Understanding Cassiopea andromeda (Scyphozoa) Invasiveness in Different Habitats: A Multiple Biomarker Comparison
Abstract
1. Introduction
2. Materials and Methods
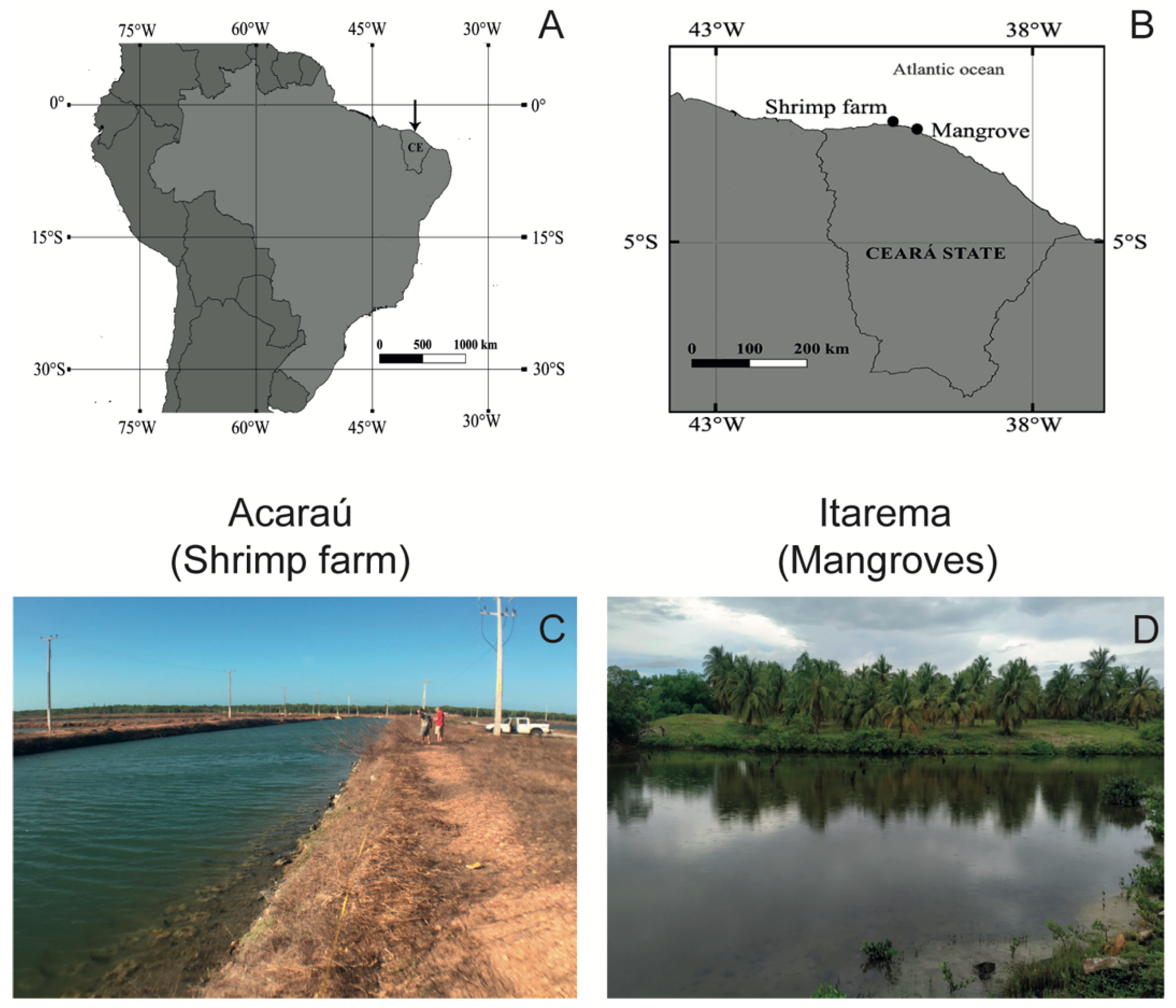
2.1. Study Area
2.2. Sample Collection and Preservation
2.3. Biomarker Analysis
2.3.1. Organic Matter
2.3.2. Total Carbohydrate Content
2.3.3. Total Lipid Content
2.3.4. C:N Ratios, δ15N and δ13C Composition
2.3.5. Statistical Analysis
3. Results
3.1. Organic Matter
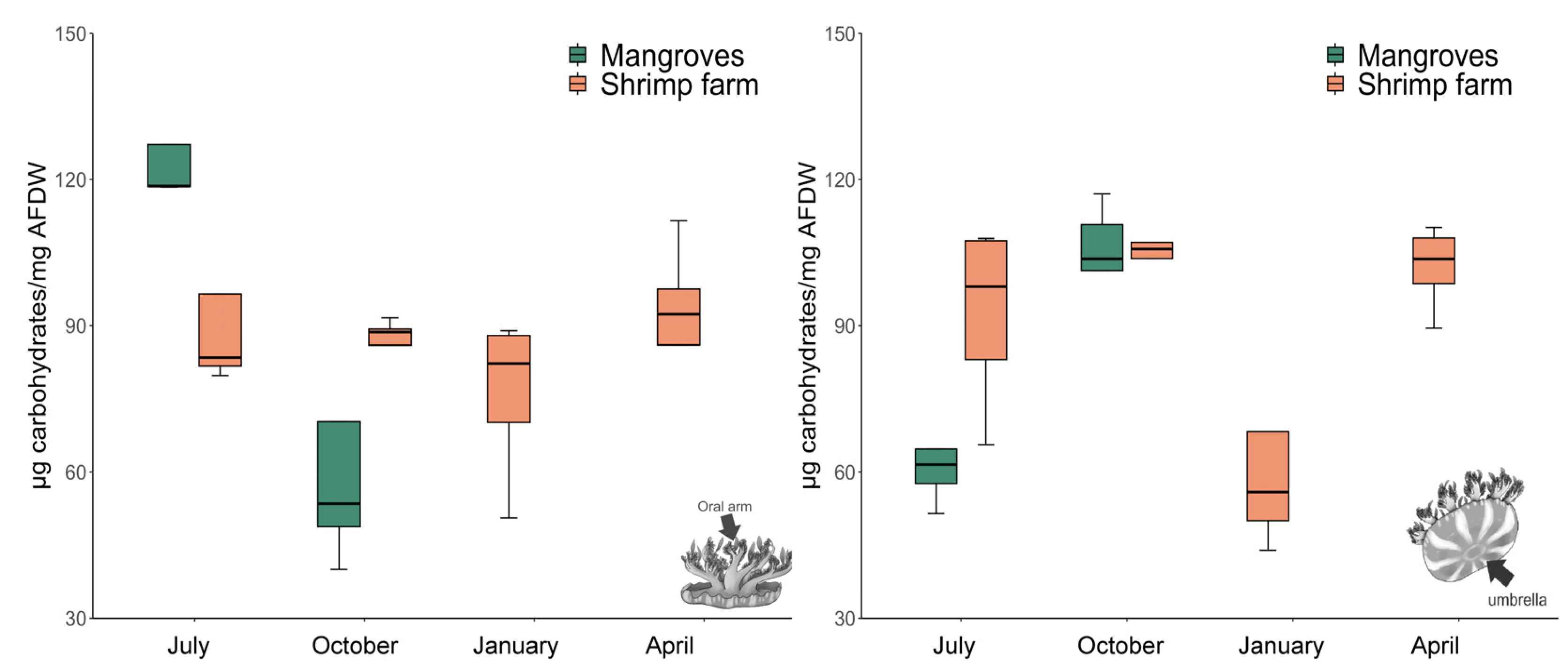
3.2. Total Carbohydrate Content
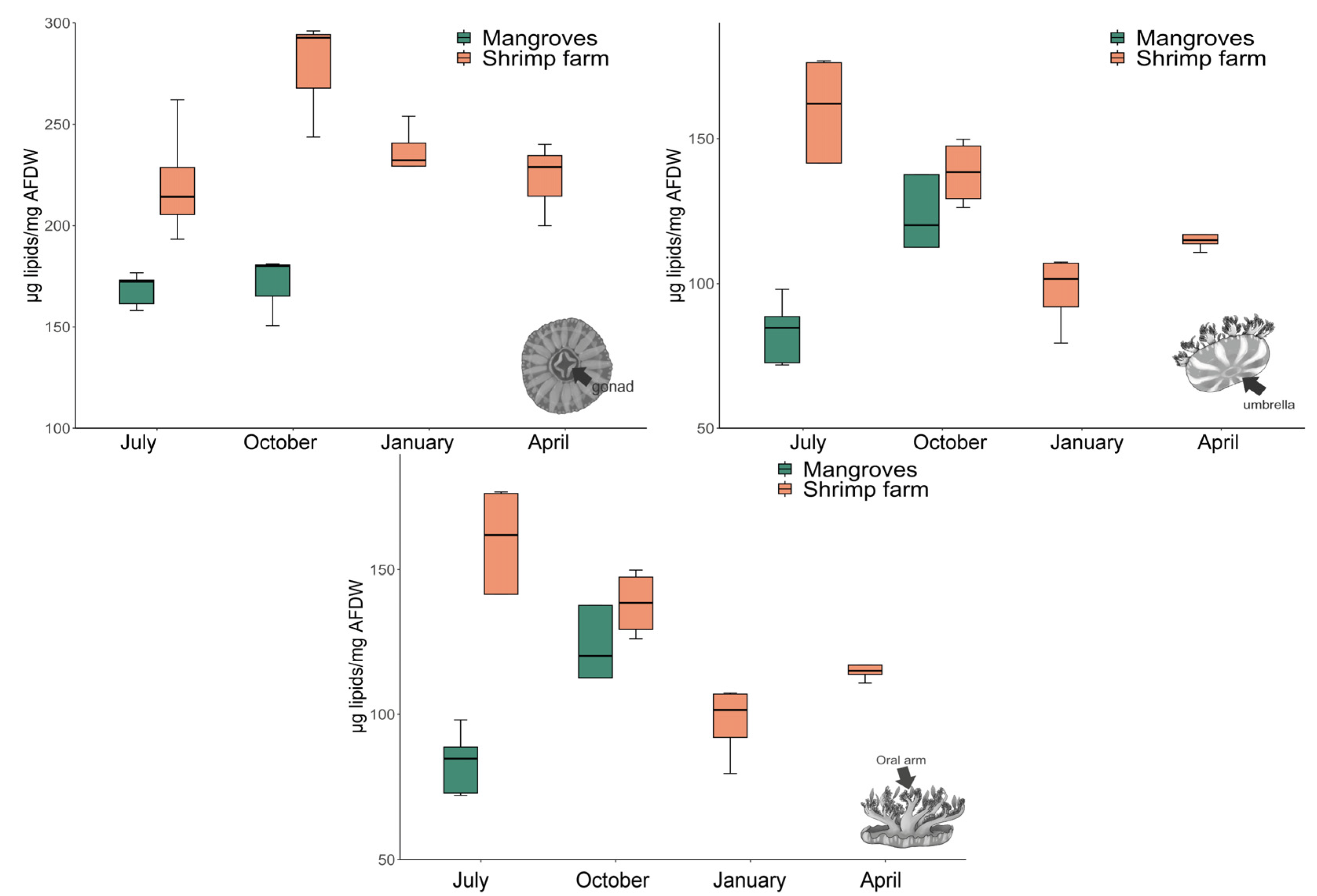
3.3. Total Lipid Content
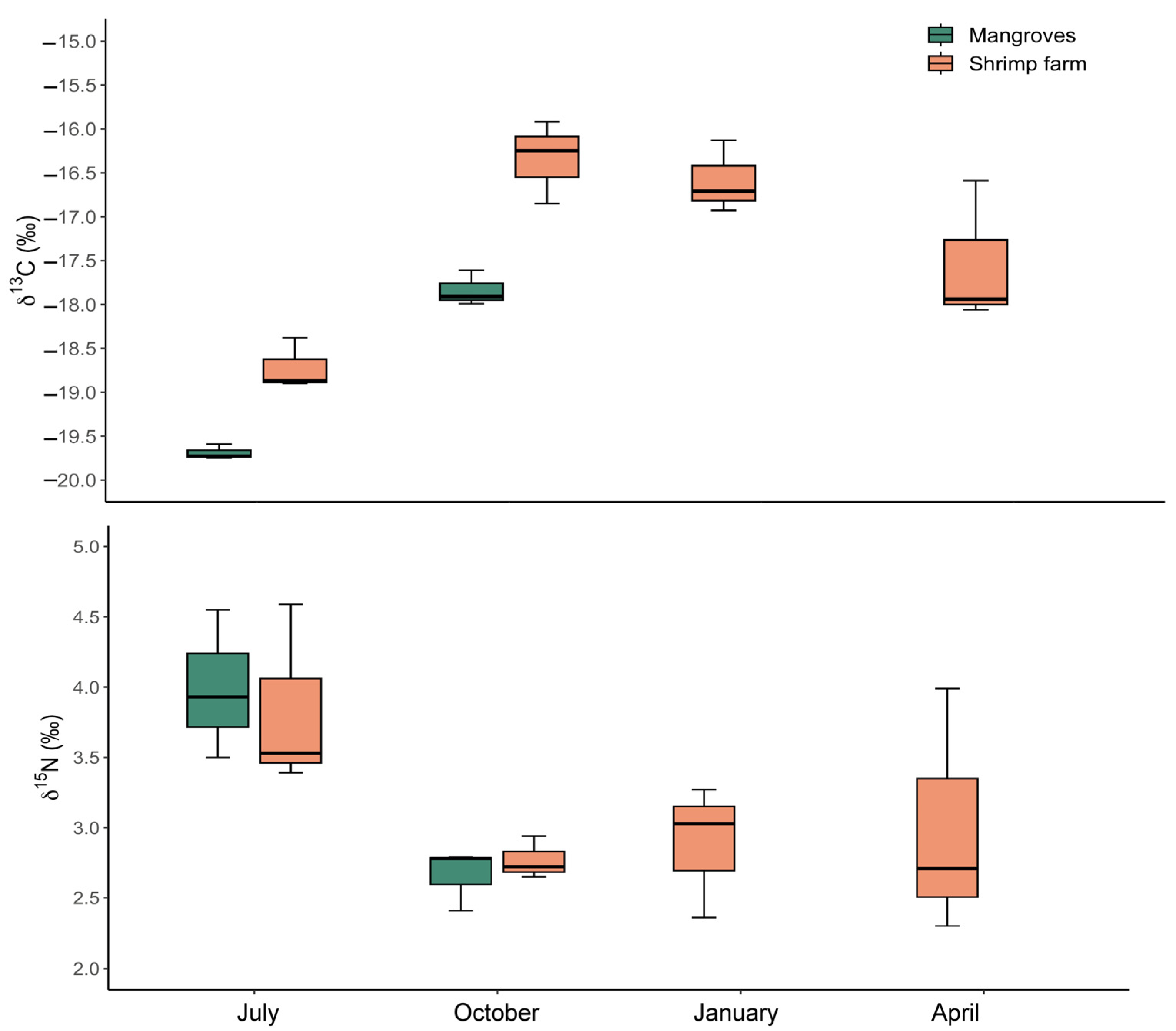
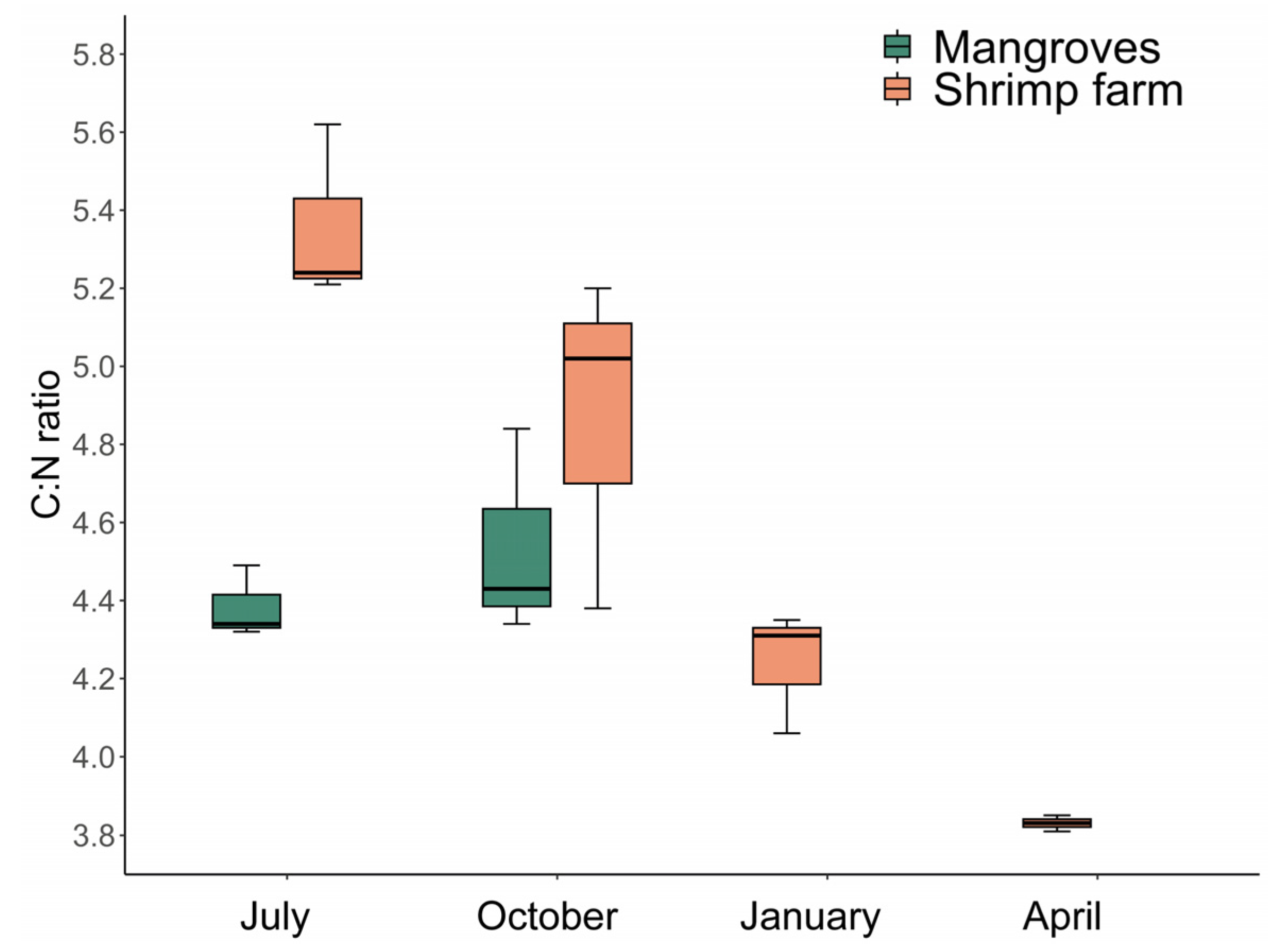
3.4. Stable Isotopes Analysis and C:N Ratio
4. Discussion
4.1. Nutritional Condition of Cassiopea andromeda
4.2. Trophic Strategy of Cassiopea andromeda
4.3. Why Did Cassiopea andromeda Disappear in the Mangroves?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Purcell, J.E.; Uye, S.I.; Lo, W.T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Progr. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Bayha, K.M.; Graham, W.M. Nonindigenous Marine Jellyfish: Invasiveness, Invasibility, and Impacts. In Jellyfish Blooms; Pitt, K.A., Lucas, C.H., Eds.; Springer Science: Berlin/Heidelberg, Germany, 2014; Volume 3, pp. 45–77. [Google Scholar] [CrossRef]
- Stoner, E.W.; Archer, S.K.; Layman, C.A. Increased nutrient availability correlates with increased growth of the benthic jellyfish Cassiopea spp. Food Webs 2022, 31, e00231. [Google Scholar] [CrossRef]
- Peron, F.; Lesueur, C.A. Tableau des caractères generiques et specifiques de toutes les espèces de méduses connues jusqùà ce jour. Ann. Muséum D’histoire Nat. 1810, 14, 325–366. [Google Scholar]
- Holland, B.S.; Dawson, M.N.; Crow, G.L.; Hofmann, D.K. Global phylogeography of Cassiopea (Scyphozoa: Rhizostomeae): Molecular evidence for cryptic species and multiple invasions of the Hawaiian Islands. Mar. Biol. 2004, 145, 1119–1128. [Google Scholar] [CrossRef]
- Ohdera, A.H.; Abrams, M.J.; Ames, C.L.; Baker, D.M.; Suescún-Bolívar, L.P.; Collins, A.G.; Freeman, C.J.; Gamero-Mora, E.; Goulet, T.L.; Hofmann, D.K.; et al. Upside-Down but Headed in the Right Direction: Review of the Highly Versatile Cassiopea xamachana System. Front. Ecol. Devol. 2018, 6, 35. [Google Scholar] [CrossRef]
- Thé, J.; Gamero-Mora, E.; Silva, M.V.C.; Morandini, A.C.; Rossi, S.; Soares, M.O. Non-indigenous upside-down jellyfish Cassiopea andromeda in shrimp farms (Brazil). Aquaculture 2020, 532, 735999. [Google Scholar] [CrossRef]
- Cillari, T.; Andaloro, F.; Castriota, L. First documented record of Cassiopea cf andromeda (Cnidaria: Scyphozoa) in Italian waters. Cah. Biol. Mar. 2018, 59, 193–195. [Google Scholar] [CrossRef]
- Deidun, A.; Sciberras, A.; Formosa, J.; Zava, B.; Insacco, G.; Corsini-Foka, M.; Crandall, K.A. Invasion by non-indigenous freshwater decapods of Malta and Sicily, central Mediterranean Sea. J. Crustac. Biol. 2018, 38, 748–753. [Google Scholar] [CrossRef]
- Morandini, A.C.; Stampar, S.N.; Maronna, M.M.; Silveira, F.L. All non-indigenous species were introduced recently? The case study of Cassiopea (Cnidaria: Scyphozoa) in Brazilian waters. J. Mar. Biolog. Assoc. U. K. 2017, 97, 321–328. [Google Scholar] [CrossRef]
- Stampar, S.N.; Gamero-Mora, E.; Maronna, M.M.; Fritscher, J.M.; Oliveira, B.S.P.; Sampaio, C.L.S.; Morandini, A.C. The puzzling occurrence of the upside-down jellyfish Cassiopea (Cnidaria: Scyphozoa) along the Brazilian coast: A result of several invasion events? Zoologia 2020, 37, 1–10. [Google Scholar] [CrossRef]
- Mortillaro, J.M.; Pitt, K.A.; Lee, S.Y.; Meziane, T. Light intensity influences the production and translocation of fatty acids by zooxanthellae in the jellyfish Cassiopea sp. J. Exp. Mar. Bio. Ecol. 2009, 378, 22–30. [Google Scholar] [CrossRef]
- Verde, E.; McCloskey, L. Production, respiration, and photophysiology of the mangrove jellyfish Cassiopea xamachana symbiotic with zooxanthellae: Effect of jellyfish size and season. Mar. Ecol. Progr. Ser. 1998, 168, 147–162. [Google Scholar] [CrossRef]
- Mammone, M.; Ferrier-Pages, C.; Lavorano, S.; Rizzo, L.; Piraino, S.; Rossi, S. High photosynthetic plasticity may reinforce invasiveness of upside-down zooxanthellate jellyfish in Mediterranean coastal waters. PLoS ONE 2021, 16, e0248814. [Google Scholar] [CrossRef]
- Djeghri, N.; Pondaven, P.; le Grand, F.; Bideau, A.; Duquesne, N.; Stockenreiter, M.; Behl, S.; Huang, J.; Hansen, T.; Patris, S.; et al. High trophic plasticity in the mixotrophic Mastigias papua-Symbiodiniaceae holobiont: Implications for the ecology of zooxanthellate jellyfishes. Mar. Ecol. Progr. Ser. 2021, 666, 73–88. [Google Scholar] [CrossRef]
- Drazen, J.C.; Phleger, C.F.; Guest, M.A.; Nichols, P.D. Lipid, sterols and fatty acid composition of abyssal holothurians and ophiuroids from the north-east Pacific Ocean: Food web implications. Comp. Biochem. Physiol. 2008, 151, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Scheibling, R.E. Fatty acids as dietary tracers in benthic food webs. Mar. Ecol. Progr. Ser. 2012, 446, 1–22. [Google Scholar] [CrossRef]
- Leal, M.C.; Ferrier-Pagès, C. Stable isotope as tracers of trophic interactions in marine mutualistic symbiosis. Ecol. Evol. 2018, 9, 723–740. [Google Scholar] [CrossRef]
- Milisenda, G.; Rossi, S.; Vizzini, S.; Fuentes, V.L.; Purcell, J.E.; Tilves, U.; Piraino, S. Seasonal variability of diet and trophic level of the gelatinous predator Pelagia noctiluca (Scyphozoa). Sci. Rep. 2018, 8, 12140. [Google Scholar] [CrossRef]
- Nagata, R.M.; Moreira, M.Z.; Pimentel, C.R.; Morandini, A.C. Food web characterization based on δ15N and δ13C reveals isotopic niche partitioning between fish and jellyfish in a relatively pristine ecosystem. Mar. Eco. Pro. Ser. 2015, 519, 13–27. [Google Scholar] [CrossRef]
- Purcell, J.E. Extension of methods for jellyfish and ctenophore trophic ecology to large-scale research. Hydrobiologia 2009, 616, 23–50. [Google Scholar] [CrossRef]
- Alfaro, A.C.; Thomas, F.; Sergent, L.; Duxbury, M. Identification of trophic interactions within an estuarine food web (northern New Zealand) using fatty acid biomarkers and stable isotopes. Estuar. Coast. 2006, 70, 271–286. [Google Scholar] [CrossRef]
- Calado, R.; Leal, M.C. Trophic Ecology of Benthic Marine Invertebrates with Bi-Phasic Life Cycles: What Are We Still Missing? In Advances in Marine Biology; Academic Press: Orlando, FL, USA, 2015; Volume 71, pp. 1–70. [Google Scholar] [CrossRef]
- Freeman, C.J.; Stoner, E.W.; Easson, C.G.; Matterson, K.O.; Baker, D.M. Symbiont carbon and nitrogen assimilation in the Cassiopea-Symbiodinium mutualism. Mar. Ecol. Progr. Ser. 2016, 544, 281–286. [Google Scholar] [CrossRef]
- Freeman, C.J.; Stoner, E.W.; Easson, C.G.; Matterson, K.O.; Baker, D.M. Variation in δ13C and δ15N values suggests a coupling of host and symbiont metabolism in the Symbiodinium-Cassiopea mutualism. Mar. Ecol. Progr. Ser. 2017, 571, 245–251. [Google Scholar] [CrossRef]
- Leal, M.C.; Ferrier-Pagès, C. Molecular trophic markers in marine food webs and their potential use for coral ecology. Mar. Genom. 2016, 29, 1–7. [Google Scholar] [CrossRef]
- Pitt, K.A.; Clement, A.L.; Connolly, R.M.; Thibault-Botha, D. Predation by jellyfish on large and emergent zooplankton: Implications for benthic pelagic coupling. Estuar. Coast. Shelf Sci. 2018, 76, 827–833. [Google Scholar] [CrossRef]
- Rossi, S.; Coppari, M.; Viladrich, N. Benthic-Pelagic Coupling: New Perspectives in the Animal Forests. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 855–886. [Google Scholar]
- Houlbrèque, F. Interactions between zooplankton feeding, photosynthesis and skeletal growth in the scleractinian coral Stylophora pistillata. J. Exp. Biol. 2004, 207, 1461–1469. [Google Scholar] [CrossRef]
- Ying, C.; Ying, W.; Jing, Z.; Na, W. Potential dietary influence on the stable isotopes and fatty acid compositions of jellyfish in the Yellow Sea. J. Mar. Biol. Assoc. U. K. 2012, 92, 1325–1333. [Google Scholar] [CrossRef]
- Brodeur, R.D.; Sugisaki, H.; Hunt, G.L., Jr. Increases in jellyfish biomass in the Bering Sea: Implications for the ecosystem. Mar. Ecol. Prog. Ser. 2002, 233, 89–103. [Google Scholar] [CrossRef]
- Milisenda, G. Ecophysiology, Trophic Ecology, Reproductive Biology and Bioenergetics of Pelagia noctiluca (Forskål, 1775). Ph.D. Thesis, Universitá del Salento, Lecce, Italy, 2014. [Google Scholar]
- Tilves, U.; Fuentes, V.L.; Milisenda, G.; Parrish, C.C.; Vizzini, S.; Sabatés, A. Trophic interactions of the jellyfish Pelagia noctiluca in the NW Mediterranean: Evidence from stable isotope signatures and fatty acid composition). Mar. Ecol. Progr. Ser. 2018, 591, 101–116. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Borge, R.; Ferreira, A.C. Neotropical mangroves: Conservation and sustainable use in a scenario of global climate change. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1347–1364. [Google Scholar] [CrossRef]
- Queiroz, L.; Rossi, S.; Meireles, J.; Coelho, J. Shrimp aquaculture in the state of Ceará during the period 1970–2012: Trends of the privatization of mangrove forest in Brazil. Ocean Coast. Manag. 2013, 73, 54–62. [Google Scholar] [CrossRef]
- Sousa, O.V.; Macrae, A.; Menezes, F.G.R.; Gomes, N.C.M.; Vieira, R.H.S.F.; Mendonça-Hagler, L.C.S. The impact of shrimp farming effluent on bacterial communities in mangrove waters, Ceará, Brazil. Mar. Pollut. Bull. 2006, 52, 1725–1734. [Google Scholar] [CrossRef]
- Thé, J.; Barroso, H.; Mammone, M.; Viana, M.; Melo, C.; Banha, T.; Mies, M.; Morandini, A.C.; Rossi, S.; Soares, M.O. Aquaculture facilities promote population stability throughout seasons and increase medusae size for the invasive jellyfish Cassiopea andromeda. Mar. Environ. Res. 2020, 162, 105161. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Rossi, S.; Martins, F.; Carneiro, P. The forgotten reefs: Benthic assemblage coverage on a sandstone reef (Tropical South-western Atlantic). J. Mar. Biol. Assoc. U. K. 2017, 97, 1585–1592. [Google Scholar] [CrossRef]
- Soares, M.O.; Campos, C.C.; Carneiro, P.B.M.; Barroso, H.S.; Marins, R.V.; Teixeira, C.E.P.; Menezes, M.O.B.; Pinheiro, L.S.; Viana, M.B.; Feitosa, C.V.; et al. The Brazilian semi-arid coast in times of global environmental changes. Perspect. Ecol. Conserv. 2021, 19, 267–278. [Google Scholar] [CrossRef]
- Barroso, H.S.; Tavares, T.C.L.; Soares, M.O.; Garcia, T.M.; Rozendo, B.; Vieira, A.S.C.; Viana, B.; Pontes, T.M.; Ferreira, T.J.; Filho, J.P.; et al. Intra-annual variability of phytoplankton biomass and nutrients in a tropical estuary during a severe drought. Estuar. Coast. 2018, 213, 283–293. [Google Scholar] [CrossRef]
- Slattery, M.; Mcclintock, J.B. Population structure and feeding deterrence in three shallow-water antarctic soft corals. Mar. Biol. 1995, 122, 461–470. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for the determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Rossi, S.; Sabatés, A.; Latasa, M.; Reyes, E. Lipid biomarkers and trophic linkages between phytoplankton, zooplankton and anchovy (Engraulis encrasicolus) larvae in the NW Mediterranean. J. Plankton Res. 2006, 28, 551–562. [Google Scholar] [CrossRef]
- Barnes, H.; Blackstock, J. Estimation of lipids in marine animal tissues: Detailed investigation of the sulphophosphovanillin method for ‘‘total’’ lipids. J. Exp. Mar. Biol. Ecol. 1973, 2, 103–118. [Google Scholar] [CrossRef]
- Rossi, S.; Gili, J.M.; Coma, R.; Linares, C.; Gori, A.; Vert, N. Temporal variation in protein, carbohydrate, and lipid concentrations in Paramuricea clavate (Anthozoa, Octocorallia): Evidence for summer–autumn feeding constraints. Mar. Biol. 2006, 149, 643–651. [Google Scholar] [CrossRef]
- D’Ambra, I.; Carmichael, R.H.; Graham, W.M. Determination of δ13C and δ15N and trophic fractionation in jellyfish: Implications for food web ecology. Mar. Biol. 2014, 161, 473–480. [Google Scholar] [CrossRef]
- Lampert, K.P.; Bürger, P.; Striewski, S.; Tollrian, R. Lack of association between color morphs of the Jellyfish Cassiopea andromeda and zooxanthella clade. Mar. Ecol. 2012, 33, 364–369. [Google Scholar] [CrossRef]
- Mammone, M.; Bosch-Belmar, M.; Milisenda, G.; Castriota, L.; Sinopoli, M.; Allegra, A.; Falautano, M.; Maggio, T.; Rossi, S.; Piraino, S. Reproductive cycle and gonadal output of the Lessepsian jellyfish Cassiopea andromeda in NW Sicily (Central Mediterranean Sea). PLoS ONE 2023, 18, e0281787. [Google Scholar] [CrossRef]
- Stoner, E.W.; Yeager, L.A.; Sweatman, J.L.; Sebilian, S.S.; Layman, C.A. Modification of a seagrass community by benthic jellyfish blooms and nutrient enrichment. J. Exp. Mar. Biol. Ecol. 2014, 461, 185–192. [Google Scholar] [CrossRef]
- Welsh, D.T.; Dunn, R.J.K.; Meziane, T. Oxygen and nutrient dynamics of the upside-down jellyfish (Cassiopea sp.) and its influence on benthic nutrient exchanges and primary production. Hydrobiologia 2009, 635, 351–362. [Google Scholar] [CrossRef]
- Zarnoch, C.B.; Hossain, N.; Fusco, E.; Alldred, M.; Hoellein, T.J.; Perdikaris, S. Size and density of upside-down jellyfish, Cassiopea sp., and their impact on benthic fluxes in a Caribbean lagoon. Mar. Environ. Res. 2020, 154, 104845. [Google Scholar] [CrossRef]
- Todd, B.D.; Thornhill, D.J.; Fitt, W.K. Patterns of inorganic phosphate uptake in Cassiopea xamachana: A bioindicator species. Mar. Pollut. Bull. 2006, 52, 515–521. [Google Scholar] [CrossRef]
- Jantzen, C.; Wild, C.; Rasheed, M.; El-Zibdah, M.; Richter, C. Enhanced pore-water nutrient fluxes by the upside-down jellyfish Cassiopea sp. in a Red Sea coral reef. Mar. Ecol. Progr. Ser. 2010, 411, 117–125. [Google Scholar] [CrossRef]
- Pitt, K.A.; Connolly, R.M.; Meziane, T. Stable isotope and fatty acid tracers in energy and nutrient studies of jellyfish: A review. Hydrobiologia 2009, 616, 119–132. [Google Scholar] [CrossRef]
- Djeghri, N.; Stibor, H.; Lebeau, O.; Pondaven, P. δ13C, δ15N, and C:N ratios as nutrition indicators of zooxanthellate jellyfish: Insights from an experimental approach. J. Exp. Mar. Biol. Ecol. 2020, 522, 151257. [Google Scholar] [CrossRef]
- Phillips, D.L. Converting isotope values to diet composition: The use of mixing models. J. Mammal. 2012, 93, 342–352. [Google Scholar] [CrossRef]
- Giarrizzo, T.; Schwamborn, R.; Saint-Paul, U. Utilization of carbon sources in a northern Brazilian mangrove ecosystem. Estuar. Coast. Shelf Sci. 2011, 95, 447–457. [Google Scholar] [CrossRef]
- Junior, A.P.B.; Flickinger, D.L.; Henry-Silva, G.G. Sedimentation rates of nutrients and particulate material in pond mariculture of shrimp (Litopenaeus vannamei) carried out with different management regimes. Aquaculture 2021, 534, 736307. [Google Scholar] [CrossRef]
- Poersch, L.H.; Bauer, W.; Kersanach, M.W.; Wasielesky, W. Assessment of trace metals, total organic carbon and total nitrogen of a shrimp farm system in Southern Brazil. Reg. Stud. Mar. Sci. 2020, 39, 101452. [Google Scholar] [CrossRef]
- Barcellos, D.; Queiroz, H.M.; Nóbrega, G.N.; Filho, R.L.O.; Santaella, S.T.; Otero, X.L.; Ferreira, T.O. Phosphorus enriched effluents increase eutrophication risks for mangrove systems in northeastern Brazil. Mar. Pollut. Bull. 2019, 142, 58–63. [Google Scholar] [CrossRef]
- Marins, R.V.; Lacerda, L.D.; Araújo, C.S.; Fonseca, L.V.; Silva, F.A.T.F. Phosphorus and suspended matter retention in mangroves affected by shrimp farms effluents in NE Brazil. An. Acad. Bras. Ciênc. 2020, 92, e20200758. [Google Scholar] [CrossRef] [PubMed]
- Tacon, A.G.J.; Jory, D.; Nunes, A. Shrimp feed management: Issues and perspectives. In On-Farm Feeding and Feed Management in Aquaculture; Hasan, M.R., News, M.B., Eds.; FAO Fisheries and Aquaculture Technical Paper: Rome, Italy, 2013; pp. 481–488. [Google Scholar]
- Baker, D.M.; Kim, K.; Andras, J.P.; Sparks, J.P. Light-mediated 15N fractionation in Caribbean gorgonian octocorals: Implications for pollution monitoring. Coral. Reefs 2011, 30, 709–717. [Google Scholar] [CrossRef]
- Heikoop, J.M.; Dunn, J.J.; Risk, M.J.; Sandeman, I.M.; Schwartz, H.P.; Waltho, N. Relationship between light and the S15N of coral tissue: Examples from Jamaica and Zanzibar. Limnol. Oceanog. 1998, 43, 909–920. [Google Scholar] [CrossRef]
| Temperature °C | pH | Salinity | TN (µM) | TP (µM) | |
|---|---|---|---|---|---|
| Shrimp farm | 27.8–31.5 | 7.9–8.4 | 31.6–46.2 | 13–42 | 0.3–1.8 |
| Mangroves | 30.4–34 | 7.9–8.3 | 24.4–46.9 | 42–64 | 1.8–2.8 |
| Analysis | Total N | N Replicates/Treatment | Month and Area | Body Part |
|---|---|---|---|---|
| Stable isotopes | 18 | 3 | J (SF, M); O (SF, M); Ja (SF); A (SF) | U |
| Organic matter | 88 | 5 * | J (SF, M); O (SF, M); Ja (SF); A (SF) | U, OA, G |
| Carbohydrates | 60 | 5 | J (SF, M); O (SF, M); Ja (SF); A (SF) | U, OA |
| Lipids | 88 | 5 * | J (SF, M); O (SF, M); Ja (SF); A (SF) | U, OA, G |
| Fatty acids | 10 | 5 | O (SF, M) | G |
| Location | Month | Umbrella (%) | Oral Arms (%) | Gonads (%) |
|---|---|---|---|---|
| Sf | Jul | 39 ± 2 | 40 ± 3 | 78 ± 7 |
| M | Jul | 39 ± 4 | 48 ± 2 | 63 ± 6 |
| Sf | Oct | 46 ± 13 | 42 ± 7 | 77 ± 1 |
| M | Oct | 52 ± 15 | 37 ± 7 | 79 ± 4 |
| Sf | Jan | 28 ± 2 | 28 ± 5 | 79 ± 3 |
| M | Jan | absent | absent | absent |
| Sf | Apr | 41 ± 4 | 44 ± 7 | 69 ± 1 |
| M | Apr | absent | absent | absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thé, J.; Mammone, M.; Piraino, S.; Pennetta, A.; De Benedetto, G.E.; Garcia, T.M.; de Oliveira Soares, M.; Rossi, S. Understanding Cassiopea andromeda (Scyphozoa) Invasiveness in Different Habitats: A Multiple Biomarker Comparison. Water 2023, 15, 2599. https://doi.org/10.3390/w15142599
Thé J, Mammone M, Piraino S, Pennetta A, De Benedetto GE, Garcia TM, de Oliveira Soares M, Rossi S. Understanding Cassiopea andromeda (Scyphozoa) Invasiveness in Different Habitats: A Multiple Biomarker Comparison. Water. 2023; 15(14):2599. https://doi.org/10.3390/w15142599
Chicago/Turabian StyleThé, Jorge, Marta Mammone, Stefano Piraino, Antonio Pennetta, Giuseppe Egidio De Benedetto, Tatiane Martins Garcia, Marcelo de Oliveira Soares, and Sergio Rossi. 2023. "Understanding Cassiopea andromeda (Scyphozoa) Invasiveness in Different Habitats: A Multiple Biomarker Comparison" Water 15, no. 14: 2599. https://doi.org/10.3390/w15142599
APA StyleThé, J., Mammone, M., Piraino, S., Pennetta, A., De Benedetto, G. E., Garcia, T. M., de Oliveira Soares, M., & Rossi, S. (2023). Understanding Cassiopea andromeda (Scyphozoa) Invasiveness in Different Habitats: A Multiple Biomarker Comparison. Water, 15(14), 2599. https://doi.org/10.3390/w15142599








