What Are the Relationships between Plankton and Macroinvertebrates in Reservoir Systems?
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Areas
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Water Parameters
3.2. Characteristics of Different Assemblages
3.3. Concordance in Different Assemblages
3.4. Relationships among Different Assemblages
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Reservoirs | Storage Capacity (×104 m3) | Size | Location | Sampling Period |
|---|---|---|---|---|
| Daxi | 11,300 | Large | Changzhou | Spring, Summer |
| Qiansong | 1416 | Medium | Changzhou | Spring, Summer |
| Shahe | 10,900 | Large | Changzhou | Spring, Summer |
| Tangma | 1236 | Medium | Changzhou | Spring, Summer |
| Guiwu | 2620 | Medium | Huaian | Spring, Summer |
| Hongqi | 4119 | Medium | Huaian | Spring, Summer |
| Hualong | 4131 | Medium | Huaian | Spring, Summer |
| Longwangshan | 9099 | Medium | Huaian | Spring, Summer |
| Anfengshan | 12,000 | Large | Lianyungang | Spring, Summer |
| Batiaolu | 2143 | Medium | Lianyungang | Spring, Summer |
| Changli | 1405 | Medium | Lianyungang | Spring, Summer |
| Dashibu | 1930 | Medium | Lianyungang | Spring, Summer |
| Fangshan | 2218 | Medium | Lianyungang | Spring, Summer |
| Henggou | 2529 | Medium | Lianyungang | Spring, Summer |
| Huozhuang | 2480 | Medium | Lianyungang | Spring, Summer |
| Shilianghe | 53,100 | Large | Lianyungang | Spring, Summer |
| Xiaotashan | 28,200 | Large | Lianyungang | Spring, Summer |
| Xishuanghu | 1954 | Medium | Lianyungang | Spring, Summer |
| Yushan | 1225 | Medium | Lianyungang | Spring, Summer |
| Daheqiao | 1692 | Medium | Nanjing | Spring, Summer |
| Daquan | 1270 | Medium | Nanjing | Spring, Summer |
| Fangbian | 5070 | Medium | Nanjing | Spring, Summer |
| Heiwangba | 2216 | Medium | Nanjing | Spring, Summer |
| Jinniushan | 9286 | Medium | Nanjing | Spring, Summer |
| Laoyaba | 1136 | Medium | Nanjing | Spring, Summer |
| Longdunhe | 1124 | Medium | Nanjing | Spring, Summer |
| Sanyou | 435 | Small | Nanjing | Summer |
| Shanhong | 1048 | Medium | Nanjing | Spring, Summer |
| Shanhu | 2357 | Medium | Nanjing | Spring, Summer |
| Wolong | 1277 | Medium | Nanjing | Spring, Summer |
| Yaojia | 1108.4 | Medium | Nanjing | Spring, Summer |
| Zhaocun | 1034.2 | Medium | Nanjing | Spring, Summer |
| Zheshantou | 1138 | Medium | Nanjing | Spring, Summer |
| Zhongshan | 2868 | Medium | Nanjing | Spring, Summer |
| Hengshan | 11,200 | Large | Wuxi | Spring, Summer |
| Cuihuozhuang | 3388 | Medium | Xuzhou | Spring, Summer |
| Dalongkou | 465 | Small | Xuzhou | Spring, Summer |
| Erhu | 4094 | Medium | Xuzhou | Summer |
| Gaotang | 3815 | Medium | Xuzhou | Spring, Summer |
| Qingan | 6030 | Medium | Xuzhou | Spring, Summer |
| Yunlonghu | 4229 | Medium | Xuzhou | Spring, Summer |
| Beishan | 8156 | Medium | Zhengjiang | Spring, Summer |
| Ershen | 5720 | Medium | Zhengjiang | Spring, Summer |
| Jurong | 2859 | Medium | Zhengjiang | Spring, Summer |
| Lintang | 1492 | Medium | Zhengjiang | Spring, Summer |
| Lunshan | 2704 | Medium | Zhengjiang | Spring, Summer |
| Maodong | 1800 | Medium | Zhengjiang | Spring, Summer |
| Maoshan | 2178 | Medium | Zhengjiang | Spring, Summer |
| Mudong | 1176 | Medium | Zhengjiang | Spring, Summer |
| Yuetang | 1789.5 | Medium | Zhengjiang | Spring, Summer |
References
- Paavola, R.; Muotka, T.; Virtanen, R.; Heino, J.; Kreivi, P. Are Biological Classifications of Headwater Streams Concordant across Multiple Taxonomic Groups? Freshw. Biol. 2003, 48, 1912–1923. [Google Scholar] [CrossRef]
- Paszkowski, C.A.; Tonn, W.M. Community Concordance between the Fish and Aquatic Birds of Lakes in Northern Alberta, Canada: The Relative Importance of Environmental and Biotic Factors. Freshw. Biol. 2000, 43, 421–437. [Google Scholar] [CrossRef]
- Jackson, D.A. PROTEST: A PROcrustean Randomization TEST of Community Environment Concordance. Ecoscience 1995, 2, 297–303. [Google Scholar] [CrossRef]
- Grenouillet, G.; Brosse, S.; Tudesque, L.; Lek, S.; Baraillé, Y.; Loot, G. Concordance among Stream Assemblages and Spatial Autocorrelation along a Fragmented Gradient. Divers. Distrib. 2008, 14, 592–603. [Google Scholar] [CrossRef]
- Padial, A.A.; Siqueira, T.; Heino, J.; Vieira, L.C.G.; Bonecker, C.C.; Lansac-Tôha, F.A.; Rodrigues, L.C.; Takeda, A.M.; Train, S.; Velho, L.F.M. Relationships between Multiple Biological Groups and Classification Schemes in a Neotropical Floodplain. Ecol. Indic. 2012, 13, 55–65. [Google Scholar] [CrossRef]
- Vieira, L.C.G.; Padial, A.A.; Velho, L.F.M.; Carvalho, P.; Bini, L.M. Concordance among Zooplankton Groups in a Near-Pristine Floodplain System. Ecol. Indic. 2015, 58, 374–381. [Google Scholar] [CrossRef]
- Jackson, D.A.; Harvey, H.H. Fish and Benthic Invertebrates: Community Concordance and Community–Environment Relationships. Can. J. Fish. Aquat. Sci. 1993, 50, 2641–2651. [Google Scholar] [CrossRef]
- Allen, A.P.; Whittier, T.R.; Kaufmann, P.R.; Larsen, D.P.; O’Connor, R.J.; Hughes, R.M.; Stemberger, R.S.; Dixit, S.S.; Brinkhurst, R.O.; Herlihy, A.T.; et al. Concordance of Taxonomic Richness Patterns across Multiple Assemblages in Lakes of the Northeastern United States. Can. J. Fish. Aquat. Sci. 1999, 56, 739–747. [Google Scholar] [CrossRef]
- Allen, A.P.; Whittier, T.R.; Larsen, D.P.; Kaufmann, P.R.; O’Connor, R.J.; Hughes, R.M.; Stemberger, R.S.; Dixit, S.S.; Brinkhurst, R.O.; Herlihy, A.T. Concordance of Taxonomic Composition Patterns across Multiple Lake Assemblages: Effects of Scale, Body Size, and Land Use. Can. J. Fish. Aquat. Sci. 1999, 56, 2029–2040. [Google Scholar] [CrossRef]
- Velghe, K.; Gregory-Eaves, I. Body Size Is a Significant Predictor of Congruency in Species Richness Patterns: A Meta-Analysis of Aquatic Studies. PLoS ONE 2013, 8, e57019. [Google Scholar] [CrossRef] [Green Version]
- Trigal, C.; Fernandez-Alaez, C.; Fernandez-Alaez, M. Congruence between Functional and Taxonomic Patterns of Benthic and Planktonic Assemblages in Flatland Ponds. Aquat. Sci. 2014, 76, 61–72. [Google Scholar] [CrossRef]
- Hanson, M.A.; Maurer, K. Co-Correspondence among Aquatic Invertebrates, Fish, and Submerged Aquatic Plants in Shallow Lakes. Freshw. Sci. 2015, 34, 953–964. [Google Scholar] [CrossRef]
- Wan, C.; Wu, X.; Hu, C.; Yu, T.; Guo, H.; Zhu, D. Investigation and Comprehensive Assessment of Zoobenthos of Reservoirs in Jiangsu Province. J. Lake Sci. 2004, 16, 43–48. (In Chinese) [Google Scholar]
- Morse, J.C.; Yang, L.; Tian, L. Aquatic Insects of China Useful for Monitoring Water Quality, 1st ed.; Hohai University Press: Nanjing, China, 1994. [Google Scholar]
- Hu, H.J.; Li, R.Y.; Wei, Y.X.; Zhu, H.Z.; Chen, J.Y.; Shi, Z.X. Freshwater Algae in China; Scientific and Technical Publisher: Shanghai, China, 1980. (In Chinese) [Google Scholar]
- Zhuge, Y. Study on the Typical Area of China Rotifers. Ph.D. Thesis, Institute of Hydrobiology, Chinese Academy of Sciences, Beijing, China, 1997. (In Chinese). [Google Scholar]
- Shen, J. Fauna Sinica-Freshwater Copepods; Science Press: Beijing, China, 1979. (In Chinese) [Google Scholar]
- Jiang, X.; Du, N. Fauna Sinica—Freshwater Cladocera; Science Press: Beijing, China, 1979. (In Chinese) [Google Scholar]
- Malmqvist, B.; Hoffsten, P.-O. Influence of Drainage from Old Mine Deposits on Benthic Macroinvertebrate Communities in Central Swedish Streams. Water Res. 1999, 33, 2415–2423. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Jackson, D.A. How Well Do Multivariate Data Sets Match? The Advantages of a Procrustean Superimposition Approach over the Mantel Test. Oecologia 2001, 129, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Saito, V.S.; Fonseca-Gessner, A.A.; Siqueira, T. How Should Ecologists Define Sampling Effort? The Potential of Procrustes Analysis for Studying Variation in Community Composition. Biotropica 2015, 47, 399–402. [Google Scholar] [CrossRef]
- Afthanorhan, W.M.A.B.W. Hierarchical Component Using Reflective-Formative Measurement Model In Partial Least Square Structural Equation Modeling (Pls-Sem). Int. J. Math. Stat. Invent. 2014, 2, 55–71. [Google Scholar]
- Wong, K.K. Partial Least Square Structural Equation Modeling (PLS-SEM) Techniques Using SmartPLS. Mark. Bull. 2013, 24, 1–32. [Google Scholar]
- Zhao, M.; Lei, L.; Han, B. Seasonal Change in Phytoplankton Communities in Tangxi Reservoir and the Effecting Factors. J. Trop. Subtrop. Bot. 2005, 13, 386–392. (In Chinese) [Google Scholar]
- Zhang, T.; Li, L.; Song, L. Annual Dynamics of Phytoplankton Abundance and Community Structure in the Xionghe Reservoir. Acta Ecol. Sin. 2009, 29, 2971–2979. (In Chinese) [Google Scholar]
- Guo, F.; Zhang, Y.; Zhao, G.; Ao, M.; Lei, D.; Xiong, B.; Ma, X. Community Structure of Zooplankton in the Jinshahe Reservoir and Its Relationship with Environmental Factors. Chin. J. Ecol. 2016, 35, 2116–2208. (In Chinese) [Google Scholar]
- Ma, X.; Xiong, B.; Wang, W.; Wang, M.; Liu, X. Community Structure and Species Diversity of Zooplankton in Daoguanhe Reservoir, Hubei Province. J. Huazhong Agric. 2005, 24, 63–67. (In Chinese) [Google Scholar]
- Vaughn, C.C.; Hakenkamp, C.C. The Functional Role of Burrowing Bivalves in Freshwater Ecosystems. Freshw. Biol. 2001, 46, 1431–1446. [Google Scholar] [CrossRef] [Green Version]
- Saddozai, S.; Baloch, W.A.; Achakzai, W.M.; Memon, N. Population Dynamics and Ecology of Freshwater Gastropods in Manchar Lake Sindh, Pakistan. J. Anim. Plant Sci. 2013, 23, 1089–1093. [Google Scholar]
- Yang, L.; Chen, S. Progress in Chironomid-Based Studies of Past Water Environment in the Lacustrine System. J. Jiangsu Norm. Univ. (Nat. Sci. Ed.) 2016, 34, 11–15. (In Chinese) [Google Scholar]
- Yu, J.; Li, Y.; Liu, X.; Li, K.; Chen, F.; Gulati, R.; Liu, Z. The Fate of Cyanobacterial Detritus in the Food Web of Lake Taihu: A Mesocosm Study Using 13C and 15N Labeling. Hydrobiologia 2013, 710, 39–46. [Google Scholar] [CrossRef]
- Moore, J.W. Importance of Algae in the Diet of the Oligochaetes Lumbriculus Variegatus (MÜller) and Rhyacodrilus Sodalis (Eisen). Oecologia 1978, 35, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhang, Y.; Yu, H.; Wang, B. Concordance among Different Aquatic Insect Assemblages and the Relative Role of Spatial and Environmental Variables. Biodivers. Sci. 2013, 21, 326–333. [Google Scholar] [CrossRef]
- Soininen, J.; Paavola, R.; Kwandrans, J.; Muotka, T. Diatoms: Unicellular Surrogates for Macroalgal Community Structure in Streams? Biodivers. Conserv. 2009, 18, 79–89. [Google Scholar] [CrossRef]
- Heino, J.; Tolonen, K.T.; Kotanen, J.; Paasivirta, L. Indicator Groups and Congruence of Assemblage Similarity, Species Richness and Environmental Relationships in Littoral Macroinvertebrates. Biodivers. Conserv. 2009, 18, 3085–3098. [Google Scholar] [CrossRef]
- Rossaro, B.; Marziali, L.; Cardoso, A.C.; Solimini, A.; Free, G.; Giacchini, R. A Biotic Index Using Benthic Macroinvertebrates for Italian Lakes. Ecol. Indic. 2007, 7, 412–429. [Google Scholar] [CrossRef]

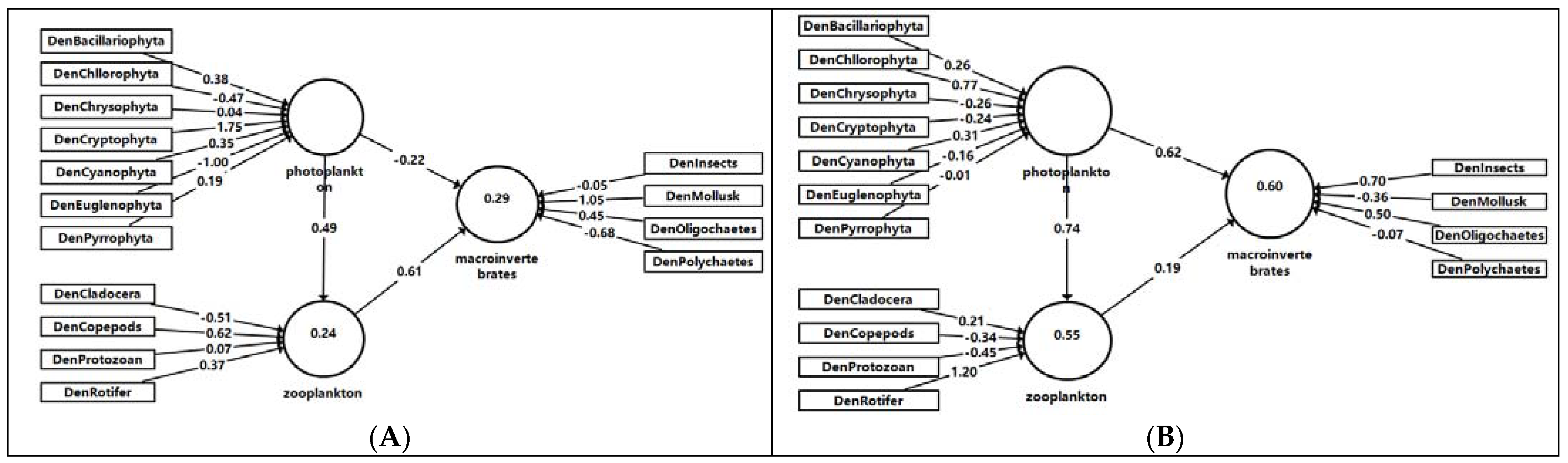
| Season | Water Temperature (°C) | Transparency (cm) | pH | Total Nitrogen (mg/L) | Total Phosphorus (mg/L) |
|---|---|---|---|---|---|
| Spring | 22.2 (17.5–27.5) a | 131.4 (20–420) a | 8.2 (7.7–9.2) a | 1.22 (0.31–2.88) a | 0.225 (0.020–5.830) a |
| Summer | 29.0 (25.0–32.5) b | 103.4 (30–300) b | 8.4 (7.8–9.1) b | 0.98 (0.20–8.27) b | 0.146 (0.026–0.773) a |
| Seasons | Pairs of Assemblages | rp | p |
|---|---|---|---|
| Spring | Phytoplankton vs. Macroinvertebrate | 0.260 | 0.083 |
| Phytoplankton vs. Zooplankton | 0.365 | 0.003 | |
| Zooplankton vs. Macroinvertebrate | 0.342 | 0.014 | |
| Summer | Phytoplankton vs. Macroinvertebrate | 0.225 | 0.185 |
| Phytoplankton vs. Zooplankton | 0.156 | 0.536 | |
| Zooplankton vs. Macroinvertebrate | 0.164 | 0.510 |
| Variable | Specific Interpretation |
|---|---|
| DenMollusk | Density of mollusks |
| DenInsects | Density of insects |
| DenOligochaetes | Density of oligochaetes |
| DenTotal | Density of macroinvertebrate |
| DenPhytoplankton | Density of phytoplankton |
| DenPyrrophyta | Density of Pyrrophyta |
| DenEuglenophyta | Density of Euglenophyta |
| DenBacillariophyta | Density of bacillariophyta |
| DenCryptophyta | Density of Cryptophyta |
| DenChrysophyta | Density of Chrysophyta |
| DenChlorophyta | Density of Chlorophyta |
| DenCyanophyta | Density of Cyanophyta |
| DenZooplankton | Density of zooplankton |
| DenProtozoan | Density of protozoan |
| DenCopepods | Density of copepods |
| DenRotifer | Density of rotifer |
| DenCladoceran | Density of cladoceran |
| Seasons | Explained Y | RX2 | RY2 | Adjusted R2 |
|---|---|---|---|---|
| Spring | DenMollusk | 0.181 | 0.161 | 0.143 |
| DenInsects | 0.286 | 0.175 | 0.157 | |
| DenOligochaetes | 0.322 | 0.165 | 0.147 | |
| DenTotal | 0.300 | 0.170 | 0.152 | |
| Summer | DenMollusk | 0.195 | 0.309 | 0.294 |
| DenInsects | 0.360 | 0.350 | 0.337 | |
| DenOligochaetes | 0.242 | 0.287 | 0.273 | |
| DenTotal | 0.359 | 0.349 | 0.336 |
| Spring | Summer | |||||||
|---|---|---|---|---|---|---|---|---|
| DenMollusk | DenInsects | DenOligochaetes | DenTotal | DenMollusk | DenInsects | DenOligochaetes | DenTotal | |
| DenPhytoplankton | −0.270 | 0.196 | 0.324 | 0.222 | −0.200 | 0.444 | 0.123 | 0.421 |
| DenPyrrophyta | −0.196 | −0.110 | 0.069 | −0.096 | 0.076 | −0.133 | 0.289 | −0.081 |
| DenEuglenophyta | −0.133 | 0.551 | 0.378 | 0.517 | 0.265 | 0.036 | 0.071 | 0.037 |
| DenBacillariophyta | −0.191 | −0.024 | 0.173 | 0.018 | −0.153 | 0.329 | 0.018 | 0.301 |
| DenCryptophyta | −0.055 | 0.441 | 0.347 | 0.434 | 0.253 | 0.117 | −0.156 | 0.079 |
| DenChrysophyta | −0.098 | −0.14 | 0.023 | −0.123 | 0.783 | −0.048 | 0.003 | −0.035 |
| DenChlorophyta | −0.233 | 0.467 | 0.505 | 0.499 | −0.248 | 0.312 | 0.866 | 0.424 |
| DenCyanophyta | −0.249 | 0.341 | 0.291 | 0.319 | −0.230 | 0.421 | 0.083 | 0.393 |
| DenZooplankton | 0.246 | 0.073 | 0.245 | 0.149 | −0.054 | 0.255 | 0.015 | 0.241 |
| DenProtozoan | −0.032 | 0.196 | 0.273 | 0.245 | −0.033 | 0.221 | −0.008 | 0.206 |
| DenCopepods | 0.398 | 0.034 | 0.178 | 0.091 | 0.096 | −0.040 | −0.208 | −0.062 |
| DenRotifer | 0.691 | −0.216 | 0.069 | −0.121 | −0.226 | 0.518 | 0.264 | 0.527 |
| DenCladoceran | −0.093 | −0.071 | −0.291 | −0.118 | −0.065 | −0.038 | 0.017 | −0.031 |
| Seasons | Pairs of Assemblages | Original Sample (O) | Sample Mean (M) | Standard Deviation (STDEV) | T Statistics (|O/STDEV|) | p Values |
|---|---|---|---|---|---|---|
| Spring | Phytoplankton->Macroinvertebrate | −0.22 | 0.27 | 0.59 | 0.38 | 0.71 |
| Phytoplankton->Zooplankton | 0.49 | 0.60 | 0.32 | 1.54 | 0.13 | |
| Zooplankton->Macroinvertebrate | 0.61 | 0.23 | 0.45 | 1.35 | 0.18 | |
| Summer | Phytoplankton->Macroinvertebrate | 0.62 | 0.47 | 0.57 | 1.09 | 0.27 |
| Phytoplankton->Zooplankton | 0.74 | 0.77 | 0.13 | 5.72 | <0.001 | |
| Zooplankton->Macroinvertebrate | 0.19 | 0.17 | 0.35 | 0.55 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, S.; Hu, J.; Li, M.; Wan, C. What Are the Relationships between Plankton and Macroinvertebrates in Reservoir Systems? Water 2023, 15, 2682. https://doi.org/10.3390/w15152682
Chi S, Hu J, Li M, Wan C. What Are the Relationships between Plankton and Macroinvertebrates in Reservoir Systems? Water. 2023; 15(15):2682. https://doi.org/10.3390/w15152682
Chicago/Turabian StyleChi, Shiyun, Jun Hu, Ming Li, and Chenyan Wan. 2023. "What Are the Relationships between Plankton and Macroinvertebrates in Reservoir Systems?" Water 15, no. 15: 2682. https://doi.org/10.3390/w15152682
APA StyleChi, S., Hu, J., Li, M., & Wan, C. (2023). What Are the Relationships between Plankton and Macroinvertebrates in Reservoir Systems? Water, 15(15), 2682. https://doi.org/10.3390/w15152682





