Greenhouse Gases Emissions of Constructed Wetlands: Mechanisms and Affecting Factors
Abstract
:1. Introduction
2. CWs and Their Source-Sink Effects on GHGs
2.1. Overview of CWs
2.1.1. Free Water Surface Flow Constructed Wetlands (FWS CWs)
2.1.2. Horizontal Subsurface Flow Constructed Wetlands (HSSF CWs)
2.1.3. Vertical Subsurface Flow Constructed Wetlands (VSSF CWs)
2.2. CWs’ Source-Sink Effects on GHGs
3. GHG emissions Mechanisms of CWs
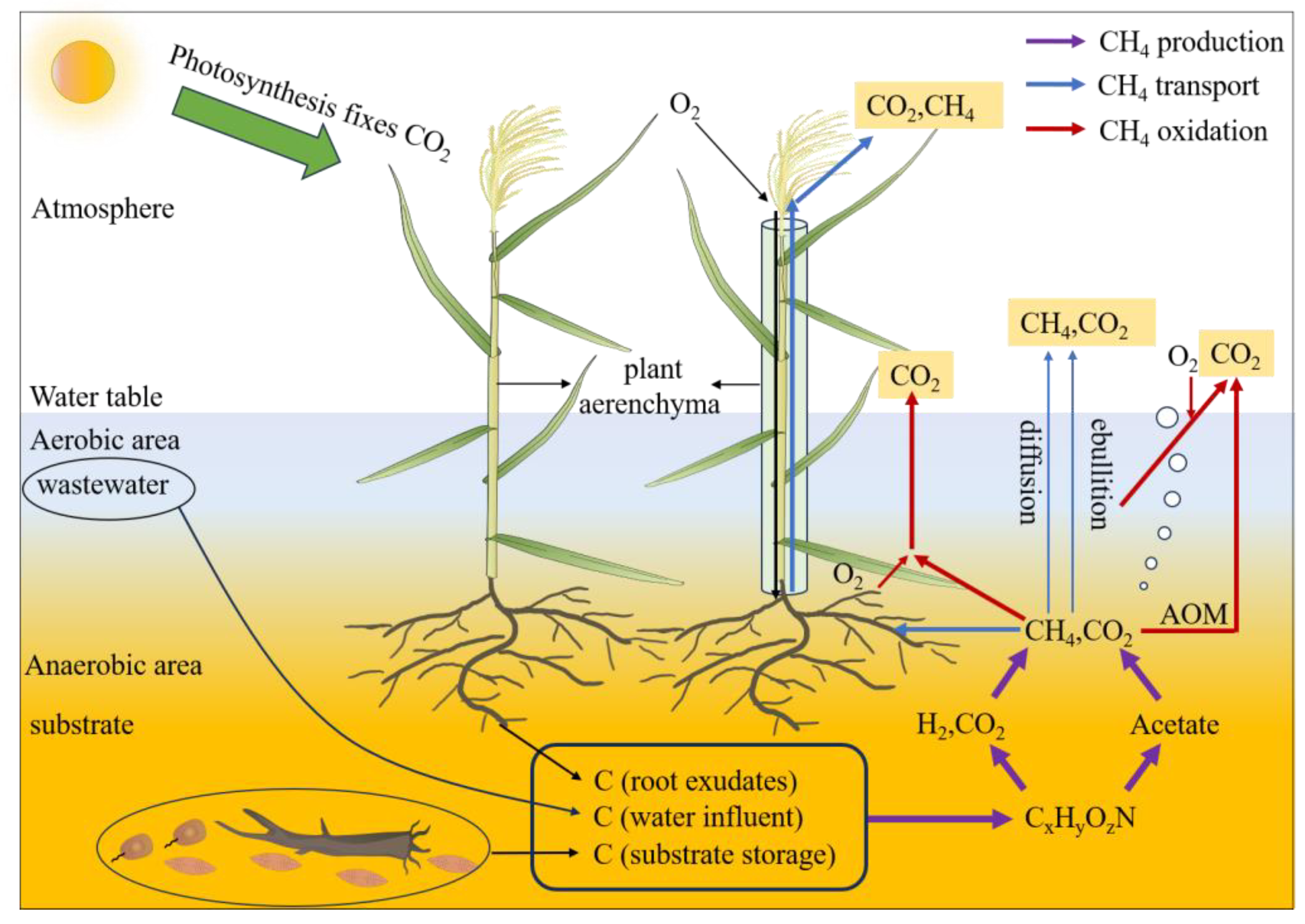
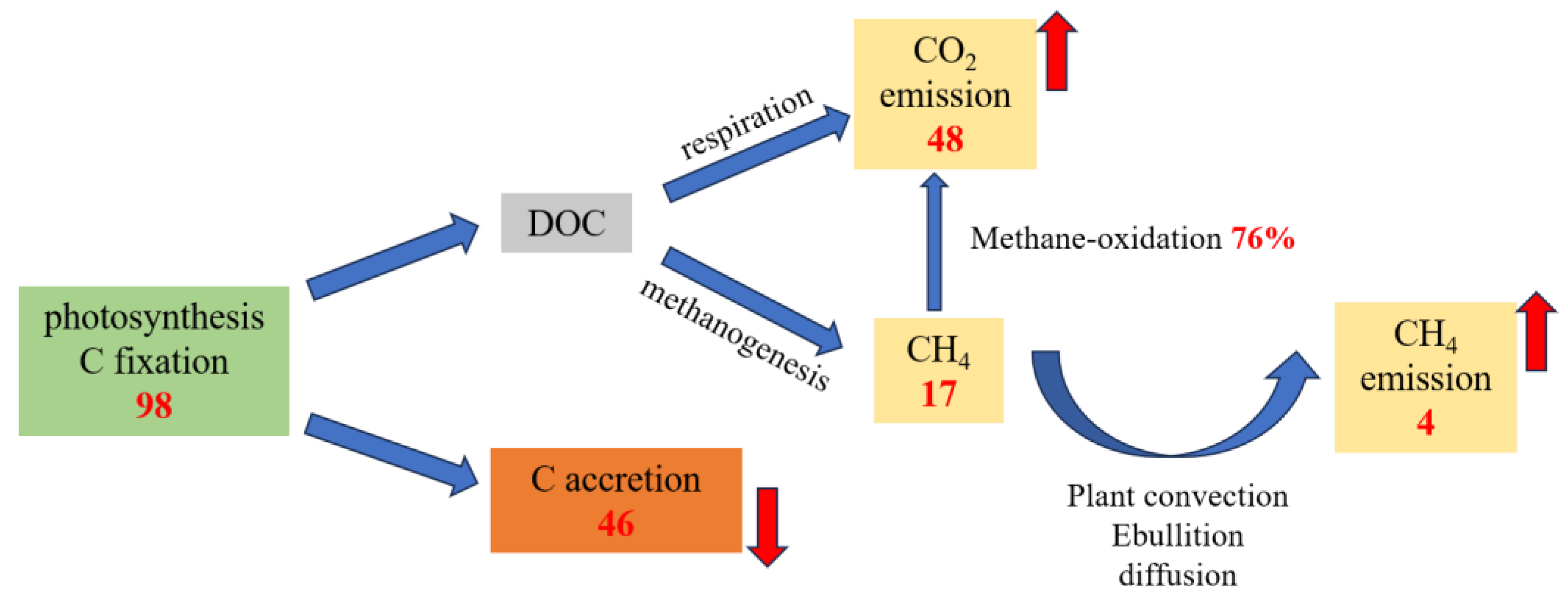
3.1. Emission of CH4 and CO2 in CWs
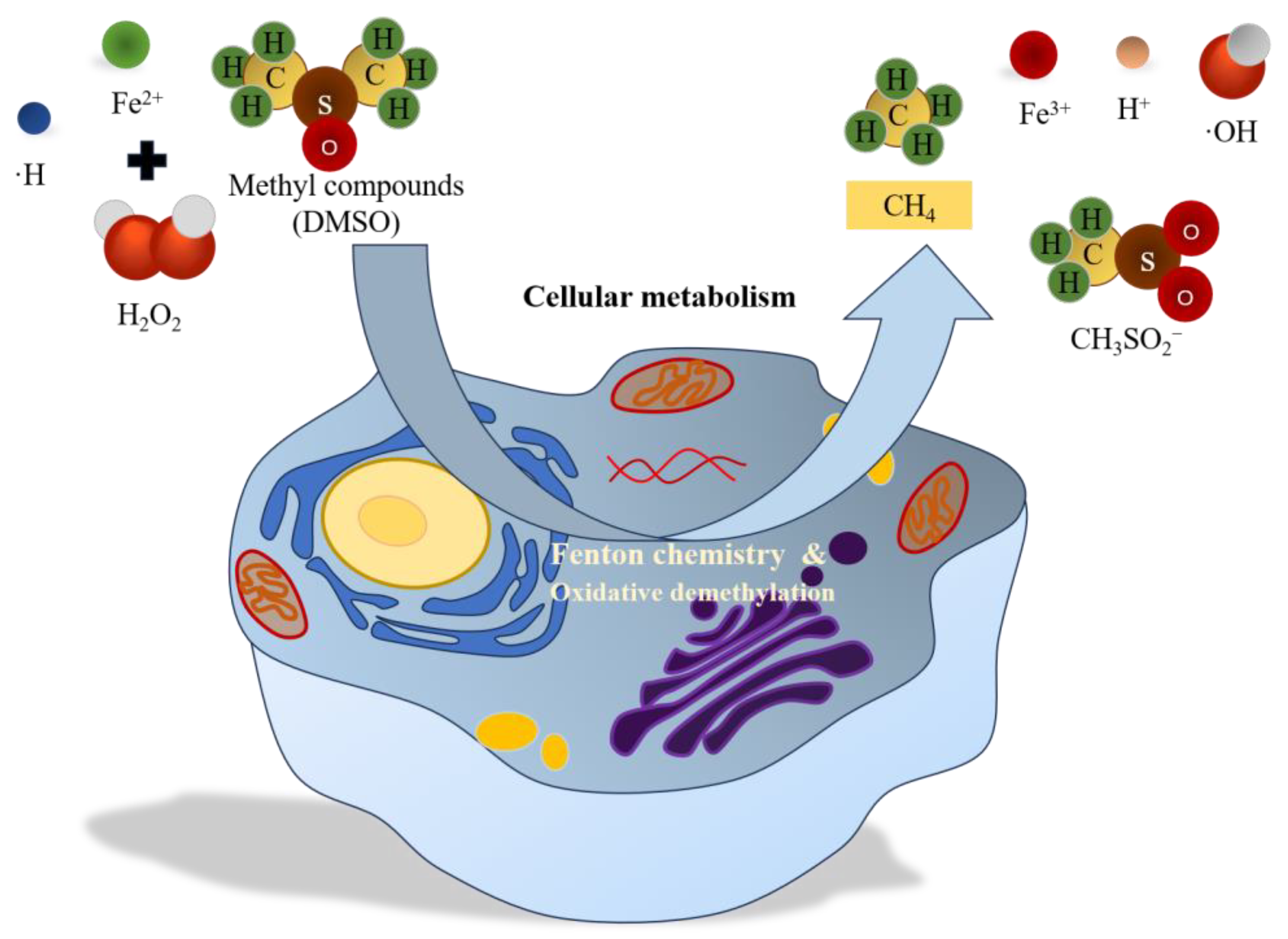
3.1.1. Production of CH4 in CWs
3.1.2. Transport of CH4 in CWs
3.1.3. Oxidation of CH4 in CWs
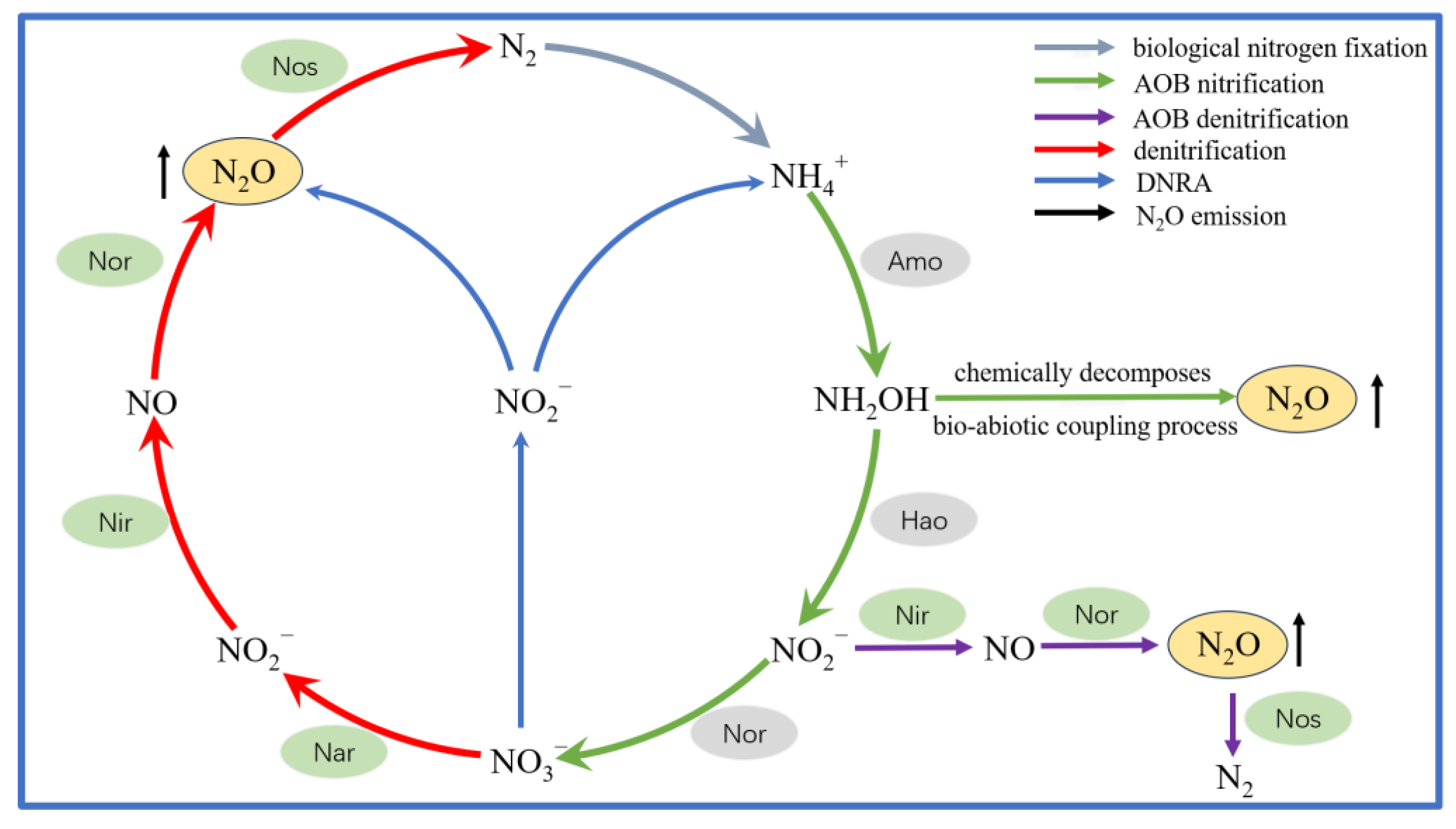
3.2. Emission of N2O in CWs
4. Affecting Factors of GHG Emissions in CWs
4.1. Type and Age of CWs
4.2. Plant
4.3. Substrate
4.4. Temperature
4.5. Influent Characteristics
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.; Zhao, J.Y. Attentional and perceptual biases of climate change. Curr. Opin. Behav. Sci. 2021, 42, 22–26. [Google Scholar] [CrossRef]
- Asaad, S.M.; Inayat, A.; Rocha-Meneses, L.; Jamil, F.; Ghenai, C.; Shanableh, A. Prospective of Response Surface Methodology as an Optimization Tool for Biomass Gasification Process. Energies 2023, 16, 40. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, B.; Wu, X.; Chen, Y.; Guo, J.; Ma, Z.; Bao, S.; Jiang, X.; Chen, L.; Shu, K.; et al. Research on photocatalytic CO2 conversion to renewable synthetic fuels based on localized surface plasmon resonance: Current progress and future perspectives. Catal. Sci. Technol. 2023, 13, 1932–1975. [Google Scholar] [CrossRef]
- Peter, R.; Kuttippurath, J.; Chakraborty, K.; Sunanda, N. A high concentration CO2 pool over the Indo-Pacific Warm Pool. Sci. Rep. 2023, 13, 4314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xing, A.; Shen, H. Effects of nitrogen addition on the combined global warming potential of three major soil greenhouse gases: A global meta-analysis. Environ. Pollut. 2023, 334, 121848. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Andres, R.J.; Boden, T.A.; Breon, F.M.; Ciais, P.; Davis, S.; Erickson, D.; Gregg, J.S.; Jacobson, A.; Marland, G.; Miller, J.; et al. A synthesis of carbon dioxide emissions from fossil-fuel combustion. Biogeosciences 2012, 9, 1845–1871. [Google Scholar] [CrossRef] [Green Version]
- Riris, H.; Numata, K.; Wu, S.; Gonzalez, B.; Rodriguez, M.; Molly, F.; Yu, A.; Stephen, M.; Mao, J. A Methane Lidar for Greenhouse Gas Measurements. In Proceedings of the Conference on Lasers and Electro-Optics (CLEO), San Jose, CA, USA, 5–10 June 2016. [Google Scholar]
- Dreyfus, G.B.; Xu, Y.; Shindell, D.T.; Zaelke, D.; Ramanathan, V. Mitigating climate disruption in time: A self-consistent approach for avoiding both near-term and long-term global warming. Proc. Natl. Acad. Sci. USA 2022, 119, e2123536119. [Google Scholar] [CrossRef]
- Khodayari, A.; Olsen, S.C.; Wuebbles, D.J.; Phoenix, D.B. Aviation NOx-induced CH4 effect: Fixed mixing ratio boundary conditions versus flux boundary conditions. Atmos. Environ. 2015, 113, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Cruz Copetti, A.C.; da Silva, L.S.; Drescher, G.L.; Mueller, E.A.; Busanello, R.L.; Beber Vieira, F.C. Effect of rice straw and nitrate levels in soil sollution on nitrous oxide emission. Rev. Bras. Cienc. Solo 2015, 39, 458–465. [Google Scholar] [CrossRef]
- Xie, G.-Z.; Zhang, L.-P.; Li, C.-Y.; Sun, W.-D. Accelerated methane emission from permafrost regions since the 20th century. Deep.-Sea Res. Part I-Oceanogr. Res. Pap. 2023, 195, 103981. [Google Scholar] [CrossRef]
- Perugini, L.; Pellis, G.; Grassi, G.; Ciais, P.; Dolman, H.; House, J.I.; Peters, G.P.; Smith, P.; Guenther, D.; Peylin, P. Emerging reporting and verification needs under the Paris Agreement: How can the research community effectively contribute? Environ. Sci. Policy 2021, 122, 116–126. [Google Scholar] [CrossRef]
- Nam-Chol, O.; Kim, H.; Kim, G.-S.; Ri, G.-S. Characterisation analysis of national greenhouse gas emissions in DPR of Korea: Emission trends for a 12-year period. Int. J. Glob. Warm. 2019, 17, 401–416. [Google Scholar]
- Jonas, M.; Bun, R.; Nahorski, Z.; Marland, G.; Gusti, M.; Danylo, O. Quantifying greenhouse gas emissions. Mitig. Adapt. Strateg. Glob. Chang. 2019, 24, 839–852. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Xiong, T.; Liu, Y.; Yang, C.; Cheng, Q.; Lin, S. Research overview of urban carbon emission measurement and future prospect for GHG monitoring network. Energy Rep. 2023, 9, 231–242. [Google Scholar] [CrossRef]
- Bai, M.; Wang, Z.; Lloyd, J.; Seneviratne, D.; Flesch, T.; Yuan, Z.; Chen, D. Long-term onsite monitoring of a sewage sludge drying pan finds methane emissions consistent with IPCC default emission factor. Water Res. X 2023, 19, 100184. [Google Scholar] [CrossRef]
- Cui, C.; Wang, Z.; Cai, B.; Peng, S.; Wang, Y.; Xu, C. Evolution-based CO2 emission baseline scenarios of Chinese cities in 2025. Appl. Energy 2021, 281, 116116. [Google Scholar] [CrossRef]
- Long, W.; Wang, S.; Lu, C.; Xue, R.; Liang, T.; Jiang, N.; Zhang, R. Quantitative assessment of energy conservation potential and environmental benefits of an iron and steel plant in China. J. Clean. Prod. 2020, 273, 123163. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, R.; Majeed, M.T.; Usman, A. Clean energy consumption and CO2 emissions: Does China reduce some pollution burdens through environmental regulation? Environ. Sci. Pollut. Res. 2022, 29, 79156–79167. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, X.; Ding, H.; Yang, Y.; Li, D.; Wang, J.; Han, J. Cleaner production and carbon reduction target: Analysis of sewage treatment plants in Nort-Central China. Energy Sources Part A-Recovery Util. Environ. Eff. 2022, 44, 2770–2781. [Google Scholar] [CrossRef]
- Zhang, Y.-h.; Feng, T.-t. How does the design of personal carbon trading system affect willingness to participate under carbon neutrality goal?-evidence from a choice experiment. Environ. Sci. Pollut. Res. 2022, 29, 81970–81992. [Google Scholar] [CrossRef]
- Wu, L. How can carbon trading price distortion be corrected? An empirical study from China’s carbon trading pilot markets. Environ. Sci. Pollut. Res. 2021, 28, 66253–66271. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, J. Research on the Application of GA-ELM Model in Carbon Trading Price-An Example of Beijing. Pol. J. Environ. Stud. 2022, 31, 149–161. [Google Scholar] [CrossRef]
- Chen, X.; Qin, X.; Li, Y.; Wan, Y.; Liao, Y.; Lu, Y.; Wang, B.; Chen, H.; Wang, K. Residential and agricultural soils dominate soil organic matter loss in a typical agricultural watershed of subtropical China. Agric. Ecosyst. Environ. 2022, 338, 108100. [Google Scholar] [CrossRef]
- Chen, W.; Tang, H.; He, L.; Zhang, Y.; Ma, W. Co-effect assessment on regional air quality: A perspective of policies and measures with greenhouse gas reduction potential. Sci. Total Environ. 2022, 851, 158119. [Google Scholar] [CrossRef]
- Yanez, E.; Ramirez, A.; Nunez-Lopez, V.; Castillo, E.; Faaij, A. Exploring the potential of carbon capture and storage-enhanced oil recovery as a mitigation strategy in the Colombian oil industry. Int. J. Greenh. Gas Control 2020, 94, 102938. [Google Scholar] [CrossRef]
- Rashid, M.I.; Benhelal, E.; Rafiq, S. Reduction of Greenhouse Gas Emissions from Gas, Oil, and Coal Power Plants in Pakistan by Carbon Capture and Storage (CCS): A Review. Chem. Eng. Technol. 2020, 43, 2140–2148. [Google Scholar] [CrossRef]
- Paltsev, S.; Morris, J.; Kheshgi, H.; Herzog, H. Hard-to-Abate Sectors: The role of industrial carbon capture and storage (CCS) in emission mitigation. Appl. Energy 2021, 300, 117322. [Google Scholar] [CrossRef]
- Ali, N.B.H.; Abichou, T.; Green, R. Comparing estimates of fugitive landfill methane emissions using inverse plume modeling obtained with Surface Emission Monitoring (SEM), Drone Emission Monitoring (DEM), and Downwind Plume Emission Monitoring (DWPEM). J. Air Waste Manag. Assoc. 2020, 70, 410–424. [Google Scholar]
- Bastos, A.; Ciais, P.; Sitch, S.; Aragao, L.E.O.C.; Chevallier, F.; Fawcett, D.; Rosan, T.M.; Saunois, M.; Guenther, D.; Perugini, L.; et al. On the use of Earth Observation to support estimates of national greenhouse gas emissions and sinks for the Global stocktake process: Lessons learned from ESA-CCI RECCAP2 COMMENT. Carbon Balance Manag. 2022, 17, 15. [Google Scholar] [CrossRef]
- Zaborowska, E.; Czerwionka, K.; Makinia, J. Integrated plant-wide modelling for evaluation of the energy balance and greenhouse gas footprint in large wastewater treatment plants. Appl. Energy 2021, 282, 116126. [Google Scholar] [CrossRef]
- Stadler, C.; Fuse, V.S.; Linares, S.; Juliarena, P. Estimation of methane emission from an urban wastewater treatment plant applying inverse Gaussian model. Environ. Monit. Assess. 2022, 194, 27. [Google Scholar] [CrossRef] [PubMed]
- Thalasso, F.; Sepulveda-Jauregui, A.; Cabrol, L.; Lavergne, C.; Olgun, N.; Martinez-Cruz, K.; Aguilar-Munoz, P.; Calle, N.; Mansilla, A.; Soledad Astorga-Espana, M. Methane and carbon dioxide cycles in lakes of the King George Island, maritime Antarctica. Sci. Total Environ. 2022, 848, 157485. [Google Scholar] [CrossRef] [PubMed]
- Moomaw, W.R.; Law, B.E.; Goetz, S.J. Focus on the role of forests and soils in meeting climate change mitigation goals: Summary. Environ. Res. Lett. 2020, 15, 045009. [Google Scholar] [CrossRef]
- Martin-Pozas, T.; Cuezva, S.; Fernandez-Cortes, A.; Carlos Canaveras, J.; Benavente, D.; Jurado, V.; Saiz-Jimenez, C.; Janssens, I.; Seijas, N.; Sanchez-Moral, S. Role of subterranean microbiota in the carbon cycle and greenhouse gas dynamics. Sci. Total Environ. 2022, 831, 154921. [Google Scholar] [CrossRef]
- Bonetti, G.; Trevathan-Tackett, S.M.; Carnell, P.E.; Macreadie, P.I. The potential of viruses to influence the magnitude of greenhouse gas emissions in an inland wetland. Water Res. 2021, 193, 116875. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xiong, P.; Wu, Y.; Dong, Y. Forecasting greenhouse gas emissions with the new information priority generalized accumulative grey model. Sci. Total Environ. 2022, 807, 150859. [Google Scholar] [CrossRef]
- Li, J.; Sun, S.; Sharma, D.; Ho, M.S.; Liu, H. Tracking the drivers of global greenhouse gas emissions with spillover effects in the post-financial crisis era. Energy Policy 2023, 174, 113464. [Google Scholar] [CrossRef]
- Kutlu, L. Greenhouse Gas Emission Efficiencies of World Countries. Int. J. Environ. Res. Public Health 2020, 17, 8771. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.C.; Zheng, Y.; Dzakpasu, M. Removal of pharmaceutical active compounds in wastewater by constructed wetlands: Performance and mechanisms. J. Environ. Manag. 2023, 325, 116478. [Google Scholar] [CrossRef]
- Cui, E.; Zhou, Z.; Gao, F.; Chen, H.; Li, J. Roles of substrates in removing antibiotics and antibiotic resistance genes in constructed wetlands: A review. Sci. Total Environ. 2023, 859, 160257. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, J.-C.; Sun, S.; Rehman, M.M.U.; He, S. Depth-specific distribution and significance of nitrite-dependent anaerobic methane oxidation process in tidal flow constructed wetlands used for treating river water. Sci. Total Environ. 2020, 716, 137054. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Xu, Z.; Zhang, L.; Dai, Y.; Tang, X.; Tao, R.; Li, R.; Yang, Y.; Tai, Y. Effect of heavy metals in mixed domestic-industrial wastewater on performance of recirculating standing hybrid constructed wetlands (RSHCWs) and their removal. Chem. Eng. J. 2020, 379, 122363. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, P.; Zhang, N.; Zhang, Z.; Guo, Q.; Chen, C.; Cui, L. Effects of matrix modification and bacteria amendment on the treatment efficiency of municipal tailwater pollutants by modified vertical flow constructed wetland. J. Environ. Manag. 2021, 281, 111920. [Google Scholar] [CrossRef]
- Lu, Q.; Li, Q.; An, Y.; Duan, X.; Zhao, R.; Zhao, D.; An, S. Evaluation of constructed wetland systems toward naturalization of the elemental composition and microbial community in wastewater treatment plant effluent. J. Clean. Prod. 2022, 376, 134117. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, M.I.; Martin de la Vega, P.T.; Jaramillo-Moran, M.A.; Garrido, M. Hybrid Constructed Wetland to Improve Organic Matter and Nutrient Removal. Water 2020, 12, 2023. [Google Scholar] [CrossRef]
- Xu, G.; Li, Y.; Hou, W.; Wang, S.; Kong, F. Effects of substrate type on enhancing pollutant removal performance and reducing greenhouse gas emission in vertical subsurface flow constructed wetland. J. Environ. Manag. 2021, 280, 111674. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Sun, H.; Wang, J.; Wang, N.; Zuo, Y.; Mosa, A.A.; Yin, X. Global trends and current advances regarding greenhouse gases in constructed wetlands: A bibliometric-based quantitative review over the last 40 years. Ecol. Eng. 2023, 193, 107018. [Google Scholar] [CrossRef]
- Mao, X.; Wei, X.; Engel, B.; Wei, X.; Zhang, Z.; Tao, Y.; Wang, W. Network-based perspective on water-air interface GHGs flux on a cascade surface-flow constructed wetland in Qinghai-Tibet Plateau, China. Ecol. Eng. 2020, 151, 105862. [Google Scholar] [CrossRef]
- Tsai, C.-P.; Huang, C.-M.; Yuan, C.-S.; Yang, L. Seasonal and diurnal variations of greenhouse gas emissions from a saline mangrove constructed wetland by using an in situ continuous GHG monitoring system. Environ. Sci. Pollut. Res. 2020, 27, 15824–15834. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Yan, B.; Shutes, B.; Xing, D.; Banuelos, G.; Cheng, R.; Wang, X. Greenhouse gas emissions and wastewater treatment performance by three plant species in subsurface flow constructed wetland mesocosms. Chemosphere 2020, 239, 124795. [Google Scholar] [CrossRef]
- Mander, Ü.; Dotro, G.; Ebie, Y.; Towprayoon, S.; Chiemchaisri, C.; Nogueira, S.F.; Jamsranjav, B.; Kasak, K.; Truu, J.; Tournebize, J.; et al. Greenhouse gas emission in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2014, 66, 19–35. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Ge, Z.; Zhou, X.; Li, S.; Li, X.; Tang, J. Conversion of coastal wetlands, riparian wetlands, and peatlands increases greenhouse gas emissions: A global meta-analysis. Glob. Change Biol. 2020, 26, 1638–1653. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.G.; Wang, J.H.; Li, H.F.; Zhang, J.; Qi, P.Y.; Hu, Z. Nitrous oxide and methane emissions from different treatment processes in full-scale municipal wastewater treatment plants. Environ. Technol. 2013, 34, 2917–2927. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, L.; Liu, J. Characteristics of greenhouse gas emission in three full-scale wastewater treatment processes. J. Environ. Sci. 2014, 26, 256–263. [Google Scholar] [CrossRef]
- Bao, Z.; Sun, S.; Sun, D. Assessment of greenhouse gas emission from A/O and SBR wastewater treatment plants in Beijing, China. Int. Biodeterior. Biodegrad. 2016, 108, 108–114. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, X.; Zhang, X. The 2 degrees C Global Temperature Target and the Evolution of the Long-Term Goal of Addressing Climate Change-From the United Nations Framework Convention on Climate Change to the Paris Agreement. Engineering 2017, 3, 272–278. [Google Scholar] [CrossRef]
- Maucieri, C.; Salvato, M.; Borin, M. Vegetation contribution on phosphorus removal in constructed wetlands. Ecol. Eng. 2020, 152, 105853. [Google Scholar] [CrossRef]
- Verduzo Garibay, M.; Fernández del Castillo, A.; de Anda, J.; Senés-Guerrero, C.; Gradilla-Hernández, M.S. Structure and activity of microbial communities in response to environmental, operational, and design factors in constructed wetlands. Int. J. Environ. Sci. Technol. 2021, 19, 11587–11612. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Tang, Y.; Jiang, Y.; Xie, S.; Zhang, Y.; Qin, Y. Selection and optimization of the substrate in constructed wetland: A review. J. Water Process Eng. 2022, 49, 103140. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef] [Green Version]
- Maine, M.A.; Sanchez, G.C.; Hadad, H.R.; Caffaratti, S.E.; Pedro, M.d.C.; Di Luca, G.A.; Mufarrege, M.d.l.M.; Nocetti, E. Hybrid wetland system for a pet-care center wastewater treatment. Ecol. Eng. 2022, 182, 106700. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Levesque, V.; Antoun, H.; Rochette, P.; Dorais, M. Type of constructed wetlands influence nutrient removal and nitrous oxide emissions from greenhouse wastewater. Eur. J. Hortic. Sci. 2020, 85, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wen, Y.; Zhou, Q.; Vymazal, J. Effects of plant biomass on nitrogen transformation in subsurface-batch constructed wetlands: A stable isotope and mass balance assessment. Water Res. 2014, 63, 158–167. [Google Scholar] [CrossRef]
- Bakhshoodeh, R.; Alavi, N.; Oldham, C.; Santos, R.M.; Babaei, A.A.; Vymazal, J.; Paydary, P. Constructed wetlands for landfill leachate treatment: A review. Ecol. Eng. 2020, 146, 105725. [Google Scholar] [CrossRef]
- Vymazal, J. Plants used in constructed wetlands with horizontal subsurface flow: A review. Hydrobiologia 2011, 674, 133–156. [Google Scholar] [CrossRef]
- Engida, T.; Wu, J.M.; Xu, D.; Wu, Z.B. Review paper on horizontal substurface flow constructed wetlands: Potential for their use in climate change mitigation and treatment of wastewater. Appl. Ecol. Environ. Res. 2020, 18, 1051–1089. [Google Scholar] [CrossRef]
- Ranieri, E.; Young, T.M. Clogging influence on metals migration and removal in sub-surface flow constructed wetlands. J. Contam. Hydrol. 2012, 129, 38–45. [Google Scholar] [CrossRef]
- Shahid, M.J.; Al-surhanee, A.A.; Kouadri, F.; Ali, S.; Nawaz, N.; Afzal, M.; Rizwan, M.; Ali, B.; Soliman, M.H. Role of Microorganisms in the Remediation of Wastewater in Floating Treatment Wetlands: A Review. Sustainability 2020, 12, 5559. [Google Scholar] [CrossRef]
- Mitter, B.; Brader, G.; Afzal, M.; Compant, S.; Naveed, M.; Trognitz, F.; Sessitsch, A. Advances in Elucidating Beneficial Interactions Between Plants, Soil, and Bacteria. Adv. Agron. 2013, 121, 381–445. [Google Scholar]
- Vymazal, J. Long-term performance of constructed wetlands with horizontal sub-surface flow: Ten case studies from the Czech Republic. Ecol. Eng. 2011, 37, 54–63. [Google Scholar] [CrossRef]
- Vymazal, J. Does clogging affect long-term removal of organics and suspended solids in gravel-based horizontal subsurface flow constructed wetlands? Chem. Eng. J. 2018, 331, 663–674. [Google Scholar] [CrossRef]
- Tee, H.-C.; Lim, P.-E.; Seng, C.-E.; Nawi, M.A.M.; Adnan, R. Enhancement of azo dye Acid Orange 7 removal in newly developed horizontal subsurface-flow constructed wetland. J. Environ. Manag. 2015, 147, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Maucieri, C.; Barbera, A.C.; Vymazal, J.; Borin, M. A review on the main affecting factors of greenhouse gases emission in constructed wetlands. Agric. For. Meteorol. 2017, 236, 175–193. [Google Scholar] [CrossRef]
- Pillai, J.S.; Vijayan, N. Decentralized Greywater Treatment for Nonpotable Reuse in a Vertical Flow Constructed Wetland. In Proceedings of the International Conference on Green Technologies (ICGT), Trivandrum, India, 18–20 December 2012. [Google Scholar]
- Tsihrintzis, V.A. The use of Vertical Flow Constructed Wetlands in Wastewater Treatment. Water Resour. Manag. 2017, 31, 3245–3270. [Google Scholar] [CrossRef]
- Maucieri, C.; Mietto, A.; Barbera, A.C.; Borin, M. Treatment performance and greenhouse gas emission of a pilot hybrid constructed wetland system treating digestate liquid fraction. Ecol. Eng. 2016, 94, 406–417. [Google Scholar] [CrossRef]
- Barbera, A.C.; Borin, M.; Cirelli, G.L.; Toscano, A.; Maucieri, C. Comparison of carbon balance in Mediterranean pilot constructed wetlands vegetated with different C4 plant species. Environ. Sci. Pollut. Res. 2015, 22, 2372–2383. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Santino, M.B.d.; Bitar, A.L.; Bianchini Junior, I. Gas emission from anaerobic decomposition of plant resources. Acta Limnol. Bras. 2016, 28, e30. [Google Scholar] [CrossRef] [Green Version]
- Mitsch, W.J.; Mander, U. Wetlands and carbon revisited. Ecol. Eng. 2018, 114, 1–6. [Google Scholar] [CrossRef]
- Kayranli, B.; Scholz, M.; Mustafa, A.; Hedmark, A. Carbon Storage and Fluxes within Freshwater Wetlands: A Critical Review. Wetlands 2010, 30, 111–124. [Google Scholar] [CrossRef]
- Maucieri, C.; Salvato, M.; Tamiazzo, J.; Borin, M. Biomass production and soil organic carbon accumulation in a free water surface constructed wetland treating agricultural wastewater in North Eastern Italy. Ecol. Eng. 2014, 70, 422–428. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, C.-S. Analysis of carbon sink effects for saline constructed wetlands vegetated with mangroves to treat mariculture wastewater and sewage. Water Sci. Technol. 2019, 79, 1474–1483. [Google Scholar] [CrossRef]
- Brix, H.; Sorrell, B.K.; Lorenzen, B. Are Phragmites-dominated wetlands a net source or net sink of greenhouse gases? Aquat. Bot. 2001, 69, 313–324. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, U.; Zhang, L.; Anderson, C.J.; Jorgensen, S.E.; Brix, H. Wetlands, carbon, and climate change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Wu, H.; Lin, L.; Zhang, J.; Guo, W.; Liang, S.; Liu, H. Purification ability and carbon dioxide flux from surface flow constructed wetlands treating sewage treatment plant effluent. Bioresour. Technol. 2016, 219, 768–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Tian, Y.; Liu, H.; Zhao, X.; Peng, S. The influence of incorporating microbial fuel cells on greenhouse gas emissions from constructed wetlands. Sci. Total Environ. 2019, 656, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Singh, R.S.; Nathanail, C.P. Mine spoil acts as a sink of carbon dioxide in Indian dry tropical environment. Sci. Total Environ. 2014, 468, 1162–1171. [Google Scholar] [CrossRef]
- Liu, S.; Xue, H.; Wang, Y.; Wang, Z.; Feng, X.; Pyo, S.-H. Effects of bioelectricity generation processes on methane emission and bacterial community in wetland and carbon fate analysis. Bioresour. Bioprocess. 2022, 9, 69. [Google Scholar] [CrossRef]
- Gougoulias, C.; Clark, J.M.; Shaw, L.J. The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Zhang, J.; Yang, X.; He, Q.; Ao, L.; Chen, Y. Impact of biochar on greenhouse gas emissions from constructed wetlands under various influent chemical oxygen demand to nitrogen ratios. Bioresour. Technol. 2020, 303, 122908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.C.; Dzakpasu, M.; Cao, T.; Zhang, H.; Liu, Y.; Zheng, Y. Integrated environmental influences quantification of pilot-scale constructed wetlands based on modified ecological footprint assessment. Sci. Total Environ. 2022, 843, 157039. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Change Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, X.; Luo, H.; Li, X.; Chen, W.; Chen, J.; Mo, Y.; Wang, W. CH4 control and associated microbial process from constructed wetland (CW) by microbial fuel cells (MFC). J. Environ. Manag. 2020, 260, 110071. [Google Scholar] [CrossRef]
- Lopez, D.; Sepulveda-Mardones, M.; Ruiz-Tagle, N.; Sossa, K.; Uggetti, E.; Vidal, G. Potential methane production and molecular characterization of bacterial and archaeal communities in a horizontal subsurface flow constructed wetland under cold and warm seasons. Sci. Total Environ. 2019, 648, 1042–1051. [Google Scholar] [CrossRef]
- Lyu, Z.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, R727–R732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, S.; Takaki, Y.; Shimamura, S.; Sekine, M.; Tajima, T.; Kosugi, H.; Ichikawa, N.; Tasumi, E.; Hiraki, A.T.; Shimizu, A.; et al. Genome Sequence of a Mesophilic Hydrogenotrophic Methanogen Methanocella paludicola, the First Cultivated Representative of the Order Methanocellales. PLoS ONE 2011, 6, e22898. [Google Scholar] [CrossRef] [Green Version]
- Borrel, G.; O’Toole, P.W.; Harris, H.M.B.; Peyret, P.; Brugere, J.-F.; Gribaldo, S. Phylogenomic Data Support a Seventh Order of Methylotrophic Methanogens and Provide Insights into the Evolution of Methanogenesis. Genome Biol. Evol. 2013, 5, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Borrel, G.; Parisot, N.; Harris, H.M.B.; Peyretaillade, E.; Gaci, N.; Tottey, W.; Bardot, O.; Raymann, K.; Gribaldo, S.; Peyret, P.; et al. Comparative genomics highlights the unique biology of Methanomassiliicoccales, a Thermoplasmatales-related seventh order of methanogenic archaea that encodes pyrrolysine. BMC Genom. 2014, 15, 679. [Google Scholar] [CrossRef] [Green Version]
- Bhaduri, D.; Mandal, A.; Chakraborty, K.; Chatterjee, D.; Dey, R. Interlinked chemical-biological processes in anoxic waterlogged soil—A review. Indian J. Agric. Sci. 2017, 87, 1587–1599. [Google Scholar] [CrossRef]
- Yu, G.; Chen, J.; Wang, G.; Chen, H.; Huang, J.; Li, Y.; Wang, W.; Song, F.; Ma, Y.; Wang, Q.; et al. Recent advances in constructed wetlands methane reduction: Mechanisms and methods. Front. Microbiol. 2023, 14, 1106332. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Chen, Q.; Luo, H.; Zhu, Z.; Chen, W.; Chen, J.; Mo, Y. Effect of submerged plant species on CH4 flux and methanogenic community dynamics in a full-scale constructed wetland. Ecol. Eng. 2018, 115, 96–104. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, H.; Zhu, Z.; Chen, W.; Chen, J.; Mo, Y. CH4 flux and methanogen community dynamics from five common emergent vegetations in a full-scale constructed wetland. Environ. Sci. Pollut. Res. 2018, 25, 26433–26445. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, C.-j.; Liu, P.-f.; Fu, L.; Laso-Perez, R.; Yang, L.; Bai, L.-p.; Li, J.; Yang, M.; Lin, J.-z.; et al. Non-syntrophic methanogenic hydrocarbon degradation by an archaeal species. Nature 2022, 601, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Ernst, L.; Steinfeld, B.; Barayeu, U.; Klintzsch, T.; Kurth, M.; Grimm, D.; Dick, T.P.; Rebelein, J.G.; Bischofs, I.B.; Keppler, F. Methane formation driven by reactive oxygen species across all living organisms. Nature 2022, 603, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-B.; Sun, H.-Y.; Ge, Y.; Gu, B.-J.; Wang, H.; Chang, J. Plant species richness enhanced the methane emission in experimental microcosms. Atmos. Environ. 2012, 62, 180–183. [Google Scholar] [CrossRef]
- Wang, G.; Yu, G.; Chi, T.; Li, Y.; Zhang, Y.; Wang, J.; Li, P.; Liu, J.; Yu, Z.; Wang, Q.; et al. Insights into the enhanced effect of biochar on cadmium removal in vertical flow constructed wetlands. J. Hazard. Mater. 2023, 443, 130148. [Google Scholar] [CrossRef]
- Thangarajan, R.; Bolan, N.S.; Tian, G.; Naidu, R.; Kunhikrishnan, A. Role of organic amendment application on greenhouse gas emission from soil. Sci. Total Environ. 2013, 465, 72–96. [Google Scholar] [CrossRef]
- Liu, S.; Feng, X.; Li, X. Bioelectrochemical approach for control of methane emission from wetlands. Bioresour. Technol. 2017, 241, 812–820. [Google Scholar] [CrossRef]
- Bonetti, G.; Trevathan-Tackett, S.M.; Hebert, N.; Carnell, P.E.; Macreadie, P.I. Microbial community dynamics behind major release of methane in constructed wetlands. Appl. Soil Ecol. 2021, 167, 104163. [Google Scholar] [CrossRef]
- Lenzewski, N.; Mueller, P.; Meier, R.J.; Liebsch, G.; Jensen, K.; Koop-Jakobsen, K. Dynamics of oxygen and carbon dioxide in rhizospheres of Lobelia dortmanna—A planar optode study of belowground gas exchange between plants and sediment. New Phytol. 2018, 218, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Song, H.-L.; Singh, R.P.; Yang, Y.-L.; Xu, J.-Y.; Yang, X.-L. Simultaneous reduction of antibiotics leakage and methane emission from constructed wetland by integrating microbial fuel cell. Bioresour. Technol. 2021, 320, 124285. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Zhao, Q.; Gao, Q.Z.; Li, Y.E.; Wan, Y.F.; Li, Y.; Tian, D.; Liao, Y.L.; Fan, M.R.; Ganjurjav, H.; et al. Human Activities Inducing High CH4 Diffusive Fluxes in an Agricultural River Catchment in Subtropical China. Sustainability 2020, 12, 2114. [Google Scholar] [CrossRef] [Green Version]
- Thauer, R.K. Functionalization of Methane in Anaerobic Microorganisms. Angew. Chem.-Int. Ed. 2010, 49, 6712–6713. [Google Scholar] [CrossRef]
- Wegener, G.; Krukenberg, V.; Riedel, D.; Tegetmeyer, H.E.; Boetius, A. Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria. Nature 2015, 526, 587–590. [Google Scholar] [CrossRef]
- Guerrero-Cruz, S.; Vaksmaa, A.; Horn, M.A.; Niemann, H.; Pijuan, M.; Ho, A. Methanotrophs: Discoveries, Environmental Relevance, and a Perspective on Current and Future Applications. Front. Microbiol. 2021, 12, 678057. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; Prieto-Davo, A.; Lopez-Lozano, N.E.; Hernandez-Eligio, A.; Vega-Alvarado, L.; Juarez, K.; Sarahi Garcia-Gonzalez, A.; Lopez, M.G.; Cervantes, F.J. Anaerobic Methane Oxidation Driven by Microbial Reduction of Natural Organic Matter in a Tropical Wetland. Appl. Environ. Microbiol. 2017, 83, e00645-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, B.; Chen, J.; Li, W.; Mei, J.; Yang, Y.; Chang, J. Greenhouse gas emissions from constructed wetlands are mitigated by biochar substrates and distinctly affected by tidal flow and intermittent aeration modes. Environ. Pollut. 2021, 271, 116328. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Yan, B.; Shutes, B.; Tian, L.; Wen, H. Optimal influent COD/N ratio for obtaining low GHG emissions and high pollutant removal efficiency in constructed wetlands. J. Clean. Prod. 2020, 267, 122003. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Yamazaki, T.; Hozuki, T.; Arai, K.; Toyoda, S.; Koba, K.; Fujiwara, T.; Yoshida, N. Isotopomeric characterization of nitrous oxide produced by reaction of enzymes extracted from nitrifying and denitrifying bacteria. Biogeosciences 2014, 11, 2679–2689. [Google Scholar] [CrossRef] [Green Version]
- Schrnitz, B.W.; Kitajima, M.; Campillo, M.E.; Gerba, C.P.; Pepper, I.L. Virus Reduction during Advanced Bardenpho and Conventional Wastewater Treatment Processes. Environ. Sci. Technol. 2016, 50, 9524–9532. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Ye, L.; Erler, D.; Ni, B.-J.; Yuan, Z. Quantifying nitrous oxide production pathways in wastewater treatment systems using isotope technology—A critical review. Water Res. 2017, 122, 96–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kool, D.M.; Wrage, N.; Zechmeister-Boltenstern, S.; Pfeffer, M.; Brus, D.; Oenema, O.; Van Groenigen, J.W. Nitrifier denitrification can be a source of N2O from soil: A revised approach to the dual-isotope labelling method. Eur. J. Soil Sci. 2010, 61, 759–772. [Google Scholar] [CrossRef]
- Thakur, I.S.; Medhi, K. Nitrification and denitrification processes for mitigation of nitrous oxide from waste water treatment plants for biovalorization: Challenges and opportunities. Bioresour. Technol. 2019, 282, 502–551. [Google Scholar] [CrossRef]
- Lv, Y.-T.; Zhang, X.; Zhu, C.; Lin, L.; Sun, T.; Wang, X.; Wang, L. Micro-analysis of nitrous oxide accumulation in denitrification under acidic conditions: The role of pH and free nitrous acid. J. Water Process Eng. 2022, 47, 102767. [Google Scholar] [CrossRef]
- Li, C.; Wang, Q.; Jia, W. N2O reduction during denitrifying phosphorus removal with propionate as carbon source. Environ. Sci. Pollut. Res. 2022, 29, 12390–12398. [Google Scholar] [CrossRef]
- Carreira, C.; Pauleta, S.R.; Moura, I. The catalytic cycle of nitrous oxide reductase—The enzyme that catalyzes the last step of denitrification. J. Inorg. Biochem. 2017, 177, 423–434. [Google Scholar] [CrossRef]
- Huang, L.; Gao, X.; Guo, J.; Ma, X.; Liu, M. A review on the mechanism and affecting factors of nitrous oxide emission in constructed wetlands. Environ. Earth Sci. 2012, 68, 2171–2180. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, J.; Xie, H.; Yan, Y.; Wang, J.; Zhao, Y.; Xu, X. Effect of PHB and oxygen uptake rate on nitrous oxide emission during simultaneous nitrification denitrification process. Bioresour. Technol. 2012, 113, 232–238. [Google Scholar] [CrossRef]
- Kool, D.M.; Dolfing, J.; Wrage, N.; Van Groenigen, J.W. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol. Biochem. 2011, 43, 174–178. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, G.-M.; Miao, L.-L.; Liu, Z.-P. Marinobacter strain NNA5, a newly isolated and highly efficient aerobic denitrifier with zero N2O emission. Bioresour. Technol. 2016, 206, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Massara, T.M.; Malamis, S.; Guisasola, A.; Antonio Baeza, J.; Noutsopoulos, C.; Katsou, E. A review on nitrous oxide (N2O) emissions during biological nutrient removal from municipal wastewater and sludge reject water. Sci. Total Environ. 2017, 596, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sorensen, P.; Olesen, J.E.; Petersen, S.O. Evidence for denitrification as main source of N2O emission from residue-amended soil. Soil Biol. Biochem. 2016, 92, 153–160. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, L.; Wang, D.; Zhou, Y.; Yang, X. Recent advances in nitrous oxide production and mitigation in wastewater treatment. Water Res. 2020, 184, 116168. [Google Scholar] [CrossRef]
- Kou-Giesbrecht, S.; Menge, D. Nitrogen-fixing trees could exacerbate climate change under elevated nitrogen deposition. Nat. Commun. 2019, 10, e03415. [Google Scholar] [CrossRef] [Green Version]
- Bateganya, N.L.; Mentler, A.; Langergraber, G.; Busulwa, H.; Hein, T. Carbon and nitrogen gaseous fluxes from subsurface flow wetland buffer strips at mesocosm scale in East Africa. Ecol. Eng. 2015, 85, 173–184. [Google Scholar] [CrossRef]
- VanderZaag, A.C.; Gordon, R.J.; Burton, D.L.; Jamieson, R.C.; Stratton, G.W. Greenhouse Gas Emissions from Surface Flow and Subsurface Flow Constructed Wetlands Treating Dairy Wastewater. J. Environ. Qual. 2010, 39, 460–471. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, X.; Wang, W.; Chen, J.; Luo, H.; Chen, W.; Ma, D.; An, X.; Chen, F.; Cheng, L.; et al. Anaerobic oxidation of methane (AOM) driven by multiple electron acceptors in constructed wetland and the related mechanisms of carbon, nitrogen, sulfur cycles. Chem. Eng. J. 2022, 433, 133663. [Google Scholar] [CrossRef]
- Lopez, D.; Fuenzalida, D.; Vera, I.; Rojas, K.; Vidal, G. Relationship between the removal of organic matter and the production of methane in subsurface flow constructed wetlands designed for wastewater treatment. Ecol. Eng. 2015, 83, 296–304. [Google Scholar] [CrossRef]
- Henneberg, A.; Brix, H.; Sorrell, B.K. The interactive effect of Juncus effusus and water table position on mesocosm methanogenesis and methane emissions. Plant Soil 2016, 400, 45–54. [Google Scholar] [CrossRef]
- Saeed, T.; Afrin, R.; Al Muyeed, A.; Sun, G. Treatment of tannery wastewater in a pilot-scale hybrid constructed wetland system in Bangladesh. Chemosphere 2012, 88, 1065–1073. [Google Scholar] [CrossRef]
- Liu, C.; Xu, K.; Inamori, R.; Ebie, Y.; Liao, J.; Inamori, Y. Pilot-scale studies of domestic wastewater treatment by typical constructed wetlands and their greenhouse gas emissions. Front. Environ. Sci. Eng. China 2009, 3, 477–482. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L. A comparative estimate of life cycle greenhouse gas emissions from two types of constructed wetlands in Tianjin, China. Desalination Water Treat. 2013, 51, 2280–2293. [Google Scholar] [CrossRef]
- Wu, H.; Wang, R.; Yan, P.; Wu, S.; Chen, Z.; Zhao, Y.; Cheng, C.; Hu, Z.; Zhuang, L.; Guo, Z.; et al. Constructed wetlands for pollution control. Nat. Rev. Earth Environ. 2023, 4, 218–234. [Google Scholar] [CrossRef]
- Liikanen, A.; Huttunen, J.T.; Karjalainen, S.M.; Heikkinen, K.; Vaisanen, T.S.; Nykanen, H.; Martikainen, P.J. Temporal and seasonal changes in greenhouse gas emissions from a constructed wetland purifying peat mining runoff waters. Ecol. Eng. 2006, 26, 241–251. [Google Scholar] [CrossRef]
- Zhai, X.; Piwpuan, N.; Arias, C.A.; Headley, T.; Brix, H. Can root exudates from emergent wetland plants fuel denitrification in subsurface flow constructed wetland systems? Ecol. Eng. 2013, 61, 555–563. [Google Scholar] [CrossRef]
- Chang, J.; Fan, X.; Sun, H.; Zhang, C.; Song, C.; Chang, S.X.; Gu, B.; Liu, Y.; Li, D.; Wang, Y.; et al. Plant species richness enhances nitrous oxide emissions in microcosms of constructed wetlands. Ecol. Eng. 2014, 64, 108–115. [Google Scholar] [CrossRef]
- Turner, J.C.; Moorberg, C.J.; Wong, A.; Shea, K.; Waldrop, M.P.; Turetsky, M.R.; Neumann, R.B. Getting to the Root of Plant-Mediated Methane Emissions and Oxidation in a Thermokarst Bog. J. Geophys. Res.-Biogeosci. 2020, 125, e2020JG005825. [Google Scholar] [CrossRef]
- Picek, T.; Čížková, H.; Dušek, J. Greenhouse gas emissions from a constructed wetland—Plants as important sources of carbon. Ecol. Eng. 2007, 31, 98–106. [Google Scholar] [CrossRef]
- Chowdhury, T.R.; Dick, R.P. Ecology of aerobic methanotrophs in controlling methane fluxes from wetlands. Appl. Soil Ecol. 2013, 65, 8–22. [Google Scholar] [CrossRef]
- Gu, X.; Chen, D.; Wu, F.; Tang, L.; He, S.; Zhou, W. Function of aquatic plants on nitrogen removal and greenhouse gas emission in enhanced denitrification constructed wetlands: Iris pseudacorus for example. J. Clean. Prod. 2022, 330, 129842. [Google Scholar] [CrossRef]
- Du, Y.; Pan, K.; Yu, C.; Luo, B.; Gu, W.; Sun, H.; Min, Y.; Liu, D.; Geng, Y.; Han, W.; et al. Plant diversity decreases net global warming potential integrating multiple functions in microcosms of constructed wetlands. J. Clean. Prod. 2018, 184, 718–726. [Google Scholar] [CrossRef]
- Zhao, Z.; Chang, J.; Han, W.; Wang, M.; Ma, D.; Du, Y.; Qu, Z.; Chang, S.X.; Ge, Y. Effects of plant diversity and sand particle size on methane emission and nitrogen removal in microcosms of constructed wetlands. Ecol. Eng. 2016, 95, 390–398. [Google Scholar] [CrossRef]
- Kwon, M.J.; Beulig, F.; Ilie, I.; Wildner, M.; Kuesel, K.; Merbold, L.; Mahecha, M.D.; Zimov, N.; Zimov, S.A.; Heimann, M.; et al. Plants, microorganisms, and soil temperatures contribute to a decrease in methane fluxes on a drained Arctic floodplain. Glob. Change Biol. 2017, 23, 2396–2412. [Google Scholar] [CrossRef]
- Van der Nat, F.J.; Middelburg, J.J. Methane emission from tidal freshwater marshes. Biogeochemistry 2000, 49, 103–121. [Google Scholar] [CrossRef]
- Juutinen, S.; Larmola, T.; Remus, R.; Mirus, E.; Merbach, W.; Silvola, J.; Augustin, J. The contribution of Phragmites australis litter to methane (CH4) emission in planted and non-planted fen microcosms. Biol. Fertil. Soils 2003, 38, 10–14. [Google Scholar] [CrossRef]
- Zhu, N.; An, P.; Krishnakumar, B.; Zhao, L.; Sun, L.; Mizuochi, M.; Inamori, Y. Effect of plant harvest on methane emission from two constructed wetlands designed for the treatment of wastewater. J. Environ. Manag. 2007, 85, 936–943. [Google Scholar] [CrossRef]
- Mander, U.; Maddison, M.; Soosaar, K.; Karabelnik, K. The Impact of Pulsing Hydrology and Fluctuating Water Table on Greenhouse Gas Emissions from Constructed Wetlands. Wetlands 2011, 31, 1023–1032. [Google Scholar] [CrossRef]
- Ji, B.; Chen, J.; Mei, J.; Chang, J.; Li, X.; Jia, W.; Qu, Y. Roles of biochar media and oxygen supply strategies in treatment performance, greenhouse gas emissions, and bacterial community features of subsurface-flow constructed wetlands. Bioresour. Technol. 2020, 302, 122890. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Li, M.; Li, Y.; Zhang, L.; Xu, X.; Wu, H.; Liang, S.; Su, C.; Zhang, J. The performance and mechanism of biochar-enhanced constructed wetland for wastewater treatment. J. Water Process Eng. 2022, 45, 102522. [Google Scholar] [CrossRef]
- Cheng, S.; Qin, C.; Xie, H.; Wang, W.; Hu, Z.; Liang, S.; Feng, K. A new insight on the effects of iron oxides and dissimilated metal-reducing bacteria on CH4 emissions in constructed wetland matrix systems. Bioresour. Technol. 2021, 320, 124296. [Google Scholar] [CrossRef]
- Cheng, S.; Qin, C.; Xie, H.; Wang, W.; Zhang, J.; Hu, Z.; Liang, S. Comprehensive evaluation of manganese oxides and iron oxides as metal substrate materials for constructed wetlands from the perspective of water quality and greenhouse effect. Ecotoxicol. Environ. Saf. 2021, 221, 112451. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yu, K.; Gambrell, R.P. Effects of ferric iron reduction and regeneration on nitrous oxide and methane emissions in a rice soil. Chemosphere 2009, 74, 481–486. [Google Scholar] [CrossRef]
- Dong, C.; Li, M.; Zhuang, L.-L.; Zhang, J.; Shen, Y.; Li, X. The Improvement of Pollutant Removal in the Ferric-Carbon Micro-Electrolysis Constructed Wetland by Partial Aeration. Water 2020, 12, 389. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xu, J.; Sheng, L. Purification mechanism of sewage from constructed wetlands with zeolite substrates: A review. J. Clean. Prod. 2020, 258, 120760. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, C.; Liu, C.e.; Sun, H.; Zhang, J.; Zhang, X.; Xin, L. Nutrient removal, methane and nitrous oxide emissions in a hybrid constructed wetland treating anaerobic digestate. Sci. Total Environ. 2020, 733, 138338. [Google Scholar] [CrossRef]
- Zhao, Z.; Hao, Q.; Ma, R.; Chen, X.; Xiong, Y.; Hu, J.; Zhang, G.; Jiang, C. Ferric-carbon micro-electrolysis and zeolite reduce CH4 and N2O emissions from the aerated constructed wetland. J. Clean. Prod. 2022, 342, 130946. [Google Scholar] [CrossRef]
- Zhang, G.; Hao, Q.; Ma, R.; Luo, S.; Chen, K.; Liang, Z.; Jiang, C. Biochar and hematite amendments suppress emission of CH4 and NO2 in constructed wetlands. Sci. Total Environ. 2023, 874, 162451. [Google Scholar] [CrossRef]
- Huo, J.; Hu, X.; Cheng, S.; Xie, H.; Hu, Z.; Wu, H.; Liang, S. Effects and mechanisms of constructed wetlands with different substrates on N2O emission in wastewater treatment. Environ. Sci. Pollut. Res. Int. 2022, 29, 19045–19053. [Google Scholar] [CrossRef]
- Wu, X.L.; Chin, K.J.; Conrad, R. Effect of temperature stress on structure and function of the methanogenic archaeal community in a rice field soil. FEMS Microbiol. Ecol. 2002, 39, 211–218. [Google Scholar] [CrossRef]
- Conrad, R. Microbial ecology of methanogens and methanotrophs. Adv. Agron. 2007, 96, 1–63. [Google Scholar]
- Hoj, L.; Olsen, R.A.; Torsvik, V.L. Effects of temperature on the diversity and community structure of known methanogenic groups and other archaea in high Arctic peat. ISME J. 2008, 2, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.X.; Cai, Z.C.; Wang, D.X. Preliminary budget of methane emissions from natural wetlands in China. Atmos. Environ. 2004, 38, 751–759. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Liu, X.; Fu, X.; Luo, H.; Li, M.; Jiang, B.; Chen, J.; Chen, W.; Huang, B.; et al. Methane emissions and methanogenic community investigation from constructed wetlands in Chengdu City. Urban Clim. 2021, 39, 100956. [Google Scholar] [CrossRef]
- Maucieri, C.; Borin, M.; Milani, M.; Cirelli, G.L.; Barbera, A.C. Plant species effect on CO2 and CH4 emissions from pilot constructed wetlands in Mediterranean area. Ecol. Eng. 2019, 134, 112–117. [Google Scholar] [CrossRef]
- Shao, X.; Zhao, L.; Sheng, X.; Wu, M. Effects of influent salinity on water purification and greenhouse gas emissions in lab-scale constructed wetlands. Environ. Sci. Pollut. Res. 2020, 27, 21487–21496. [Google Scholar] [CrossRef]
- Lu, Y.; Fu, L.; Lu, Y.; Hugenholtz, F.; Ma, K. Effect of temperature on the structure and activity of a methanogenic archaeal community during rice straw decomposition. Soil Biol. Biochem. 2015, 81, 17–27. [Google Scholar] [CrossRef]
- Ström, L.; Lamppa, A.; Christensen, T.R. Greenhouse gas emissions from a constructed wetland in southern Sweden. Wetl. Ecol. Manag. 2006, 15, 43–50. [Google Scholar] [CrossRef]
- Mander, U.; Maddison, M.; Soosaar, K.; Koger, H.; Teemusk, A.; Truu, J.; Well, R.; Sebilo, M. The impact of a pulsing water table on wastewater purification and greenhouse gas emission in a horizontal subsurface flow constructed wetland. Ecol. Eng. 2015, 80, 69–78. [Google Scholar] [CrossRef]
- Maucieri, C.; Borin, M.; Barbera, A.C. Role of C3 plant species on carbon dioxide and methane emissions in Mediterranean constructed wetland. Ital. J. Agron. 2014, 9, 120–126. [Google Scholar] [CrossRef] [Green Version]
- de la Varga, D.; Ruiz, I.; Alvarez, J.A.; Soto, M. Methane and carbon dioxide emissions from constructed wetlands receiving anaerobically pretreated sewage. Sci. Total Environ. 2015, 538, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Hakobyan, A.; Glatter, T.; Paczia, N.; Liesack, W. Methylocystis sp. Strain SC2 Acclimatizes to Increasing NH4+ Levels by a Precise Rebalancing of Enzymes and Osmolyte Composition. Msystems 2022, 7, e00403-22. [Google Scholar] [CrossRef]
- Hu, S.; Zeng, R.J.; Keller, J.; Lant, P.A.; Yuan, Z. Effect of nitrate and nitrite on the selection of microorganisms in the denitrifying anaerobic methane oxidation process. Environ. Microbiol. Rep. 2011, 3, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Reay, D.S.; Nedwell, D.B. Methane oxidation in temperate soils: Effects of inorganic N. Soil Biol. Biochem. 2004, 36, 2059–2065. [Google Scholar] [CrossRef]
- Raghoebarsing, A.A.; Pol, A.; van de Pas-Schoonen, K.T.; Smolders, A.J.P.; Ettwig, K.F.; Rijpstra, W.I.C.; Schouten, S.; Damste, J.S.S.; Op den Camp, H.J.M.; Jetten, M.S.M.; et al. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 2006, 440, 918–921. [Google Scholar] [CrossRef] [Green Version]
- Ettwig, K.F.; Butler, M.K.; Le Paslier, D.; Pelletier, E.; Mangenot, S.; Kuypers, M.M.M.; Schreiber, F.; Dutilh, B.E.; Zedelius, J.; de Beer, D.; et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 2010, 464, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Nordi, K.A.; Thamdrup, B. Nitrate-dependent anaerobic methane oxidation in a freshwater sediment. Geochim. Et Cosmochim. Acta 2014, 132, 141–150. [Google Scholar] [CrossRef]
- Wunderlin, P.; Mohn, J.; Joss, A.; Emmenegger, L.; Siegrist, H. Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res. 2012, 46, 1027–1037. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Ge, Z.; Hu, C.; Zhang, H. Effects of influent C/N ratios on wastewater nutrient removal and simultaneous greenhouse gas emission from the combinations of vertical subsurface flow constructed wetlands and earthworm eco-filters for treating synthetic wastewater. Environ. Sci.-Process. Impacts 2014, 16, 567–575. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, Y.; Wu, J.; Zhang, J.; Zheng, Z. Effects of different influent C/N ratios on the performance of various earthworm eco-filter systems: Nutrient removal and greenhouse gas emission. World J. Microbiol. Biotechnol. 2014, 30, 109–118. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Jia, W.; Xie, H.; Gu, R.R.; Li, C.; Gao, B. Impact of COD/N ratio on nitrous oxide emission from microcosm wetlands and their performance in removing nitrogen from wastewater. Bioresour. Technol. 2009, 100, 2910–2917. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Jiang, C.; Xu, S.; Yu, X.; Yin, X.; Wang, J.; Maihaiti, M.; Wang, C.; Zheng, X.; Zhuang, X. Greenhouse Gases Emissions of Constructed Wetlands: Mechanisms and Affecting Factors. Water 2023, 15, 2871. https://doi.org/10.3390/w15162871
Yin X, Jiang C, Xu S, Yu X, Yin X, Wang J, Maihaiti M, Wang C, Zheng X, Zhuang X. Greenhouse Gases Emissions of Constructed Wetlands: Mechanisms and Affecting Factors. Water. 2023; 15(16):2871. https://doi.org/10.3390/w15162871
Chicago/Turabian StyleYin, Xiaoxue, Cancan Jiang, Shengjun Xu, Xiaojuan Yu, Xiaolin Yin, Jinglin Wang, Mairemu Maihaiti, Cong Wang, Xiaoxu Zheng, and Xuliang Zhuang. 2023. "Greenhouse Gases Emissions of Constructed Wetlands: Mechanisms and Affecting Factors" Water 15, no. 16: 2871. https://doi.org/10.3390/w15162871






