenic sulfuric acid in limestone catchment with that in mixed limestone–dolomite catchment; (2) quantitatively evaluate the impact of anthropogenic sulfuric acid on carbonate weathering and the associated carbon sink budget.
2. Material and Methods
2.1. Study Area and Sampling Sites
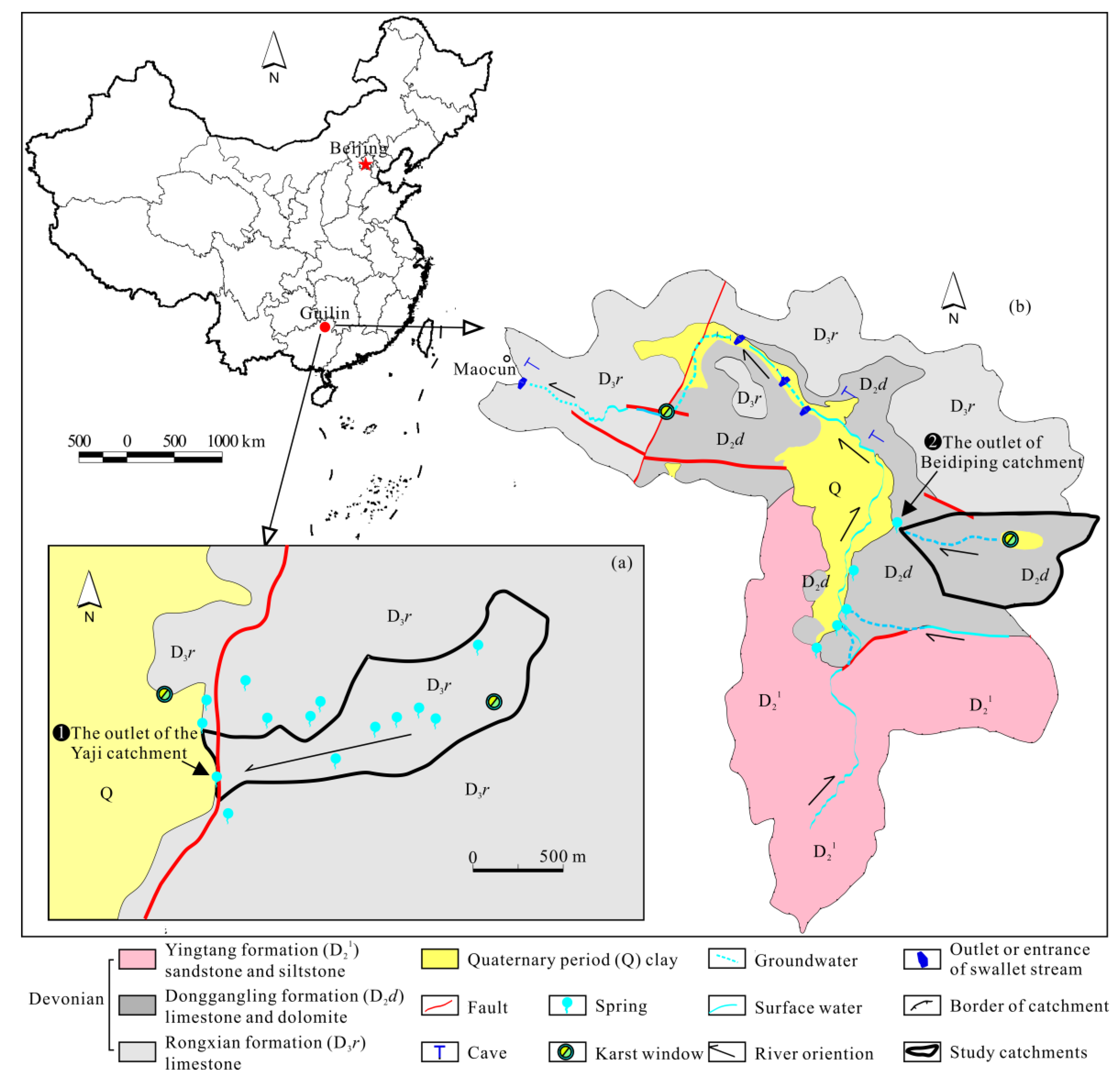
The studied region is located in the Guilin area in northern Guangxi Province, Southwest China (Figure 1). It has a subtropical monsoon climate, with the rainy season from April to August and the dry season from September to March of the following year. The mean annual air temperature, humidity and precipitation are 19.3 °C, 78% and 1900 mm, respectively; about 70% of rainfall occurs during the rainy season [32]. The region is undergoing severe acid precipitation pollution (mainly sulfuric acid) [21,32]. There are no mining activities and no evaporite rocks in the studied area (Figure 1). The two small typical karst catchments selected (Yaji and Beidiping), approximately 20 km apart, have similar drainage areas, vegetation and degree of anthropogenic influences and the same climate but different geological settings (Figure 1).
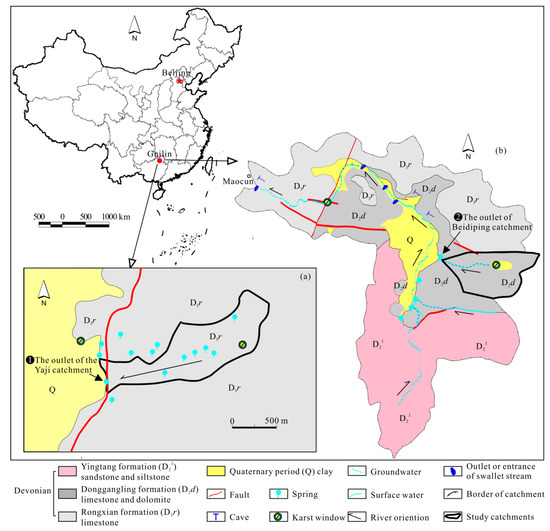
Figure 1.
Hydrogeological diagram of the Yaji (a) and Beidiping (b) karst catchments.
Yaiji karst spring catchment is located near Yaji Village, about 8 km southeast of Guilin City. It is a typical karst landscape, and the topography is dominated by karst peak-cluster depressions. The catchment encompasses an area of 1 km2. The lithology is the Devonian Rongxian Formation (D3r) pure limestone, which forms the karst aquifer (Figure 1a). The catchment is covered entirely by woodland and shrubland.
Beidiping karst spring catchment is located in Maocun Village, about 30 km southeast of Guilin City. The catchment is a typical karst landscape, and the topography is dominated by karst peak-cluster depressions. It covers an area of 0.8 km2. The lithology is the Middle Devonian Donggangling formation (D2d) limestone and dolomite, which forms the karst aquifer (Figure 1b). The vegetation type is woodland and shrubland, with a few scattered fruit trees in depressions.
2.2. Sampling and Analysis
The spring water in the two catchment outlets was sampled once per month from August 2013 to December 2014. The locations of the sampling springs are shown in Figure 1.
Water pH and temperature (T) were measured in situ by a hand-held multi-parameter analyzer (Multi3430, WTW, Oberbayern, Germany), with a resolution of 0.01 pH and 0.1 °C, respectively. The analyzer was calibrated for deployment using standard solutions with pH values (4, 7 and 10) before use. The concentrations of Ca2+ and HCO3− were measured in situ using portable testing kits (Merck Company, Darmstadt, Germany) in the field, with precisions of 1 and 6 mg/L, respectively. All samples were immediately filtered through cellulose acetate filter membranes (pore size, 0.45 μm) and kept in pre-cleaned polyethylene bottles of different sizes. One 600 mL polyethylene bottle of sample for cation analysis was acidified with ultrapurified HNO3 (1:1) to pH below 2, and the other one was untreated for anion analysis. Each sample for δ13CDIC was stored in 30 mL polyethylene bottle with air-tight cap, and 2–3 drops of saturated HgCl2 was added to prevent microbial activity. All samples were stored in a refrigerator at 4 °C until analysis.
The concentrations of K+, Na+ and Mg2+ were determined by an ICP-OES (IRIS Intrepid II XSP, Thermo Fisher Scientific, Waltham, MA, USA), and anions (Cl−, SO42− and NO3−) were measured by an ion chromatograph (861 Advanced Compact IC Metrohm, Switzerland). The uncertainties of all analyses were within ±5%. The carbon isotopic composition of DIC (δ13CDIC) was analyzed using a GasBench II interfaced with a Finnigan MAT-253 mass spectrometer (Thermo Fisher Scientific, USA) with a precision better than ±0.1‰, based on replicate measurements of an internal laboratory standard. Results are reported by the delta (δ) notation relative to the international Vienna Peedee Belemnite (VPDB) using per mil (‰), where
δ13C (‰) = (Rsample/RPDB − 1) × 1000
The normalized inorganic charge balance (NICB) value of samples was calculated using the following formula:
where TZ+ = Na+ + K+ + 2Mg2+ + 2Ca2+ and TZ− = Cl− + 2SO42− + HNO3− + NO3− in meq/L.
NICB = (TZ+ − TZ−)/(TZ+ + TZ−) × 100%
Evapotranspiration factor (Ef) was calculated using the following formula:
where P represents precipitation (mm) and E represents evaporation (mm).
Ef = P/(P − E)
2.3. pCO2 and Calcite and Dolomite Saturation Indexes
The water temperature, pH and concentrations of Ca2+, Mg2+, K+, Na+, Cl−, HCO3− and SO42− were processed with the program WATSPEC [33] to calculate the partial pressure value of CO2 (pCO2) and the saturation indexes of calcite (SIC) and dolomite (SID) in spring water. pCO2 is assumed to be in equilibrium with the sampled spring waters and was calculated using the following equation:
where K1 and Kh are the temperature-dependent dissociation constants between DIC species.
pCO2 = ([HCO3−][H+])/(K1Kh)
SIC and SID were calculated using the following equations:
where Kc and Kd are the equilibrium constants of calcite dissolution and dolomite dissolution, respectively.
SIC = log ([Ca2+][CO32−]/Kc)
SID = log (([Ca2+][Mg2+][CO32−])/Kd)
2.4. Calculation of the Effect of Anthropogenic H2SO4 on Carbonate Weathering and Carbon Sink
According to Equations (1) and (2), d1 (mmol/L) carbonic acid and d2 (mmol/L) sulfuric acid together participate in carbonate weathering and thus can be written as:
(d1 + 2d2)CaxMg1−xCO3+d2H2SO4 + d1H2CO3 → (d1 + 2d2) × Ca2+ + (d1+2d2)(1 − x)Mg2++2(d1 + d2)HCO3− +d2SO42− (0 ≤ x ≤ 1)
Therefore, the concentrations of Ca2+, Mg2+ and HCO3− (in mmol/L) produced by carbonate weathering can be expressed as:
a(Ca2++Mg2+)carbonate weathering = d1+2d2
a(HCO3−)carbonate weathering = 2d1+2d2
The value of d1 can be calculated by the following formula:
d1 = Equation (11) − Equation (10) = a(HCO3−)carbonate weathering − a(Ca2++Mg2+)carbonate weathering
The contribution rate of carbonate weathering by H2CO3 to [Ca2+ + Mg2+]carbonate weathering (k1) and [HCO3−]carbonate weathering (k2) in spring water can thus be quantified by the following formulas:
k1 = d1/a(Ca2+ + Mg2+)carbonate weathering
k2 = 2d1/a(HCO3−)carbonate weathering
The contribution rate of H2SO4 weathering of carbonate rocks to a(Ca2++Mg2+)carbonate weathering (S1) and a(HCO3−)carbonate weathering (S2) in spring water can thus be quantified by the following formulas:
S1 = 1 − d1/a(Ca2+ + Mg2+)carbonate weathering
S2 = 1 − 2d1/a(HCO3−)carbonate weathering
3. Results
The monthly major ion (Ca2+, Mg2+, K+, Na+, Cl−, SO42−, NO3−) concentrations and δ13CDIC of samples in Yaji and Beidiping are shown in Table 1. The pH values of spring water in Yaji and Beidiping ranged from 6.88 to 7.22 and from 6.93 to 7.26, with average values of 7.03 and 7.07, respectively. Thus, the pH values of the two catchments are similar and all close to or higher than 7, showing the importance of carbonate weathering. The temperatures of karst spring water in Yaji and Beidiping were 19.4–22.9 °C and 19.0–19.8 °C, respectively. Thus, the seasonal dynamic variations of water temperatures in the two catchments were both small, which were similar to the annual average air temperature of 19.6–20.4 °C in the Guilin area [34], reflecting that the temperature of karst spring water was mainly controlled by the local mean temperature. The normalized inorganic charge balance (NICB) values of all samples were within 10%, indicating that the contribution of organic ligands to the charge balance can be negligible, and the measured results were reliable [1,25]. The total cationic charges (TZ+) of samples in Yaji and Beidiping varied from 5.04 to 6.59 meq/L and from 4.68 to 7.86 meq/L, with average values of 5.79 and 6.40 meq/L, respectively, which were 4.6 times and 5.1 times higher than the average TZ+ value of the rivers in the world (1.25 meq/L) [35], respectively, reflecting strong chemical weathering. It is worth noting that the average TZ+ values of Yaji and Beidiping karst water were both significantly higher than that of the Banzhai karst underground river in Guizhou (TZ+ = 5.13 meq/L) [36] and that of the main underground rivers in Guangxi (TZ+ = 4.05 meq/L) [37] without sulfuric acid participating in carbonate weathering, while similar to that of karst water in the Pingguo area, Guangxi, where sulfuric acid was involved in carbonate weathering (TZ+ = 6.01 meq/L) [25]. This suggests that sulfuric acid may be involved in the weathering processes of carbonate rocks in Yaji and Beidiping.

Table 1.
Major ion chemical concentrations and δ13CDIC of spring water in Yaji and Beidiping karst catchments.
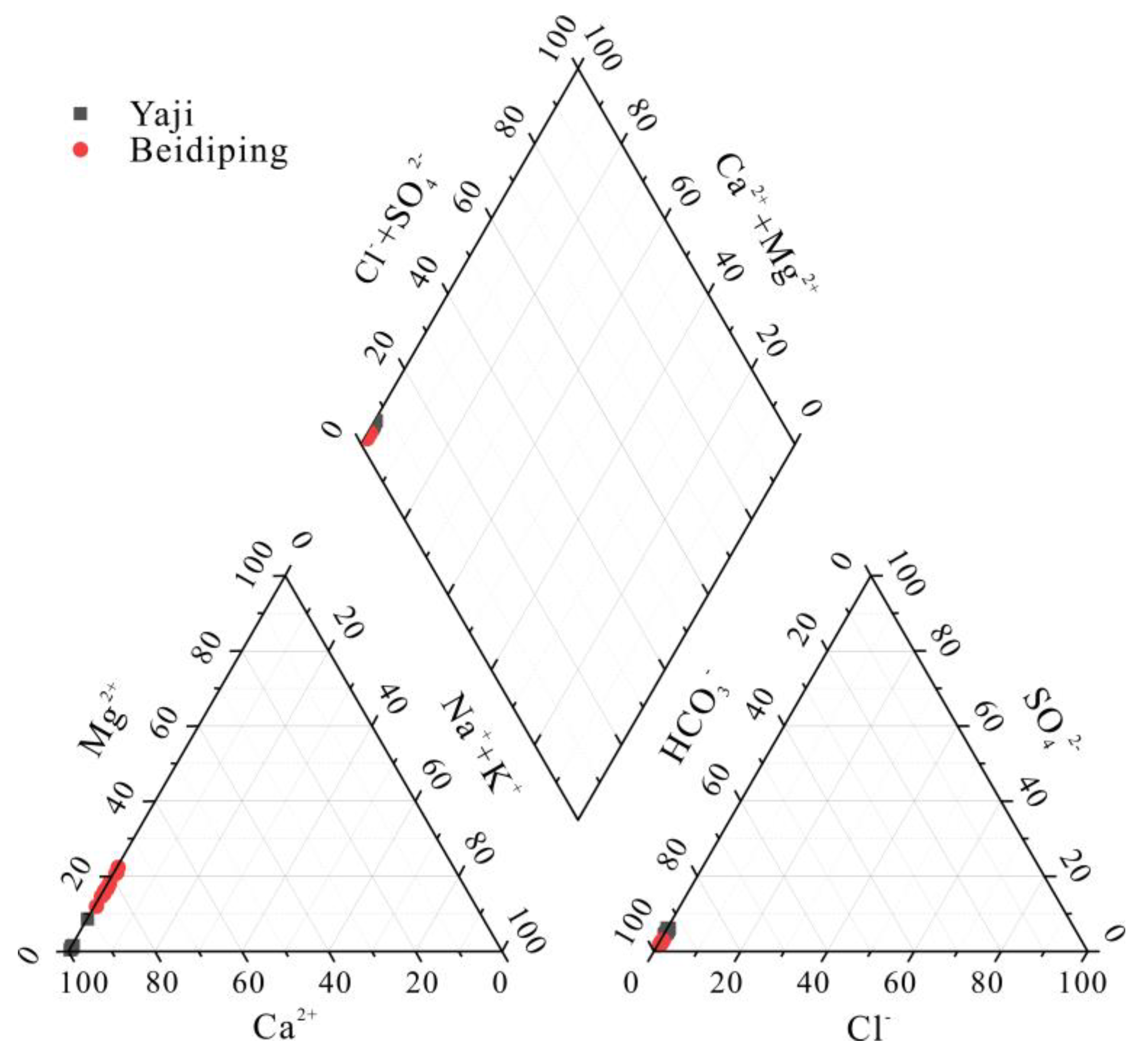
The cationic composition of spring water was mainly Ca2+, accounting for 91.2–99.3% (mean: 98.2%) and 77.3–87.6% (mean: 82.6%) of the total cationic equivalent concentration in Yaji and Beidiping, respectively (Figure 2). Mg2+ was the second most abundant cation in spring water, accounting for 4.24–6.15% (mean: 5.24%) and 6.75–17.7% (mean: 13.1%) of the total cationic equivalent concentration in Yaji and Beidiping, respectively (Figure 2). HCO3− was the main anion in spring water, accounting for 92.4–95.1% (mean: 93.7%) and 94.9–97.2% (mean: 96.4%) of the total anionic equivalent concentration in Yaji and Beidiping, respectively (Figure 2). The contents of other anions and cations were all very low (Figure 2). The hydrochemical composition in Yaji and Beidiping reflected that the typical hydrochemical characteristics were controlled by carbonate weathering [38].
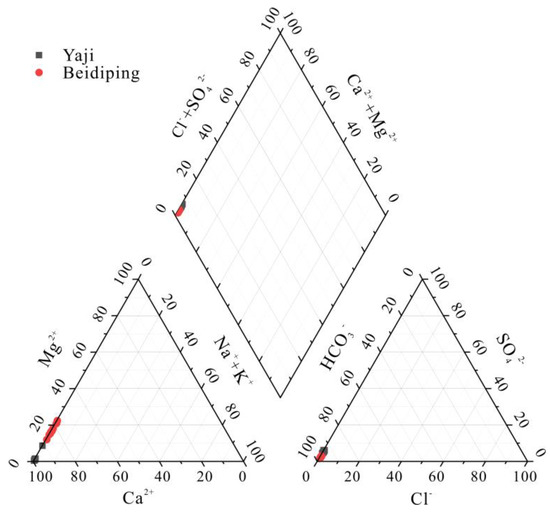
Figure 2.
Piper diagram showing the relative proportions of major ions in the Yaji and Beidiping karst catchments.
The δ13CDIC values of samples in Yaji and Beidiping varied from −17.0 to −14.7‰ and from −16.1 to −15.3‰, with average values of −16.2‰ and −15.8‰, respectively.
4. Discussion
4.1. Sources of Dissolved Load
In general, dissolved loads in water systems mainly come from the weathering of minerals, atmospheric deposition and anthropogenic inputs [1,25,35,39]. All samples in Yaji and Beidiping plotted in the range of rock weathering (Figure 3), indicating that the hydrochemical composition of spring water was mainly controlled by rock weathering. The aquifers in the Yaji and Beidiping catchments are all composed of carbonate rocks; thus, carbonate weathering controlled the hydrochemical composition of spring water. Generally, the input pathways of human activities to river solutes mainly include atmospheric acid deposition, domestic sewage and waste discharge from agricultural and industrial activities [1,40,41]. However, there are no industrial activities in the Yaji and Beidiping catchments; thus, industrial waste discharge can be ruled out. In addition, on the one hand, there are no human habitation and farmland distributions in the Yaji catchment, which excludes the inputs of agricultural activities and domestic sewage. There is only a small amount of farmland and human habitation in the Beidiping catchment; thus, the impacts of agricultural activities and the inputs of domestic sewage on the spring water chemistry were relatively very small and thus can be ignored, and on the other hand, the studied area is located in the area with severe acid rain pollution [21,32,42]. Therefore, human activities mainly affect the dissolved loads in the spring water through atmospheric acid deposition [15,25]. Thus, the dissolved ions in spring water in Yaji and Beidiping mainly come from atmospheric inputs and carbonate weathering.
The straightforward method [43] was employed to calculate the contributions of carbonate weathering and atmospheric inputs to the chemical composition of the spring water. For any element X, the following mass budget equation in spring water can be assumed as:
where [X] stands for concentration of ions in mmol/L, atm refers to atmospheric inputs, carb refers to carbonate weathering.
[X]spring = [X]atm + [X]carb
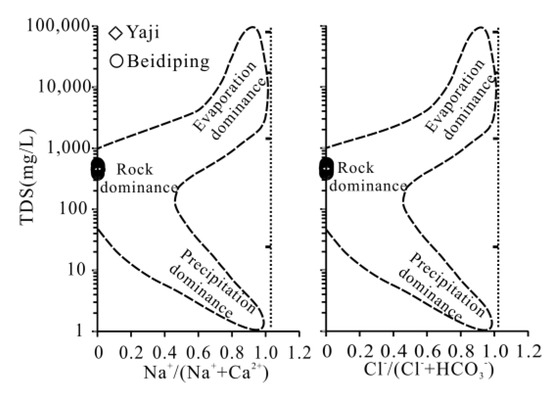
Figure 3.
Gibbs chart of spring water in the Yaji and Beidiping karst catchments. The figure is adapted from [44]. Total dissolved solid (TDS) was calculated using equation: TDS = Ca2+ + Mg2+ + Na+ + K+ + Cl− + SO42− + HCO3− + NO3− (in mg/L).
Figure 3.
Gibbs chart of spring water in the Yaji and Beidiping karst catchments. The figure is adapted from [44]. Total dissolved solid (TDS) was calculated using equation: TDS = Ca2+ + Mg2+ + Na+ + K+ + Cl− + SO42− + HCO3− + NO3− (in mg/L).
Chloride is the most commonly used reference element to calculate the atmospheric inputs to the hydrogeochemical composition [1]. The annual average precipitation (P) and evaporation (E) of the studied area are 1900 mm [21] and 1255 mm [45], respectively, according to Equation (5), the evapotranspiration factor (Ef) is 2.9. Yu [42] and Zhu [21] reported that the Cl− concentration of rainwater in the studied area ranged from 2.34 to 224 μmol/L. According to the formula:
where [X]rain stands for the concentration of element X in rainwater.
[X]atm = (Ef) × [X]rain = 2.9 × [X]rain
Thus, the Cl− concentration from atmospheric inputs in spring water was calculated to be 6.79–650 μmol/L. In the studied catchments, the Cl− concentrations of spring water ranged from 12.6 to 43.4 μmol/L; the values were all within the range of that from atmospheric inputs. Additionally, in the two catchments, there are no evaporite rocks, and the foregoing discussion shows that human activities mainly affect the dissolved loads in the spring water through atmospheric acid deposition. Thus, the Cl− in the spring water was only derived from atmospheric inputs [46,47]. Similar to Cl−, the K+ (1.11–18.1 μmol/L) and Na+ (8.1–26.5 μmol/L) contents in the spring water were within the ranges of K+ (3.42–99.6 μmol/L) and Na+ (1.89–329 μmol/L) contents from atmospheric inputs [21,42], respectively. Therefore, all K+ and Na+ in the spring water came from atmospheric inputs. Thus, Equation (17) can be simplified as follows:
[Cl−]spring = [Cl−]atm
[Na+]spring = [Na+]atm
[K+]spring = [K+]atm
[Ca2+]spring = [Ca2+]atm + [Ca2+]carb
[Mg2+]spring = [Mg2+]atm + [Mg2+]carb
The Ca2+ and Mg2+ from atmospheric inputs in spring water were calculated by using the average molar ratios of precipitation in the Guilin area (Ca2+/Cl− = 2.21, Mg2+/Cl− = 0.34) [21]. Thus, [Ca2+]atm and [Mg2+]atm can be calculated as follows:
[Ca2+]atm = (Ca2+/Cl−)× [Cl−]atm=2.21 × [Cl−]atm
[Mg2+]atm = (Mg2+/Cl−) × [Cl−]atm =0.34 × [Cl−]atm
After the atmospheric input correction, the rest of the Ca2+ and Mg2+ came from carbonate weathering. Thus, [Ca2+]carb and [Mg2+]carb can be simplified as follows:
[Ca2+]carb = [Ca2+]spring − [Ca2+]atm = [Ca2+]spring − 2.21 × [Cl−]atm
[Mg2+]carb = [Mg2+]spring − [Mg2+]atm = [Mg2+]spring − 0.34 × [Cl−]atm
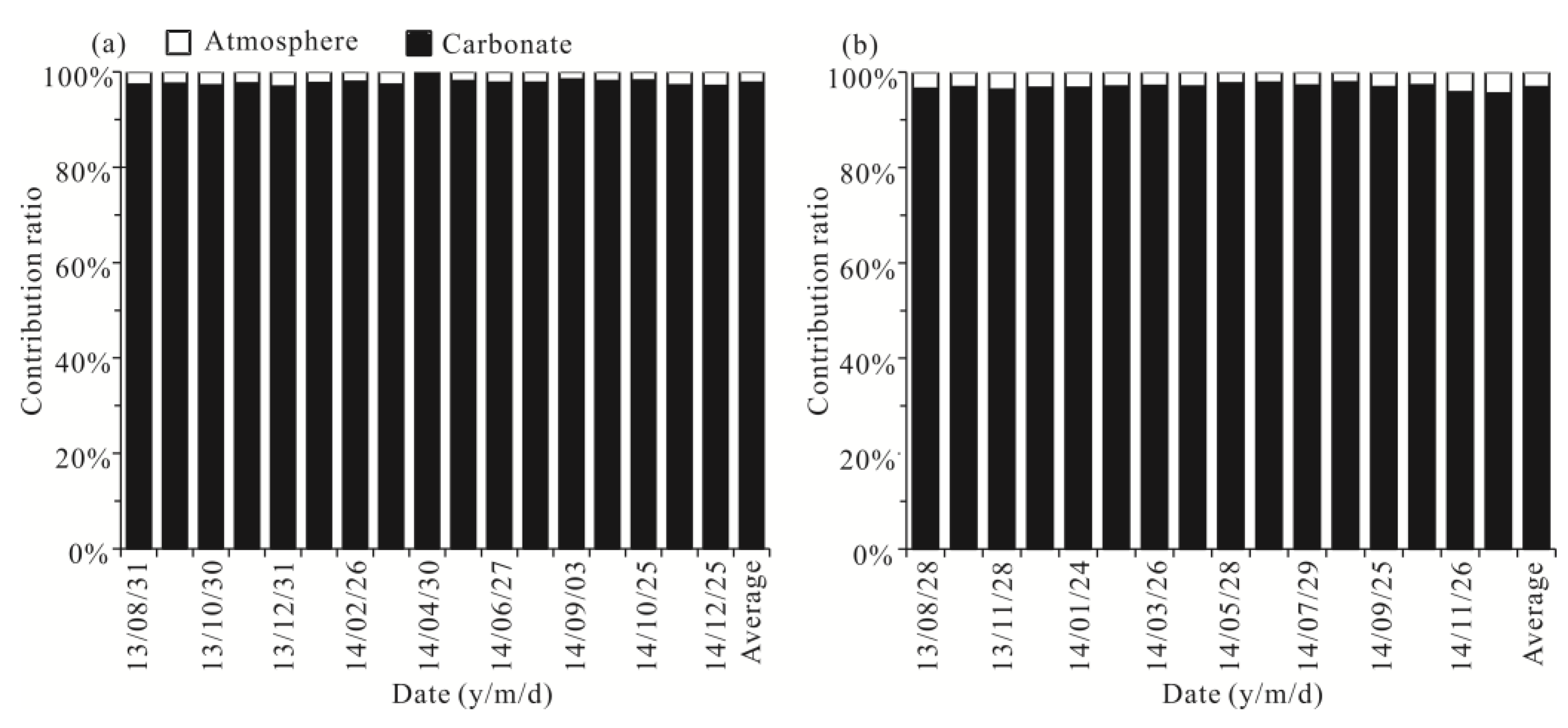
Therefore, the contributions of atmospheric inputs and carbonate weathering to the dissolved cationic TDS (mg/L) in spring water can be calculated (Figure 4). The contributions of cations from atmospheric inputs and carbonate weathering in Yaji and Beidiping varied from 0.26 to 3.05% (mean: 2.24%) and from 97.0 to 99.7% (mean: 97.8%) and from 2.12 to 4.45% (mean: 3.09%) and from 94.5 to 97.9% (mean: 96.9%), respectively. The results fully proved that the dissolved loads of the karst spring water were controlled by carbonate weathering, which is consistent with the results of the Gibbs diagram (Figure 3) and the fact that aquifer lithology is composed of carbonate rocks in the two catchments.
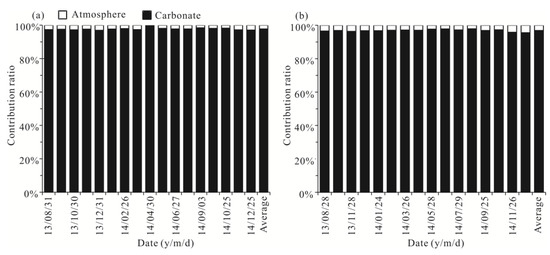
Figure 4.
The contributions of different sources (atmosphere, carbonate) to the total dissolved cations of the samples in the Yaji (a) and Beidiping (b) karst catchments.
4.2. Anthropogenic Source of H2SO4
Generally, SO42− in karst water is derived from atmospheric deposition, anthropogenic inputs, sulfide oxidation and dissolution of evaporites [22,25,31,41]. However, sulfate and sulfide minerals were not found in our study catchment, which is consistent with the reports of previous research in the study area [32,48]. The influences of sulfide oxidation and dissolution of evaporites on the chemical composition of spring water, thus, can be ruled out. According to the discussion above, the impacts of anthropogenic inputs, such as waste discharge from agricultural and industrial activities and domestic sewage inputs to the spring water, can be neglected. According to a previous study [14], sulfuric acid in precipitation mainly comes from the reaction between free radicals (OH, H2O2) and SO2 from automobile exhaust and coal combustion in the atmosphere. In the studied area, the SO42− of precipitation was identified as the most important acidogenic anion and varied from 1.64 to 90.0 mg/L [21,42]. The SO42− in spring water in the studied catchments varied from 4.88 to 17.7 mg/L (Table 1), which were all within that range. Therefore, H2SO4 in spring water mainly comes from atmospheric acid precipitation. This is consistent with the fact that the study area was significantly affected by sulfuric acid precipitation [21,32,42]. Still, δ34SSO4 and δ18OSO4 will help to improve interpretations about the sources of SO42−, and it will be useful to study additional isotopic tracers (δ34SSO4 and δ18OSO4) in the future.
4.3. Hydrochemical Evidence of Sulfuric Acid Participation in Carbonate Weathering
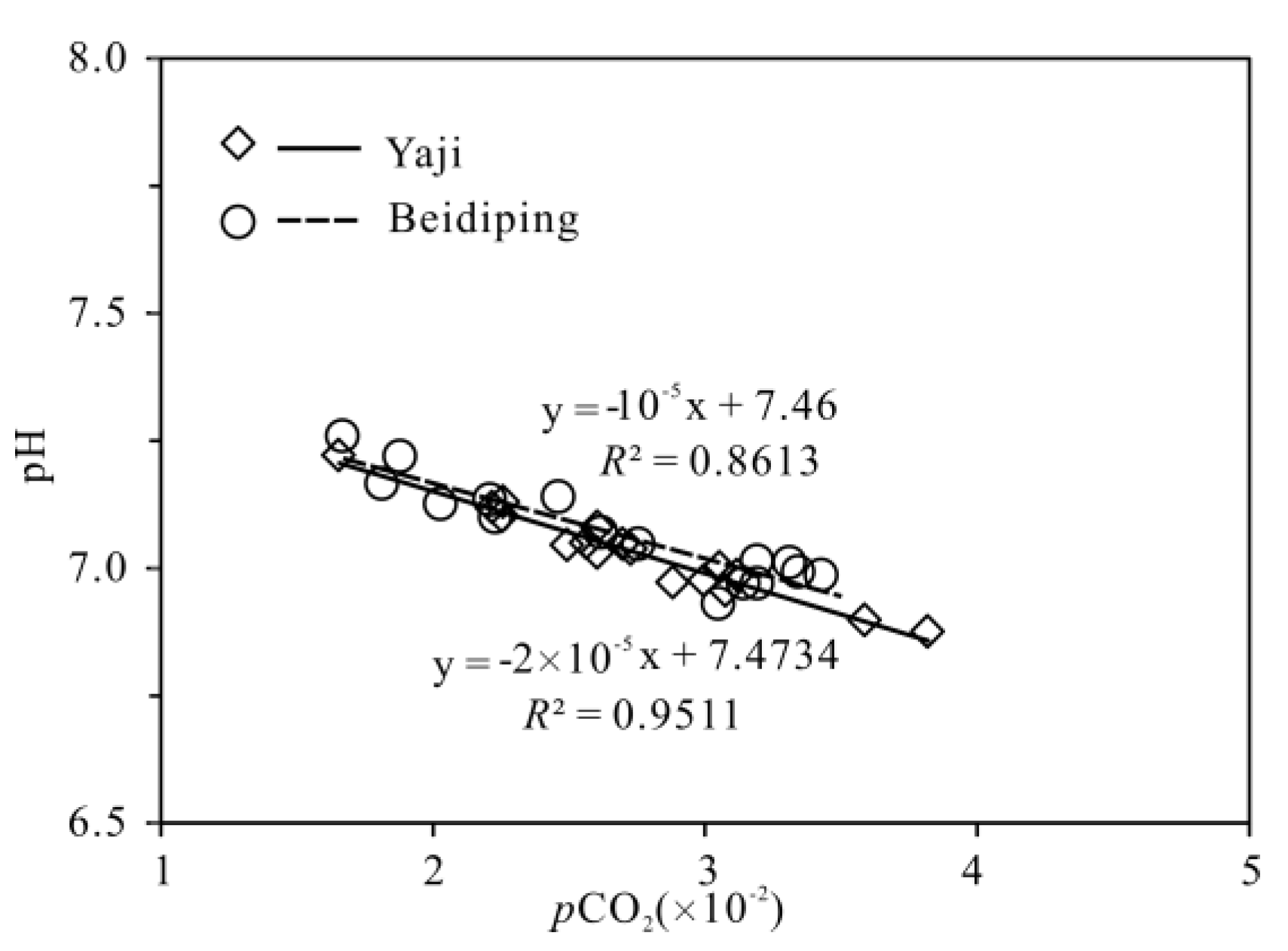
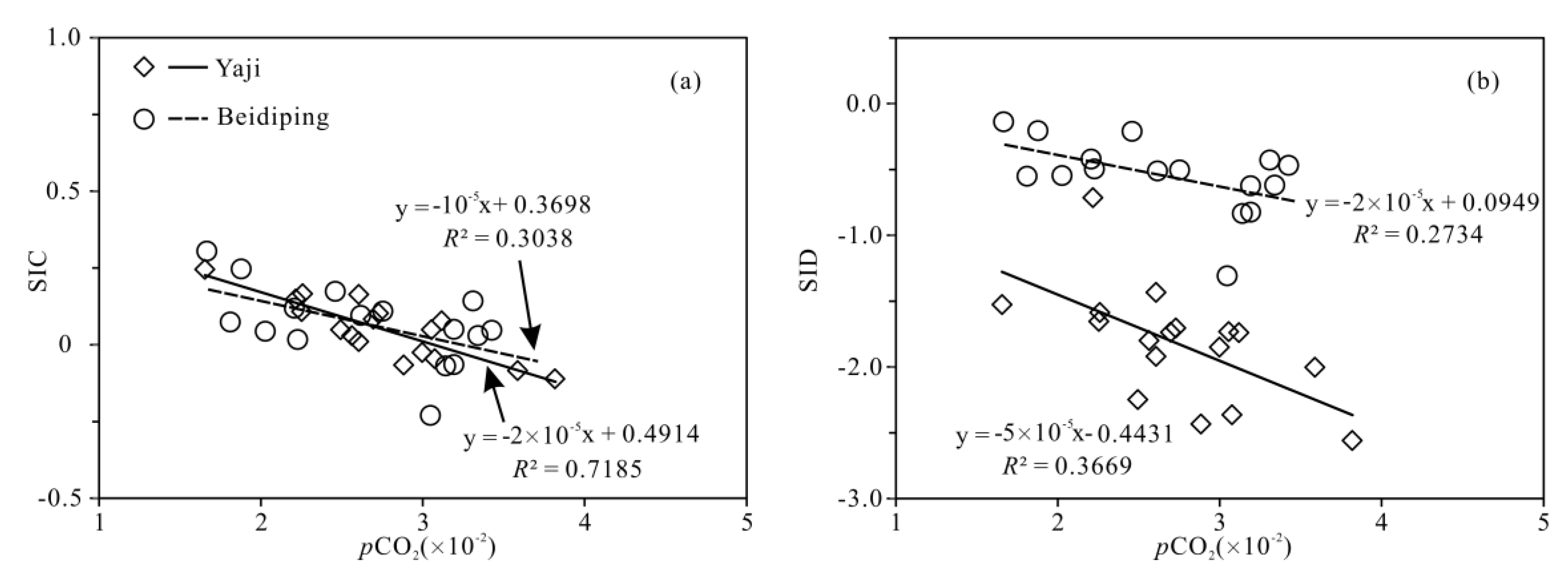
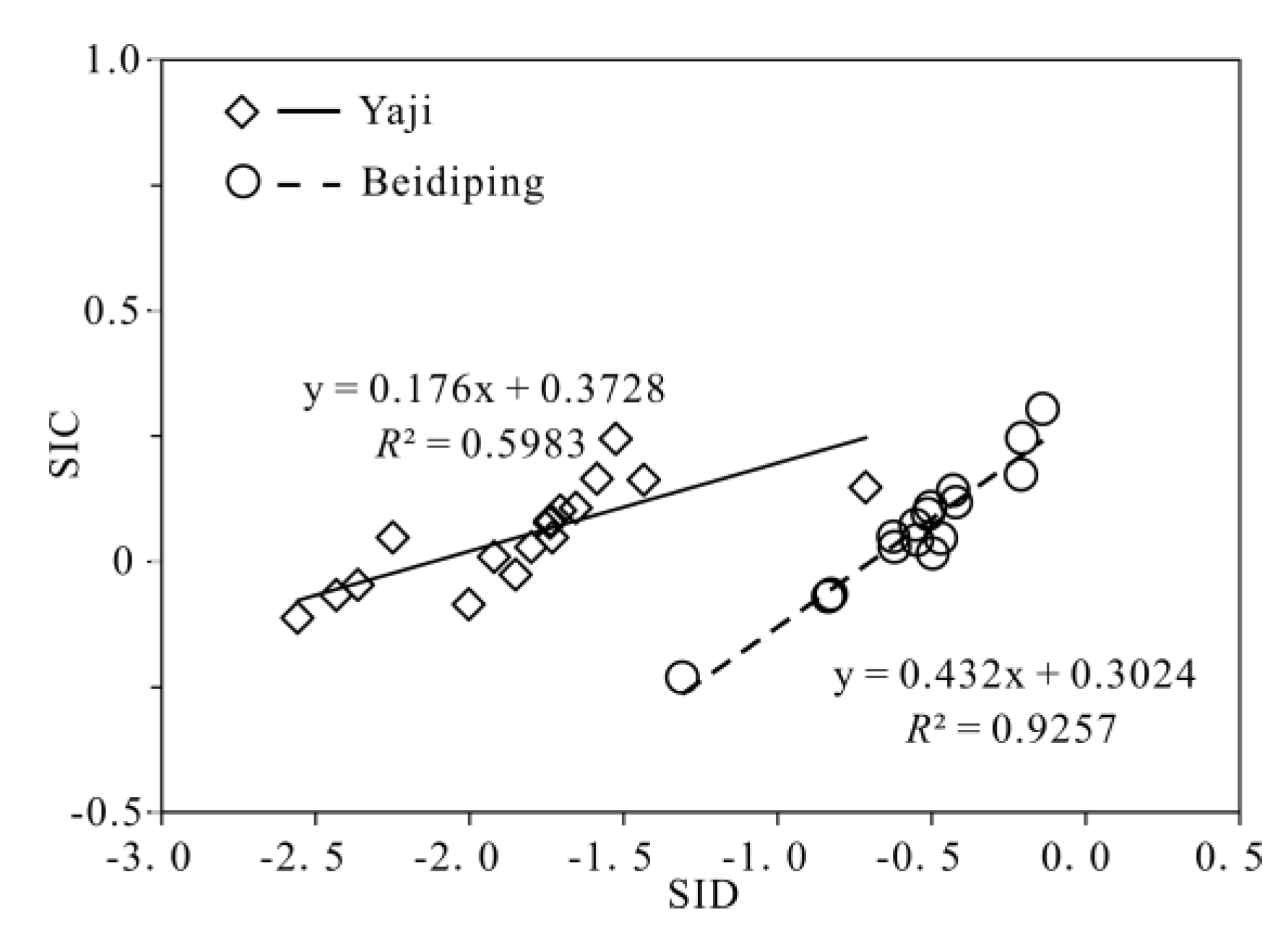
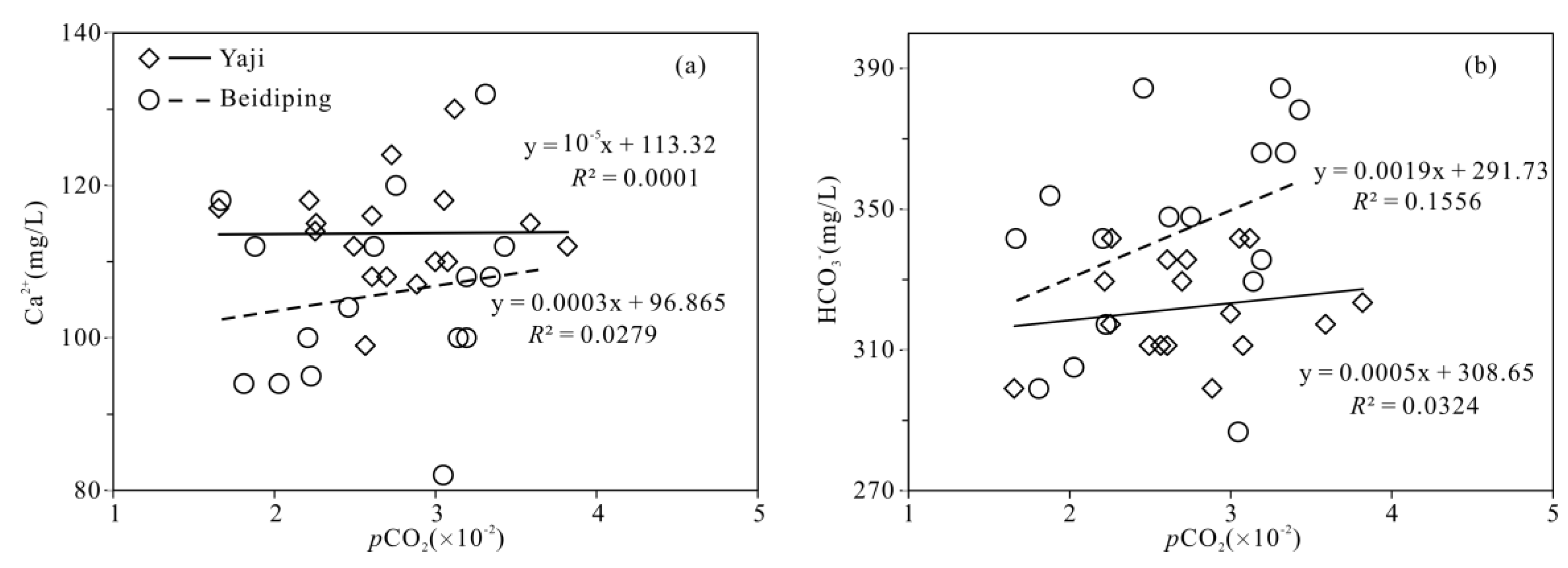
The partial pressure of CO2 (pCO2) values in spring water ranged from 16,558 to 38,194 ppmv, which were 39.4–90.9 times higher than that in atmosphere (about 420 ppmv, https://keelingcurve.ucsd.edu/, accessed on 6 June 2023), and the pCO2 values in spring water were within the range of that in soil in karst area (1057–80,000 ppmv) [34,49,50], showing that the spring water equilibrated with pCO2 in soil rather than atmosphere. Therefore, karst spring water HCO3− was likely to come from the soil CO2 and carbonate rocks. The pCO2 in the karst dynamic system not only controls the pH but also drives the karstification process [10,51]. Generally, when CO2 enters a karst dynamic system, the increased pCO2 promotes carbonate weathering, resulting in a decrease in pH value and an increase in Ca2+ and HCO3− concentrations in karst water, otherwise carbonate minerals will deposit, resulting in an increase in pH and a decrease in Ca2+ and HCO3− concentrations in karst water [2]. In the studied karst catchments, the pH values of spring water had a negative correlation with pCO2 values (Figure 5), indicating the control of CO2 concentration on the pCO2 and pH values of karst spring water [2]. As shown in Figure 6, the pCO2 had a negative correlation with SIC and SID, respectively, suggesting the control of pCO2 on calcite and dolomite dissolution in spring water. Meanwhile, the SIC had a significant positive correlation with SID in the spring water (Figure 7), showing that calcite and dolomite dissolution may be synchronous in the catchments. All of the SIC values of samples were around 0 (Table 1), reflecting that the calcite dissolution in spring water was sufficient and reached the saturation state, which means that the “transport-limited” regime dominated the hydrochemical evolution in the catchments [52]. Therefore, runoff was a restrictive factor for calcite dissolution in the studied catchments due to the subtropical monsoon climate. The calcite dissolution products are easily transported to the karst water system in the monsoon season when rainfall is relatively heavy. Unlike the SIC, the SID values of karst spring water in Yaji and Beidiping were all less than 0 (Figure 7), indicating that the dolomite dissolution was insufficient and in an undersaturated state, which means that the “kinetic-limited” regime dominated the dolomite dissolution in the spring water [52]. This is consistent with the fact that the dissolution rate of dolomite is much lower than that of calcite [28]. It is worth noting that the pCO2 of karst spring water had no correlation with Ca2+ and HCO3−, respectively (Figure 8), which was different from the obvious positive correlation between Ca2 and HCO3− from carbonate weathering by carbonic acid and pCO2 of karst water, respectively [36,53,54], showing that the Ca2+ and HCO3− in karst spring water were not only from carbonate weathering by carbonic acid but also come from gypsum mineral weathering and/or carbonate weathering by anthropogenic acids [25,31]. Considering that there is no gypsum mineral in the stratum of the study area [32,48], anthropogenic acids may participate in carbonate weathering. Anthropogenic acids that dissolve carbonate rocks include sulfuric acid and nitric acid [25,41]. As the NO3− only accounted for 0.33–1.64% of the total anionic equivalent concentration and the corresponding concentrations were very low (≤6.65 mg/L) in the studied karst spring water, the contribution of nitric acid to carbonate weathering in spring water is negligible; thus, sulfuric acid may participate in carbonate weathering.
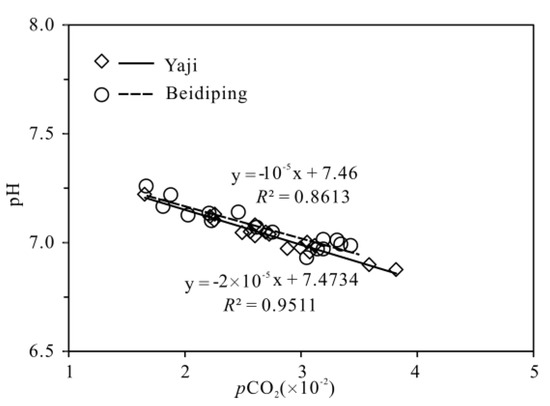
Figure 5.
The relationship between pH and pCO2 of karst spring in the Yaji and Beidiping karst catchments.
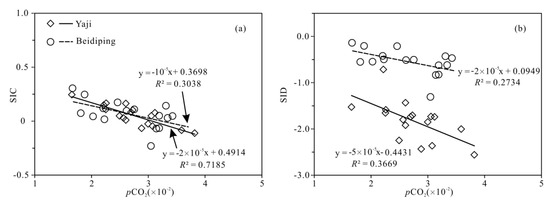
Figure 6.
Relationships of SIC vs. pCO2 (a) and SID vs. pCO2 (b) in the Yaji and Beidiping karst catchments.
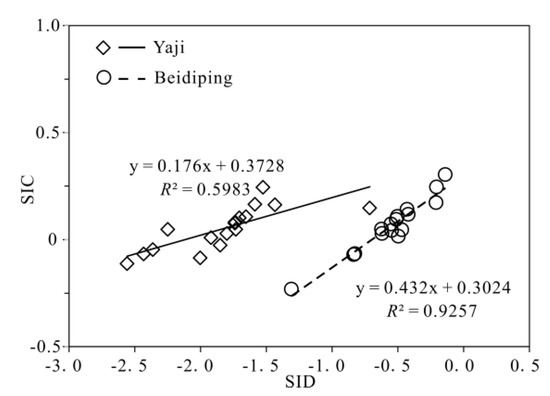
Figure 7.
Relationships of SIC and SID in the Yaji and Beidiping karst catchments.
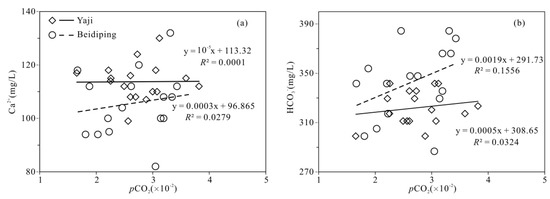
Figure 8.
Relationships of Ca2+ vs. pCO2 (a) and HCO3− vs. pCO2 (b) in the Yaji and Beidiping karst catchments.
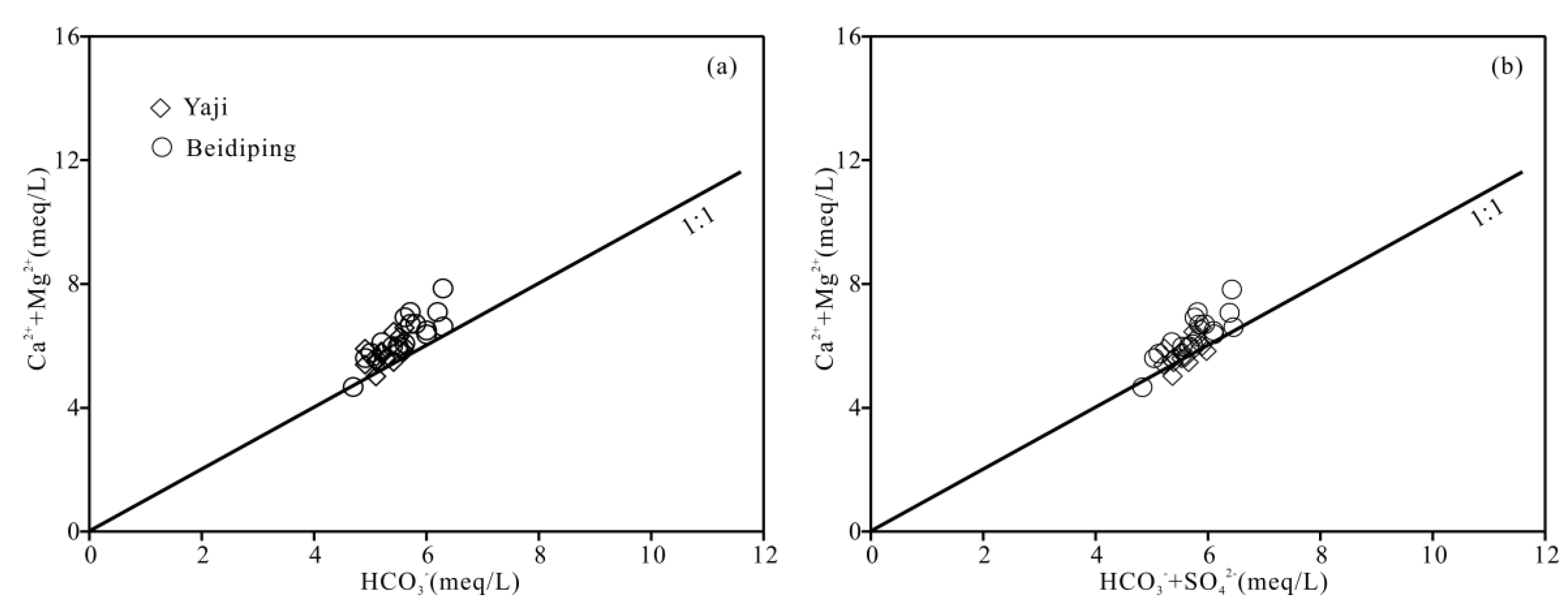
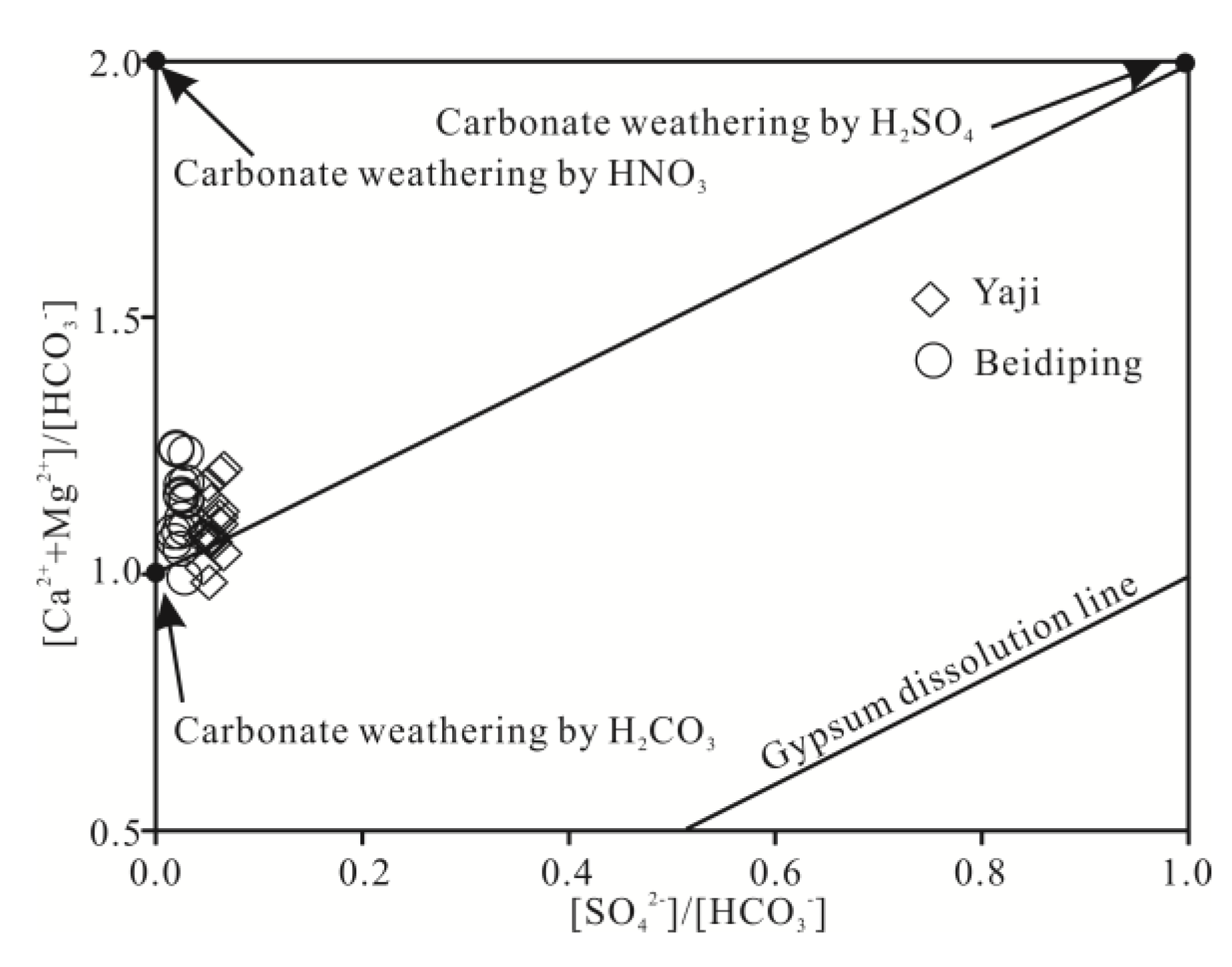
The foregoing discussion shows that carbonate weathering controls the chemical composition of the spring water. According to Equation (1), when carbonate rocks are only weathered by carbonic acid, the equivalent ratio of [Ca2+ + Mg2+]/[HCO3−] in water should be equal to 1. However, the ratios of spring water samples, except for two values (0.98 and 0.99), varied from 1.01 to 1.25 (Figure 9a), showing that the carbonate rocks were not weathered by carbonic acid only. Other acids, thus, should be involved in carbonate weathering. Generally, except carbonic acid, nitric acid and sulfuric acid play an important role in carbonate weathering in karst areas [25,31,41,55,56]. However, as mentioned above, the contribution of nitric acid to carbonate weathering in spring water can be negligible; thus, sulfuric acid may be involved in carbonate weathering. According to Equations (1) and (2), if carbonate weathering is controlled by both carbonic acid and sulfuric acid, the [Ca2+ + Mg2+]/[HCO3− + SO42−] equivalent ratio should be equal to 1. Figure 9b shows the [Ca2+ + Mg2+]/[HCO3− + SO42−] equivalent ratios of samples were all close to 1, thereby implying that sulfuric acid was also involved in the carbonate weathering. As depicted in Figure 10, the data distribution was mainly controlled by the two end-members of carbonate weathering by sulfuric acid and carbonic acid, proving that the influence of carbonate weathering by sulfuric acid on the chemical composition of spring water was considerable. Therefore, sulfuric acid participated in carbonate weathering in the Yaji and Beidiping catchments. In addition, the data distributions of the samples were all far away from the gypsum dissolution line (Figure 10), which coincides with the fact that no evaporite minerals such as gypsum exist in the studied catchments.
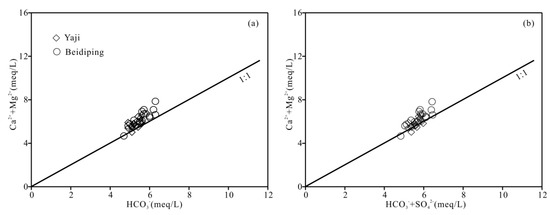
Figure 9.
Relationships of HCO3− vs. Ca2++ Mg2+ (a) and HCO3− + SO42− vs. Ca2+ + Mg2+ (b) in the Yaji and Beidiping karst catchments.
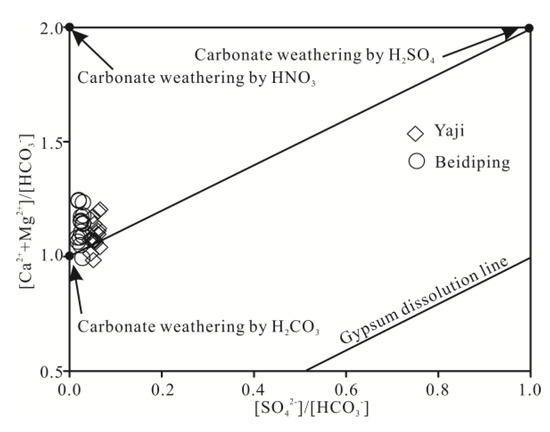
Figure 10.
Equivalent ratios of [Ca2+ + Mg2+]/[HCO3−] vs. [SO42−]/[HCO3−] in springs draining the Yaji and Beidiping karst catchments.
4.4. Carbon Isotopic (13CDIC) Evidence of Sulfuric Acid Weathering of Carbonate Rocks
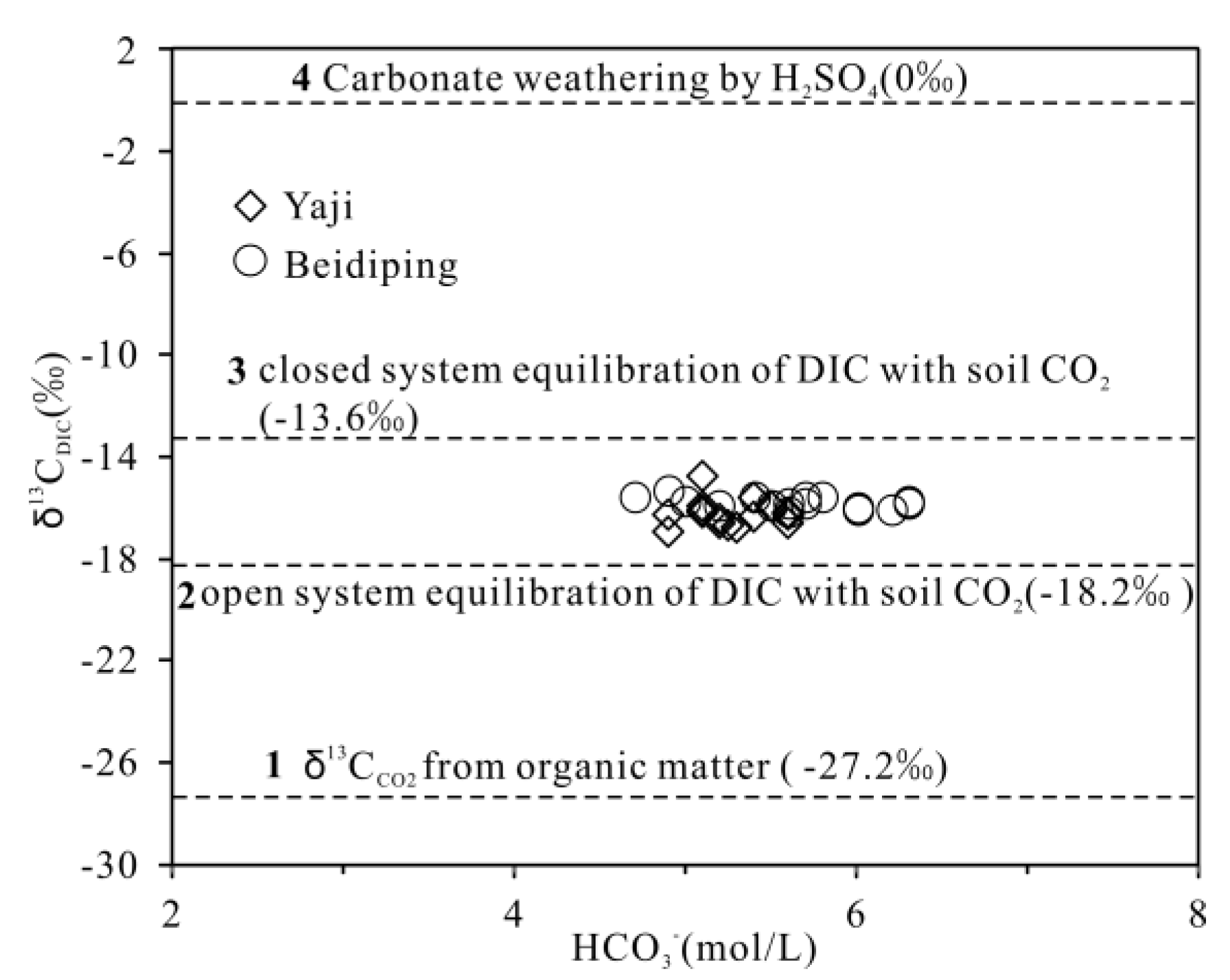
As discussed above, the spring water HCO3− in the studied catchments originated from the soil CO2 and carbonate weathering. The δ13C value of soil CO2 in karst areas ranged from −18.3 to −36‰ [34,57,58,59] with a median value of −27.2‰ (Figure 11). On the other hand, the δ13C value of marine carbonate rocks is about 0‰ [56,59,60]. Due to the low solubility of CO2 in pure water [61], the theoretical HCO3− concentration in pure water at normal temperature and pressure is about 0.6 mg/L [61]; thus, its contribution to spring water HCO3−can be ignored because the HCO3− concentrations in spring water were three–four orders of magnitude higher than 0.6 mg/L. In a closed system, according to Equation (1), the theoretical δ13C value of HCO3− formed by carbonate weathering by carbonic acid only is −13.6‰ (−27.2‰/2) (Figure 11). While in an open system, the fractionation of δ13C is about +9‰ between soil CO2 and HCO3− [62,63]; thus, the theoretical δ13CDIC value is −18.2‰ (−27.2‰ + 9‰) (Figure 11). According to Equation (2), the theoretical end-member value of carbonate weathering by sulfuric acid is δ13CDIC = 0‰. In the studied catchments, the HCO3− concentrations of the spring water were plotted in Figure 11; the characteristics of all samples are high HCO3− concentrations, and the carbonate weathering will not produce the observed high concentrations of HCO3− in a closed system [64]. Moreover, the pCO2 in spring water in Yaji and Beidiping is much higher than that in the atmosphere, indicating adequate CO2 [65,66]. These results suggest that carbonated weathering is probably completed in an open system [64]. This is why all δ13CDIC values in spring water are significantly lower than −13.6‰ (Figure 11). As shown in Figure 11, the sampling points were all located between the two end-members of sulfuric acid and carbonic acid weathering of carbonate rocks in an open system, fully proving that sulfuric acid participated in carbonate weathering.
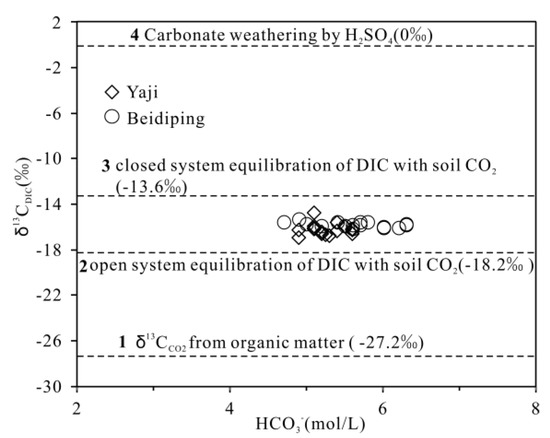
Figure 11.
Variation of δ13CDIC vs. HCO3− shows the response of δ13CDIC to HCO3− evolution in springs draining the Yaji and Beidiping karst catchments. These include: (1) soil CO2 originating from degradation of organic matter with δ13Cco2=−27.2‰ [34,57,58,59]; (2) open system equilibration of HCO3− with soil CO2 originating from degradation of organic matter; (3) carbonate weathering by H2CO3 produced from soil zone CO2 in closed system; and (4) carbonate weathering by H2SO4 according to the δ13C value of marine carbonate rock (0‰) [56,59,60].
In order to quantify the contributions of soil CO2 and carbonate rocks to the HCO3− in the spring water, using the isotope mass-balance bimodal mixing model, we assumed that the isotopic composition ratios of soil CO2 and carbonate rocks were x and 1 − x, respectively. Therefore, the δ13CDIC value of spring water can be expressed as:
δ13CDIC-spring water = x × δ13Csoil CO2 + (1 − x) × δ13Ccarbonate rocks
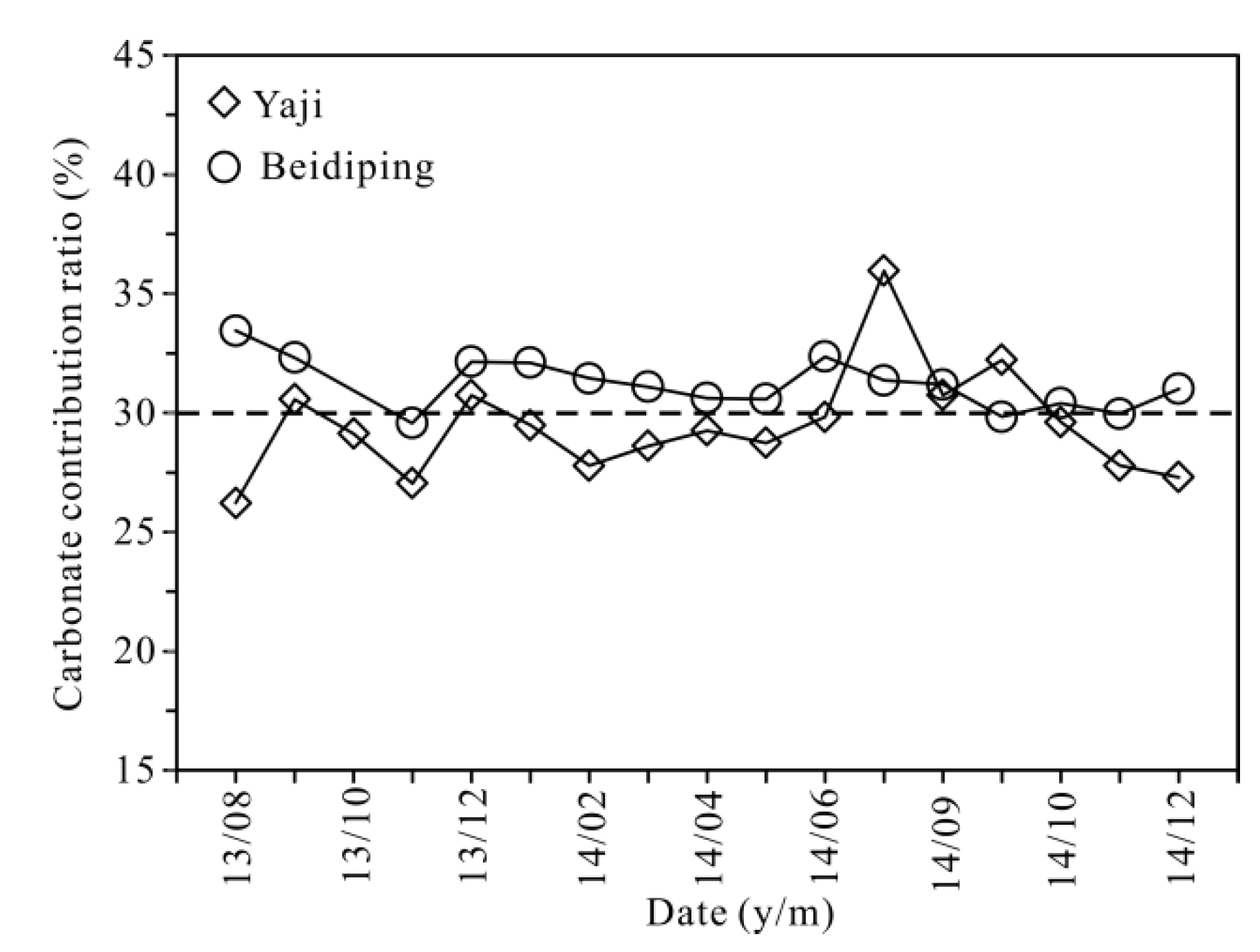
In the studied catchments and other karst areas of Guangxi and Southwest China, the mean δ13C value of karst soil CO2 is about −23% [56,67], while the δ13C value of marine carbonate rocks is around 0‰ [56,60]. Based on the two end-members of δ13C values, the calculated results show (Figure 12) that carbon from carbonate rocks in Yaji and Beidiping accounted for 26–36% and 30–33% of the HCO3− in spring water, with average values of 29% and 31%, respectively. Many studies have shown that more soil CO2 converted into carbonic acid participates in carbonate weathering in the rainy season than that in the dry season due to enhanced root respiration and microbial activities in the warm and wet summer season [36,68,69], resulting in a relatively lower proportion of carbonate rocks than that of soil CO2 participating in carbonate weathering. However, the average proportions of carbon from carbonate rocks in the rainy season accounted for 30% and 32% of the spring water HCO3−, and the proportions in the dry season were 29% and 31% in Yaji and Beidiping, respectively, reflecting the contribution of carbonate rocks to the spring water HCO3− in the rainy season almost coincided with that in the dry season. The seasonal variation characteristics are probably linked to the sulfuric acid involved in carbonate weathering. As discussed above, sulfuric acid in the studied catchments mainly came from acid precipitation. Moreover, about 70% of rainfall occurs during the rainy season [32]. Therefore, more sulfuric acid participates in the weathering process of carbonate rocks in the rainy season than that in the dry season, finally resulting in nearly identical contributions of carbonate rocks to the spring water HCO3− in both rainy and dry seasons. Yet, it is worth noting that about 70% of rainfall occurs during the rainy season, and the mean frequency of acid precipitation is up to 66.3% [21], meaning that monsoon acidic rainfall can significantly enhance the weathering of carbonate rocks.
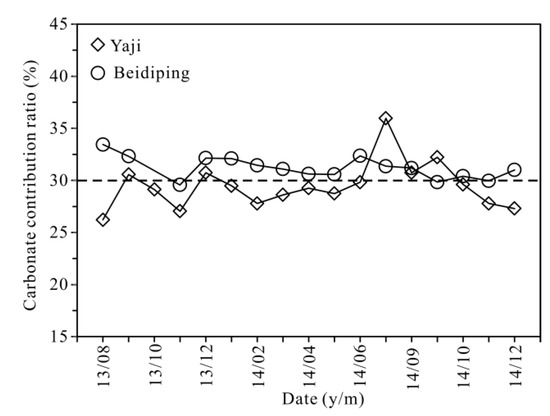
Figure 12.
Carbonate contribution ratio versus date in the Yaji and Beidiping karst catchments.
4.5. Effect of Anthropogenic H2SO4 on Carbonate Weathering and Carbon Sink
Since the study area is severely affected by acid precipitation and the HCO3− concentrations in precipitation are almost non-existent [21,32,42], precipitation contributes little to the spring water HCO3−. Therefore, the HCO3− in spring water was mainly controlled by carbonate weathering.
According to Equations (12)–(16), the quantified results are shown in Table 1 (see S1 and S2 parameters). The contribution rate of H2CO3 weathering of carbonate rocks to the spring water [Ca2+ + Mg2+]carbonate weathering and [HCO3−]carbonate weathering in Yaji and Beidiping ranged from 70 to 100% and from 82 to 100%, and from 63 to 100% and from 77 to 100%, with average values of 87% and 93%, and 81% and 89%, respectively. The contribution rate of H2SO4 weathering of carbonate rocks to the spring water [Ca2+ + Mg2+]carbonate weathering and [HCO3−]carbonate weathering in Yaji and Beidiping ranged from 0 to 30% and from 0 to 18%, and from 0 to 37% and from 0 to 23%, with average values of 14% and 7%, and 19% and 11%, respectively. Therefore, the participation of sulfuric acid in carbonate weathering increased the weathering rate by 14% and 19% while decreasing the karst carbon sink by 7% and 11% in Yaji and Beidiping, respectively. These different effects of anthropogenic H2SO4 on carbonate weathering and karst carbon sink are probably linked to the different lithological carbonate weathering in the Yaji and Beidiping karst catchments (Figure 1). Many studies have shown that the carbonate weathering within karst aquifers and karst catchments is remarkably influenced by different carbonate minerals; that is, under normal pressure and temperature, the solubility of dolomite is usually 20–30% higher than that of limestone [6,28], but the dissolution rate of dolomite is only 1/3–1/60 of that of limestone [28], resulting in varying degrees of water–rock interactions. The aquifer lithology in the Yaji karst catchment is only composed of the D3r pure limestone (Figure 1), and the SIC values of the spring water ranged from −0.11 to 0.24 with an average value of 0.05, indicating that the CaCO3-CO2-H2O system within the karst aquifer had reached equilibrium and the water–rock interaction was sufficient. In contrast, the aquifer lithology in the Beidiping karst catchment is composed of the D2d limestone and dolomite, and the dolomite is an important part of the karst aquifer. However, the SID values of the spring water in Beidiping were all less than 0, with SIC values around 0 (Figure 7), suggesting that the CaMg(CO3)2-CO2-H2O system within the karst aquifer had not reached equilibrium and the water–rock interaction was insufficient. As a result, the weathering intensity of carbonate rocks in Beidiping was less than that in Yaji. Therefore, in the Yaji and Beidiping karst catchments with similar climate, hydrology, vegetation coverage, acid rain pollution and drainage area, with the participation of anthropogenic sulfuric acid from acid precipitation, the increased proportion of carbonate weathering rate and the reduced proportion of karst carbon sink flux in Yaji were both lower than those in Beidiping. Theoretically, if the CaMg(CO3)2-CO2-H2O system within the karst aquifer reaches equilibrium, the karst carbon sink effect produced by weathering of dolomite with higher solubility is stronger than that of limestone [68,70,71]. Then, under similar conditions, when the same amount of anthropogenic sulfuric acid participates in carbonate weathering, the reduced proportion of the karst carbon sink effect in the limestone catchment should be higher than that in the dolomite catchment. Considering that carbonate rocks are composed of limestone, dolomite and a series of transitional types of mixed limestone and dolomite, and the distribution of dolomite is as common as that of limestone [26,27], simply replacing carbonate rocks with a type of carbonate rock, thus, can lead to increased uncertainty in the assessment of the chemical weathering flux induced by anthropogenic sulfuric acid. Therefore, it is imperative to understand more comprehensively the impact process and mechanism of anthropogenic acids on karst carbon sinks from the perspective of carbonate lithology, further improving the accuracy of the assessment of karst carbon sink flux in karst catchments in future studies.
The Yaji and Beidiping karst catchments are fairly typical in the karst region of Southwest China, and anthropogenic sulfuric acid from acid precipitation participating in carbonate weathering is extensively reported in many other karst systems in the world [15,72,73]; thus, the results of this research are probably applied to most karst systems. If the Yaji and Beidiping karst catchments are taken as end-members of the typical limestone catchment and the mixed limestone and dolomite catchment, respectively, the sulfuric acid from acid precipitation can decrease the karst carbon sink flux by 7–11% by carbonate weathering, which is consistent with the previous findings of about 4.5–19.3% reduction in the karst carbon sink caused by sulfuric acid precipitation in Southwest China [22,23,24,25], showing that anthropogenic sulfuric acid plays an important role in the weathering processes of carbonate rocks. At present, the effects of anthropogenic acids on the carbon cycle of the earth’s surface have been widely reported worldwide [24,25,31,74,75]. Considering that the karst area occupies 15% of the world’s total land area [76], the effects of anthropogenic acids on the chemical weathering of carbonate rocks in karst systems should not be ignored, especially in the karst areas with the extensive use of fertilizers, the large discharge of sewage and severe acid precipitation, otherwise the actual karst carbon sink budget will be overestimated.
5. Conclusions
In this study, data about hydrogeochemical compositions and δ13CDIC of spring water samples were collected monthly in two typical karst catchments with distinct carbonate lithology (Yaji limestone catchment, Beidiping mixed limestone–dolomite catchment) in Southwest China. The average contribution rates of atmospheric inputs and carbonate weathering to the total cationic load in spring water were 2.24% and 97.8% in Yaji, and 3.09% and 96.9% in Beidiping, respectively. Carbonate weathering was caused not only by carbonic acid but also by sulfuric acid from acid precipitation. The participation of sulfuric acid in carbonate weathering increased the weathering rate and decreased the karst carbon sink by 14% and 7% in Yaji (composed of limestone) and by 19% and 11% in Beidiping (composed of limestone–dolomite), respectively. Thus, simply replacing carbonate rocks with a type of carbonate rock can increase uncertainty in the assessment of the chemical weathering rate and karst carbon sink budget induced by sulfuric acid. The participation of anthropogenic sulfuric acid can increase the rate of carbonate weathering by 14–19% and decrease the karst carbon sink flux by 7–11% in Southwest China. Therefore, anthropogenic acids influence the global carbon cycle and climate change by carbonate weathering due to the large karst areas in the world. On the one hand, when accurately calculating the karst carbon sink flux, the contribution of anthropogenic acids to carbonate weathering should be reasonably deducted; otherwise, the actual karst carbon sink budget will be overestimated. On the other hand, understanding more comprehensively the impact process and mechanism of anthropogenic acids on karst carbon sinks from the perspective of carbonate lithology can further improve the accuracy of the assessment of karst carbon sink budget in karst catchments.
Author Contributions
Y.X.: conceptualization, formal analysis, investigation, data curation, software, writing—original draft, writing—review and editing; Y.H. and J.L.: methodology, supervision, formal analysis; Y.G. and F.H.: formal analysis; Q.X.: writing—review and editing, formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Guangxi Natural Science Foundation (2021GXNSFBA220065, GuikeAB21196050, 2022GXNSFAA035569 and 2021GXNSFBA075013), the Institute of Karst Geology Fundamental Research Project (2017025 and 2021001), the National Natural Science Foundation of China (42177075) and the China Geological Survey (12120113005300 and DD20230547).
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data supporting reported results can be found in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dreybrodt, W.; Wang, H. A new direction in effective accounting for the atmospheric CO2 budget: Considering the combined action of carbonate dissolution, the global water cycle and photosynthetic uptake of DIC by aquatic organisms. Earth-Sci. Rev. 2010, 99, 162–172. [Google Scholar] [CrossRef]
- Liu, Z.H.; Macphersonb, G.L.; Groves, C.; Martin, J.B.; Yuan, D.X.; Zeng, S.B. Large and active CO2 uptake by coupled carbonate weathering. Earth-Sci. Rev. 2018, 182, 42–49. [Google Scholar] [CrossRef]
- Berner, R.A. A model for atmospheric CO2 over Phanerozoic time. Am. J. Sci. 1991, 291, 339–376. [Google Scholar] [CrossRef]
- IPCC. Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Dreybrodt, W. Processes in Karst Systems (Series in Physical Environment); Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1988; p. 140. [Google Scholar]
- Morse, J.W.; Arvidson, R.S. The dissolution kinetics of major sedimentary carbonate minerals. Earth Sci. Rev. 2002, 58, 51–84. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dreybrodt, W.; Liu, H. Atmospheric CO2 sink: Silicate weathering or carbonate weathering. Appl. Geochem. 2011, 26, S292–S294. [Google Scholar] [CrossRef]
- Apollaro, C.; Fuoco, I.; Gennaro, E.; Giuliani, L.; Iezzi, G.; Marini, L.; Radica, F.; Di Luccio, F.; Ventura, G.; Vespasiano, G. Advanced argillic alteration at Cave di Caolino, Lipari, Aeolian Islands (Italy): Implications for the mitigation of volcanic risks and the exploitation of geothermal resources. Sci. Total Environ. 2023, 889, 164333. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.X. Contribution of IGCP 379 “Karst Processes and Carbon Cycle” to global change. Episodes 1998, 21, 198. [Google Scholar]
- Liu, Z.H.; Dreybrodt, W. Significance of the carbon sink produced by H2O-carbonate-CO2-aquatic phototroph interaction on land. Sci. Bull. 2015, 60, 182–191. [Google Scholar] [CrossRef]
- Detwiler, R.P.; Charles, A.S.H. Tropical forests and the global carbon cycle. Science 1988, 239, 42–47. [Google Scholar] [CrossRef]
- Tans, P.P.; Fung, I.; Takahashi, T. Observational constraints on the global atmospheric CO2 budget. Science 1990, 247, 1431–1438. [Google Scholar] [CrossRef]
- Berner, E.K.; Berner, R.A. The Global Water Cycle: Geochemistry and Environment; Prentice-Hall: New York, NY, USA, 1987; p. 394. [Google Scholar]
- Huang, T.M.; Fan, Y.F.; Long, Y.; Pang, Z.H. Quantitative calculation for the contribution of acid rain to carbonate weathering. J. Hydrol. 2019, 568, 360–371. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.S.; Liang, P.; Zhou, Y.; Chai, F.H.; Mo, Z.Y.; Chen, Z.M.; Li, H. Pollution characteristics and control countermeasures of acid rain in Guilin. Res. Environ. Sci. 2020, 33, 1393–1401. [Google Scholar]
- Zhang, G.Z.; Liu, D.Y.; He, X.H.; Yu, D.Y.; Pu, M.J. Acid rain in Jiangsu province, Eastern China: Tempo-spatial variations features and analysis. Atmos. Pollut. Res. 2017, 8, 1031–1043. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.L.; Wu, Q.X.; Tang, Y. Effects of agricultural alkaline substances on reducing the rainwater acidification: Insight from chemical compositions and calcium isotopes in a karst forests area. Agric. Ecosyst. Environ. 2020, 290, 106782. [Google Scholar] [CrossRef]
- Xie, S.Y.; Wang, S.J.; Yu, Y.; Liu, L.; Zhang, F.Y. Analysis on acid rain status and its change trends in China from 2003 to 2018. Environ. Monit. China 2020, 36, 80–88. [Google Scholar]
- Zhou, X.D.; Xu, Z.F.; Liu, W.J.; Wu, Y.; Zhao, T.; Jiang, H. Progress in the studies of precipitation chemistry in acid rain areas of Southwest China. Environ. Sci. 2017, 38, 4438–4446. [Google Scholar]
- Zhu, H.Y. Analysis on Cause and Source of Acid Precipitation in Guilin; Northwest Normal University: Lanzhou, China, 2020; pp. 1–62. [Google Scholar]
- Li, S.L.; Calmels, D.; Han, G.L.; Gaillardet, J.; Liu, C.Q. Sulfuric acid as an agent of carbonate weathering constrained by δ13CDIC: Examples from Southwest China. Earth Planet. Sci. Lett. 2008, 270, 180–199. [Google Scholar] [CrossRef]
- Ding, H.; Lang, Y.C.; Liu, C.Q.; Liu, T.Z. Chemical characteristics and δ34S-SO42− of acid rain: Anthropogenic sulfate deposition and its impacts on CO2 consumption in the rural karst area of southwest China. Geochem. J. 2013, 47, 625–638. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Wu, L.J.; Xin, C.L.; Yu, S.; Guo, Y.S.; Wang, J.J. Impact of anthropogenic sulfate deposition via precipitation of carbonate weathering in a typical industrial city in a karst basin of southwest China: A case study in Liuzhou. Appl. Geochem. 2019, 110, 104417. [Google Scholar] [CrossRef]
- Xie, Y.C.; Huang, F.; Yang, H.; Yu, S. Role of anthropogenic sulfuric and nitric acids in carbonate weathering and associated carbon sink budget in a karst catchment (Guohua), southwestern China. J. Hydrol. 2021, 599, 126287. [Google Scholar] [CrossRef]
- Li, D.T.; Luo, Y. Measurement of carbonate rocks distribution area in China. Carsologica Sin. 1983, 2, 147–150. [Google Scholar]
- Li, H.W.; Wang, S.J.; Bai, X.Y.; Cao, Y.; Wu, L.H. Spatiotemporal evolution of carbon sequestration of limestone weathering in China. Sci. China. Earth. Sci. 2019, 62, 974–991. [Google Scholar] [CrossRef]
- Liu, Z.H.; Dreybrodt, W.; Li, H.J. Comparison of dissolution rate-determining mechanisms between limestone and dolomite. Earth Sci.-J. China Univ. Geosci. 2006, 31, 411–416. [Google Scholar]
- Blasco, M.; Auqué, L.F.; Gimeno, M.J.; Acero, P.; Asta, M.P. Geochemistry, geothermometry and influence of the concentration of mobile elements in the chemical characteristics of carbonate-evaporitic thermal systems. The case of the Tiermas geothermal system (Spain). Chem. Geol. 2017, 466, 696–709. [Google Scholar] [CrossRef]
- Kanduč, T.; Geršl, M.; Geršlová, E.; McIntosh, J. Temporal and seasonal variations of silicate Svratka River and sediment characterization, Czech Republic: Geochemical and isotopic approach. Aquat. Geochem. 2023, 29, 145–171. [Google Scholar] [CrossRef]
- Han, X.K.; Yan, Z.L.; Lang, Y.C.; Hu, D.; Guo, Q.J.; Li, S.L. Enhanced sulfide oxidation by monsoon rainfall in a small typical karstic catchment of Southwest China. J. Hydrol. 2022, 615, 128682. [Google Scholar] [CrossRef]
- Huang, Q.B.; Wu, H.Y.; Chen, R.R.; Li, T.F.; Luo, F.; Zhao, G.S.; Li, X.P. Buffering effect of lime soil on acid rain and its influence on the evaluation of the karst carbon sink effect. Acta Geosci. Sin. 2022, 43, 461–471. [Google Scholar]
- Wigley, T.M.L. WATSPEC: A computer program for determining the equilibrium speciation of aqueous solutions. Br. Geo Morphol. Res. Group Tech. Bull. 1977, 20, 1–46. [Google Scholar]
- Cao, J.H.; Zhou, L.; Yang, H.; Lu, Q.; Kang, Z.Q. Comparison of carbon transfer between forest soils in karst and clasolite areas and the karst carbon sink effect in Maocun village of Guilin. Quat. Sci. 2011, 31, 431–437. [Google Scholar]
- Meybeck, M. Pathways of major elements from land to oceans through rivers. In River Inputs to Ocean Systems; Martin, J.M., Burton, J.D., Eisma, D., Eds.; United Nations Press: New York, NY, USA, 1981; pp. 18–30. [Google Scholar]
- Zhao, M.; Liu, Z.H.; Li, H.C.; Zeng, C.; Yang, R.; Chen, B.; Yan, H. Response of dissolved inorganic carbon (DIC) and δ13CDic to changes in climate and land cover in SW China karst catchments. Geochim. Cosmochim. Acta 2015, 165, 123–136. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, G.H.; Pei, J.G.; Zhang, C. Assessment on the water qualities of major subterranean rivers in Guangxi and their changing trend. Carsologica Sin. 2002, 21, 195–201. [Google Scholar]
- Fuoco, I.; Figoli, A.; Criscuoli, A.; Brozzo, G.; De Rosa, R.; Gabriele, B.; Apollaro, C. Geochemical modeling of chromium release in natural waters and treatment by RO/NF membrane processes. Chemosphere 2020, 254, 126696. [Google Scholar] [CrossRef]
- Xie, Y.C.; Yu, S.; Miao, X.Y.; Li, J.; He, S.Y.; Sun, P.A. Chemical weathering and CO2 consumption flux in Tibetan Plateau: A case of Lhasa River Basin. Earth Sci. Front. 2023, 37, 688. [Google Scholar] [CrossRef]
- Roy, S.; Gaillardet, J.; Allègre, C.J. Geochemistry of dissolved and suspended load of the Seine river, France: Anthropogenic impact, carbonate and silicate weathering. Geochim. Cosmochim. Acta 1999, 63, 1277–1292. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Jiang, Y.J.; Yuan, D.X.; Cui, J.; Li, Y.; Yang, J.; Cao, M. Source and flux of anthropogenically enhanced dissolved inorganic carbon: A comparative study of urban and forest karst catchments in Southwest China. Sci. Total Environ. 2020, 725, 138255. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Kuo, Y.M.; Du, W.; He, S.Y.; Sun, P.A.; Yuan, Y.Q.; Li, R.; Li, Y.S. The hydrochemistry properties of precipitation in karst tourism city (Guilin), Southwest China. Environ. Earth Sci. 2015, 74, 1061–1069. [Google Scholar] [CrossRef]
- Galy, A.; France-Lanord, C. Weathering processes in the Ganges-Brahmaputra basin and the riverine alkalinity budget. Chem. Geol. 1999, 159, 31–60. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mehanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Dai, J.F.; Rong, F.Y.; Du, J.; Han, P.L.; Guan, B.L. Study on temporal and spatial variability of pan evaporation in Guangxi province. China Rural. Water Hydropower 2013, 1, 11–14. [Google Scholar]
- Grosbois, C.; Négrel, P.H.; Fouillac, C.; Grimaud, D. Chemical and isotopic characterization of the dissolved load of the Loire River. Chem. Geol. 2000, 170, 179–201. [Google Scholar] [CrossRef]
- Moquet, J.S.; Crave, A.; Viers, J.; Seyler, P.; Guyot, J.L. Chemical weathering and atmospheric/soil CO2 uptake in the Andean and Foreland Amazon basins. Chem. Geol. 2011, 287, 1–26. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, C.L.; Xie, Y.C.; Li, L.; Cao, J.H. Inorganic carbon flux and its source in the karst catchment of Maocun, Guilin, China. Environ. Earth Sci. 2015, 74, 1079–1089. [Google Scholar] [CrossRef]
- Liu, Z.H.; He, S.Y.; Yuan, D.X.; Zhao, J.B. CO2 in the soil and its function on karsts process. Hydrol. Geol. Eng. Geol. 1998, 4, 42–45. [Google Scholar]
- He, S.Y.; Pan, G.X.; Cao, J.H.; Tao, Y.X.; Teng, Y.Z. Research on characteristics of carbon cycle in epikarst ecological system. Quat. Sci. 2000, 20, 383–390. [Google Scholar]
- Yuan, D.X. Scientific innovation in karst resources and environment research field of China. Carsologica Sin. 2015, 34, 98–100. [Google Scholar]
- Yu, Z.L.; Yan, N.; Wu, G.J.; Xu, T.L.; Li, F. Chemical weathering in the upstream and midstream reaches of the Yarlung Tsangpo basin, southern Tibetan Plateau. Chem. Geol. 2021, 559, 119906. [Google Scholar] [CrossRef]
- Liu, Z.H.; Li, Q.; Sun, H.L.; Wang, J.L. Seasonal, diurnal and storm-scale hydrochemical variations of typical epikarst springs in subtropical karst areas of SW China: Soil CO2 and dilution effects. J. Hydrol. 2007, 337, 207–223. [Google Scholar] [CrossRef]
- Li, S.L.; Liu, C.Q.; Li, J.; Lang, Y.C.; Hu, D.; Li, L.B. Geochemistry of dissolved inorganic carbon and carbonate weathering in a small typical karstic catchment of Southwest China: Isotopic and chemical constraints. Chem. Geol. 2010, 277, 301–309. [Google Scholar] [CrossRef]
- Perrin, A.S.; Probst, A.; Probst, J.L. Impact of nitrogenous fertilizers on carbonate dissolution in small agricultural catchments: Implications for weathering CO2 uptake at regional and global scales. Geochim. Cosmochim. Acta 2008, 72, 3105–3123. [Google Scholar] [CrossRef]
- Jiang, Y.J. The contribution of human activities to dissolved inorganic carbon fluxes in a karst underground river system: Evidence from major elements and δ13CDIC in Nandong, Southwest China. J. Contam. Hydrol. 2013, 152C, 1–11. [Google Scholar] [CrossRef]
- Smith, B.N. General characteristics of terrestrial plants (agronomic and forests)-C3, C4 and crustacean acid metabolism. In Basic Principles; Zaborsky, R., Ed.; CRC Handbook of Biosolar Resources: Boca Raton, FL, USA, 1982; pp. 99–118. [Google Scholar]
- Zheng, L.P. The stable carbon isotopic composition of soil CO2 in the karst, the middle parts of Guizhou province. Sci. China Ser. D Earth Sci. 1999, 29, 514–520. [Google Scholar]
- Kanduč, T.; Mori, N.; Kocman, D.; Stibilj, V.; Grassa, F. Hydrogeochemistry of Alpine springs from North Slovenia: Insights from stable isotopes. Chem. Geol. 2012, 300–301, 40–54. [Google Scholar] [CrossRef]
- Clark, I.; Fritz, P. Environmental Isotopes in Hydrogeology; Lewis Press: Boca Raton, FL, USA, 1997; pp. 111–169. [Google Scholar]
- Karim, A.; Veizer, J. Weathering processes in the Indus River Basin: Implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chem. Geol. 2000, 170, 153–177. [Google Scholar] [CrossRef]
- Deines, P. Carbon isotope effects in carbonate systems. Geochim. Cosmochim. Acta 2004, 68, 2659–2679. [Google Scholar] [CrossRef]
- Zhang, J.; Quay, P.D.; Wilbur, D.O. Carbon isotope fractionation during gas-water exchange and dissolution of CO2. Geochim. Cosmochim. Acta 1995, 59, 107–114. [Google Scholar] [CrossRef]
- Liu, J.; Han, G. Effects of chemical weathering and CO2 outgassing on δ13CDIC signals in a karst watershed. J. Hydrol. 2020, 589, 125192. [Google Scholar] [CrossRef]
- Xie, Y.C.; Zhu, T.B.; Yang, H.; Huang, F.; Zhang, C.L.; Luo, W.Q.; Huang, Q.B. Chemical weathering of carbonate rocks by sulfur acid in typical karst catchment and its implication for carbon cycle: A case study in Pingguo, Guangxi province, southwest China. Quat. Sci. 2017, 37, 1271–1282. [Google Scholar]
- Qin, C.; Li, S.L.; Yue, F.J.; Xu, S.; Ding, H. Spatiotemporal variations of dissolved inorganic carbon and controlling factors in a small karstic catchment, Southwestern China. Earth Surf. Proc. Land. 2019, 44, 2423–2436. [Google Scholar] [CrossRef]
- Wang, Z.J.; Yin, J.J.; Pu, J.B.; Wang, P.; Liang, X.; Yang, P.H.; He, Q.F.; Gou, P.F.; Yuan, D. Integrated understanding of the critical zone processes in a subtropical karst watershed (Qingmuguan, Southwestern China): Hydrochemical and isotopic constraints. Sci. Total Environ. 2020, 749, 141257. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, Z.; Zhao, M.; Yang, R. Hydrologically-driven variations in the karstrelated carbon sink fluxes: Insights from high-resolution monitoring of three karst catchments in Southwest China. J. Hydrol. 2016, 533, 74–90. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, Z.; Chen, B.; Hu, Y.; Zeng, S.; Zeng, C.; Yang, R.; He, H.; Zhu, H.; Cai, X.; et al. Carbonate weathering-related carbon sink fluxes under different land uses: A case study from the Shawan Simulation Test Site, Puding, Southwest China. Chem. Geol. 2017, 474, 58–71. [Google Scholar] [CrossRef]
- Williams, E.L.; Szramek, K.J.; Jin, L.X.; Timothy, C.W.K.; Lynn, M. The carbonate system geochemistry of shallow groundwater surface water systems in temperate glaciated watersheds (Michigan, USA): Significance of open system dolomite weathering. Geol. Soc. Am. Bull. 2007, 119, 515–528. [Google Scholar] [CrossRef]
- Szramek, K.; Walter, L.M.; Kandu, T.; Ogrinc, N. Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soca Rivers, Slovenia). Aquat. Geochem. 2011, 17, 357–396. [Google Scholar] [CrossRef]
- Lerman, A.; Wu, L.; Mackenzie, F.T. CO2 and H2SO4 consumption in weathering and material transport to the ocean, and their role in the global carbon balance. Mar. Chem. 2007, 106, 326–350. [Google Scholar] [CrossRef]
- Martin, J.B. Carbonate minerals in the global carbon cycle. Chem. Geol. 2017, 449, 58–72. [Google Scholar] [CrossRef]
- Calmels, D.; Gaillardet, J.; Brenot, A.; France-Lanord, C. Sustained sulfide oxidation by physical erosion processes in the Mackenzie River basin: Climatic perspectives. Geology 2007, 35, 1003–1006. [Google Scholar] [CrossRef]
- Ali, H.N.; Atekwana, E.A. The effect of sulfuric acid neutralization on carbonate and stable carbon isotope evolution of shallow groundwater. Chem. Geol. 2011, 284, 217–228. [Google Scholar] [CrossRef]
- Goldscheider, N.; Chen, Z.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Hartmann, J.; Jiang, G.H.; Moosdorf, N.; Stevanovic, Z.; et al. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 2020, 28, 1661–1677. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).