BENFEP Spheres: New Porous Adsorbent Material for Arsenic Removal from Natural Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Analysis
2.3. Determination of Surface Area and Porosity
2.4. Point of Zero Charge (PZC) Analysis
2.5. Batch Adsorption Experiments
2.6. Thermodynamic Studies
2.7. Water Sampling
3. Results and Discussion
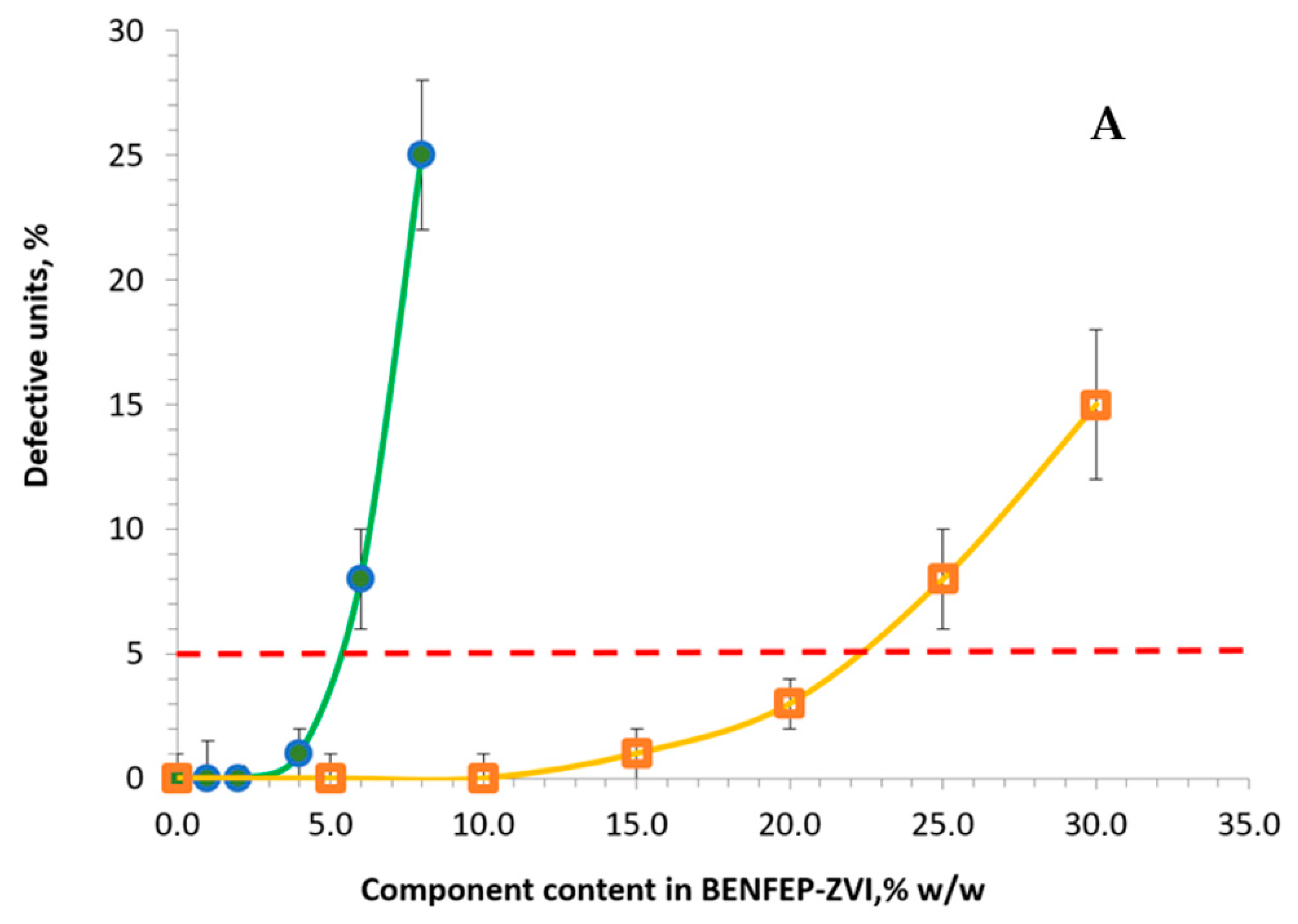
3.1. Synthesis and Characterization of Materials
3.2. Porosity and Surface Area
3.3. pH Effect
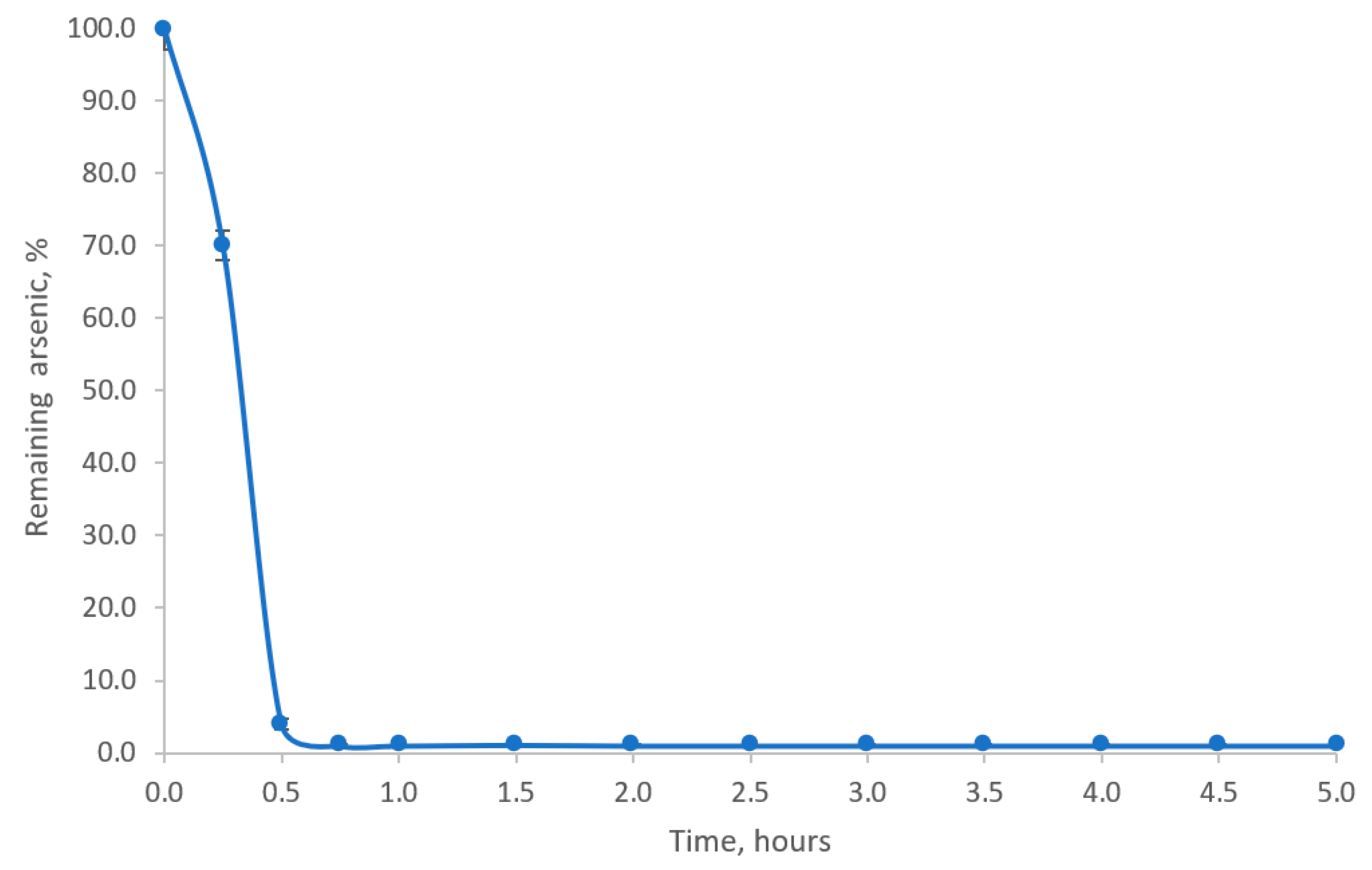
3.4. Adsorption Isotherm Studies
3.5. Thermodynamic Studies
3.6. Adsorbent Regeneration
3.7. Natural Water Sample Test
| Parameters | Unit | Before Treatment | After Treatment | Test Method * | |
|---|---|---|---|---|---|
| Electrical conductivity | mS/cm | 1.90 ± 0.02 | 1.94 ± 0.03 | SMWW 2510B | |
| Sulfates | SO42− | mg/L | 340 ± 10 | 325 ± 8 | SMWW 4500-SO42-D |
| Chloride | Cl− | mg/L | 350 ± 5 | 332 ± 6 | SMWW 4500-Cl-B |
| Calcium | Ca | mg/L | 310 ± 3 | 323 ± 3 | SMWW 3111B |
| Iron | Fe | mg/L | <0.1 | <0.1 | SMWW 3111B |
| Manganese | Mn | mg/L | <0.1 | <0.1 | SMWW 3111B |
| Copper | Cu | mg/L | <0.1 | <0.1 | SMWW 3111B |
| Zinc | Zn | mg/L | <0.1 | <0.1 | SMWW 3111B |
| Lead | Pb | mg/L | <0.1 | <0.1 | SMWW 3111B |
| Chromium | Cr | mg/L | <0.1 | <0.1 | SMWW 3111B |
| Total dissolved solids | mg/L | 1750 ± 6 | 1823 ± 9 | SISS-ME-31-2007 | |
| Boron | B | mg/L | 3.1 ± 0.2 | 2.8 ± 0.15 | ISO9390.1990 |
| Total arsenic | As | mg/L | 1.0 ± | <0.010 ± 0.009 | SMWW 3114C |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lan, L.E.; Reina, F.D.; De Seta, G.E.; Meichtry, J.M.; Litter, M.I. Comparison between Different Technologies (Zerovalent Iron, Coagulation-Flocculation, Adsorption) for Arsenic Treatment at High Concentrations. Water 2023, 15, 1481. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- National Academy of Sciences (NAS). Arsenic in Drinking Water; National Academy of Sciences: Washington, DC, USA, 2001. [Google Scholar]
- Nordstrom, D.; Alpers, C. Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site California. Proc. Natl. Acad. Sci. USA 1999, 96, 3455–3462. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry, ATSDR, Addendum to the Toxicological Profile for Arsenic. 2016. Available online: https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=3 (accessed on 12 June 2023).
- Abejon, R.; Garea, A. A bibliometric analysis of research on arsenic in drinking water during the 1992–2012 period: An outlook to treatment alternatives for arsenic removal. J. Water Process. Eng. 2015, 6, 105–119. [Google Scholar] [CrossRef]
- European Union, EU. 2014. European Union (Drinking Water) Regulations 2014. S.I. No. 122 of 2014. Available online: https://www.ecolex.org/details/legislation/european-union-drinking-water-regulations-2014-si-no-122-of-2014-lex-faoc134269/ (accessed on 12 June 2023).
- Kumar, A.; Thakur, A.; Panesar, P.S. A review on the industrial wastewater with the efficient treatment techniques. Chem. Pap. 2023, 77, 4131–4163. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. USEPA, USEPA Integrated Risk Information System (IRIS) Online Database. 2016. Available online: http://www.epa.gov/iris (accessed on 12 June 2023).
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/bitstream/handle/10665/254637/9789241549950-eng.pdf;jsessionid=A03BF55AAAB65FDEE990ED0001BCF997?sequence=1/ (accessed on 12 June 2023).
- Al Samman, M.T.; Sotelo, S.; Sánchez, J.; Rivas, B.L. Arsenic oxidation and its subsequent removal from water: An overview. Sep. Purif. Technol. 2023, 123055, 1383–5866. [Google Scholar] [CrossRef]
- Cornejo, L.; Acarapi, J.; Arenas, M. Development and validation of a method for simultaneous arsenic, antimony, selenium and mercury determination in plants by energy dispersive X ray fluorescence spectrometry. Interciencia 2018, 43, 425–433. [Google Scholar]
- Gamboa, J.; Cornejo, L.; Acarapi, J.; Squella, J. Determination of arsenic (III) by differential pulse polarography in the waters of Camarones area, Chile. J. Chil. Chem. Soc. 2013, 53, 2031–2034. [Google Scholar] [CrossRef] [Green Version]
- Akram, Z.; Jalali, S.; Shami, S.A.; Ahmad, L.; Batool, S.; Kalsoom, O. Adverse effects of arsenic exposure on uterine function and structure in female rat. Exp. Toxicol. Pathol. 2010, 62, 451–459. [Google Scholar] [CrossRef]
- Cornejo, L.; Lienqueo, H.; Arenas, M.; Acarapi, J.; Yañez, J.; Mansilla, H. In field arsenic removal from natural water by zero valent iron. Environ. Pollut. 2008, 156, 827–831. [Google Scholar] [CrossRef]
- Cornejo, L.; Acarapi, J. Fractionation and bioavailability of arsenic in agricultural soils: Solvent extraction tests and their relevance in risk assessment. J. Environ. Sci. Health Part A 2011, 46, 1247–1258. [Google Scholar] [CrossRef]
- Marcela, S.; Eric, L.; Lorena, C.; Jorge, A. Elemental characterization of prehispanic rock art and arsenic in northern Chile. J. Rock Art Res. 2012, 29, 93–107. [Google Scholar]
- International Agency for Research on Cancer, IARC. 2016. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volumes 1–115. Available online: http://monographs.iarc.fr/ENG/Classification/latest_classif.php (accessed on 20 June 2021).
- Marshall, G.; Ferreccio, C.; Yuan, Y.; Michael, N.B.; Steinmaus, C.; Selvin, S.; Liaw, J.; Smith, H.A. Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J. Natl. Cancer Inst. 2007, 99, 920–928. [Google Scholar] [CrossRef] [Green Version]
- Lisabeth, L.D.; Hyeong, A.J.; Chen, J.J.; Shawnita, S.J.; Burke, J.F.; Meliker, J.R. Arsenic in drinking water and stroke hospitalizations in Michigan. Stroke 2010, 41, 2499–2504. [Google Scholar] [CrossRef] [Green Version]
- Islam, R.; Zhao, L.; Zhang, X.; Liu, L.Z. MiR-218-5p/EGFR Signaling in Arsenic-Induced Carcinogenesis. Cancers 2023, 15, 1204. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Laing, G.D. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chem. Eng. J. 2012, 198, 219–227. [Google Scholar] [CrossRef]
- Awual, M.R.; Hossain, M.A.; Shenashen, M.A.; Yaita, T.; Suzuki, S.; Jyo, A. Evaluating of arsenic(V) removal from water by weak-base anion exchange adsorbents. Environ. Sci. Pollut. R. 2013, 20, 421–430. [Google Scholar] [CrossRef]
- Jain, C.; Singh, R. Technological options for the removal of arsenic with special reference to South East Asia. J. Environ. Manag. 2012, 107, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Dilpazeer, F.; Munir, M.; Baloch, M.Y.J.; Shafiq, I.; Iqbal, J.; Saeed, M.; Abbas, M.M.; Shafique, S.; Aziz, K.H.H.; Mustafa, A.; et al. A Comprehensive Review of the Latest Advancements in Controlling Arsenic Contaminants in Groundwater. Water 2023, 15, 478. [Google Scholar] [CrossRef]
- Oscarson, D.; Huang, P.; Liaw, W.; Hammer, U. Kinetics of oxidation of arsenite by various manganese dioxides. Soil Sci. Soc. Am. J. 1983, 47, 644–648. [Google Scholar] [CrossRef]
- Ferguson, J.; Gavis, J. Review of the arsenic cycle in natural waters. Water Res. 1972, 6, 1259–1274. [Google Scholar] [CrossRef]
- Hassan, H. A review on different arsenic removal techniques used for decontamination of drinking water. Environ. Pollut. Bioavailab. 2023, 35, 2165964. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents. A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Murphy, T.; Guo, J. Aquatic Arsenic Toxicity and Treatment; Backhuys Publishers: Leiden, The Netherlands, 2003. [Google Scholar]
- Dutta, S.; Manna, K.; Srivastava, S.K.; Gupta, A.K.; Yadav, M.K. Hollow polyaniline Microsphere/Fe3O4 nanocomposite as an Effective Adsorbent for Removal of Arsenic from Water. Sci. Rep. 2020, 10, 4982. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Feng, Z.; He, Y.; Hou, Y.; Ren, H.; Su, M.; Ni, L.; Liu, Z. Pyrolysis self-activation: An environmentally friendly method to transform biowaste into activated carbon for arsenic removal. Bioresour. Technol. 2023, 368, 128353. [Google Scholar] [CrossRef]
- Grba, N.; Baldermann, A.; Dietzel, M. Novel green technology for wastewater treatment: Geo-material/geopolymer applications for heavy metal removal from aquatic media. Int. J. Sediment Res. 2023, 38, 33–48. [Google Scholar] [CrossRef]
- Thirunavukkarasu, O.; Viraraghavan, T.; Subramanian, K. Arsenic removal from drinking water using granular ferric hydroxide. Water SA 2003, 29, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Altundogan, H.; Altundogan, S.; Tumen, F.; Bildik, M. Arsenic adsorption from aqueous solutions by activated red mud. Waste Manage. 2002, 22, 357–363. [Google Scholar] [CrossRef]
- Do, D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998. [Google Scholar]
- Livesey, N.; Huang, P. Adsorption of arsenate by soils and its relation to selected chemical properties and anions. Soil Sci. 1981, 131, 88–94. [Google Scholar] [CrossRef]
- Rosenblum, E.; Clifford, D. Equilibrium Arsenic Capacity of Activated Alumina [EPA-600/2-83-107]; U.S. Environmental Protection Agency: Washington, DC, USA, 1983; p. 89.
- Subramanian, K.; Viraraghavan, T.; Phommavong, T.; Tanjore, S. Manganese greensand for removal of arsenic in drinking water. Water Qual. Res. J. Can. 1997, 32, 551–561. [Google Scholar] [CrossRef]
- Zialame, A.; Jamshidi-Zanjani, A.; Darban, A.K.; Homaee, M. Arsenic removal from solution using nano-magnetic compound: Optimization modeling by response surface method. Environ. Monit. Assess 2023, 195, 136. [Google Scholar] [CrossRef]
- Gupta, S.; Chen, K. Arsenic removal by adsorption. J. Water Pollut. Control Fed. 1978, 50, 493–506. [Google Scholar]
- Han, X.; Wang, F.; Zhao, Y.; Meng, J.; Tian, G.; Wang, L.; Liang, J. Recycling of iron ore tailings into magnetic nanoparticles and nanoporous materials for the remediation of water, air and soil: A review. Environ. Chem. Lett. 2023, 21, 1005–1028. [Google Scholar] [CrossRef]
- Maeda, S.; Ohki, A.; Saikoji, S.; Naka, K. Iron(III) hydroxide-loaded coral limestone as an adsorbent for arsenic(III) and arsenic(V). Sep. Sci. Technol. 1992, 27, 681–689. [Google Scholar] [CrossRef]
- Manning, B.; Goldberg, S. Modeling arsenate competitive adsorption on kaolinite, montmorillonite and illite. Clay Clay Miner. 1996, 44, 609–623. [Google Scholar] [CrossRef]
- Das, T.; Bezbaruah, A. Comparative study of arsenic removal by iron-based nanomaterials: Potential candidates for field applications. Sci. Total Environ. 2021, 764, 142914. [Google Scholar] [CrossRef]
- Cundy, A.B.; Hopkinson, L.; Whitby, R.L.D. Use of iron-based technologies in contaminated land and groundwater remediation: A review. Sci. Total Environ. 2008, 400, 42–51. [Google Scholar] [CrossRef]
- Jin, H.Z.; Qiu, C.X.; Li, Y.S.; Liu, B.; Liu, J.Y.; Chen, Q.; Lu, X.F.; Li, C.X.; Wamg, Q.K. Structural and functional design of geopolymer adsorbents: A review. Tungsten 2023. [Google Scholar] [CrossRef]
- Diya’uddeen, B.; Mohammed, I.; Jibril, B.; Bugaje, I. Comparison of Bet and Water Adsorption Techniques. Am. J. Eng. Appl. Sci. 2013, 6, 282–285. [Google Scholar] [CrossRef]
- Ahmedna, M.; Johns, M.; Clark, S.; Marshall, W.; Rao, R. Potential of agricultural byproduct- based activated carbons for use in raw sugar decolourisation. J. Sci. Food Agric. 1997, 75, 117–124. [Google Scholar] [CrossRef]
- Akinyemi, O.P.; Taiwo, E.A. Production of activated carbon from agricultural wastes. In Proceedings of the Nigerian Society of Chemical Engineers, Warri, Nigeria, 20–24 September 2004; pp. 59–62. [Google Scholar]
- Jaramillo-Fierro, X.; Alvarado, H.; Montesdeoca, F.; Valarezo, E. Faujasite-Type Zeolite Obtained from Ecuadorian Clay as a Support of ZnTiO3/TiO2 NPs for Cyanide Removal in Aqueous Solutions. Int. J. Mol. Sci. 2023, 24, 9281. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Md. Hasan Susan, A.B.; Carabineiro, S.A.C. Adsorption of cationic dyes, drugs and metal from aqueous solutions using a polymer composite of magnetic/β-cyclodextrin/activated charcoal/Na alginate: Isotherm, kinetics and regeneration studies. J. Hazard. Mater. 2021, 409, 124840. [Google Scholar] [CrossRef]
- Adefila, S.S.; Aderemi, B.O.; Ajayi, O.A.; Baderin, D.A. Comparative surface area determination using water adsorption method. Niger. J. Eng. Fac. Eng. Ahmadu Bello Univ. Zaria 2003, 11, 88–95. [Google Scholar]
- Mandal, S.; Kumar, M.; Kishore, R. Adsorption studies of arsenic(III) removal from water by zirconium polyacrylamide hybrid material (ZrPACM-43). Water Resour. Ind. 2013, 4, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Thirunavukkarasu, O.; Viraraghavan, T.; Subramanian, K. Removal of arsenic in drinkingwater by iron oxide-coated sand and ferrihydrite—Batch studies. Water Qual. Res. J. Can. 2001, 36, 55–70. [Google Scholar] [CrossRef]
- Triszcz, M.; Porta, A.; Einschlag, F. Effect of operating conditions on iron corrosion rates in zero-valent iron systems for arsenic removal. Chem. Eng. J. 2009, 150, 431–439. [Google Scholar] [CrossRef]
- Giasuddin, A.; Kanel, S.; Choi, H. Adsorption of humic acid onto nanoscale zerovalent iron and its effect on arsenic removal. Environ. Sci. Technol. 2007, 41, 2022–2027. [Google Scholar] [CrossRef]
- Katsoyiannis, I.O.; Ruettimann, T.; Hug, S. pH Dependence of Fenton Reagent Generation and As(III) Oxidation and Removal by Corrosion of Zero Valent Iron in Aerated Water. Environ. Sci. Technol. 2008, 42, 7424–7430. [Google Scholar] [CrossRef]
- Smedley, P.; Kinniburgh, D. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Ye, F.; Ren, X.; Zhao, D.; Sheng, G.; Li, H.; Ma, J.; Wang, X.; Huang, Y. X-ray absorption fine structure study of enhanced sequestration of U(VI) and Se(IV) by montmorillonite decorated zerovalent iron nanoparticles. Environ. Sci. Nano 2016, 3, 1460–1472. [Google Scholar] [CrossRef]
- Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale Zero Valent Iron and Bimetallic Particles for Contaminated Site Remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Wang, Y.; Morin, G.; Ona-nguema, G.; Brown, G. Arsenic(III) and Arsenic(V) Speciation during Transformation of Lepidocrocite to Magnetite. Environ. Sci. Technol. 2014, 48, 14282–14290. [Google Scholar] [CrossRef]
- Wu, C.; Tu, J.; Liu, W.; Zhang, J.; Chu, S.; Lu, G.; Lin, Z.; Danga, Z. The double influence mechanism of pH to the arsenic removal 2 by nano zero valent iron: Electrostatic interactions and the corrosion of Fe0. Environ. Sci. Nano 2017, 4, 1544–1552. [Google Scholar] [CrossRef]
- Guaya, D.; Maza, L.; Angamarca, A.; Mendoza, E.; García, L.; Valderrama, C.; Cortina, J.L. Fe3+/Mn2+ Oxy)Hydroxide Nanoparticles Loaded onto Muscovite/Zeolite Composites (Powder, Pellets and Monoliths): Phosphate Carriers from Urban Wastewater to Soil. Nanomaterials 2022, 12, 3848. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.; Postma, D.; Jakobsen, R. Release of arsenic associated with the reduction and transformation of iron oxides. Geochim. Cosmochim. Acta 2006, 70, 4116–4129. [Google Scholar] [CrossRef]
- Alarcon, M.; Lopez, M. Technical feasibility of using magnetic nanoparticles obtained from metallic wool for arsenite (As (III)) removal from aqueous solutions. J. Nanosci. Technol. 2016, 4, 35–43. [Google Scholar]
- Shrestha, R.M.; Yadav, A.P.; Pokharel, B.P.; Rajbhandari, R. Preparation and Characterization of Activated Carbon from Lapsi (Choerospondias axillaris) seed stone by chemical activation with phosphoric acid. Res. J. Chem. Sci. 2012, 2, 80–86. [Google Scholar]
- Rahman, S.; Yanful, E. Arsenic and chromium removal by mixed magnetite-maghemite nanoparticles and the effect of phosphate on removal. J. Environ. Manag. 2010, 11, 2238–2247. [Google Scholar]
- Figueroa, D.; Moreno, A.; Hormaza, A. Equilibrio, termodinámica y modelos cinéticos en la adsorción de Rojo 40 sobre tuza de maíz. Rev. Medellín 2015, 14, 105–120. [Google Scholar] [CrossRef]
- Aksu, Z.; Tatlı, A.İ.; Tunç, Ö. A comparative adsorption/biosorption study of Acid Blue 161: Effect of temperature on equilibrium and kinetic parameters. Chem. Eng. J. 2008, 142, 23–39. [Google Scholar] [CrossRef]
- Sorg, T.; Wang, L.; Chen, A. Costs of Small Drinking Water Systems Removing Arsenic from Groundwater. J. Water Supply Res. Technol.—AQUA 2015, 64, 219. [Google Scholar] [CrossRef]
- Wang, L.; Chen, A. Costs of Arsenic Removal Technologies for Small Water Systems: U.S. EPA Arsenic Removal Technology Demonstration Program; EPA/600/R-11/090; National Risk Management Research Laboratory: Cincinnati, OH, USA, 2011. [Google Scholar]
- Chaudhary, B.; Farrell, J. Understanding Regeneration of Arsenate-Loaded Ferric Hydroxide-Based Adsorbents. Environ. Eng. Sci. 2015, 32, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Sorg, T.; Chen, A.; Wang, L.; Kolisz, R. Regenerating an Arsenic Removal Iron-Bed Adsorptive Media System, Part 1: The Regeneration Process. J. Am. Water Work. Assoc. 2017, 109, 13–24. [Google Scholar] [CrossRef]
- Bundschuh, J.; Litter, M.; Parvez, F.; Román-Ross, G.; Nicolli, H.; Jeanc, J.; Liuj Ch López, D.; Armiental, M.; Guilherme, L.; Gomez, A.; et al. One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries. Sci. Total Environ. 2012, 429, 2–35. [Google Scholar] [CrossRef] [PubMed]
- Yañez, J.; Fierro, V.; Mansilla, H.; Cornejo, L.; Figueroa, L.; Barnes, R. Arsenic speciation in human hair: A new perspective for epidemiological assessment in chronic arsenicism. J. Environ. Monit. 2005, 7, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Lara, F.; Cornejo, L.; Yañez, J.; Freer, J.; Mansilla, H.D. Solar-light assisted removal of Arsenic from natural water: Effect of iron and citrate concentrations. J. Chem. Technol. Biotechnol. 2006, 81, 1282–1287. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Braun-Howland, E.B., Baxter, T.E., Eds.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]






| X-ray Diffraction * | |||
|---|---|---|---|
| Bentonite | BENFEP-ZVI | ||
| Montmorillonite | 22.58 | 21.92 | |
| Quartz | 4.27 | 24.92 | |
| Anhydrite | 10.02 | 16.87 | |
| Magnetite | <1 | 8.43 | |
| Magnesioferrite | <1 | 12.33 | |
| Cristobalita | <1 | 1.78 | |
| Hematite | <1 | 13.72 | |
| Andesita | 13.31 | <1 | |
| Muscovite | 41.78 | <1 | |
| X-ray Fluorescence (EDXRF) * | |||
| Bentonite | ZVI | Polyurethane (PU) | |
| Silicon | 79.70 | <0.001 | <0.001 |
| Sulfur | 7.91 | <0.001 | <0.001 |
| Potassium | 4.35 | <0.001 | <0.001 |
| Iron | 3.81 | 98.80 | <0.001 |
| Calcium | 1.98 | <0.001 | 0.16 |
| Titanium | 1.07 | <0.001 | <0.001 |
| Manganese | <0.001 | 0.94 | <0.001 |
| Strontium | 0.76 | <0.001 | <0.001 |
| Arsenic | 0.25 | <0.001 | <0.001 |
| Lead | <0.001 | <0.001 | <0.001 |
| (kJ/mol) at Studied Temperature (K) | ||||
|---|---|---|---|---|
(kJ/mol) | (kJ/mol ∗ K) | 298 | 313 | 333 |
| −12.66 | −0.002 | −12.19 | −12.15 | −12.13 |
| Sample | NaOH | First Regeneration | Second Regeneration | Third Regeneration |
|---|---|---|---|---|
| (% w/v) | (%) | (%) | (%) | |
| 1 | 83.2 | 81.1 | 80.2 | |
| BENFEP 20 | 3 | 93.0 | 92.3 | 90.1 |
| 5 | 99.3 | 99.0 | 98.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acarapi-Cartes, J.; Cornejo-Ponce, L. BENFEP Spheres: New Porous Adsorbent Material for Arsenic Removal from Natural Waters. Water 2023, 15, 2887. https://doi.org/10.3390/w15162887
Acarapi-Cartes J, Cornejo-Ponce L. BENFEP Spheres: New Porous Adsorbent Material for Arsenic Removal from Natural Waters. Water. 2023; 15(16):2887. https://doi.org/10.3390/w15162887
Chicago/Turabian StyleAcarapi-Cartes, Jorge, and Lorena Cornejo-Ponce. 2023. "BENFEP Spheres: New Porous Adsorbent Material for Arsenic Removal from Natural Waters" Water 15, no. 16: 2887. https://doi.org/10.3390/w15162887
APA StyleAcarapi-Cartes, J., & Cornejo-Ponce, L. (2023). BENFEP Spheres: New Porous Adsorbent Material for Arsenic Removal from Natural Waters. Water, 15(16), 2887. https://doi.org/10.3390/w15162887






