Synthesis of a Chemically Modified Biosorbent Based on the Invasive Plant Leucaena leucocephala and Its Application in Metformin Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. L. leucocephala Seed Preparation
2.2. LLMO Characterization
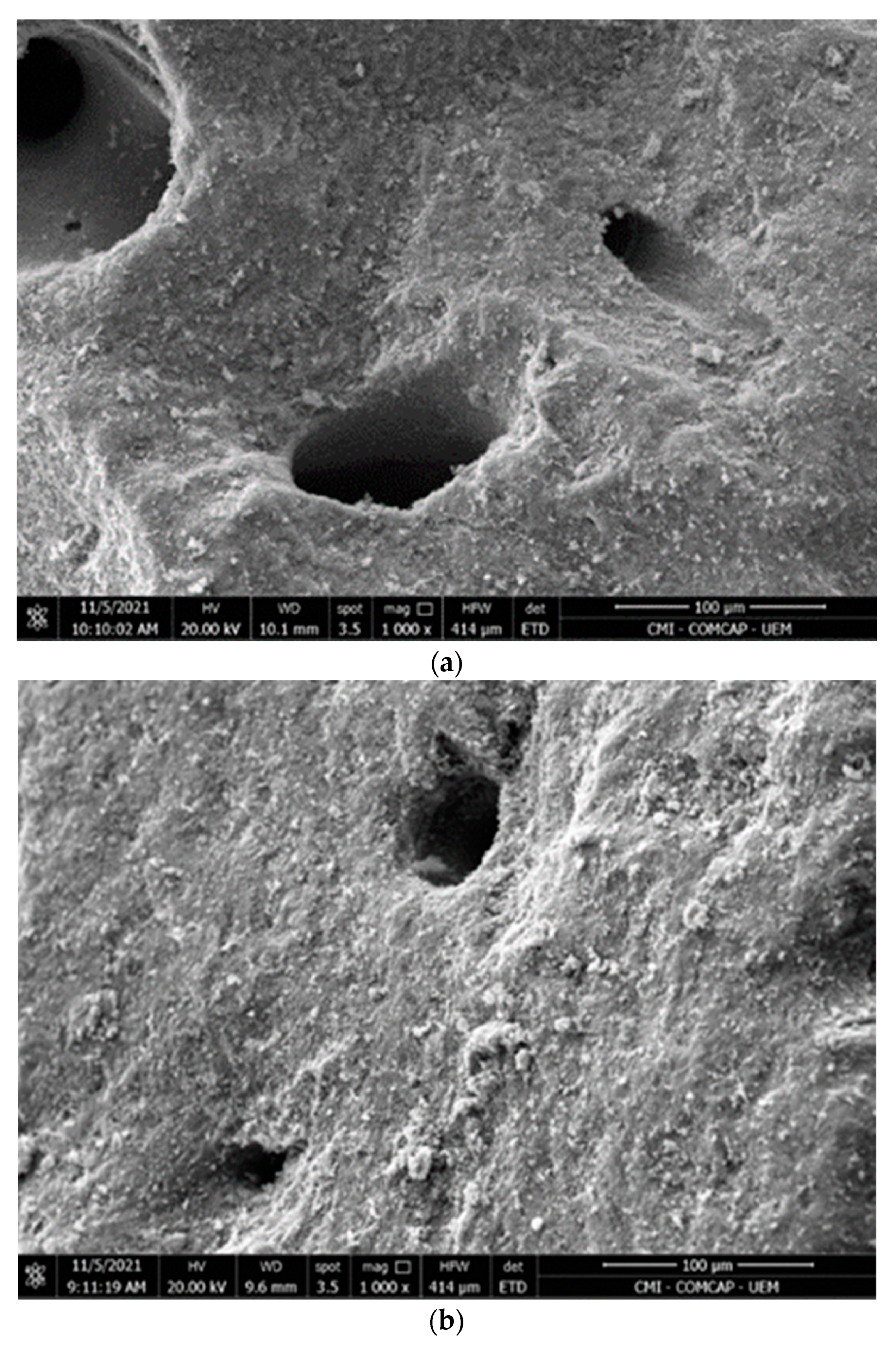
2.2.1. SEM
2.2.2. Zeta Potential Analyzer
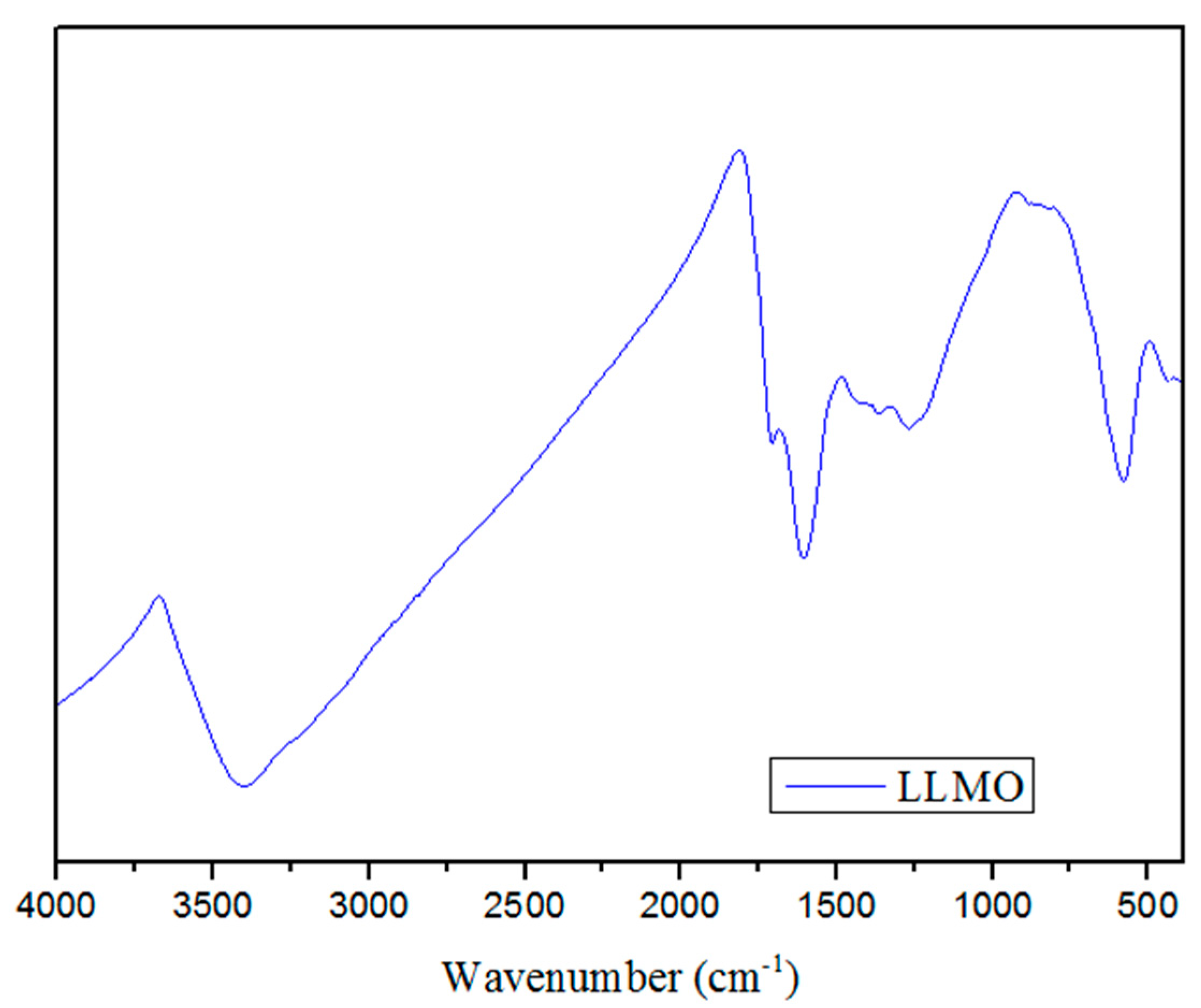
2.2.3. FTIR and BET
2.3. Metformin Samples
2.3.1. Adsorption Experiments
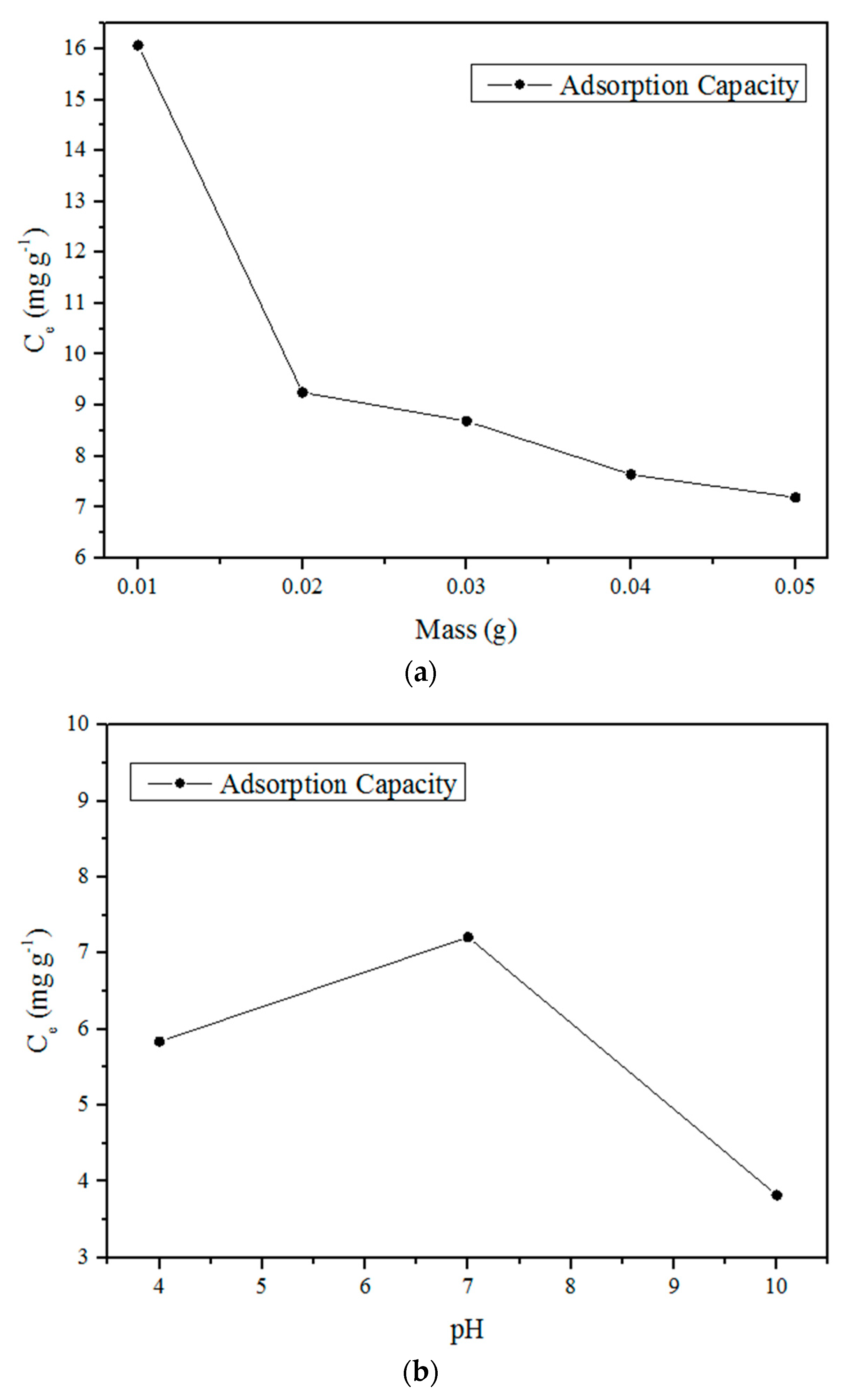
2.3.2. Effect of Biosorbent Concentration and pH
2.3.3. Kinetic and Equilibrium Study
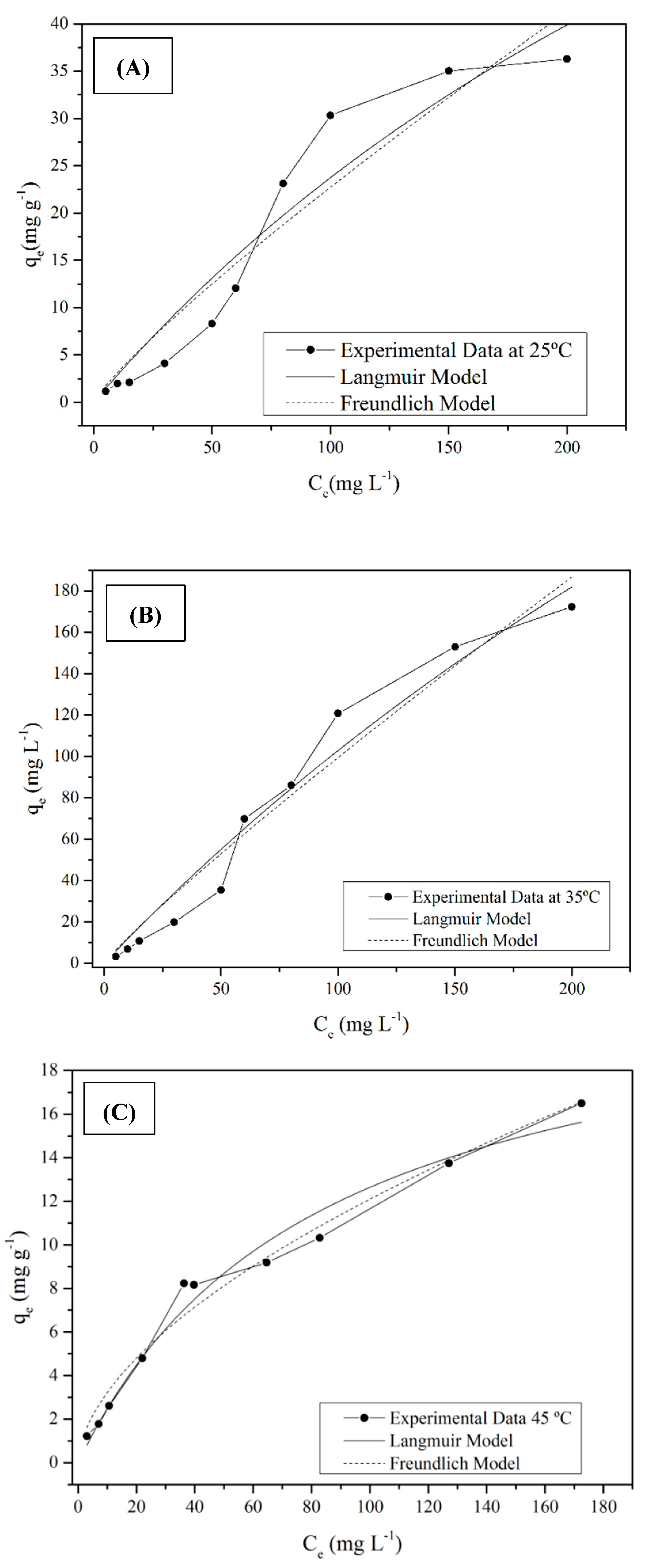
2.4. Adsorption Isotherms
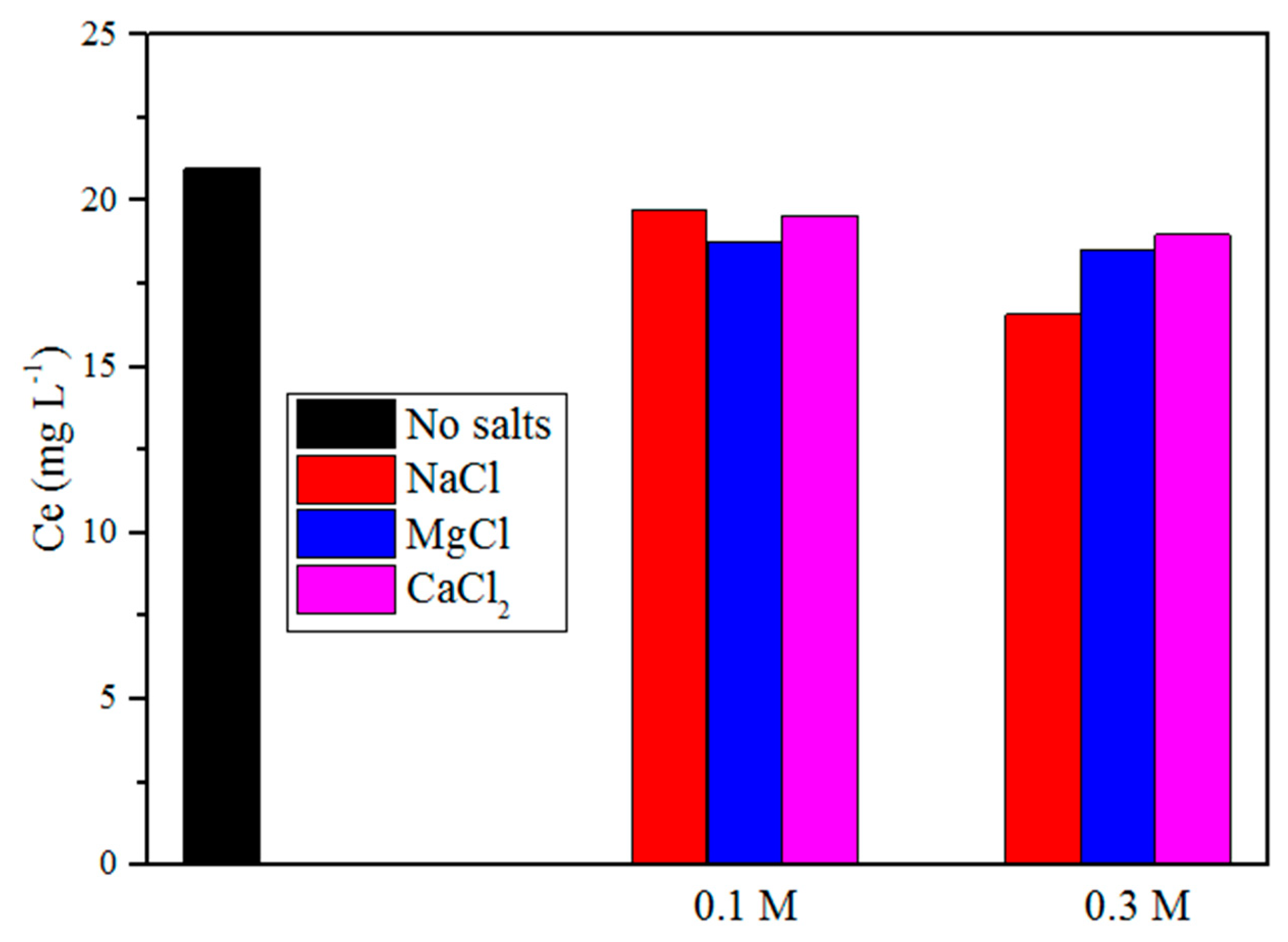
2.5. Effect of Ionic Strength
3. Results and Discussion
3.1. Biosorbent Characterization
3.2. Adsorption Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahammad, N.A.; Ahmad, M.A.; Hameed, B.H.; Mohd Din, A.T. A Mini Review of Recent Progress in the Removal of Emerging Contaminants from Pharmaceutical Waste Using Various Adsorbents. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- O’Flynn, D.; Lawler, J.; Yusuf, A.; Parle-Mcdermott, A.; Harold, D.; Mc Cloughlin, T.; Holland, L.; Regan, F.; White, B. A Review of Pharmaceutical Occurrence and Pathways in the Aquatic Environment in the Context of a Changing Climate and the COVID-19 Pandemic. Anal. Methods 2021, 13, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Masanabo, N.; Orimolade, B.; Idris, A.O.; Nkambule, T.T.I.; Mamba, B.B.; Feleni, U. Advances in Polymer-Based Detection of Environmental Ibuprofen in Wastewater. Environ. Sci. Pollut. Res. 2023, 30, 14062–14090. [Google Scholar] [CrossRef] [PubMed]
- Eniola, J.O.; Kumar, R.; Barakat, M.A.; Rashid, J. A Review on Conventional and Advanced Hybrid Technologies for Pharmaceutical Wastewater Treatment. J. Clean. Prod. 2022, 356, 131826. [Google Scholar] [CrossRef]
- Foretz, M.; Viollet, B. New Promises for Metformin: Advances in the Understanding of Its Mechanisms of Action. Med. Sci. 2014, 30, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Scheurer, M.; Michel, A.; Brauch, H.J.; Ruck, W.; Sacher, F. Occurrence and Fate of the Antidiabetic Drug Metformin and Its Metabolite Guanylurea in the Environment and during Drinking Water Treatment. Water Res. 2012, 46, 4790–4802. [Google Scholar] [CrossRef]
- Yan, J.H.; Xiao, Y.; Tan, D.Q.; Shao, X.T.; Wang, Z.; Wang, D.G. Wastewater Analysis Reveals Spatial Pattern in Consumption of Anti-Diabetes Drug Metformin in China. Chemosphere 2019, 222, 688–695. [Google Scholar] [CrossRef]
- Ambrosio-Albuquerque, E.P.; Cusioli, L.F.; Bergamasco, R.; Sinópolis Gigliolli, A.A.; Lupepsa, L.; Paupitz, B.R.; Barbieri, P.A.; Borin-Carvalho, L.A.; de Brito Portela-Castro, A.L. Metformin Environmental Exposure: A Systematic Review. Environ. Toxicol. Pharmacol. 2021, 83, 103588. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Y.G.; Liu, S.B.; Zeng, G.M.; Jiang, L.H.; Tan, X.F.; Zhou, L.; Zeng, W.; Li, T.T.; Yang, C.P. Adsorption of Emerging Contaminant Metformin Using Graphene Oxide. Chemosphere 2017, 179, 20–28. [Google Scholar] [CrossRef]
- Ussery, E.; Bridges, K.N.; Pandelides, Z.; Kirkwood, A.E.; Bonetta, D.; Venables, B.J.; Guchardi, J.; Holdway, D. Effects of Environmentally Relevant Metformin Exposure on Japanese Medaka (Oryzias latipes). Aquat. Toxicol. 2018, 205, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Sahay, P.; Mohite, D.; Arya, S.; Dalmia, K.; Khan, Z.; Kumar, A. Removal of the Emergent Pollutants (Hormones and Antibiotics) from Wastewater Using Different Kinds of Biosorbent—A Review. Emergent Mater. 2023, 6, 373–404. [Google Scholar] [CrossRef]
- de Souza Leite, L.; Hoffmann, M.T.; de Vicente, F.S.; dos Santos, D.V.; Daniel, L.A. Adsorption of Algal Organic Matter on Activated Carbons from Alternative Sources: Influence of Physico-Chemical Parameters. J. Water Process Eng. 2021, 44, 102435. [Google Scholar] [CrossRef]
- Zaman, D.; Tiwari, M.K.; Mishra, S. Low-cost adsorptive removal techniques for pharmaceuticals and personal care products. In Energy, Environment, and Sustainability; Springer: Singapore, 2020. [Google Scholar]
- Cusioli, L.F.; Quesada, H.B.; de Brito Portela Castro, A.L.; Gomes, R.G.; Bergamasco, R. Development of a New Low-Cost Adsorbent Functionalized with Iron Nanoparticles for Removal of Metformin from Contaminated Water. Chemosphere 2020, 247, 125852. [Google Scholar] [CrossRef] [PubMed]
- Kalumpha, M.; Guyo, U.; Zinyama, N.P.; Vakira, F.M.; Nyamunda, B.C. Adsorptive Potential of Zea Mays Tassel Activated Carbon towards the Removal of Metformin Hydrochloride from Pharmaceutical Effluent. Int. J. Phytoremediat. 2020, 22, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, O.S.; Ibrahim, A.O.; Bello, O.S. Metformin Adsorption onto Activated Carbon Prepared by Acid Activation and Carbonization of Orange Peel. Int. J. Phytoremediat. 2023, 25, 125–136. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface Water Pollution by Pharmaceuticals and an Alternative of Removal by Low-Cost Adsorbents: A Review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Wardatun, S.; Harahap, Y.; Mun’im, A.; Saputri, F.C.; Sutandyo, N. Leucaena leucocephala (Lam.) de Wit Seeds: A New Potential Source of Sulfhydryl Compounds. Pharmacogn. J. 2020, 12, 298–302. [Google Scholar] [CrossRef] [Green Version]
- Magalhães-Ghiotto, G.A.V.; de Oliveira, A.M.; Natal, J.P.S.; Bergamasco, R.; Gomes, R.G. Green Nanoparticles in Water Treatment: A Review of Research Trends, Applications, Environmental Aspects and Large-Scale Production. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100526. [Google Scholar] [CrossRef]
- Hermes Ulises, R.S.; Montiel Aida Lucia, F.; Alma Delia, O.B.; Villaseñor Odila, D.L.T. Impacts of Climate Change on the Water Sector in Mexico. Asian J. Environ. Ecol. 2022, 17, 37–57. [Google Scholar] [CrossRef]
- Ismawati, R. Zeolite: Structure and potential in agriculture. J. Pena Sains 2018, 5, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Cimá-Mukul, C.A.; Olguín, M.T.; Abatal, M.; Vargas, J.; Barrón-Zambrano, J.A.; Ávila-Ortega, A.; Santiago, A.A. Assessment of Leucaena leucocephala as Bio-Based Adsorbent for the Removal of Pb2+, Cd2+ and Ni2+ from Water. Desalin. Water Treat. 2020, 173, 331–342. [Google Scholar] [CrossRef]
- Wan Ibrahim, W.M.H.; Mohamad Amini, M.H.; Sulaiman, N.S.; Kadir, W.R.A. Powdered Activated Carbon Prepared from Leucaena leucocephala Biomass for Cadmium Removal in Water Purification Process. Arab J. Basic Appl. Sci. 2019, 26, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.C.; Amini, M.H.M.; Sulaiman, N.S.; Mazlan, M.; Boon, J.G. Batch Adsorption and Isothermic Studies of Malachite Green Dye Adsorption Using Leucaena leucocephala Biomass as Potential Adsorbent in Water Treatment. Songklanakarin J. Sci. Technol. 2018, 40, 563–569. [Google Scholar] [CrossRef]
- Yusuff, A.S. Adsorption of Hexavalent Chromium from Aqueous Solution by Leucaena leucocephala Seed Pod Activated Carbon: Equilibrium, Kinetic and Thermodynamic Studies. Arab J. Basic Appl. Sci. 2019, 26, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Alnajjar, M.; Hethnawi, A.; Nafie, G.; Hassan, A.; Vitale, G.; Nassar, N.N. Silica-Alumina Composite as an Effective Adsorbent for the Removal of Metformin from Water. J. Environ. Chem. Eng. 2019, 7, 102994. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Solid Substance. Handlinger 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Ameen, F.; AlNadhari, S.; Al-Homaidan, A.A. Marine Microorganisms as an Untapped Source of Bioactive Compounds. Saudi J. Biol. Sci. 2021, 28, 224–231. [Google Scholar]
- Cusioli, L.F.; Quesada, H.B.; Baptista, A.T.A.; Gomes, R.G.; Bergamasco, R. Soybean Hulls as A Low-Cost Biosorbent for Removal of Methylene Blue Contaminant. Environ. Prog. Sustain. Energy 2019, 39, e13328. [Google Scholar] [CrossRef]
- Cusioli, L.F.; Quesada, H.B.; Barbosa de Andrade, M.; Gomes, R.G.; Bergamasco, R. Application of a Novel Low-Cost Adsorbent Functioned with Iron Oxide Nanoparticles for the Removal of Triclosan Present in Contaminated Water. Microporous Mesoporous Mater. 2021, 325, 111328. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as Emerging Contaminants and Their Removal from Water. A Review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [PubMed]
- Araújo, C.S.T.; Alves, V.N.; Rezende, H.C.; Almeida, I.L.S.; De Assunção, R.M.N.; Tarley, C.R.T.; Segatelli, M.G.; Coelho, N.M.M. Characterization and Use of Moringa Oleifera Seeds as Biosorbent for Removing Metal Ions from Aqueous Effluents. Water Sci. Technol. 2010, 62, 2198–2203. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Sandle, N.K. Effect of Different Oxidizing Agent Treatments on the Surface Properties of Activated Carbons. Carbon N. Y. 1999, 37, 1323–1332. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, L.A.; Rouquerol, J.; Siemieniewska, T. International Union of Pure and Applied Chemistry Physical Chemistry Division Reporting Physisorption Data for Gas/Soils Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 31, 579–638. [Google Scholar] [CrossRef]
- Adewuyi, A. Chemically Modified Biosorbents and Their Role in the Removal of Emerging Pharmaceuticalwaste in the Water System. Water 2020, 12, 1551. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mittal, A.; Jain, R.; Mathur, M.; Sikarwar, S. Adsorption of Safranin-T from Wastewater Using Waste Materials—Activated Carbon and Activated Rice Husks. J. Colloid Interface Sci. 2006, 303, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Abdulhameed, A.S. Mesoporous Iraqi Red Kaolin Clay as an Efficient Adsorbent for Methylene Blue Dye: Adsorption Kinetic, Isotherm and Mechanism Study. Surf. Interfaces 2020, 18, 100422. [Google Scholar] [CrossRef]
- Hayward, D.O.; Trapnell, B.M.W. Chemisorption, 2nd ed.; Butterworths: Washington, DC, USA, 1964. [Google Scholar]
- Ali, A.M.; Rønning, H.T.; Al Arif, W.M.; Kallenborn, R.; Kallenborn, R. Occurrence of Pharmaceuticals and Personal Care Products in Effluent-Dominated Saudi Arabian Coastal Waters of the Red Sea. Chemosphere 2017, 175, 505–513. [Google Scholar] [CrossRef]
- Elliott, J.R.; Lira, C.T. Introductory Chemical Engineering Thermodynamics; Prentice Hall: Upper Saddle River, NJ, USA, 2012. [Google Scholar]
- Crini, G. Non-Conventional Low-Cost Adsorbents for Dye Removal: A Review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J.C. Thermodynamic Study of Triclosan Adsorption from Aqueous Solutions on Activated Carbon. J. Therm. Anal. Calorim. 2020, 139, 913–921. [Google Scholar] [CrossRef]
- Sanchez-Silva, J.M.; Collins-Martínez, V.H.; Padilla-Ortega, E.; Aguilar-Aguilar, A.; Labrada-Delgado, G.J.; Gonzalez-Ortega, O.; Palestino-Escobedo, G.; Ocampo-Pérez, R. Characterization and Transformation of Nanche Stone (Byrsonima crassifolia) in an Activated Hydrochar with High Adsorption Capacity towards Metformin in Aqueous Solution. Chem. Eng. Res. Des. 2022, 183, 580–594. [Google Scholar] [CrossRef]
- Balasubramani, K.; Sivarajasekar, N.; Sarojini, G.; Naushad, M. Removal of Antidiabetic Pharmaceutical (Metformin) Using Graphene Oxide Microcrystalline Cellulose (GOMCC): Insights to Process Optimization, Equilibrium, Kinetics, And Machine Learning. Ind. Eng. Chem. Res. 2022, 62, 4713–4728. [Google Scholar] [CrossRef]
- Mohammad, A.H.; Radovic, I.; Ivanović, M.; Kijevčanin, M. Adsorption of Metformin on Activated Carbon Produced from the Water Hyacinth Biowaste Using H3PO4 as a Chemical Activator. Sustainability 2022, 14, 11144. [Google Scholar] [CrossRef]
- Hellmann, L.; Schmitz, A.P.D.O.; Módenes, A.N.; Hinterholz, C.L.; Antoniolli, C.D.A. Peach Pit Chemically Treated Biomass as a Biosorbent for Metformin Hydrochloride Removal: Modeling and Sorption Mechanisms. Eng. Agric. 2021, 41, 181–195. [Google Scholar] [CrossRef]
- Mustafa, S.; Bhatti, H.N.; Maqbool, M.; Iqbal, M. Microalgae Biosorption, Bioaccumulation and Biodegradation Efficiency for the Remediation of Wastewater and Carbon Dioxide Mitigation: Prospects, Challenges and Opportunities. J. Water Process Eng. 2021, 41, 102009. [Google Scholar]
- Mahmoud, M.E.; El-Ghanam, A.M.; Saad, S.R.; Mohamed, R.H.A. Promoted Removal of Metformin Hydrochloride Anti-Diabetic Drug from Water by Fabricated and Modified Nanobiochar from Artichoke Leaves. Sustain. Chem. Pharm. 2020, 18, 100336. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Banana Peel as a Biosorbent for the Decontamination of Water Pollutants. A Review. Environ. Chem. Lett. 2020, 18, 1085–1112. [Google Scholar]
- Shaikhiev, I.G.; Kraysman, N.V.; Sverguzova, S.V. Review of Peach (Prúnus pérsica) Shell Use to Remove Pollutants from Aquatic Environments. Biointerface Res. Appl. Chem. 2023, 13, 459. [Google Scholar]

| pH | Temperature (°C) | Metformin Concentration (mg L−1) | Adsorbent Mass (g) | Stirring Speed (rpm) |
|---|---|---|---|---|
| 7 | 25 | 10 | 0.01, 0.02, 0.03, 0.04 and 0.05 | 150 |
| 4, 7, and 10 | 25 | 10 | 0.03 | 150 |
| 7 | 25 | 10 | 0.03 | 150 |
| 7 | 25, 35, and 45 | 5–200 | 0.03 | 150 |
| Parameters | LLMO |
|---|---|
| BET Specific surface area (m² g−1) | 5.32 |
| Average pore diameter (Å) | 14.79 |
| Total pore volume (cm³ g−1) | 0.0892 |
| Micropore volume (cm³ g−1) | 0.0874 |
| Mesopore volume (cm³ g−1) | 0.0018 |
| Models | Parameters | LLMO |
|---|---|---|
| Pseudo-first-order (PFO) | qe (mg g−1) | 7.825 |
| k1 (min−1) | 0.008 | |
| R2 | 0.980 | |
| χ2 | 0.097 | |
| Pseudo-second-order (PSO) | qe (mg g−1) | 11.422 |
| k2 (g mg−1 min−1) | 0.003 | |
| R2 | 0.974 | |
| χ2 | 0.173 |
| Models | Parameters | 293 K | 303 K | 313 K |
|---|---|---|---|---|
| Langmuir | qmáx (mg g−1) | 14.64 | 39.79 | 56.18 |
| KL (L mg−1) | 0.024 | 0.057 | 0.073 | |
| R2 | 0.920 | 0.964 | 0.975 | |
| Freundlich | KF [(mg/g)/(mg/L)1/n] | 19.60 | 14.82 | 11.67 |
| nF | 1.160 | 1.012 | 0.945 | |
| R2 | 0.905 | 0.926 | 0.913 |
| T (°C) | T (K) | (ΔG) (KJ mol−1) | (ΔH) (KJ mol−1) | (ΔS) (KJ mol−1) |
|---|---|---|---|---|
| 25 | 298 | −22.36 | −30.39 | 0.027 |
| 35 | 308 | −21.31 | ||
| 45 | 318 | −20.79 |
| Adsorbent | qe (mg g−1) | Mass (g) | pH | Temperature (K) | Reference |
|---|---|---|---|---|---|
| Acid modification of orange peels (OPAC) | 50.99 | 0.1 | 7 | 323 | [17] |
| Byrsonima crassifolia activated hydrochar | 113.6 | 2.0 | 7 | 293 | [48] |
| Graphene oxide microcrystalline cellulose (GOMCC) | 132.10 | 0.5 | 8.5 | 31.8 | [49] |
| Activated Carbon chemical activation | 122.47 | 1.0 | 8.5 | 318 | [50] |
| Peach pit chemically treated biomass | 82.54 | 0.5 | 8 | 323 | [51] |
| Moringa seed husks are functionalized with nanoparticles | 65.01 | 0.03 | 7 | 298 | [15] |
| Microalgae | 45.67 | 1 | 6.3 | 298 | [52] |
| Artichoke Leaves | 17.1 | 0.01 | 2–12 | 333 | [53] |
| Banana peel | 43.28 | 1.5 | 4 | 298 | [54] |
| Peach (Prúnus pérsica) | 38.97 | 0.5 | 8 | 303 | [55] |
| LLMO | 56.18 | 0.03 | 7 | 298 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusioli, L.F.; Nishi, L.; Beltran, L.B.; Ribeiro, A.C.; Bergamasco, R.; Bulla, M.K.; Facina, R.K.; Mateus, G.A.P. Synthesis of a Chemically Modified Biosorbent Based on the Invasive Plant Leucaena leucocephala and Its Application in Metformin Removal. Water 2023, 15, 2600. https://doi.org/10.3390/w15142600
Cusioli LF, Nishi L, Beltran LB, Ribeiro AC, Bergamasco R, Bulla MK, Facina RK, Mateus GAP. Synthesis of a Chemically Modified Biosorbent Based on the Invasive Plant Leucaena leucocephala and Its Application in Metformin Removal. Water. 2023; 15(14):2600. https://doi.org/10.3390/w15142600
Chicago/Turabian StyleCusioli, Luís Fernando, Letícia Nishi, Laiza Bergamasco Beltran, Anna Carla Ribeiro, Rosângela Bergamasco, Milena Keller Bulla, Rhana Keterly Facina, and Gustavo Affonso Pisano Mateus. 2023. "Synthesis of a Chemically Modified Biosorbent Based on the Invasive Plant Leucaena leucocephala and Its Application in Metformin Removal" Water 15, no. 14: 2600. https://doi.org/10.3390/w15142600





