Effect of Subsurface Drainage Combined with Biochar on the Bacterial Community Composition of Coastal Saline Soil
Abstract
1. Introduction
2. Materials and Methods
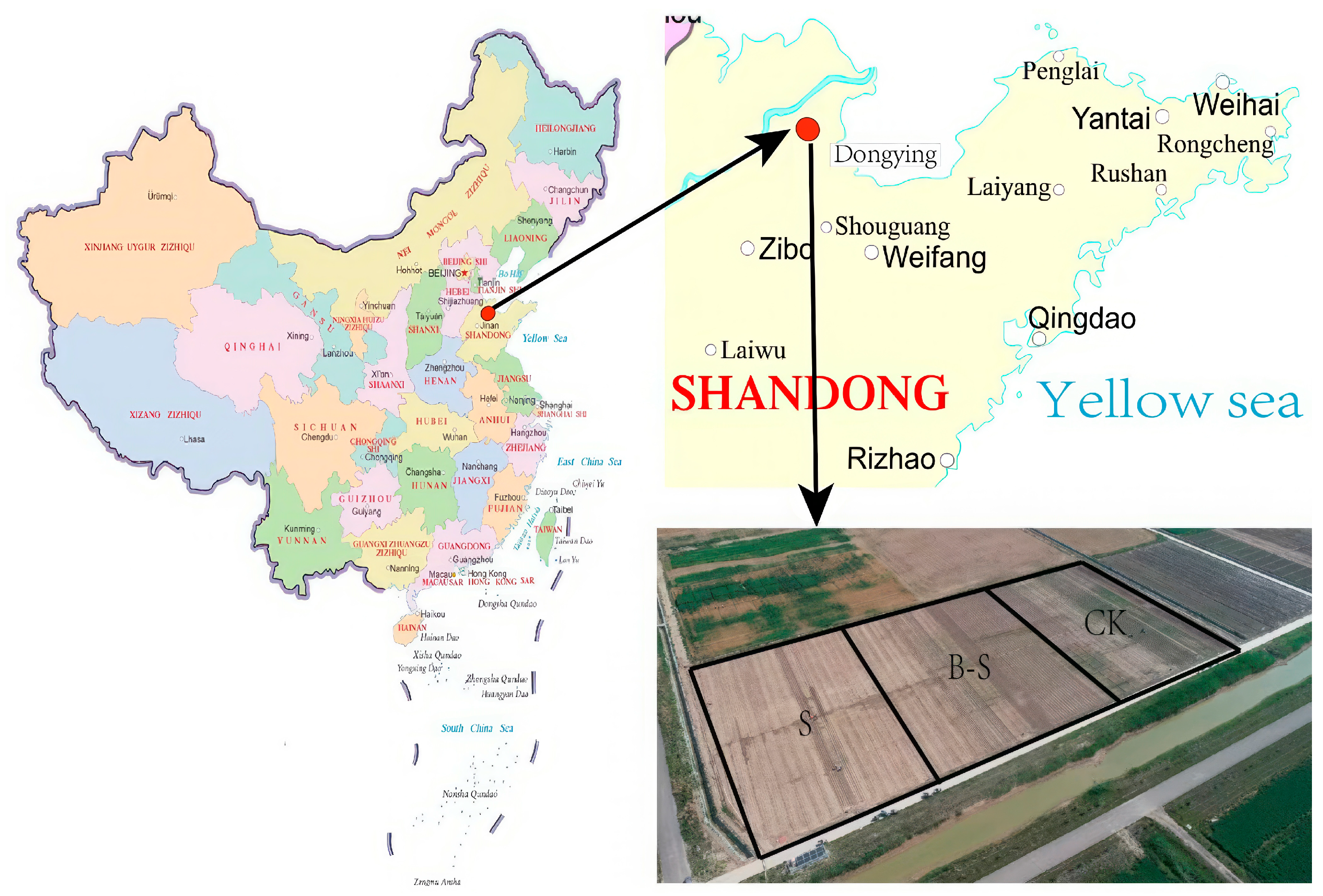
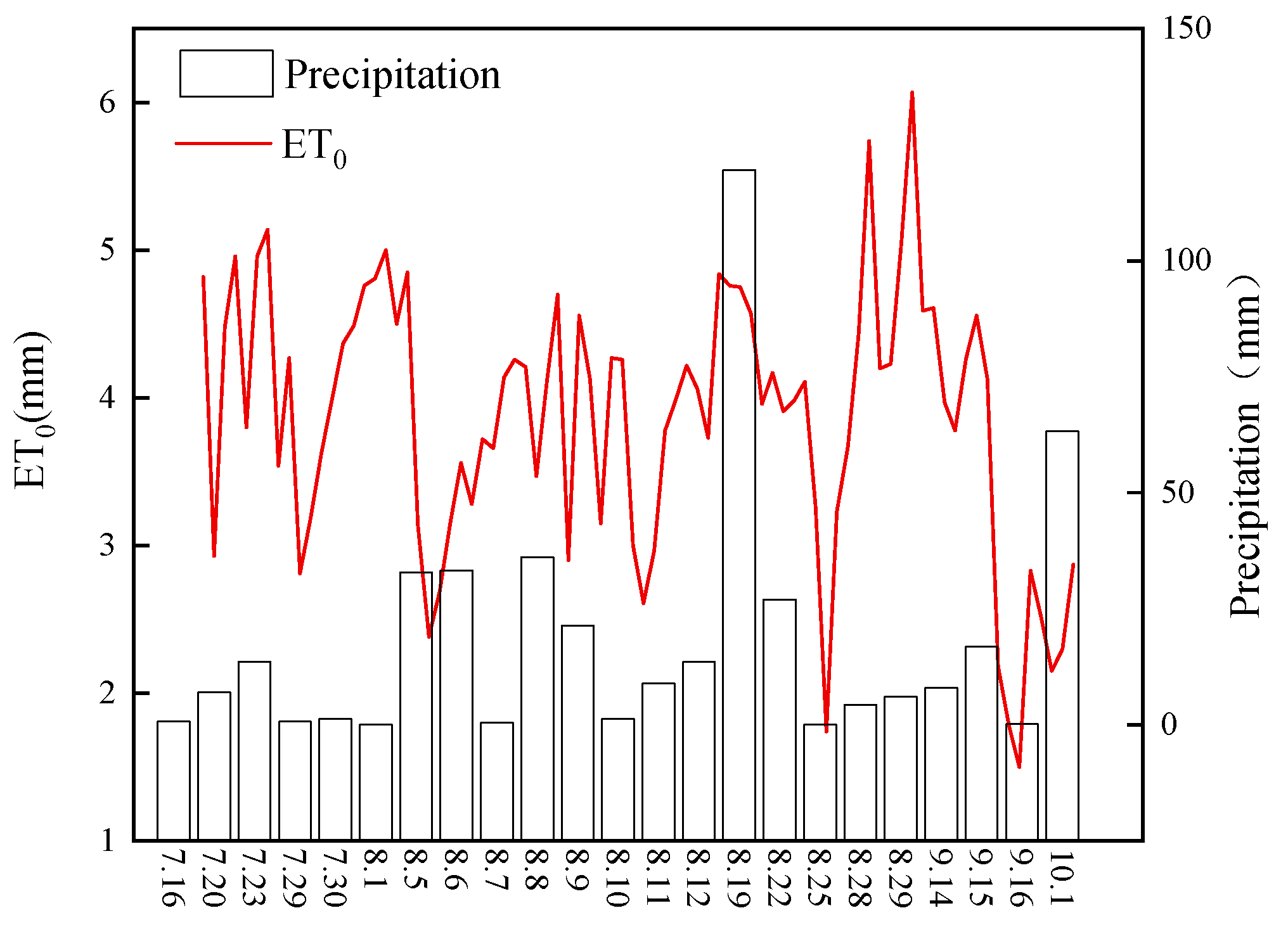
2.1. Site Description
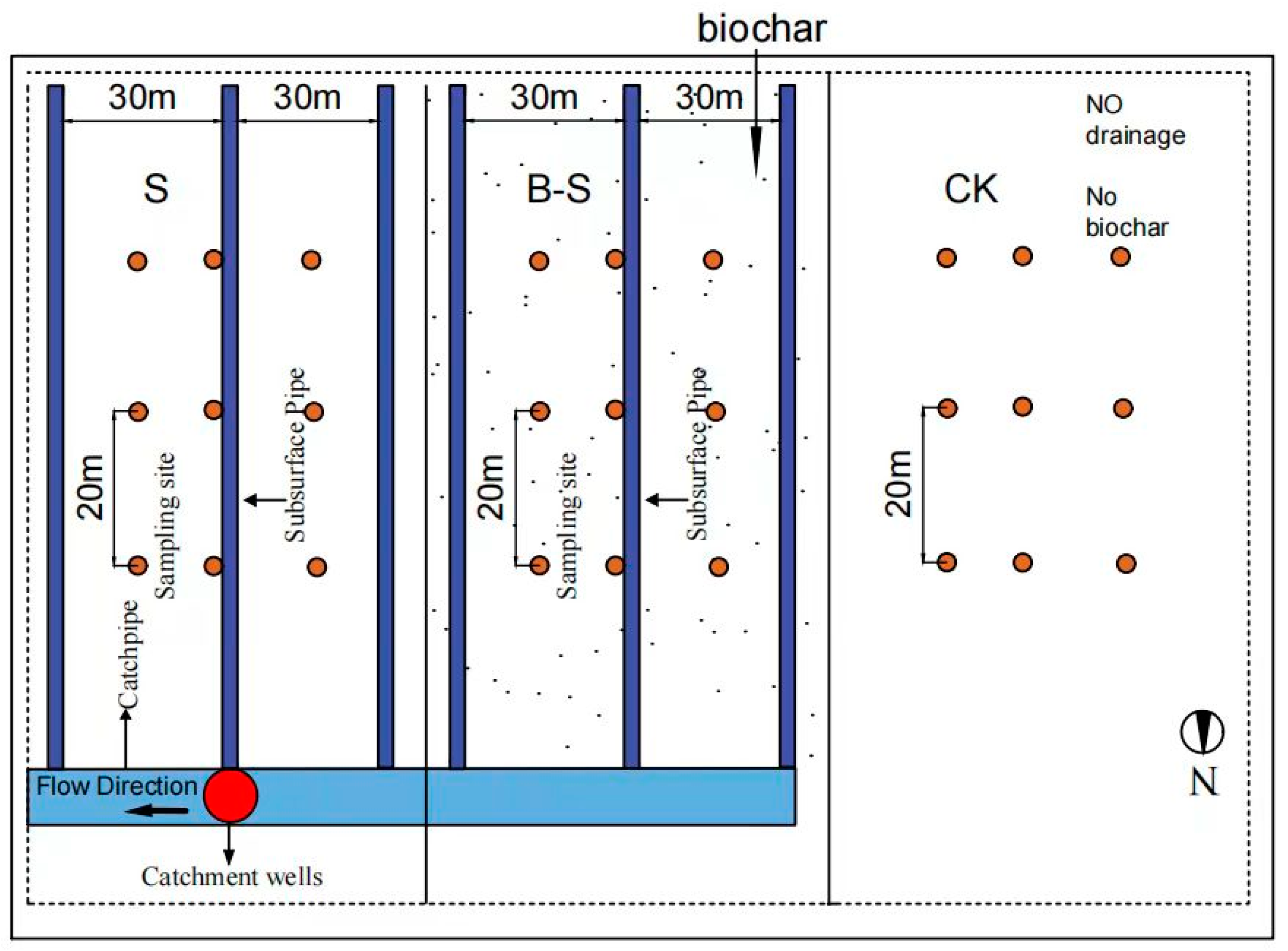
2.2. Experiment Design
2.3. Sampling and Measurement of Soil Properties
2.4. Soil Enzyme Activities
2.5. Soil DNA Extraction and Sequencing
2.6. Statistical Analyses
3. Results
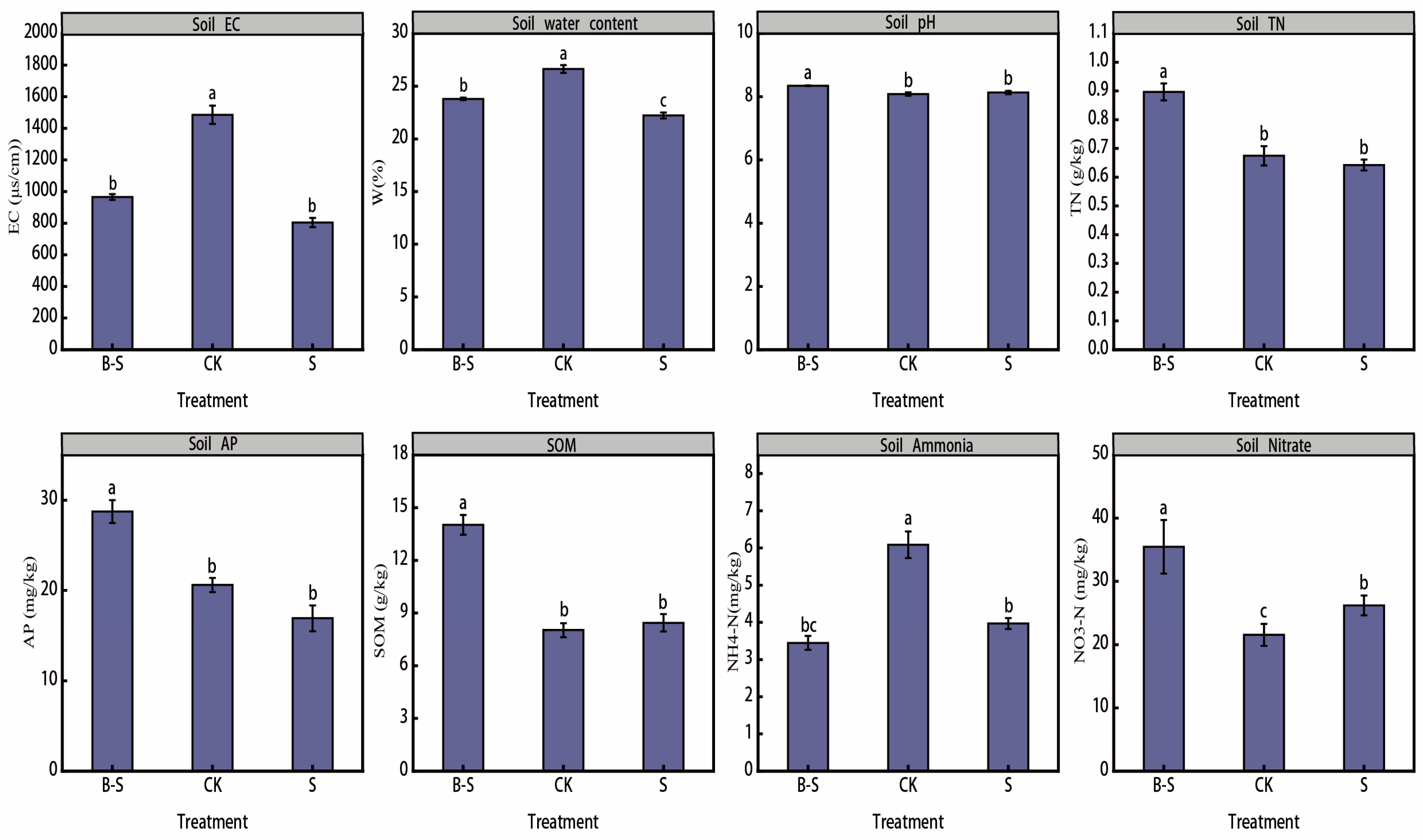
3.1. Soil Physical and Chemical Properties
3.2. Changes in Soil Enzyme Activity
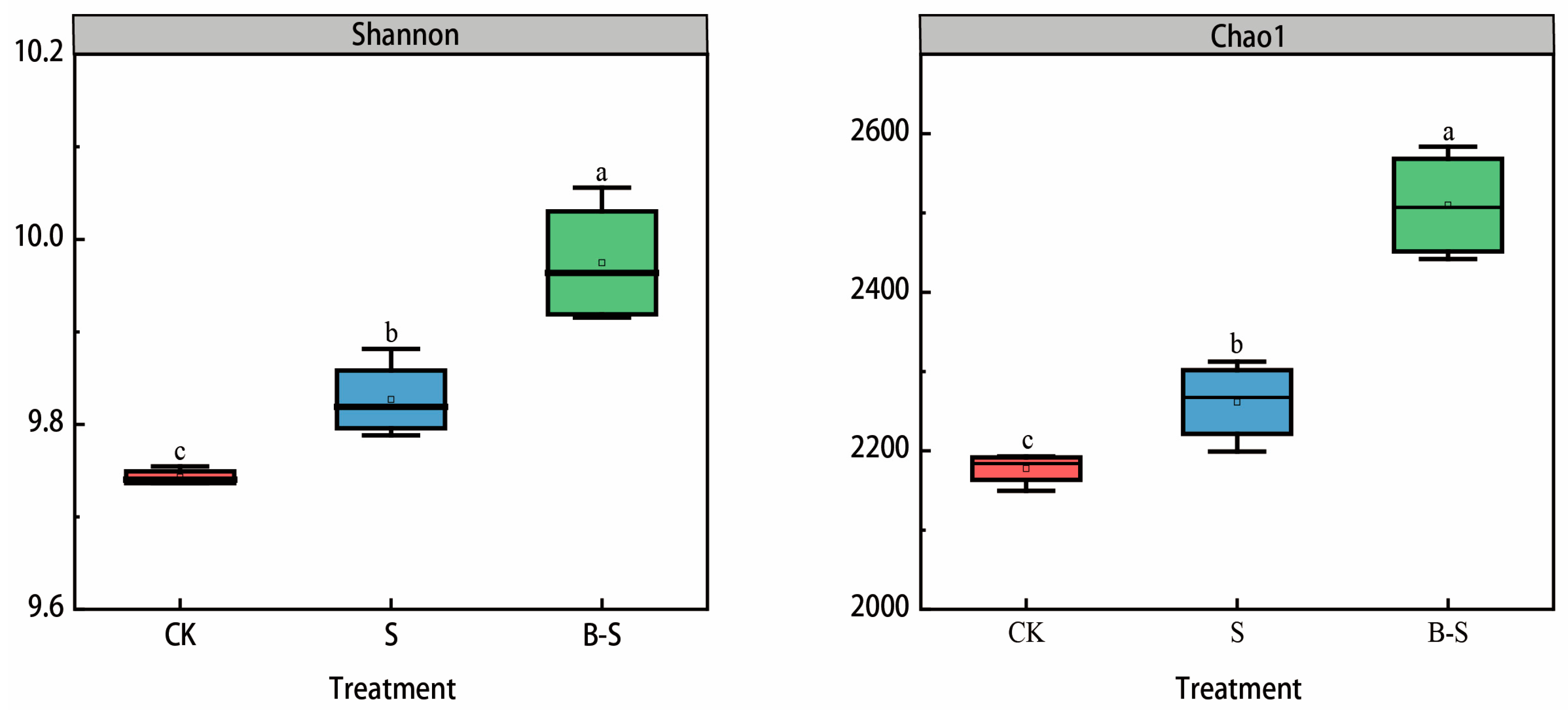
3.3. Bacterial Community Diversity
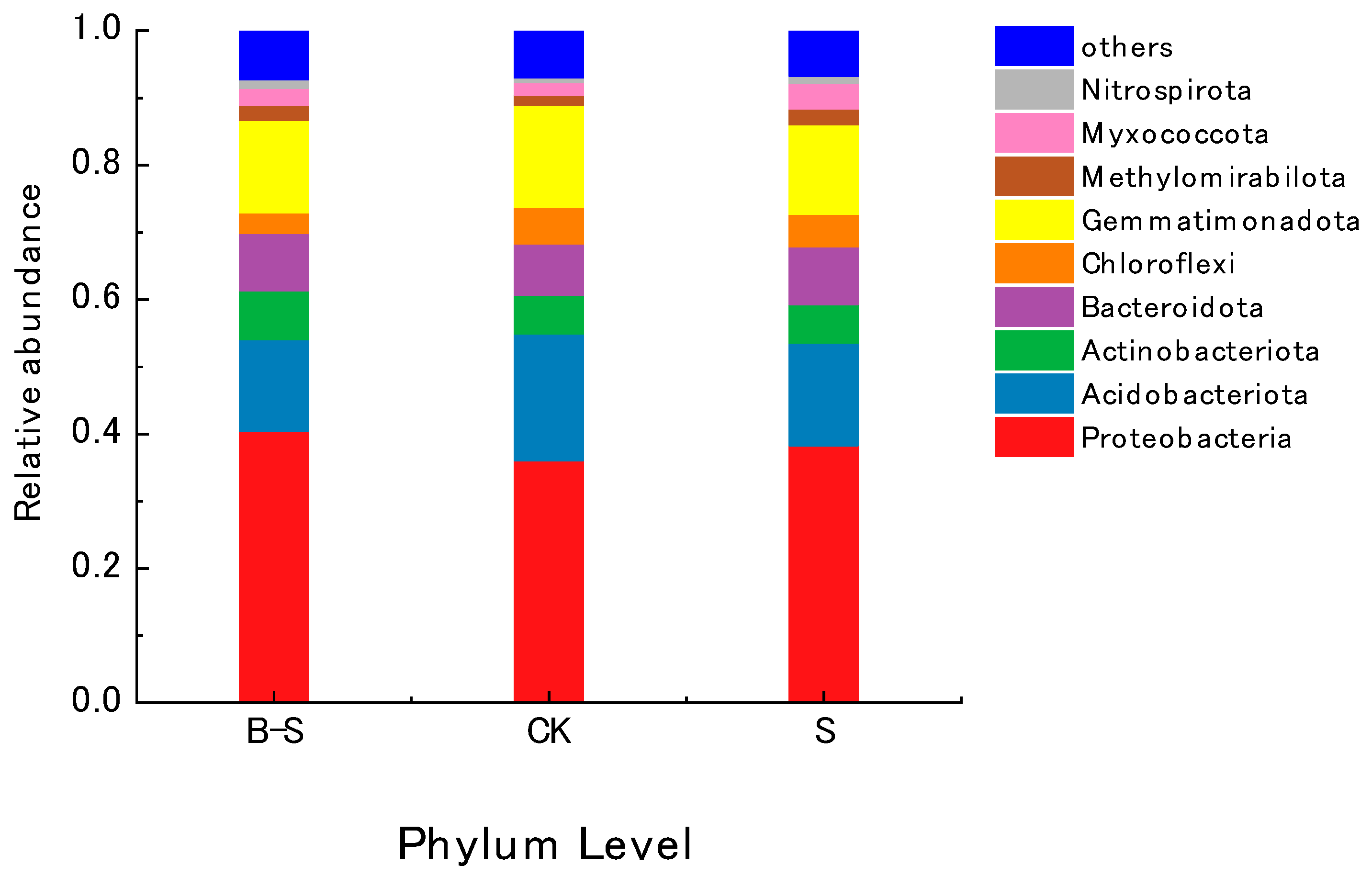
3.4. Changes in the Abundance and Composition of Microbial Communities
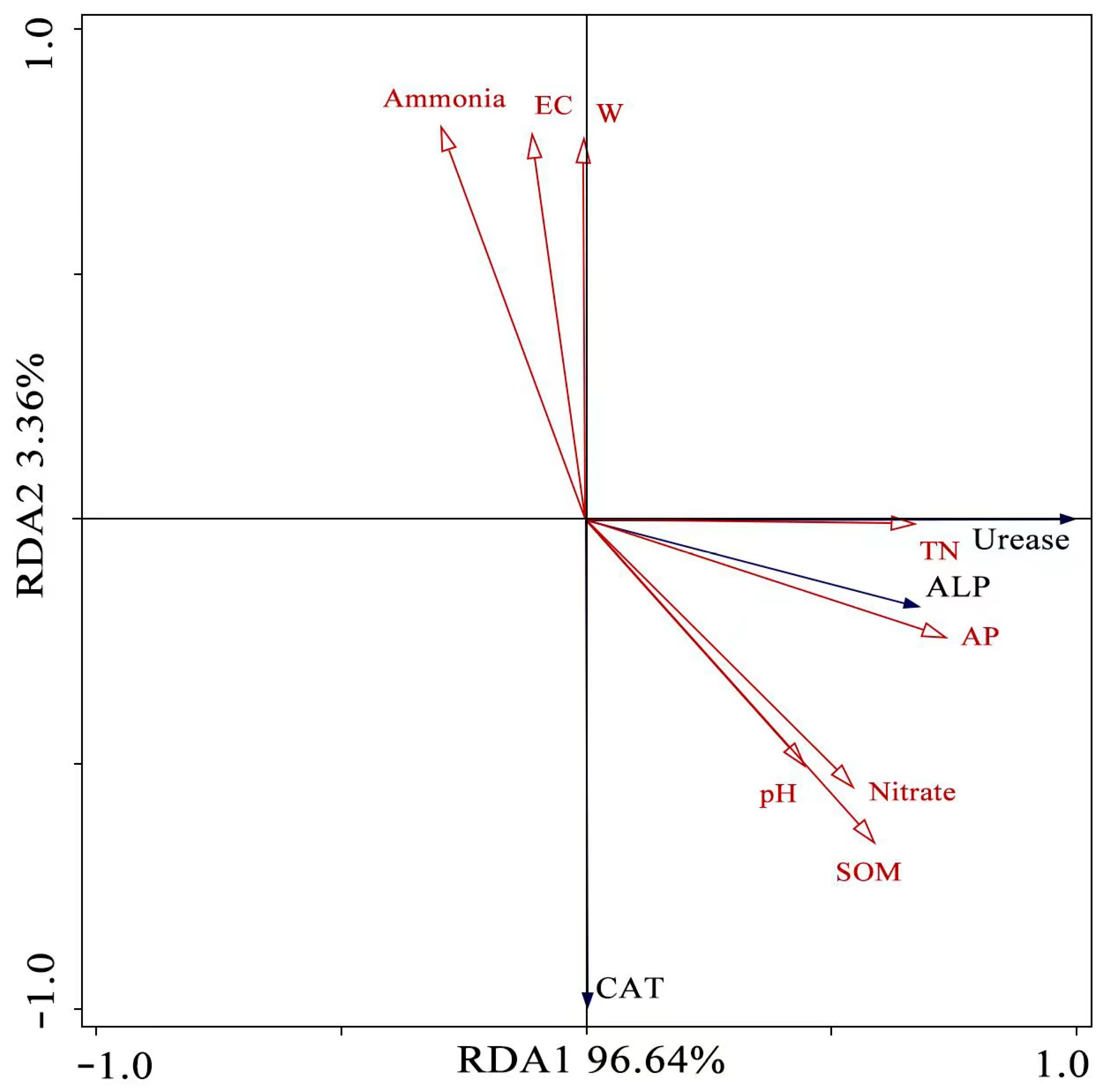
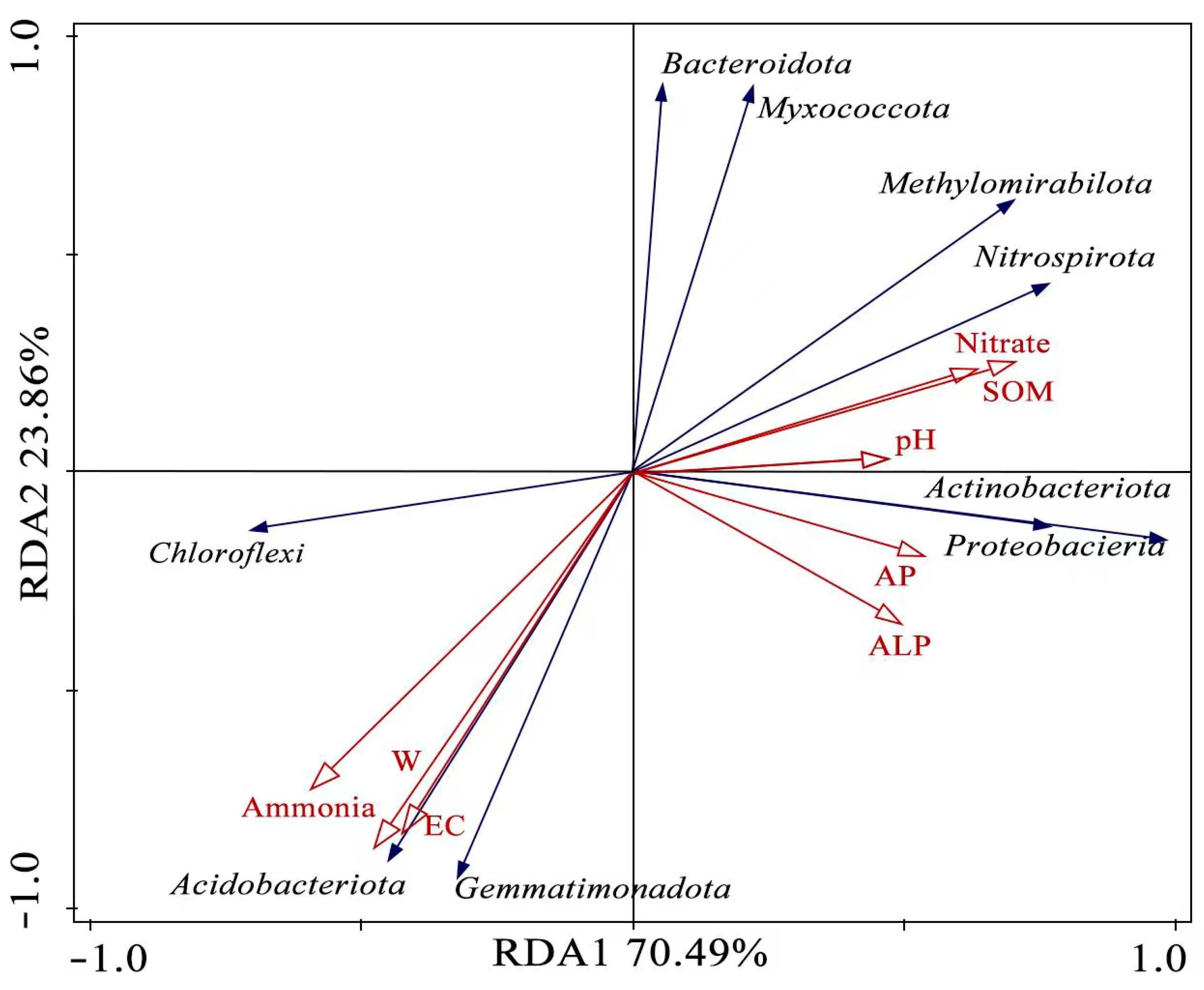
3.5. The Relationship between Soil Properties and Bacterial Abundance
4. Discussion
4.1. Changes in Soil Physical and Chemical Properties
4.2. The Relationship between Soil Properties, Bacterial Abundance, and Soil Enzymes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tahjib-Ul-Arif, M.; Hasan, M.T.; Rahman, M.A.; Nuruzzaman, M.; Rahman, A.M.S.; Hasanuzzaman, M.; Haque, M.R.; Hossain, M.A.; Abdel Latef, A.A.H.; Murata, Y.; et al. Plant response to combined salinity and waterlogging stress: Current research progress and future prospects. Plant Stress 2023, 7, 100137. [Google Scholar]
- Van Veen, H.; Mustroph, A.; Barding, G.A.; Vergeer-van Eijk, M.; Welschen-Evertman, R.A.; Pedersen, O.; Visser, E.J.; Larive, C.K.; Pierik, R.; Bailey-Serres, J.; et al. Two rumex species from contrasting hydrological niches regulate flooding tolerance through distinct mechanisms. Plant Cell 2013, 25, 4691–4707. [Google Scholar] [CrossRef]
- Valipour, M. Drainage, waterlogging, and salinity. Arch. Agron. Soil Sci. 2014, 60, 1625–1640. [Google Scholar] [CrossRef]
- Singh, A. Poor quality water utilization for agricultural production: An environmental perspective. Land Use Policy 2015, 43, 259–262. [Google Scholar]
- Gao, Y.; Xia, J.; Chen, Y.; Zhao, Y.; Kong, Q.; Lang, Y. Effects of Extreme Soil Water Stress on Photosynthetic Efficiency and Water Consumption Characteristics of Tamarix chinensis in China’s Yellow River Delta. J. For. Res. 2016, 28, 491–501. [Google Scholar] [CrossRef]
- Osman, K.T. Saline and Sodic Soils. In Management of Soil Problems; Springer: Berlin/Heidelberg, Germany, 2018; pp. 255–298. [Google Scholar]
- Ferronato, C.; Marinari, S.; Francioso, O.; Bello, D.; Trasar-Cepeda, C.; Antisari, L.V. Effect of Waterlogging on Soil Biochemical Properties and Organic Matter Quality in Different Salt Marsh Systems. Geoderma 2019, 338, 302–312. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Jia, J.; Wang, W.; Wang, D.; Zhao, Q.; Wang, C.; Chen, G. Soil Microbial Communities Regulate the Threshold Effect of Salinity Stress on Som Decomposition in Coastal Salt Marshes. Fundam. Res. 2023. [Google Scholar] [CrossRef]
- Iwai, C.B.; Oo, A.N.; Topark-ngarm, B. Soil Property and Microbial Activity in Natural Salt Affected Soils in an Alternating Wet–Dry Tropical Climate. Geoderma 2012, 189–190, 144–152. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Li, Q.; Ma, D.; Lu, H.; Feng, W.; Xie, Y.; Zhu, Y.; Guo, T. Effects of Different Irrigation and Nitrogen Regimes on Root Growth and Its Correlation with above-Ground Plant Parts in High-Yielding Wheat under Field Conditions. Field Crop. Res. 2014, 165, 138–149. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P. Microbial Activity and Biomass Recover Rapidly after Leaching of Saline Soils. Biol. Fertil. Soils 2012, 49, 367–371. [Google Scholar] [CrossRef]
- Herrmann, L.; Lesueur, D.; Robin, A.; Robain, H.; Wiriyakitnateekul, W.; Brau, L. Impact of Biochar Application Dose on Soil Microbial Communities Associated with Rubber Trees in North East Thailand. Sci. Total Environ. 2019, 689, 970–979. [Google Scholar]
- Li, G.; Niu, W.; Sun, J.; Zhang, W.; Zhang, E.; Wang, J. Soil Moisture and Nitrogen Content Influence Wheat Yield through Their Effects on the Root System and Soil Bacterial Diversity under Drip Irrigation. Land Degrad. Dev. 2021, 32, 3062–3076. [Google Scholar]
- Borowik, A.; Wyszkowska, J. Soil Moisture as a Factor Affecting the Microbiological and Biochemical Activity of Soil. Plant Soil Environ. 2016, 62, 250–255. [Google Scholar] [CrossRef]
- Rietz, D.N.; Haynes, R.J. Effects of Irrigation-Induced Salinity and Sodicity on Soil Microbial Activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Yu, S.; Hawke, B.G.; Harch, B.D. Capacity of fatty acid profiles and substrate utilization patterns to describe differences in soil microbial communities associated with increased salinity or alkalinity at three locations in South Australia. Biol. Fertil. Soils 2001, 33, 204–217. [Google Scholar] [CrossRef]
- Chen, L.J.; Feng, Q.; Wei, Y.-P.; Li, C.-S.; Zhao, Y.; Li, H.-Y.; Zhang, B.-G. Effects of Saline Water Irrigation and Fertilization Regimes on Soil Microbial Metabolic Activity. J. Soils Sediments 2016, 17, 376–383. [Google Scholar] [CrossRef]
- Muhammad, E.S.; Ibrahim, M.M.; El-Sayed, A. Effects of Drain Depth on Crop Yields and Salinity in Subsurface Drainage in Nile Delta of Egypt. Ain Shams Eng. J. 2021, 12, 1595–1606. [Google Scholar] [CrossRef]
- Askri, B.; Khodmi, S.; Bouhlila, R. Impact of Subsurface Drainage System on Waterlogged and Saline Soils in a Saharan Palm Grove. Catena 2022, 212, 106070. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Zhao, Y.; Tian, G.; Zhou, X.; Qiu, H.; Li, M. Subsurface Drainage Influences the Structure and Assembly of Soil Bacterial and Fungal Communities in Salinized Cotton Field. Arch. Agron. Soil Sci. 2022, 69, 1310–1326. [Google Scholar] [CrossRef]
- Qiu, S.L.; Wang, M.K.; Wang, F.; Chen, J.C.; Li, X.Y.; Lin, Q.H.; Lin, X.J. Effects of open drainage ditch design on bacterial and fungal communities of cold waterlogged paddy soils. Braz. J. Microbiol. 2013, 44, 983–991. [Google Scholar] [CrossRef]
- Heng, T.; He, X.L.; Yang, L.L.; Xu, X.; Feng, Y. Mechanism of Saline-Alkali Land Improvement Using Subsurface Pipe and Vertical Well Drainage Measures and Its Response to Agricultural Soil Ecosystem. Environ. Pollut. 2022, 293, 118583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Y.; Song, L.; Song, X.; Hänninen, H.; Wu, J. Biochar Enhances Nut Quality of Torreya grandis and Soil Fertility under Simulated Nitrogen Deposition. For. Ecol. Manag. 2017, 391, 321–329. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Herbert, S.; Xing, B. Impacts of Adding Biochar on Nitrogen Retention and Bioavailability in Agricultural Soil. Geoderma 2013, 206, 32–39. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Liu, Y.; Li, J.; Hu, H. Soil Organic Matter Contents Modulate the Effects of Bacterial Diversity on the Carbon Cycling Processes. J. Soils Sediments 2022, 23, 911–922. [Google Scholar]
- Wang, Z.H.; Yin, D.W.; Wang, H.Y.; Li, Z.T. Effects of biochar on waterlogging and the associated change in micro-ecological environment of maize rhizosphere soil in saline-alkali land. BioResources 2020, 15, 9303–9323. [Google Scholar] [CrossRef]
- Prayogo, C.; Jones, J.E.; Baeyens, J.; Bending, G.D. Impact of Biochar on Mineralisation of C and N from Soil and Willow Litter and Its Relationship with Microbial Community Biomass and Structure. Biol. Fertil. Soils 2013, 50, 695–702. [Google Scholar] [CrossRef]
- Xu, H.J.; Wang, X.H.; Li, H.; Yao, H.Y.; Su, J.Q.; Zhu, Y.G. Biochar Impacts Soil Microbial Community Composition and Nitrogen Cycling in an Acidic Soil Planted with Rape. Environ. Sci. Technol. 2014, 48, 9391–9399. [Google Scholar] [CrossRef]
- Grossman, J.M.; O’Neill, B.E.; Tsai, S.M.; Liang, B.; Neves, E.; Lehmann, J.; Thies, J.E. Amazonian Anthrosols Support Similar Microbial Communities that Differ Distinctly from Those Extant in Adjacent, Unmodified Soils of the Same Mineralogy. Microb. Ecol. 2010, 60, 192–205. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Romano, M.; Marzaioli, R.; Baglivo, I.; Baronti, S.; Miglietta, F.; Castaldi, S. Effect of Biochar Addition on Soil Microbial Community in a Wheat Crop. Eur. J. Soil Biol. 2014, 60, 9–15. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, J.; Gao, L.; Wang, R.; Gao, J.; Dai, Y.; Li, W.; Shen, G.; Kong, F.; Zhang, J. Effect of Straw Biochar Amendment on Tobacco Growth, Soil Properties, and Rhizosphere Bacterial Communities. Sci. Rep. 2021, 11, 20727. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Xiao, Q.; Zhu, L.-x.; Shen, Y.-f.; Li, S.-q. Sensitivity of Soil Water Retention and Availability to Biochar Addition in Rainfed Semi-Arid Farmland during a Three-Year Field Experiment. Field Crop. Res. 2016, 196, 284–293. [Google Scholar] [CrossRef]
- Tian, G.; Qiu, H.; Li, D.; Wang, Y.; Li, H.; Niu, Q.; Zhou, X. Little environmental adaptation and high stability of bacterial communities in rhizosphere rather than bulk soils in rice fields. Appl. Soil Ecol. 2022, 169, 104183. [Google Scholar] [CrossRef]
- Wylie, E.M.; Colletti, L.M.; Walker, L.F.; Lujan, E.J.W.; Garduno, K.; Mathew, K.J. Comparison of the davies and gray titrimetric method with potassium dichromate and ceric titrants. J. Radioanal. Nucl. Chem. 2018, 318, 227–233. [Google Scholar] [CrossRef]
- Fregerslev, S.; Blackstad, T.W.; Fredens, K.; Holm, M.J. Golgi potassium-dichromate silver-nitrate impregnation: Nature of the precipitate studied by X-ray powder diffraction methods. Histochemie 1971, 25, 63–71. [Google Scholar] [CrossRef]
- Baligar, V.C.; Staley, T.E.; Wright, R.J. Enzyme Activities in Appalachian Soils: 2. Urease. Commun. Soil Sci. Plant Anal. 2008, 22, 315–322. [Google Scholar] [CrossRef]
- Shi, Z.; Lu, Y.; Xu, Z.; Fu, S. Enzyme activities of urban soils under different land use in the Shenzhen city, China. Plant Soil Environ. 2005, 54, 341–346. [Google Scholar] [CrossRef]
- Arevalo, J.R.; Carreira, J.A.; Niell, F.X. Kinetic Parameters of Phosphatase Activity along a Xeric Dolomitic Soil Chronosequence Related to Wildfires. Geomicrobiol. J. 1993, 11, 235–245. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, K.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16s Rrna Gene Database and Workbench Compatible with Arb. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Altschul, S. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Lozupone, C.; Hamady, M.; Knight, R. Unifrac—An Online Tool for Comparing Microbial Community Diversity in a Phylogenetic Context. BMC Bioinform. 2006, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative Beta Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, D.; Liang, G.; Zhang, Q.; Ai, C.; Zhou, W. Maize Biochar Addition Rate Influences Soil Enzyme Activity and Microbial Community Composition in a Fluvo-Aquic Soil. Appl. Soil Ecol. 2015, 96, 265–272. [Google Scholar] [CrossRef]
- Hu, Q.; Zhao, Y.; Hu, X.; Qi, J.; Suo, L.; Pan, Y.; Song, B.; Chen, X. Effect of saline land reclamation by constructing the “raised field -shallow trench” pattern on agroecosystems in yellow river delta. Agric. Water Manag. 2022, 261, 107345. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- She, D.; Sun, X.; Gamareldawla, A.H.D.; Nazar, E.A.; Hu, W.; Edith, K.; Yu, S. Benefits of soil biochar amendments to tomato growth under saline water irrigation. Sci. Rep. 2018, 8, 14743. [Google Scholar] [CrossRef]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Fan, S.; Zuo, J.; Dong, H. Changes in Soil Properties and Bacterial Community Composition with Biochar Amendment after Six Years. Agronomy 2020, 10, 746. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Muller, C.; Kammann, C.I. Standard extraction methods may underestimate nitrate stocks captured by field-aged biochar. J. Environ. Qual. 2016, 45, 1196–1204. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Wang, Y.; Huang, H.; Chen, Y. Influence of pyrolysis temperature and holding time on properties of biochar derived from medicinal herb (radix isatidis) residue and its effect on soil CO2 emission. J. Anal. Appl. Pyrolysis 2014, 110, 277–284. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Mosa, A.; Zhan, L.; Gao, B. Biochar modulates mineral nitrogen dynamics in soil and terrestrial ecosystems: A critical review. Chemosphere 2021, 278, 130378. [Google Scholar] [PubMed]
- Clough, T.J.; Condron, L.M. Biochar and the nitrogen cycle: Introduction. J. Environ. Qual. 2010, 39, 1218–1223. [Google Scholar] [CrossRef]
- Peng, M.; Jia, H.; Wang, Q. The effect of land use on bacterial communities in saline-alkali soil. Curr. Microbiol. 2017, 74, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, M.; Müller, T.; Schmeisky, H.; Joergensen, R.G. Microbial performance in soils along a salinity gradient under acidic conditions. Appl. Soil Ecol. 2003, 23, 237–244. [Google Scholar] [CrossRef]
- Zhao, L.; Heng, T.; Yang, L.; Xu, X.; Feng, Y. Study on the farmland improvement effect of drainage measures under film mulch with drip irrigation in saline–alkali land in arid areas. Sustainability 2021, 13, 4159. [Google Scholar]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Hou, Y.; Zeng, W.; Hou, M.; Wang, Z.; Luo, Y.; Lei, G.; Zhou, B.; Huang, J. Responses of the soil microbial community to salinity stress in maize fields. Biology 2021, 10, 1114. [Google Scholar] [CrossRef]
- Rinnan, R.; Rousk, J.; Yergeau, E.; Kowalchuk, G.A.; Bååth, E. Temperature adaptation of soil bacterial communities along an antarctic climate gradient: Predicting responses to climate warming. Glob. Chang. Biol. 2009, 15, 2615–2625. [Google Scholar]
- Trivedi, P.; Delgado-Baquerizo, M.; Anderson, I.C.; Singh, B.K. Response of soil properties and microbial communities to agriculture: Implications for primary productivity and soil health indicators. Front. Plant Sci. 2016, 7, 990. [Google Scholar]
- Wang, C.Y.; Zhou, X.; Guo, D.; Zhao, J.H.; Yan, L.; Feng, G.Z.; Gao, Q.; Yu, H.; Zhao, L.P. Soil pH is the primary factor driving the distribution and function of microorganisms in farmland soils in northeastern China. Ann. Microbiol. 2019, 69, 1461–1473. [Google Scholar]
- Niu, J.; Tang, H.; Liu, Q.; Cheng, F.; Zhang, L.; Sang, L.; Huang, Y.; Shen, C.; Gao, B.; Niu, Z. Determinants of soil bacterial diversity in a black soil region in a large-scale area. Land 2022, 11, 731. [Google Scholar]
- Park, J.H.; Park, J.H.; Lee, S.H.; Yoon, J.J.; Kim, S.H.; Park, H.D. Metabolic flux and functional potential of microbial community in an acidogenic dynamic membrane bioreactor. Bioresour. Technol. 2020, 305, 123060. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Zhang, J.; Yin, J.; Huang, S. Bacterial community structure after long-term organic and inorganic fertilization reveals important associations between soil nutrients and specific taxa involved in nutrient transformations. Front Microbiol. 2017, 8, 187. [Google Scholar]
- Zhang, Q.; Wang, X.; Zhang, Z.; Liu, H.; Liu, Y.; Feng, Y.; Yang, G.; Ren, C.; Han, X. Linking Soil Bacterial Community Assembly with the Composition of Organic Carbon during Forest Succession. Soil Biol. Biochem. 2022, 173, 108790. [Google Scholar] [CrossRef]
- Lu, Q.; Ge, G.; Sa, D.; Wang, Z.J.; Hou, M.L.; Jia, Y.S. Effects of salt stress levels on nutritional quality and microorganisms of alfalfa-influenced soil. PeerJ 2022, 9, e11729. [Google Scholar] [CrossRef]
- Andronov, E.E.; Petrova, S.N.; Pinaev, A.G.; Pershina, E.V.; Rakhimgalieva, S.Z. Analysis of the structure of microbial community in soils with different degrees of salinization using T-RFLP and real-time PCR techniques. Eurasian Soil Sci. 2012, 45, 147–156. [Google Scholar] [CrossRef]
- Chen, L.; Feng, Q.; Li, C.; Wei, Y.P.; Zhao, Y.; Feng, Y.J.; Zheng, H.; Li, F.R.; Li, H.Y. Impacts of aquaculture wastewater irrigation on soil microbial functional diversity and community structure in arid regions. Sci. Rep. 2017, 7, 11193. [Google Scholar] [CrossRef] [PubMed]
- Wichern, J.; Wichern, F.; Joergensen, R.G. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 2006, 137, 100–108. [Google Scholar] [CrossRef]
- Doan, T.T.; Bouvier, C.; Bettarel, Y.; Bouvier, T.; Henry-des-Tureaux, T.; Janeau, J.L.; Lamballe, P.; Nguyen, B.V.; Jouquet, P. Influence of buffalo manure, compost, vermicompost and biochar amendments on bacterial and viral communities in soil and adjacent aquatic systems. Appl. Soil Ecol. 2014, 73, 78–86. [Google Scholar] [CrossRef]
- Yao, R.; Yang, J.; Wang, X.; Xie, W.P.; Zheng, F.; Li, H.Q.; Tang, C.; Zhu, H. Response of soil characteristics and bacterial communities to nitrogen fertilization gradients in a coastal salt-affected agroecosystem. Land Degrad. Dev. 2021, 32, 338–353. [Google Scholar]
- Lucas, J.A.; García-Villaraco, A.; Ramos, B.; García-Cristobal, J.; Algar, E.; Gutierrez-Mañero, J. Structural and functional study in the rhizosphere of Oryza sativa L. plants growing under biotic and abiotic stress. J. Appl. Microbiol. 2013, 115, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Krull, E.; Singh, B.; Joseph, S. Preface to special issue: Proceedings from the 1st asia-pacific biochar conference, 2009, Gold Coast, Australia. Aust. J. Soil Res. 2010, 48, i–iv. [Google Scholar]
- Bay, S.K.; Dong, X.; Bradley, J.A.; Leung, P.M.; Grinter, R.; Jirapanjawat, T.; Arndt, S.K.; Cook, P.L.M.; LaRowe, D.E.; Nauer, P.A.; et al. Trace gas oxidizers are widespread and active members of soil microbial communities. Nat. Microbiol. 2021, 6, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Huang, B.; Fernández-García, V.; Miesel, J.; Yan, L.; Lv, C.Q. Biochar and Rhizobacteria Amendments Improve Several Soil Properties and Bacterial Diversity. Microorganisms 2020, 8, 502. [Google Scholar] [CrossRef]
- Murphy, C.L.; Yang, R.; Decker, T.; Cavalliere, C.; Andreev, V.; Bircher, N.; Cornell, J.; Dohmen, R.; Pratt, C.J.; Grinnell, A.; et al. Genomes of novel myxococcota reveal severely curtailed machineries for predation and cellular differentiation. Appl. Environ. Microbiol. 2021, 87, e01706-21. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X.P. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, L.; He, N.; Gong, D.K.; Gao, H.; Ma, Z.B.; Fu, L.; Zhao, M.Z.; Wang, H.; Wang, C.H.; et al. Soil bacterial community as impacted by addition of rice straw and biochar. Sci. Rep. 2021, 11, 22185. [Google Scholar] [CrossRef]
- Calderón, K.; Spor, A.; Breuil, M.C.; Bru, D.; Bizouard, F. Effectiveness of ecological rescue for altered soil microbial communities and functions. ISME J. 2017, 11, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Gu, Y.; Wu, C.S.; Hu, W.H.; Xu, C.; Chen, X.F. Short-term straw returning improves qualityand bacteria community of black soilin northeast China. Pol. J. Environ. Stud. 2022, 31, 1869–1883. [Google Scholar]
- Lan, J.; Wang, S.; Wang, J.; Qi, X.; Long, Q.X.; Huang, M.Z. The shift of soil bacterial community after afforestation influence soil organic carbon and aggregate stability in Karst region. Front. Microbiol. 2022, 13, 901126. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Lücker, S.; Albertsen, M.; Kitzinger, K.; Herbold, C.; Spieck, E.; Nielsen, P.H.; Wagner, M.; Daims, H. Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus Nitrospira. Proc. Natl. Acad. Sci. USA 2015, 112, 11371–11376. [Google Scholar] [CrossRef]
- Umezawa, K.; Kojima, H.; Kato, Y.; Fukui, M. Dissulfurispira thermophila gen. nov., sp. nov., a thermophilic chemolithoautotroph growing by sulfur disproportionation, and proposal of novel taxa in the phylum Nitrospirota to reclassify the genus Thermodesulfovibrio. Syst. Appl. Microbiol. 2021, 44, 126184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, X.; Huang, L.; Shan, Q.Y.; Hu, A.Y.; Yan, D.Z.; Zhang, J.; Long, X.E. Soil profile rather than reclamation time drives the mudflat soil microbial community in the wheat-maize rotation system of Nantong, China. J. Soils Sediments 2021, 21, 1672–1687. [Google Scholar]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, C. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Sarikhani, M.R.; Shiri, J.; Shahbazi, F. Modeling soil enzyme activity using easily measured variables: Heuristic alternatives. Appl. Soil Ecol. 2021, 157, 103753. [Google Scholar]
- Zheng, H.; Liu, Y.; Zhang, J.; Chen, Y.M.; Yang, L.; Li, H.G.; Wang, L.F. Factors influencing soil enzyme activity in China’s forest ecosystems. Plant Ecol. 2018, 219, 31–44. [Google Scholar]
| Depth of Soil Layer (cm) | AP (mg/kg) | TN (g/kg) | SOM (g/kg) | EC (μs/cm) | pH | NO3−-N (mg/kg) | NH4+-N (mg/kg) |
|---|---|---|---|---|---|---|---|
| 0–20 | 18.00 | 0.53 | 8.22 | 1916 | 8.03 | 18.55 | 4.99 |
| Feedstock | pH | C (%) | N (%) | Specific Surface Area (m2/g) | Dry Bulk Density (g/cm3) |
|---|---|---|---|---|---|
| Peanut shell | 8.33 | 54.41 | 2.31 | 16.7 | 0.22 |
| Treatment | CAT (U/mL) | ALP (IU/L) | Urease (U/L) |
|---|---|---|---|
| B–S | 68.15 ± 6.30 a | 7.63 ± 0.24 a | 587.97 ± 30.87 a |
| CK | 47.94 ± 8.93 b | 5.45 ± 0.36 b | 433.08 ± 31.27 c |
| S | 71.85 ± 6.39 a | 4.47 ± 0.23 c | 493.67 ± 33.12 b |
| Treatment | Proteobacteria | Acidobacteriota | Actinobacteriota | Bacteroidota | Chloroflexi | Gemmatimonadota | Methylomirabilota | Myxococcota | Nitrospirota |
|---|---|---|---|---|---|---|---|---|---|
| B–S | 40.41 ± 2.27 a | 15.31 ± 0.67 b | 7.22 ± 0.35 a | 8.51 ± 0.33 a | 3.06 ± 0.23 c | 13.75 ± 0.69 b | 2.30 ± 0.14 a | 2.49 ± 0.19 b | 1.27 ± 0.17 a |
| CK | 36.12 ± 0.88 b | 18.81 ± 0.54 a | 5.77 ± 0.11 b | 7.61 ± 0.42 a | 5.46 ± 0.15 a | 15.21 ± 0.26 a | 1.51 ± 0.19 b | 1.80 ± 0.17 c | 0.72 ± 0.07 b |
| S | 38.30 ± 2.97 b | 13.73 ± 0.20 c | 5.72 ± 0.29 b | 8.54 ± 0.18 a | 4.34 ± 0.15 b | 13.33 ± 0.55 c | 2.31 ± 0.06 a | 3.79±0.29 a | 1.09 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Li, D.; Wang, Y.; Zhao, Q.; Li, Z.; Jing, R.; Zhou, X. Effect of Subsurface Drainage Combined with Biochar on the Bacterial Community Composition of Coastal Saline Soil. Water 2023, 15, 2701. https://doi.org/10.3390/w15152701
Tian Y, Li D, Wang Y, Zhao Q, Li Z, Jing R, Zhou X. Effect of Subsurface Drainage Combined with Biochar on the Bacterial Community Composition of Coastal Saline Soil. Water. 2023; 15(15):2701. https://doi.org/10.3390/w15152701
Chicago/Turabian StyleTian, Yuyu, Dongwei Li, Yuting Wang, Qingqing Zhao, Zongpeng Li, Rui Jing, and Xinguo Zhou. 2023. "Effect of Subsurface Drainage Combined with Biochar on the Bacterial Community Composition of Coastal Saline Soil" Water 15, no. 15: 2701. https://doi.org/10.3390/w15152701
APA StyleTian, Y., Li, D., Wang, Y., Zhao, Q., Li, Z., Jing, R., & Zhou, X. (2023). Effect of Subsurface Drainage Combined with Biochar on the Bacterial Community Composition of Coastal Saline Soil. Water, 15(15), 2701. https://doi.org/10.3390/w15152701










