Geochemical Investigations of the Geothermal Waters in the Kangding Area, SW China: Constraints from Hydrochemistry and D-O-T Isotopy
Abstract
:1. Introduction
2. Geothermal Geological Background
3. Sample Collection and Analytical Testing
4. Results and Discussion
4.1. Hydrochemical Characteristics
4.2. Ion Source Analysis
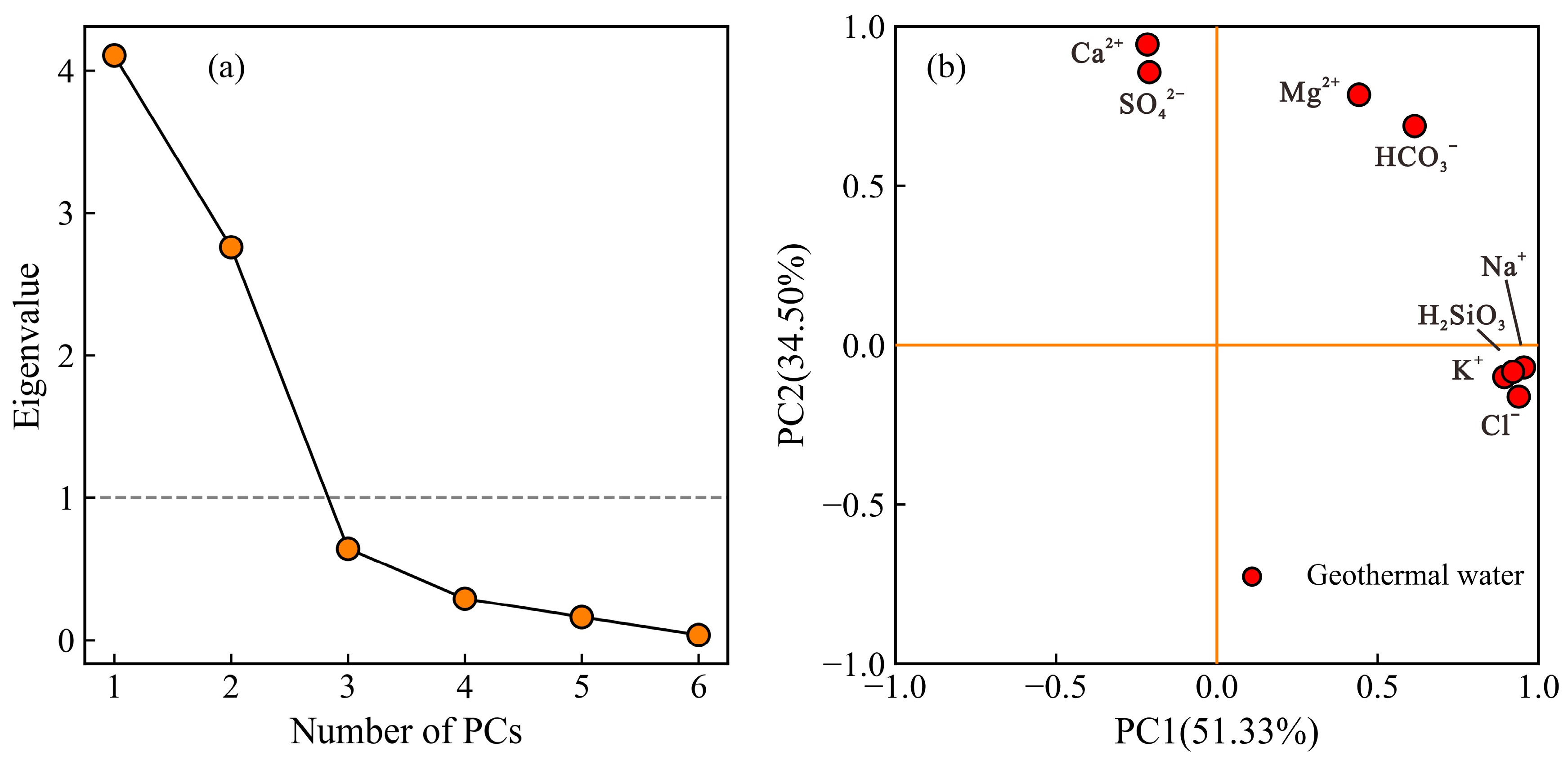
4.2.1. Principal Component Analysis
4.2.2. Cl− Correlation Analysis
4.2.3. Ion Ratio Analysis
4.3. Recharge Source and Residential Time by D-O-T Isotopes
4.3.1. Recharge Source
4.3.2. Residential Time
4.4. Geothermal Reservoir
4.4.1. Water–Rock Equilibrium State
4.4.2. Geothermal Geothermometer
- (1)
- SiO2 geothermometer
- (2)
- Geochemical thermodynamic geothermometer
4.5. Genetic Model of Geothermal Waters in the Study Area
5. Conclusions
- (1)
- The hydrochemical types of Kangding geothermal water were mainly Ca2+–Na+–HCO3− type, Na+–−–HCO3− type, and Na+–HCO3− type. The hydrochemical characteristics of geothermal water were mainly influenced by deep fluid mixing and water–rock interactions in the Kangding geothermal area.
- (2)
- The temperature of the deep geothermal reservoir was 114–219 °C, the temperature of the shallow geothermal reservoir was 50–115 °C, and the mixing ratio of cold water was 0.56–0.89.
- (3)
- The geothermal water was affected by water–rock interactions in the study area, and there was a certain degree of the “oxygen isotope drift” phenomenon. In this study, the recharge elevation of geothermal water was calculated to be 3034–3845 m, with an average of 3416 m.
- (4)
- The source of geothermal waters was atmospheric precipitation and high mountain ice and snow melt water in Kangding geothermal area. It was heated by a radioactive element decay in the granite and local melt body in the deep part, and mixed with magma water in the deep geothermal reservoir. Then, the geothermal fluid rose along the secondary fractures of Xianshui River, mixed with cold water in the shallow geothermal reservoir, and was exposed at the surface, thereby becoming springs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Muffler, L.J.P. Tectonic and hydrologic control of the nature and distribution of geothermal resources. Geo-Heat Cent. Q. Bull. 1993, 15, 1–10. [Google Scholar]
- Tang, X.; Zhang, J.; Pang, Z.; Hu, S.; Tian, J.; Bao, S. The eastern Tibetan Plateau geothermal belt, western China: Geology, geophysics, genesis, and hydrothermal system. Tectonophysics 2017, 717, 433–448. [Google Scholar] [CrossRef]
- Guo, Q.; Planer-Friedrich, B.; Liu, M.; Yan, K.; Wu, G. Magmatic fluid input explaining the geochemical anomaly of very high arsenic in some southern Tibetan geothermal waters. Chem. Geol. 2019, 513, 32–43. [Google Scholar] [CrossRef]
- Liu, M.; Guo, Q.; Wu, G.; Guo, W.; She, W.; Yan, W. Boron geochemistry of the geothermal waters from two typical hydrothermal systems in Southern Tibet (China): Daggyai and Quzhuomu. Geothermics 2019, 82, 190–202. [Google Scholar] [CrossRef]
- Tian, J.; Pang, Z.; Wang, Y.; Guo, Q. Fluid geochemistry of the Cuopu high temperature geothermal system in the eastern Himalayan syntaxis with implication on its genesis. Appl. Geochem. 2019, 110, 104422. [Google Scholar] [CrossRef]
- Apollaro, C.; Fuoco, I.; Gennaro, E.; Giuliani, L.; Iezzi, G.; Marini, L.; Radica, F.; Di Luccio, F.; Ventura, G.; Vespasiano, G. Advanced argillic alteration at Cave di Caolino, Lipari, Aeolian Islands (Italy): Implications for the mitigation of volcanic risks and the exploitation of geothermal resources. Sci. Total Environ. 2023, 889, 164333. [Google Scholar] [CrossRef]
- Fuoco, I.; Figoli, A.; Criscuoli, A.; Brozzo, G.; De Rosa, R.; Gabriele, B.; Apollaro, C. Geochemical modeling of chromium release in natural waters and treatment by RO/NF membrane processes. Chemosphere 2020, 254, 126696. [Google Scholar] [CrossRef]
- Griffiths, J.C. Statistics and Data Analysis in Geology. Technometrics 2005, 47, 526–527. [Google Scholar]
- Zhang, X.; Guo, Q.; Liu, M.; Luo, J.; Yin, Z.; Zhang, C.; Zhu, M.; Guo, W.; Li, J.; Zhou, C. Hydrogeochemical processes occurring in the hydrothermal systems of the Gonghe–Guide basin, northwestern China: Critical insights from a principal components analysis (PCA). Environ. Earth Sci. 2016, 75, 1187. [Google Scholar] [CrossRef]
- Cioni, R.; Marini, L. A Thermodynamic Approach to Water Geothermometry; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Fournier, R.O.; Truesdell, A.H. An empirical Na K Ca geothermometer for natural waters. Geochim. et Cosmochim. Acta 1973, 37, 1255–1275. [Google Scholar] [CrossRef]
- García-López, C.; Pandarinath, K.; Santoyo, E. Solute and gas geothermometry of geothermal wells: A geochemometrics study for evaluating the effectiveness of geothermometers to predict deep reservoir temperatures. Int. Geol. Rev. 2014, 56, 2015–2049. [Google Scholar] [CrossRef]
- Santoyo, E.; Díaz-González, L. A New Improved Proposal of the Na/K Geothermometer to Estimate Deep Equilibrium Temperatures and their Uncertainties in Geothermal Systems. In Proceedings of the World Geothermal Congress, Bali, Indonesia, 1 November 2009. [Google Scholar]
- Verma, S.P.; Pandarinath, K.; Santoyo, E. SolGeo: A new computer program for solute geothermometers and its application to Mexican geothermal fields. Geothermics 2008, 37, 597–621. [Google Scholar] [CrossRef]
- Verma, S.P.; Santoyo, E. New improved equations for NaK, NaLi and SiO2 geothermometers by outlier detection and rejection. J. Volcanol. Geotherm. Res. 1997, 79, 9–23. [Google Scholar] [CrossRef]
- Yi, L.; Qi, J.; Li, X.; Xu, M.; Zhang, X.; Zhang, Q.; Tang, Y. Geochemical characteristics and genesis of the high-temperature geothermal systems in the north section of the Sanjiang Orogenic belt in southeast Tibetan Plateau. J. Volcanol. Geotherm. Res. 2021, 414, 107244. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Tang, X.; Tian, J.; Wang, Y.; Guo, Q.; Pang, Z. Geothermal data analysis at the high-temperature hydrothermal area in Western Sichuan. Sci. China Earth Sci. 2017, 60, 1507–1521. [Google Scholar] [CrossRef]
- Qi, J.; Xu, M.; An, C.; Wu, M.; Zhang, Y.; Li, X.; Zhang, Q.; Lu, G. Characterizations of geothermal springs along the Moxi deep fault in the western Sichuan plateau, China. Phys. Earth Planet. Inter. 2017, 263, 12–22. [Google Scholar] [CrossRef]
- Luo, J.; Pang, Z.; Kong, Y.; Wang, Y. Geothermal potential evaluation and development prioritization based on geochemistry of geothermal waters from Kangding area, western Sichuan, China. Environ. Earth Sci. 2017, 76, 343. [Google Scholar] [CrossRef]
- Guo, Q.; Pang, Z.; Wang, Y.; Tian, J. Fluid geochemistry and geothermometry applications of the Kangding high-temperature geothermal system in eastern Himalayas. Appl. Geochem. 2017, 81, 63–75. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Liao, X.; Zhang, Y. Hydrogeochemical Characteristics and Conceptual Model of the Geothermal Waters in the Xianshuihe Fault Zone, Southwestern China. Int. J. Environ. Res. Public Health 2020, 17, 500. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, M.; Li, X.; Qi, J.; Zhang, Q.; Guo, J.; Yu, L.; Zhao, R. Hydrochemical Characteristics and Multivariate Statistical Analysis of Natural Water System: A Case Study in Kangding County, Southwestern China. Water 2018, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Roberts, N.M.W.; Searle, M.P. Zircon U–Pb–Hf constraints from Gongga Shan granites on young crustal melting in eastern Tibet. Geosci. Front. 2019, 10, 885–894. [Google Scholar] [CrossRef]
- Searle, M.P.; Roberts, N.M.W.; Chung, S.-L.; Lee, Y.-H.; Cook, K.L.; Elliott, J.R.; Weller, O.M.; St-Onge, M.R.; Xu, X.-W.; Tan, X.-B.; et al. Age and anatomy of the Gongga Shan batholith, eastern Tibetan Plateau, and its relationship to the active Xianshui-he fault. Geosphere 2016, 12, 948–970. [Google Scholar] [CrossRef] [Green Version]
- Arevalo, R.; McDonough, W.F.; Luong, M. The K/U ratio of the silicate Earth: Insights into mantle composition, structure and thermal evolution. Earth Planet. Sci. Lett. 2009, 278, 361–369. [Google Scholar] [CrossRef]
- de Sigoyer, J.; Vanderhaeghe, O.; Duchêne, S.; Billerot, A. Generation and emplacement of Triassic granitoids within the Songpan Ganze accretionary-orogenic wedge in a context of slab retreat accommodated by tear faulting, Eastern Tibetan plateau, China. J. Asian Earth Sci. 2014, 88, 192–216. [Google Scholar] [CrossRef]
- Lai, S.-C.; Zhao, S.-W. Petrogenesis of the Zheduoshan Cenozoic granites in the eastern margin of Tibet: Constraints on the initial activity of the Xianshuihe Fault. J. Geodyn. 2018, 117, 49–59. [Google Scholar] [CrossRef]
- Rybach, L. Radioactive heat production in rocks and its relation to other petrophysical parameters. Pure Appl. Geophys. 1976, 114, 309–317. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, H.; Jia, Y.; Cao, Y.; Hu, C. Principal component analysis and hierarchical cluster analyses of arsenic groundwater geochemistry in the Hetao basin, Inner Mongolia. Geochemistry 2015, 75, 197–205. [Google Scholar] [CrossRef]
- Seyedrahimi-Niaraq, M.; Mahdiyanfar, H.; Mokhtari, A.R. Integrating principal component analysis and U-statistics for mapping polluted areas in mining districts. J. Geochem. Explor. 2022, 234, 106924. [Google Scholar] [CrossRef]
- Nyakairu, G.W.A.; Kasule, J.; Ouma, O.; Bahati, G. Origin and hydrogeochemical formation processes of geothermal fluids from the Kibiro area, Western Uganda. Appl. Geochem. 2023, 152, 105648. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Z.; Xu, C.; Lv, Y.; Cheng, L.; Zhang, Y. Formation mechanism of a carbonate geothermal system and calculation of the reservoir temperature under complex conditions. Geothermics 2022, 99, 102295. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wen, H.; Hao, Y. Geochemical evidence for the nonexistence of supercritical geothermal fluids at the Yangbajing geothermal field, southern Tibet. J. Hydrol. 2022, 604, 127243. [Google Scholar] [CrossRef]
- Bärenbold, F.; Schmid, M.; Brennwald, M.S.; Kipfer, R. Missing atmospheric noble gases in a large, tropical lake: The case of Lake Kivu, East-Africa. Chem. Geol. 2020, 532, 119374. [Google Scholar] [CrossRef]
- Stefánsson, A.; Sveinbjörnsdóttir, Á.E.; Heinemeier, J.; Arnórsson, S.; Kjartansdóttir, R.; Kristmannsdóttir, H. Mantle CO2 degassing through the Icelandic crust: Evidence from carbon isotopes in groundwater. Geochim. Cosmochim. Acta 2016, 191, 300–319. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Zhang, Y.; Zhang, X.; Xiao, Y.; Duo, J.; Huang, X.; Sun, M.; Lv, G. Hydrochemical, D–O–Sr isotopic and electromagnetic characteristics of geothermal waters from the Erdaoqiao area, SW China: Insights into genetic mechanism and scaling potential. Ore Geol. Rev. 2023, 158, 105486. [Google Scholar] [CrossRef]
- Yang, P.; Luo, D.; Hong, A.; Ham, B.; Xie, S.; Ming, X.; Wang, Z.; Pang, Z. Hydrogeochemistry and geothermometry of the carbonate-evaporite aquifers controlled by deep-seated faults using major ions and environmental isotopes. J. Hydrol. 2019, 579, 124116. [Google Scholar] [CrossRef]
- Carol, E.; Kruse, E.; Mas-Pla, J. Hydrochemical and isotopical evidence of ground water salinization processes on the coastal plain of Samborombón Bay, Argentina. J. Hydrol. 2009, 365, 335–345. [Google Scholar] [CrossRef]
- Chen, L.; Wang, G.; Hu, F.; Wang, Y.; Liu, L. Groundwater hydrochemistry and isotope geochemistry in the Turpan Basin, northwestern China. J. Arid Land 2013, 6, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, Z.; Shao, J. Hydrochemistry and H-O-C-S Isotopic Geochemistry Characteristics of Geothermal Water in Nyemo-Nagqu, Tibet. Acta Geol. Sin.-Engl. Ed. 2017, 91, 644–657. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, X.; Yuan, X.; Yu, Z.; Xiao, Y.; Wang, Y.; Zhang, Y. Hydrochemical Characteristics and Genetic Mechanism of Geothermal Springs in the Aba Area, Western Sichuan Province, China. Sustainability 2022, 14, 12824. [Google Scholar] [CrossRef]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Xiangyang, S.; Genxu, W.; Juying, S.; Shouqin, S.; Zhaoyong, H.; Chunlin, S.; Shan, L. Contrasting water sources used by a coniferous forest in the high-altitude, southeastern Tibetan Plateau. Sci. Total Environ. 2022, 849, 157913. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A. The association of arsenic with redox conditions, depth, and ground-water age in the glacial aquifer system of the northern United States. In Scientific Investigations Report; US Geological Survey: Reston, VA, USA, 2007. [Google Scholar]
- Pasvanoğlu, S. Geochemical Survey of low- temperature geothermal resource of the Reşadiye Spa (Tokat, Northern Turkey). Geothermics 2021, 94, 102088. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Geothermal solute equilibria. Derivation of Na-K-Mg-Ca geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar] [CrossRef]
- Fournier, R.O.; Berger, B.R.; Bethke, P.M. The Behavior of Silica in Hydrothermal Solutions. In Geology and Geochemistry of Epithermal Systems; Society of Economic Geologists: Littleton, CO, USA, 1985; Volume 2. [Google Scholar]
- Fournier, R.O. Chemical geothermometers and mixing models for geothermal systems. Geothermics 1977, 5, 41–50. [Google Scholar] [CrossRef]
- Reed, M.H. Calculation of multicomponent chemical equilibria and reaction processes in systems involving minerals, gases and an aqueous phase. Geochim. Cosmochim. Acta 1982, 46, 513–528. [Google Scholar] [CrossRef]











| Sample ID | Sample Type | Elevation (m) | Flow (L/s) | Discharge T (°C) | pH | TDS (mg/L) | Charge Balance (%) | Hydrochemical Type | Na+ (mg/L) | K+ (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| D1 | Geothermal water | 2600 | 10.0 | 42 | 6.2 | 1262 | 0.63 | Ca2+·Na+–HCO3− | 153.50 | 20.10 |
| D2 | 2970 | 2.0 | 88 | 7.1 | 1091 | 0.65 | Na+–Cl−·HCO3− | 325.70 | 63.40 | |
| D3 | 2780 | 0.2 | 30 | 6.5 | 396 | 0.84 | Na+–HCO3− | 112.50 | 20.20 | |
| D4 | 2920 | 1.0 | 65 | 6.4 | 788 | 3.87 | Na+–Cl−·HCO3− | 246.50 | 27.50 | |
| D5 | 2912 | 1.0 | 72 | 7.1 | 1654 | 2.10 | Na+–HCO3− | 566.10 | 51.20 | |
| D6 | 2860 | 2.0 | 85 | 7.2 | 1420 | −0.61 | Na+–Cl−·HCO3− | 512.10 | 47.40 | |
| D7 | 3296 | 1.0 | 54 | 7.6 | 366 | 1.14 | Na+–HCO3− | 136.50 | 23.30 | |
| D8 | 2978 | 0.3 | 74 | 6.8 | 1332 | 2.07 | Na+–Cl−·HCO3− | 459.60 | 45.50 | |
| D9 | River water | 2968 | - | 10 | 7.5 | 109 | 0.15 | Ca2+·Mg2+–HCO3− | 10.30 | 2.70 |
| Sample ID | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | H2SiO3 | δD | δ18O | 3H | Recharge elevation |
| mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | ‰VSMOW | ‰VSMOW | TU | m | |
| D1 | 296.59 | 27.24 | 54.59 | 69.16 | 1281.42 | 58.50 | −113.2 | −15.30 | 6.89 | 3034 |
| D2 | 48.10 | 17.02 | 232.55 | 38.42 | 732.24 | 138.90 | −118.0 | −15.51 | 4.01 | 3076 |
| D3 | 23.25 | 8.76 | 61.68 | 11.53 | 317.30 | 40.70 | −112.1 | −15.43 | - | 3261 |
| D4 | 61.72 | 10.21 | 165.20 | 5.76 | 576.03 | 109.90 | −118.9 | −15.62 | 5.15 | 3296 |
| D5 | 52.10 | 34.05 | 226.88 | 3.84 | 1440.07 | 114.80 | −128.2 | −16.03 | 3.14 | 3541 |
| D6 | 13.63 | 7.30 | 266.58 | 3.84 | 1066.63 | 117.80 | −127.5 | −16.14 | 3.15 | 3626 |
| D7 | 3.21 | 0.49 | 12.05 | 1.92 | 378.32 | 40.90 | −133.2 | −18.20 | 1.73 | 3652 |
| D8 | 45.69 | 14.11 | 322.60 | 5.76 | 878.69 | 139.10 | −125.3 | −16.15 | - | 3845 |
| D9 | 20.04 | 7.30 | 4.25 | 9.61 | 109.84 | 12.00 | −110.4 | −15.26 | 5.63 | - |
| Anhydrite | Calcite | Chalcedony | Dolomite | Gypsum | Quartz | |
|---|---|---|---|---|---|---|
| D1 | −1.62 | 0.39 | −0.09 | 0.25 | −1.49 | 0.29 |
| D2 | −1.98 | 0.85 | −0.13 | 1.58 | −2.29 | 0.13 |
| D3 | −3.23 | −0.95 | −0.12 | −1.93 | −2.98 | 0.3 |
| D4 | −2.87 | −0.03 | −0.04 | −0.33 | −2.97 | 0.28 |
| D5 | −3.28 | 0.91 | −0.08 | 2.13 | −3.45 | 0.22 |
| D6 | −3.6 | 0.49 | −0.18 | 1.08 | −3.88 | 0.09 |
| D7 | −4.57 | −0.34 | −0.37 | −0.97 | −4.56 | −0.03 |
| D8 | −3.01 | 0.45 | −0.01 | 0.85 | −3.19 | 0.28 |
| Field Survey | Silica Geothermometer | Silicon Enthalpy Equation | ||||
|---|---|---|---|---|---|---|
| Sample ID | Discharge T | Quartz (No Vapor Loss) | Quartz (Maximum Vapor Loss) | Chalcedony | Cold Water Mixing Ratio | Reservoir Temperature |
| - | °C | °C | °C | °C | % | °C |
| D1 | 42 | 97 | 98 | 67 | 82 | 181 |
| D2 | 88 | 141 | 136 | 115 | 58 | 194 |
| D3 | 30 | 81 | 85 | 50 | 89 | 180 |
| D4 | 65 | 128 | 125 | 100 | 73 | 205 |
| D5 | 72 | 130 | 127 | 103 | 67 | 196 |
| D6 | 85 | 132 | 128 | 104 | 56 | 178 |
| D7 | 54 | 81 | 85 | 50 | 58 | 114 |
| D8 | 74 | 141 | 136 | 115 | 70 | 219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Deng, C.; Feng, T.; Zhang, Y. Geochemical Investigations of the Geothermal Waters in the Kangding Area, SW China: Constraints from Hydrochemistry and D-O-T Isotopy. Water 2023, 15, 2761. https://doi.org/10.3390/w15152761
Zhang X, Deng C, Feng T, Zhang Y. Geochemical Investigations of the Geothermal Waters in the Kangding Area, SW China: Constraints from Hydrochemistry and D-O-T Isotopy. Water. 2023; 15(15):2761. https://doi.org/10.3390/w15152761
Chicago/Turabian StyleZhang, Xialin, Chengdong Deng, Tao Feng, and Yunhui Zhang. 2023. "Geochemical Investigations of the Geothermal Waters in the Kangding Area, SW China: Constraints from Hydrochemistry and D-O-T Isotopy" Water 15, no. 15: 2761. https://doi.org/10.3390/w15152761
APA StyleZhang, X., Deng, C., Feng, T., & Zhang, Y. (2023). Geochemical Investigations of the Geothermal Waters in the Kangding Area, SW China: Constraints from Hydrochemistry and D-O-T Isotopy. Water, 15(15), 2761. https://doi.org/10.3390/w15152761







