Application of Chitosan and Its Derivatives as Bioflocculants for Iron and Turbidity Removal from Filter Backwash Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Water Samples
2.3. Coagulants and Flocculants—Preparation for Application
2.4. Synthesis of Chitosan Derivatives (DCT and CMC)
2.5. Degree of Substitution (DS)
2.6. Characterization of Chitosan and Its Derivatives
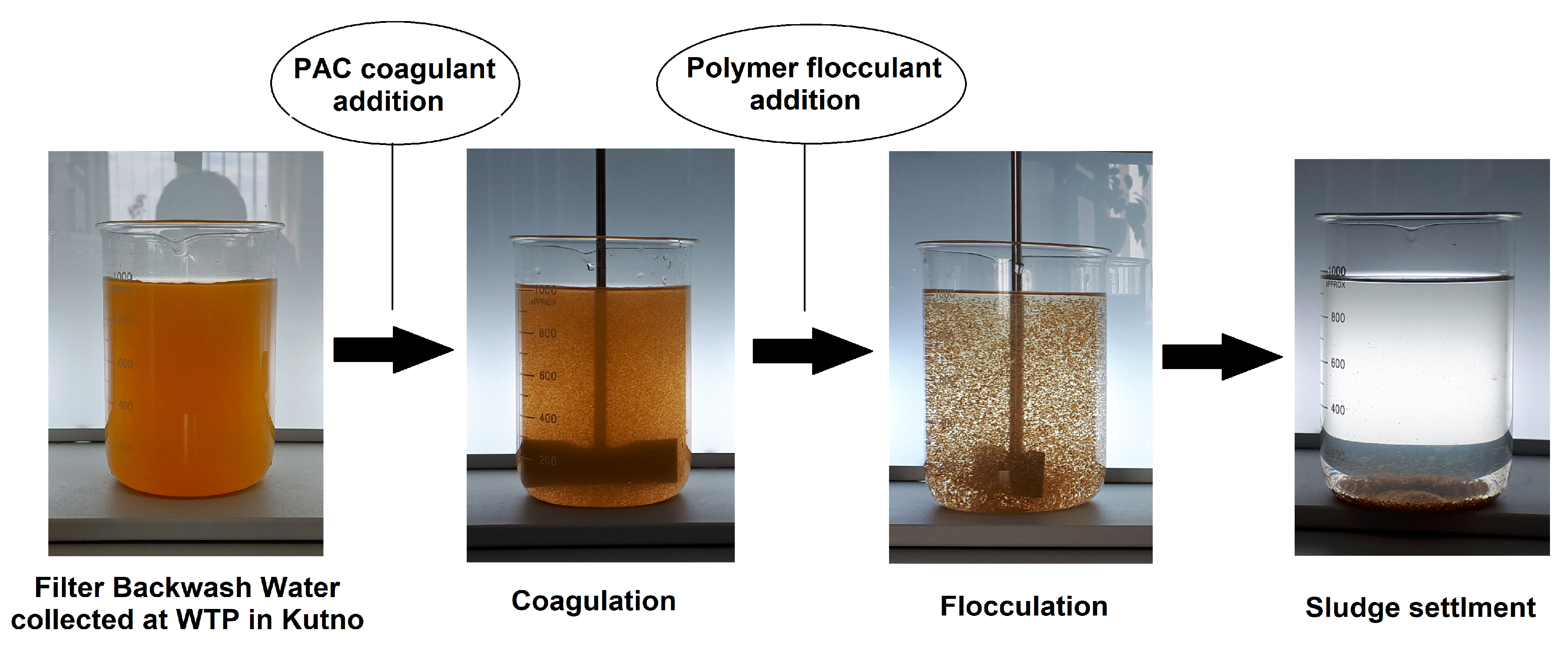
2.7. Jar Tests
- −
- Placing the samples of the tested water in equal amounts (1 L of the sample) in the beakers;
- −
- Dosing each beaker with a different dose of the appropriate chemical compound, i.e., the flocculant;
- −
- Setting the appropriate mixing speed, which should reflect the conditions prevailing in a given water treatment system (usually these values oscillate around 250 rpm for fast mixing and 25–30 rpm for slow mixing);
- −
- Subjecting the water in the beakers to the sedimentation process at a time corresponding to the actual process in the WTP;
- −
- Taking a sample from each beaker for the analysis, the results of which allow for determination of the optimal parameters of the water purification process [53].
2.8. Determination of Total Iron Concentration and Turbidity of Water Samples
- −
- Selecting the measurement method and setting the wavelength to 510 nm;
- −
- Collecting 10 mL from the water samples in a measurement cell;
- −
- Adding the reagent (FerroVer Iron Reagent Powder Pillow) to the sample cell;
- −
- Mixing the solution followed by standing for 3 min;
- −
- Performing a blank measurement without the addition of a reagent (i.e., the total iron concentration in mg/L);
- −
- After the required time, the cell is placed in the cuvette holder and the reading starts
- −
- The device performs several measurements of the placed sample and displays the finished result.
3. Results
3.1. Synthesis of Chitosan Derivatives
3.2. Degree of Substitution and the Elemental Analysis
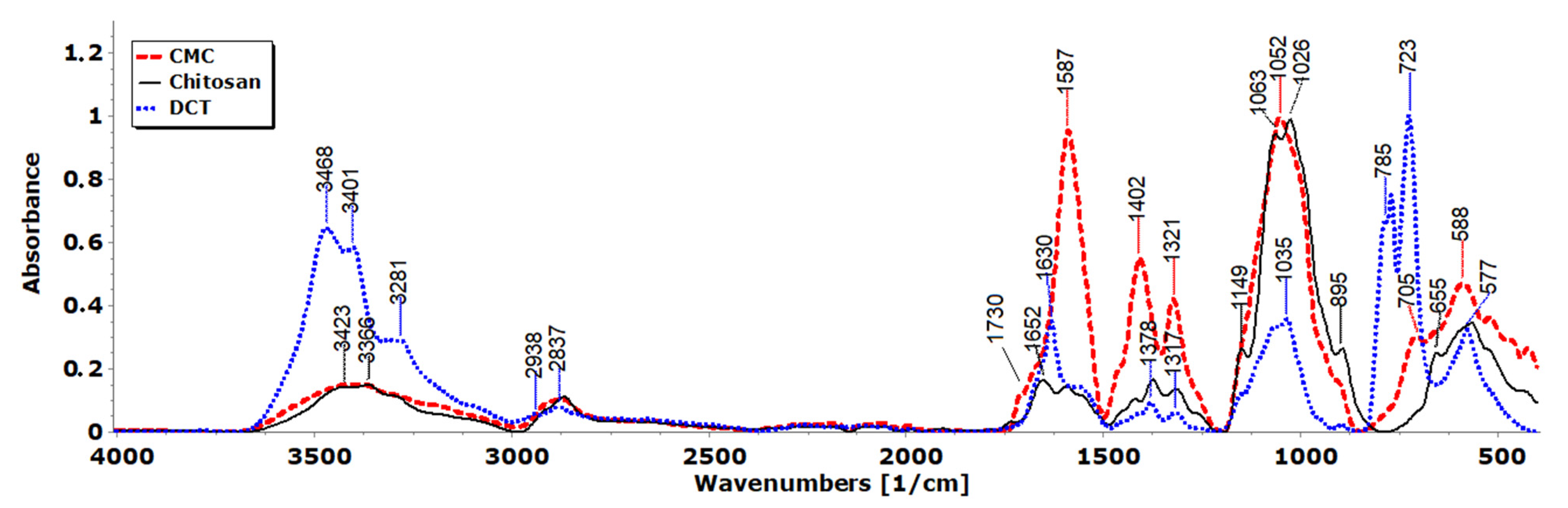
3.3. FTIR Spectra
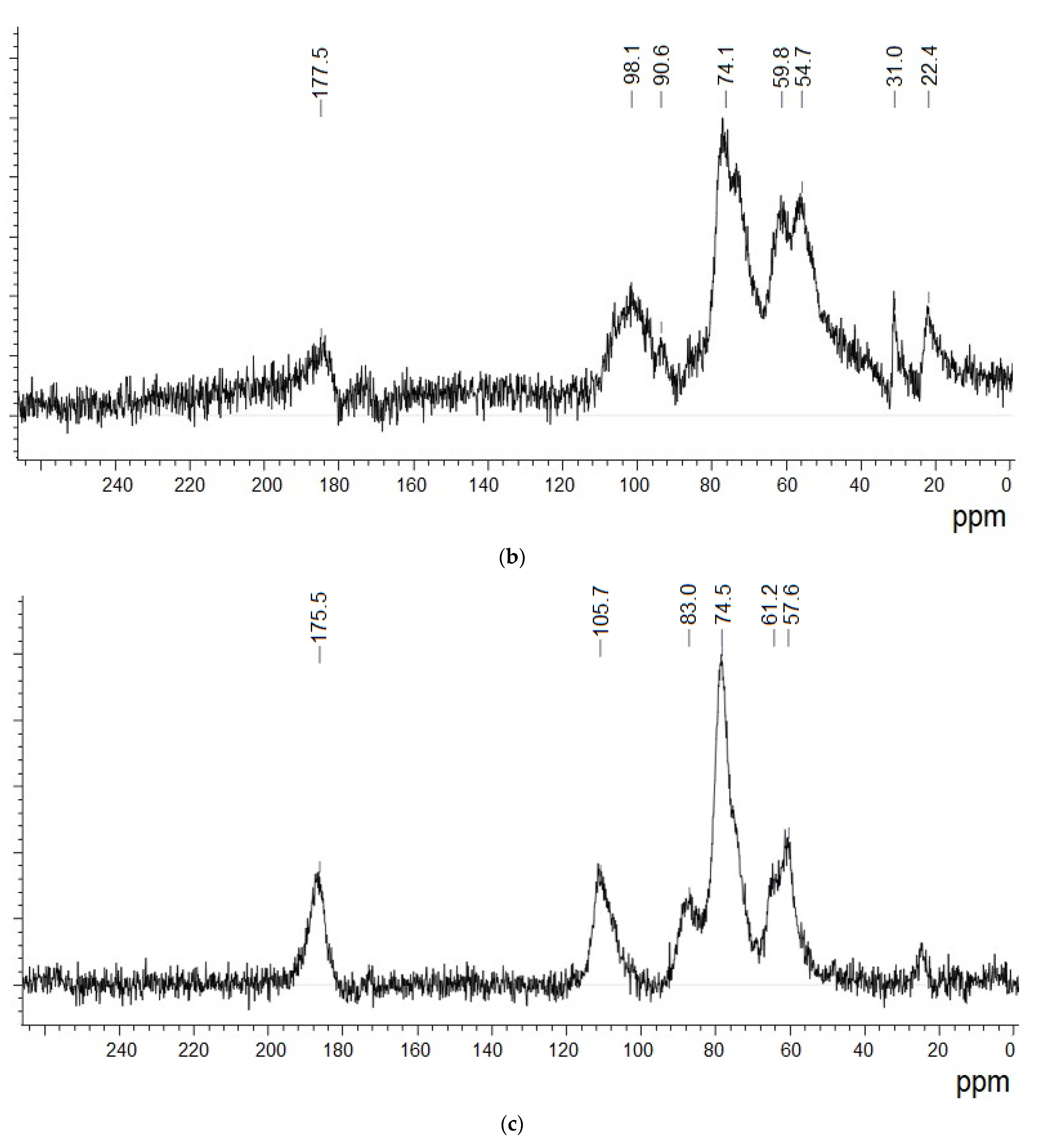
3.4. 13C NMR Data Analysis
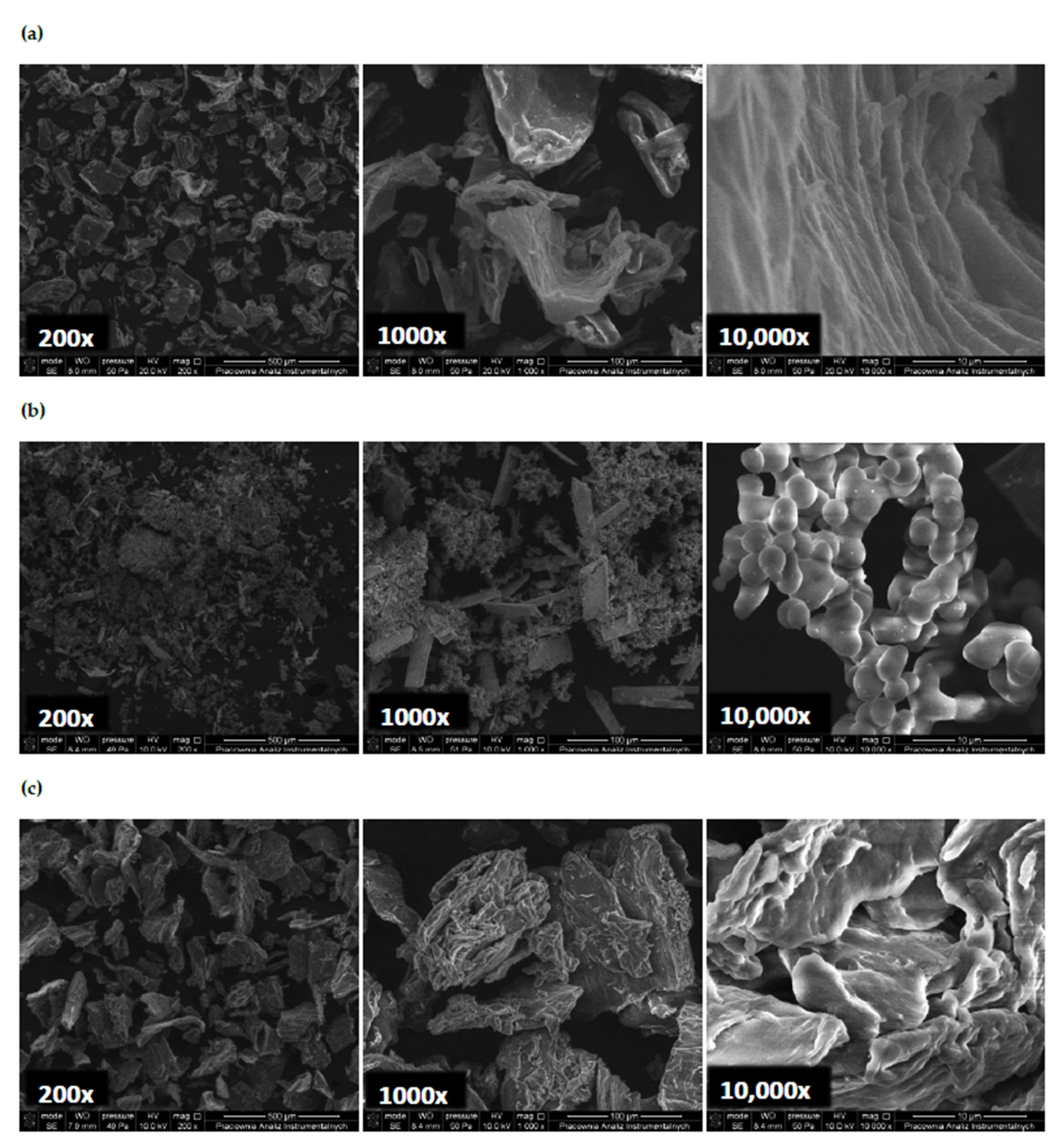
3.5. SEM Images
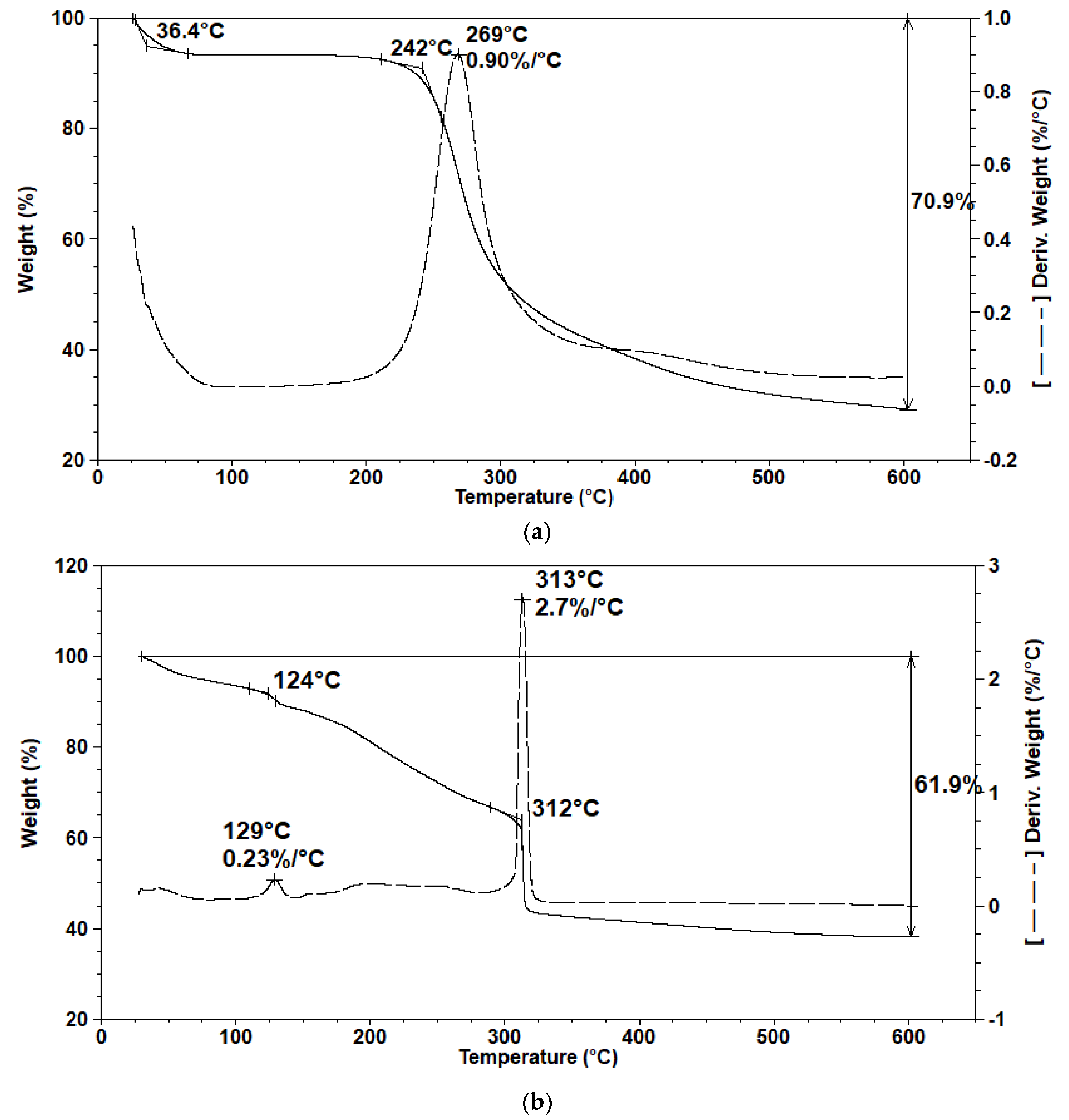
3.6. Thermal Analysis
3.7. Iron and Turbidity Removal Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El-taweel, R.M.; Mohamed, N.; Alrefaey, K.A.; Husien, S.; Abdel-Aziz, A.B.; Salim, A.I.; Mostafa, N.G.; Said, L.A.; Fahim, I.S.; Radwan, A.G. A review of coagulation explaining its definition, mechanism, coagulant types, and optimization models; RSM, and ANN. Curr. Opin. Green Sustain. Chem. 2023, 6, 100358. [Google Scholar] [CrossRef]
- Bray, R.T.; Fitobór, K. Sizes of iron hydroxide particles formed during ferric coagulation processes. Des. Water 2017, 64, 419–424. [Google Scholar] [CrossRef]
- Cui, H.; Huang, X.; Yu, Z.; Chen, P.; Cao, X. Application progress of enhanced coagulation in water treatment. RSC Adv. 2020, 10, 20231–20244. [Google Scholar] [CrossRef] [PubMed]
- Saritha, V.; Srinivas, N.; Srikanth Vuppala, N.V. Analysis and optimization of coagulation and flocculation process. Appl. Water Sci. 2017, 7, 451–460. [Google Scholar] [CrossRef]
- Naceradska, J.; Pivokonska, L.; Pivokonsky, M. On the importance of pH value in coagulation. J. Water Supply Res. Technol. Aqua 2019, 68, 222–230. [Google Scholar] [CrossRef]
- Cao, B.; Gao, B.; Xu, C.; Fu, Y.; Liu, X. Effects of pH on coagulation behavior and floc properties in Yellow River water treatment using ferric based coagulants. Chin. Sci. Bull. 2010, 55, 1382–1387. [Google Scholar] [CrossRef]
- Cao, B.; Gao, B.; Liu, X.; Wang, M.; Yang, Z.; Yue, Q. The impact of pH on floc structure characteristic of polyferric chloride in a low DOC and high alkalinity surface water treatment. Water Res. 2011, 45, 6181–6188. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.S.; Li, Y. Modeling and Optimization of New Flocculant Dosage and pH for Flocculation: Removal of Pollutants from Wastewater. Water 2013, 5, 342–355. [Google Scholar] [CrossRef]
- Martín, M.A.; González, I.; Berrios, M.; Siles, J.A.; Martín, A. Optimization of coagulation–flocculation process for wastewater derived from sauce manufacturing using factorial design of experiments. Chem. Eng. J. 2011, 172, 771–782. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Progr. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Yan, N.; Farnood, R. Recent advances in polysaccharide bio-based flocculants. Biotechnol. Adv. 2018, 62, 92–119. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Y.; Tang, X.; Jiang, J.; He, Q.; Xiong, Z.; Zheng, H. Biopolymer-based flocculants: A review of recent technologies. Environ. Sci. Pollut. Res. 2021, 28, 46934–46963. [Google Scholar] [CrossRef] [PubMed]
- Mansour, Y.H.; Othmani, B.; Rebah, F.B.; Mnif, W.; Saoudi, M.; Khadhraoui, M. Could plant-based flocculants substitute the conventional synthetic chemicals in the sludge dewatering process? Water 2023, 15, 2602. [Google Scholar] [CrossRef]
- Kolya, H.; Sasmal, D.; Tripathy, T. Novel biodegradable flocculating agents based on grafted starch family for the industrial effluent treatment. Polym. Environ. 2017, 25, 408–418. [Google Scholar] [CrossRef]
- Grenda, K.; Arnold, J.; Gamelas, A.F.; Rasteiro, M.G. Environmental friendly cellulose-based polyelectrolytes in water treatment. Water Sci. Technol. 2017, 76, 1490–1499. [Google Scholar]
- Barrero-Fernández, A.; Aguado, R.; Moral, A.; Brindley, C.; Ballesteros, M. Applications of cellulose-based agents for flocculation processes: A bibliometric analysis. Cellulose 2021, 28, 9857–9871. [Google Scholar] [CrossRef]
- Pontius, F.W. Chitosan as a drinking water treatment coagulant. Am. J. Civil Eng. 2016, 4, 205–2015. [Google Scholar] [CrossRef]
- Al-Manhel, A.J.; Al-Hilphy, A.R.S.; Niamah, A.K. Extraction of chitosan, characterisation and its use for water purification. J. Saudi Soc. Agricultur. Sci. 2018, 17, 186–190. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Lu, W.; Liu, Z.; Xu, C.; Chen, Y.; Zheng, H. Preparation of a graft modified flocculant based on chitosan by ultrasonic initiation and its synergistic effect with kaolin for the improvement of acid blue 83 (AB 83) removal. Int. J. Biol. Macromol. 2020, 150, 617–630. [Google Scholar] [CrossRef]
- Mohammadi, E.; Daraei, H.; Ghanbari, R.; Athar, S.D.; Zandsalimi, Y.; Ziaee, A.; Maleki, A.; Yetilmezsoy, K. Synthesis of carboxylated chitosan modified with ferromagnetic nanoparticles for adsorptive removal of fluoride, nitrate, and phosphate anions from aqueous solutions. J. Mol. Liquids. 2019, 273, 116–124. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, A.; Pan, S.; Sun, W.; Zhu, C.; Shah, K.J.; Zheng, H. Novel chitosan-based flocculants for chromium and nickle removal in wastewater via integrated chelation and flocculation. J. Environ. Management 2019, 248, 109241. [Google Scholar] [CrossRef] [PubMed]
- Renault, F.; Sancey, B.; Badot, P.-M.; Crini, G. Chitosan for coagulation/flocculation processes—An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Lan, L.E.; Reina, F.D.; De Seta, G.E.; Meichtry, J.M.; Litter, M.I. Comparison between different technologies (zerovalent iron, coagulation-flocculation adsorption) for arsenic treatment at high concentration. Water 2023, 15, 1481. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, Y.; Zhou, J.; Chen, A.; Shah, K.J. preparation and characterization of high-efficiency magnetic heavy metal capture flocculation. Water 2021, 13, 1732. [Google Scholar] [CrossRef]
- Xu, Y.; Gan, K.; Liang, S.; Liu, H.; Wang, Q. Investigation and optimization of chitosan performance in flocculating kaolin suspensions using a real-time suspending solid concentration measuring method. Water 2021, 13, 513. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, L.; Sang, X.; Shi, G.; Ni, C. Preparation and flocculation performance study of a novel amphoteric alginate flocculant. J. Phys. Chem. Solids 2020, 141, 109408. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Song, G.; Lou, T. Microwave assisted copolymerization of sodium alginate and dimethyl diallyl ammonium chloride as flocculant for dye removal. Int. J. Biol. Macromol. 2020, 156, 585–590. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.-F.; Lam, S.S.; Sonne, C.; Yuan, T.-Q.; Song, G.-Y.; Sun, R.-C. A review on production of lignin-based flocculants: Sustainable feedstock and low carbon footprint applications. Renewable Sustainable Energy Rev. 2020, 134, 110384. [Google Scholar] [CrossRef]
- Patra, P.; Patra, A.S.; Mukherjee, A.K.; Pal, S. Development of a highly efficient selective flocculant based on functionalized β-cyclodextrin toward beneficiation of low-quality iron ore. Polym. Adv. Technol. 2021, 32, 2169–2175. [Google Scholar] [CrossRef]
- Baran, T.; Menteş, A.; Arslan, H. Synthesis and characterization of water soluble O-carboxymethyl chitosan Schiff bases and Cu(II) complexes. Int. J. Biol. Macromol. 2015, 72, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Jain, S.K. A new horizon in modifications of chitosan: Syntheses and applications. Crit. Rev. Ther. Drug 2013, 30, 91–181. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.L.; Du, Y.L.; Wang, C.M. Synthesis of carboxymethylated chitosan and its adsorption properties for cadmium (II), lead (II) and copper (II) from aqueous solutions. Water Sci. Technol. 2009, 60, 467–474. [Google Scholar] [CrossRef]
- Mondal, S.K.; Wu, C.; Nwadire, F.C.; Rownaghi, A.; Kumar, A.; Adewuyi, Y.; Okoronkwo, M.U. Examining the effect of chitosan biopolymer on alkali-activated inorganic material for aqueous Pb (II) and Zn (II) sorption. Langmuir 2022, 38, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska-Czubenko, J.; Piróg, M.; Gierszewska, M. Modification of chitosan—A concise overview. Wiad.Chem. 2016, 70, 9–10. [Google Scholar]
- Kelar, K. Modyfikacja Polimerów; Wydawnictwo Politechniki Poznańskiej: Poznań, Poland, 1991. [Google Scholar]
- Braun, D.; Cherdron, H.; Rehahn, M.; Ritter, H.; Voit, B. Polymer Synthesis: Theory and Practice. Fundamentals, Methods, Experiments; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Wang, B.; Zhang, Y.; Miao, C. Preparation of cationic chitosan-polyacrylamide flocculant and its properties in wastewater treatment. J. Ocean. Univ. China 2011, 10, 42–46. [Google Scholar] [CrossRef]
- Yang, Z.; Shang, Y.; Lu, Y.; Chen, Y.; Huang, X.; Chen, A.; Jiang, Y.; Gu, W.; Qian, X.; Yang, H.; et al. Flocculation properties of biodegradable amphoteric chitosan-based flocculants. Chem. Eng. J. 2011, 172, 287–295. [Google Scholar] [CrossRef]
- Tao, L.; Xuejun, W.; Guojun, S.; Guangpeng, C. Synthesis and flocculation performance of a chitosan-acrylamide-fulvic acid ternary copolymer. Carbohydr. Polym. 2017, 170, 182–189. [Google Scholar]
- You, L.; Lu, F.; Li, D.; Qiao, Z.; Yin, Y. Preparation and flocculation properties of cationic starch/chitosan crosslinking-copolymer. J. Hazard. Mater. 2009, 172, 38–45. [Google Scholar] [CrossRef]
- Wei, T.; Wu, L.; Yu, F.; Lv, Y.; Chen, L.; Shi, Y.; Dai, B. pH-responsive chitosan-based flocculant for precise dye flocculation control and the recycling of textile dyeing effluents. RSC Adv. 2018, 8, 39334–39340. [Google Scholar] [CrossRef]
- Chen, X.G.; Park, H.J. Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydr. Polym. 2003, 53, 355–359. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Application of carboxymethyl polysaccharides as bio-sorbents for the sequestration of heavy metals in aquatic environments. Carbohydr. Polym. 2020, 237, 116142. [Google Scholar] [CrossRef] [PubMed]
- Mucha, M. Chitozan Wszechstronny Polimer ze Źródeł Odnawialnych, 1st ed.; WNT: Warszawa, Poland, 2010; pp. 91–96. [Google Scholar]
- Agbovi, H.K.; Wilson, L.D. Design of amphoteric chitosan flocculants for phosphate and turbidity removal in wastewater. Carbohydr. Polym. 2018, 189, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C. Improvement of aquaculture wastewater using chitosan of different degrees of deacetylation. Environ. Technol. 2006, 27, 1199–1208. [Google Scholar] [CrossRef]
- Dong, C.; Chen, W.; Liu, C. Flocculation of algal cells by amphoteric chitosan-based flocculant. Bioresour. Technol. 2014, 170, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.-Y.; Ma, C.-X.; Wen-Rong Hu, W.-R.; Sun, F. The behaviors of Microcystis aeruginosa cells and extracellular microcystins during chitosan flocculation and flocs storage processes. Bioresour. Technol. 2014, 151, 314–322. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Widayat, W.; Christwardana, M.; Monica Pratiwi, M.E. The flocculation process of Chlorella sp. using chitosan as a bio-flocculant: Optimization of operating conditions by response surface methodology. Curr. Res. Green Sustain. Chem. 2022, 5, 100291. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Yan, N. Recent advances in extracellular biopolymer flocculants. Biotechnol. Adv. 2014, 32, 1506–1522. [Google Scholar] [CrossRef]
- Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M.; Węgrzynowska-Drzymalska, K.; Burkowska-But, A. The use of chitosan and starch-based flocculants for filter backwash water treatment. Materials 2022, 15, 1056. [Google Scholar] [CrossRef]
- Zane Satterfield, P.E. Jar Testing; Tech Brief, Publisher by The National Environmental Services Center, Spring. 2005, Volume 5. Issue 1. Available online: https://www.nesc.wvu.edu/files/d/3cf372e5-ba40-450c-a3ad-cd774f4c3345/jar-testing.pdf (accessed on 22 June 2023).
- Hach Company. Hach Method 8008. USEPA FerroVer®Method; DOC316.53.01053; Hach Company: Ames, IA, USA, 2014. [Google Scholar]
- Method 180.1: Determination of Turbidity by Nephelometry. Available online: https://www.epa.gov/sites/production/files/2015-08/documents/method_180-1_1993.pdf (accessed on 26 March 2023).
- Heinze, T.; Koschella, A. Carboxymethyl ethers of cellulose and starch—Review. Macromol. Symp. 2005, 223, 13–40. [Google Scholar] [CrossRef]
- Węgrzynowska-Drzymalska, K.; Gebicka, P.; Młynarczyk, D.T.; Chełminiak-Dudkiewiecz, D.; Kaczmarek, H.; Gośliński, T.; Ziegler-Borowska, M. Crosslinking of chitosan with dialdehyde chitosan as a new approach for biomedical applications. Materials 2020, 13, 3413. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Zhou, J.; Yang, G.; Du, Y. Blend membranes from carboxymethylated chitosan/alginate in aqueous solution. J. App. Polym. Sci. 2000, 77, 610–616. [Google Scholar] [CrossRef]
- Zhao, X.; Kato, K.; Fukumoto, Y.; Nakamae, K. Synthesis of bioadhesive hydrogels from chitin derivatives. Int. J. Adhes. Adhes. 2001, 21, 227–232. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N.; Tiwari, A. Carboxymethyl chitosan and its applications. Adv. Mater. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Sun, L.; Du, Y.; Fan, L.; Chen, X.; Yang, J. Preparation, characterization and antimicrobial activity of quaternized carboxymethyl chitosan and application as pulp-cap. Polymer 2006, 47, 1796–1804. [Google Scholar] [CrossRef]
- Zheng, M.; Han, B.; Yang, Y.; Liu, W. Syntheis, characterization and biological safety of O-carboxymethyl chitosan used to treat Sarcoma 180 tumor. Carbohydr. Polym. 2011, 86, 231–238. [Google Scholar] [CrossRef]
- Almeida, V.; Frollini, E.; Castellan, A.; Coma, V. Chitosan, sisal cellulose, and biocomposite chitosan/sisal cellulose films prepared from thiourea/NaOH aqueous solution. Carbohydr. Polym. 2010, 80, 655–664. [Google Scholar] [CrossRef]
- Dao, V.H.; Cameron, N.R.; Saito, K. Synthesis, properties and performances of organic polymers employed in flocculation applications. Polym. Chem. 2016, 7, 11–25. [Google Scholar] [CrossRef]
- Bolto, B.; Gregory, J. Organic polyelectrolytes in water treatment. Water Res. 2007, 41, 2301–2324. [Google Scholar] [CrossRef] [PubMed]
- Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M. Recent achievements in polymer-based flocculants for water treatment. Materials 2020, 13, 3951. [Google Scholar] [CrossRef] [PubMed]
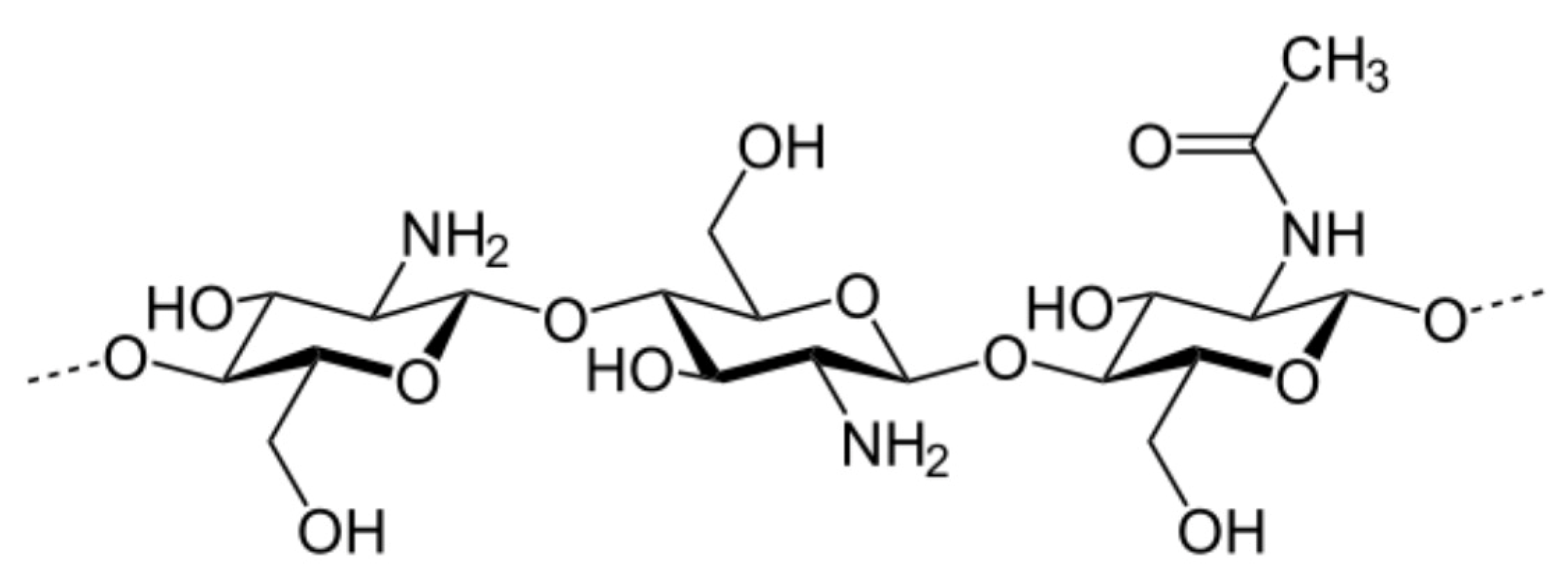
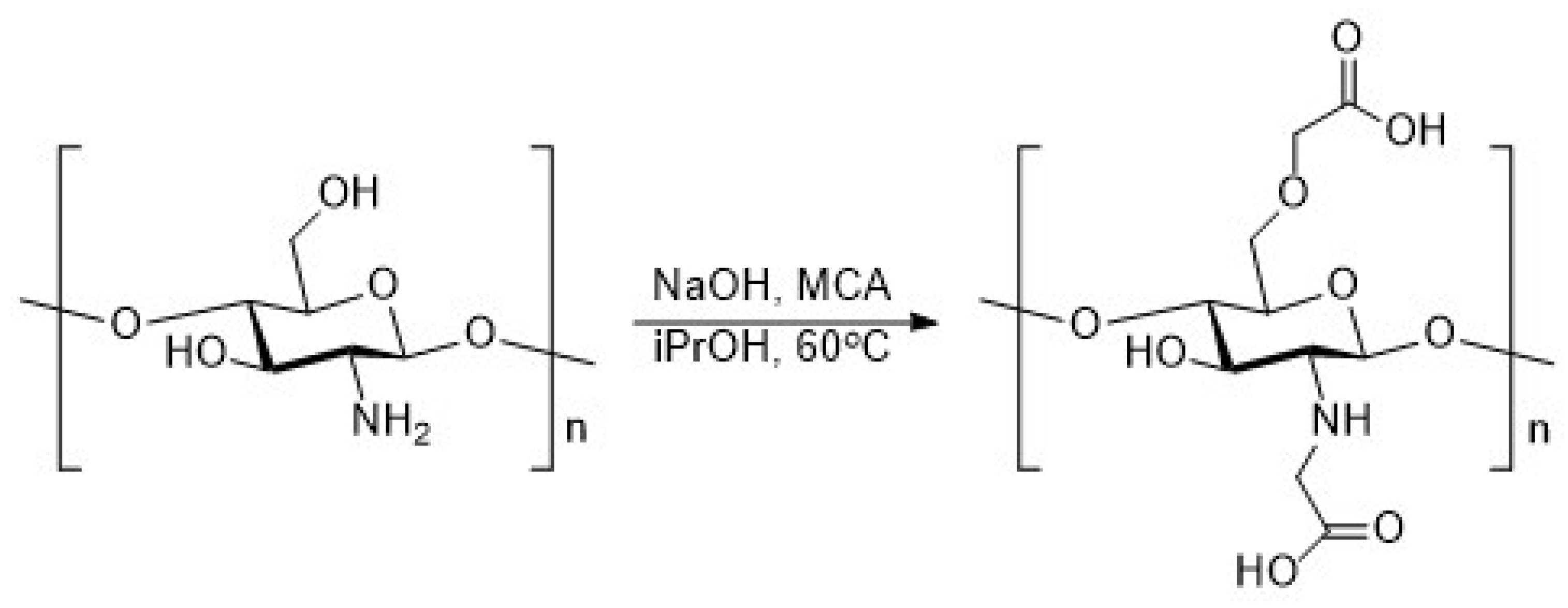

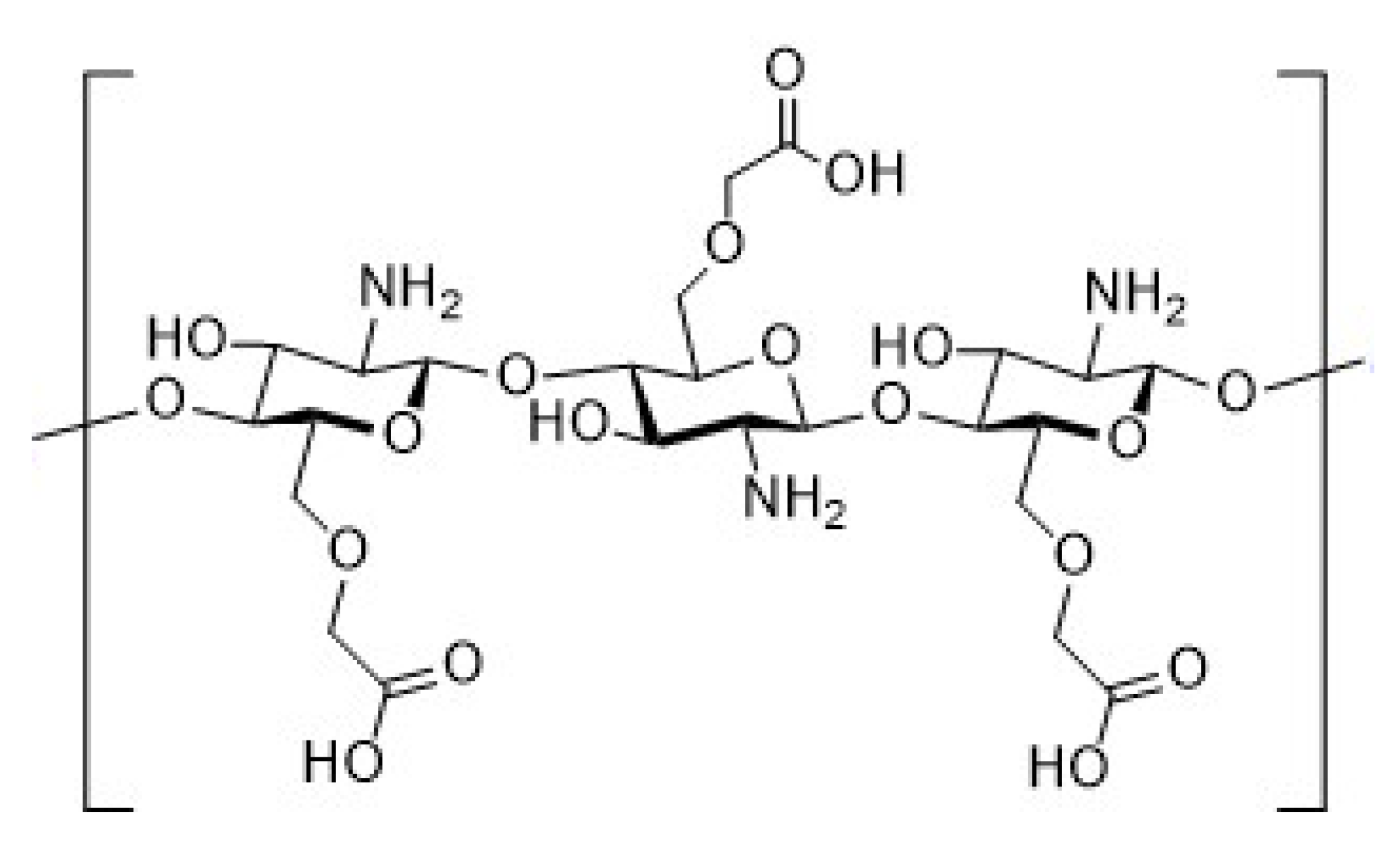

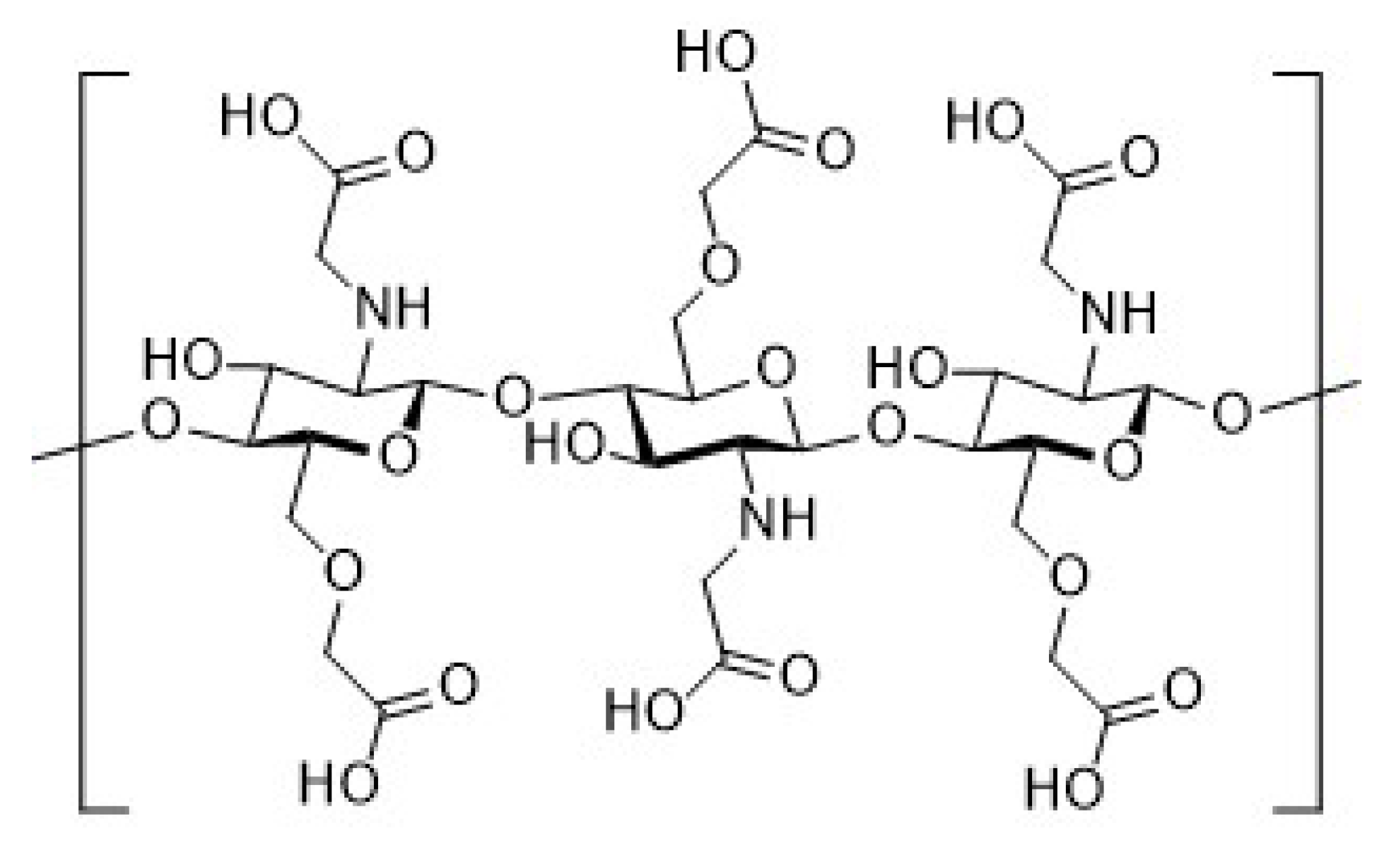




| Sample | Theoretical Values [%] | Elemental Analysis [%] | Empirical Formula | DS [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | C | H | N | O | |||
| Chitosan | 44.72 | 6.83 | 8.70 | 39.75 | 40.57 | 5.21 | 7.07 | 47.15 | C6.6H10.2NO5.8 | - |
| DCT | 45.00 | 5.00 | 0.00 | 50.00 | 29.52 | 3.75 | 3.86 | 62.80 | C8.8H13.4NO14 | 44.29 |
| CMC | 42.86 | 5.36 | 4.17 | 47.62 | 35.40 | 5.99 | 5.12 | 53.49 | C8H16.2NO9 | 49.55 |
| Water Sample | Total Iron Concentration [mg Fe/L] | Turbidity [NTU] | Temperature [°C] | pH |
|---|---|---|---|---|
| Raw ground water | 5.41 | 6.48 | 9.80 | 6.94 |
| Filter Backwash Water | 29.59 | 260.33 | 13.40 | 7.56 |
| Sample | Optimal Dose [mg/L] | Removal Efficiency [%] | |
|---|---|---|---|
| Iron Concentration | Turbidity | ||
| Chitosan | 0.2 | 98.11 ± 0.17 | 96.76 ± 0.65 |
| DCT | 1.0 | 93.19 ± 0.98 | 93.51 ± 1.01 |
| CMC | 0.2 | 98.97 ± 0.13 | 98.26 ± 0.10 |
| Superfloc A100PWG * | 0.5 | 92.17 ± 0,37 | 93.89 ± 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M. Application of Chitosan and Its Derivatives as Bioflocculants for Iron and Turbidity Removal from Filter Backwash Water. Water 2023, 15, 2913. https://doi.org/10.3390/w15162913
Maćczak P, Kaczmarek H, Ziegler-Borowska M. Application of Chitosan and Its Derivatives as Bioflocculants for Iron and Turbidity Removal from Filter Backwash Water. Water. 2023; 15(16):2913. https://doi.org/10.3390/w15162913
Chicago/Turabian StyleMaćczak, Piotr, Halina Kaczmarek, and Marta Ziegler-Borowska. 2023. "Application of Chitosan and Its Derivatives as Bioflocculants for Iron and Turbidity Removal from Filter Backwash Water" Water 15, no. 16: 2913. https://doi.org/10.3390/w15162913
APA StyleMaćczak, P., Kaczmarek, H., & Ziegler-Borowska, M. (2023). Application of Chitosan and Its Derivatives as Bioflocculants for Iron and Turbidity Removal from Filter Backwash Water. Water, 15(16), 2913. https://doi.org/10.3390/w15162913







