Abstract
Due to globalisation and anthropopressure (intensification of shipping, creation of water corridors connecting seas, cultivation of commercial species), the movement of aquatic species has increased in recent years. The determination of trends in the movement of aquatic species in their geographical distribution over time is important because it may help in the management of a species in aquatic ecosystems. There are also knowledge gaps on the long-term trends in the movements of Southern European aquatic alien invertebrates. The study provides the first evidence of both northward and southward movements of these species based on available observations from 1940 to 2021, using meta-analyses and GAM modelling. To date, the majority (98%) of analysed Southern European aquatic alien invertebrates of Mediterranean and Ponto-Caspian origin have moved to the north. Among them, 61% are Ponto-Caspian aquatic alien invertebrates that moved only to the north, and 4% are Mediterranean aquatic alien invertebrates that moved only to the north; the rest include species that moved to the north and south: 27% are Ponto-Caspian aquatic alien invertebrates, and 6% are Mediterranean aquatic alien invertebrates. The one-way movement to the south was observed only in 2% of Mediterranean aquatic alien species. The study will help in understanding the movement patterns of Southern European aquatic alien invertebrates and in the effective management of aquatic ecosystems that allow for the co-existence of people and the rest of biodiversity.
1. Introduction
There is often a knowledge gap about where and why species move. This knowledge is important to understand species distribution patterns [1,2]. Animals usually move to find more favourable conditions [3,4]. Many drivers affect the movement of organisms, e.g., climate change, disturbances in the natural habitats of organisms, etc. [5]. Among these drivers, shipping is considered the largest vector for the movement of aquatic species across the globe [6]. In the XVIIIth and XIXth centuries, important new waterways were opened. Numerous canals connecting the Mediterranean and the Ponto-Caspian areas with other parts of Europe were created as a result of industrial and economic human activity. Man-made interconnections of river basins (water corridors) have caused the movement of many aquatic species in Europe [7,8]. Thanks to these connections, the movement of, e.g., Dreissena polymorpha (Pallas, 1771) has started with climate suitability and the ability of species to successfully establish themselves [9], influencing the distribution of species [10].
It is common for species to move into cooler areas to escape warming [11,12]. Many studies have shown the northward movement of organisms, e.g., [13,14,15]. However, northward movement is usually considered in the context of native species, e.g., [16], despite the fact that human-mediated movement of species to the north has also been documented, e.g., [17,18,19,20,21]. Global warming is increasing, and it is estimated that by the end of the century, the average temperature on Earth will rise by 2.7 °C [22]. Temperature is a major factor in determining the geographical distribution of species [23,24,25,26,27,28]. Globalisation facilitates the movement of species. But are alien species moving only northward in a changing world? Is there only one trend in movement?
The movement of species may have unpredictable consequences for ecosystems, and it is not possible to predict whether changes in the distribution will have a positive or negative effect. It is very important in species conservation and management to incorporate species movements into management objectives. This knowledge can be used to develop management strategies that may improve the effectiveness of management actions [2]. Animal movement is a core component of an ecosystem and may be vital for sustaining ecosystem processes such as trophic and species interactions [29,30,31]. Aquatic invertebrates constitute a significant diet source for many fish and water birds [32], so changes in their distribution may have important consequences for consumers. Some of these species are invasive (e.g., Dikerogammarus villosus (Sowinsky, 1894)) [33,34], so their possible presence and coexistence with other species [35], as well as a possible replacement of natives and/or threat to humans [36,37], may be important information for management actions.
However, there are knowledge gaps in documented evidence of long-term changes in the distribution of Southern European aquatic alien invertebrate species based on available observations, so the aim of this study is to analyse movement in the geographical distribution of these species.
The study addresses the gaps in knowledge by:
- (1)
- analyses of long-term trends in the movement of Mediterranean and Ponto-Caspian aquatic alien invertebrate species;
- (2)
- discussion on the responses of Southern European aquatic alien invertebrate species to changing conditions and management implications.
2. Materials and Methods
The list of all aquatic invertebrate species from the database GRIIS (Global Register of Introduced and Invasive Species) [38] with habitat and country of occurrence was downloaded. The geographical origin of aquatic invertebrate taxa was searched in the literature and in the databases AquaNIS (Information System On Aquatic Non-indigenous and Cryptogenic Species) [39] and GBIF (Global Biodiversity Information Facility) [40]. All Southern European aquatic alien invertebrate species (those living in freshwater, less than 0.5 ppt; brackish, 0.5–30 ppt; and marine waters, greater than 30 ppt) [41] of Mediterranean and Ponto-Caspian origin were selected.
Later, the occurrence records of Mediterranean and Ponto-Caspian aquatic alien invertebrate species were collected from GBIF [40]. Spatial records from GBIF are commonly used in decision-making processes and large-scale biogeography research [42].
Thus, the available occurrence data from 1940 to 2021, depending on research/monitoring/reporting efforts, were processed. The trends of movement over time were determined using the maximum latitude in a year (northern extent) and/or the minimum latitude in a year (southern extent) (among all records of distributions in the GBIF database) of Mediterranean and Ponto-Caspian aquatic alien invertebrate species. Using occurrence data (degrees), a generalised additive model (GAM) approach was used to determine changes in the maximum/minimum latitude of occurrence of a species in the analysed years for evidence of species movement. The GAM models provide a useful tool to visualise the nature of the relationship between changes in the geographical distribution of a species over time. Latitudinal movements of the northern and southern limits were noticed (for species that moved north and south). For changes in geographical occurrences of particular species over time, the gamma distribution with log link function and categorisation was used. Estimates of movement (if sufficient data were available) were prepared due to the described methodology [43,44]. This GAM-based analysis was prepared using the Statistica 10 version. The shapes of the movements for the species were plotted. Additionally, the direction of species movement (northward and/or southward) was indicated based on information based on country of occurrence from GRIIS (Global Register of Introduced and Invasive Species) [38].
3. Results
3.1. General Results on the Movement of Southern European Aquatic Alien Invertebrate Species
The geographical occurrences of Southern European aquatic alien invertebrate species were analysed, and directions of movement were determined (Figure 1; Table 1, Table 2, Table 3 and Table 4), depending on available data and research/monitoring/reporting efforts. Despite the fact that geographical distribution data are incomplete in GBIF, changes in the geographical distribution of Southern European aquatic alien invertebrate species over time were observed (Figure 2, Figure 3 and Figure 4). To date, we have observed that the majority (98%) of the analysed Southern European aquatic alien invertebrate species (Mediterranean and Ponto-Caspian aquatic alien invertebrates) moved to the north. Among them, 61% are Ponto-Caspian aquatic invertebrates that moved only to the north and 4% are Mediterranean aquatic alien invertebrates that moved only to the north; the rest include 27% of Ponto-Caspian aquatic alien invertebrates that moved to the north and south and 6% of Mediterranean aquatic alien invertebrates that moved to the north and south. The one-way movement into the south (into warmer areas) was observed only in the case of 2% of species from Southern Europe (only Mediterranean aquatic alien invertebrates).
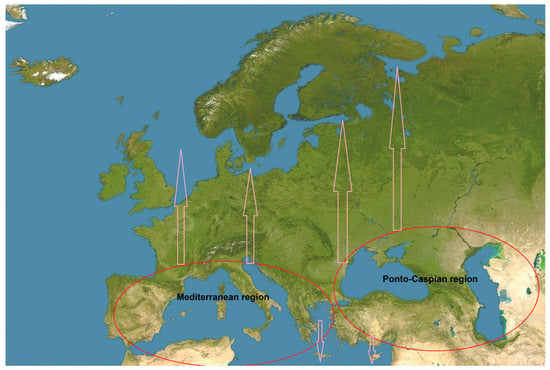
Figure 1.
Location of the Mediterranean and Ponto-Caspian regions and directions of movements of species.

Table 1.
Mediterranean aquatic alien invertebrate species that are characterised by movement, based on GBIF (Global Biodiversity Information Facility) [38] and GRIIS (Global Register of Introduced and Invasive Species) [40].

Table 2.
Ponto-Caspian aquatic alien invertebrate species that are characterised by movement, based on GBIF (Global Biodiversity Information Facility) [38] and GRIIS (Global Register of Introduced and Invasive Species) [40].

Table 3.
Statistics of GAMs of Mediterranean aquatic alien invertebrate species movement, including significance levels (p-values) and degrees of freedom (d.f.) only for species with significant differences at the 0.05 level.

Table 4.
Statistics of GAMs of Ponto-Caspian aquatic alien invertebrate species movement, including significance levels (p-value) and degrees of freedom (d.f.) only for species with significant differences at the 0.05 level.
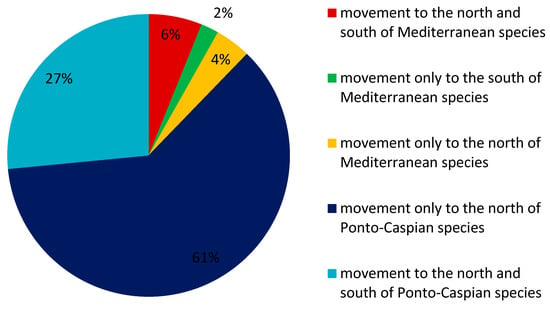
Figure 2.
Movement of Southern European aquatic alien invertebrate species to the north and south (based on data from GBIF (Global Biodiversity Information Facility) [38] and GRIIS (Global Register of Introduced and Invasive Species) [40].
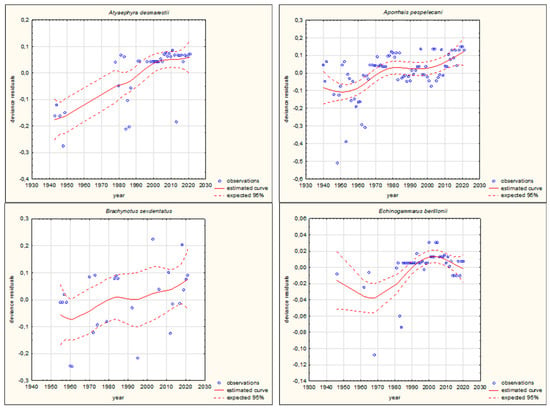
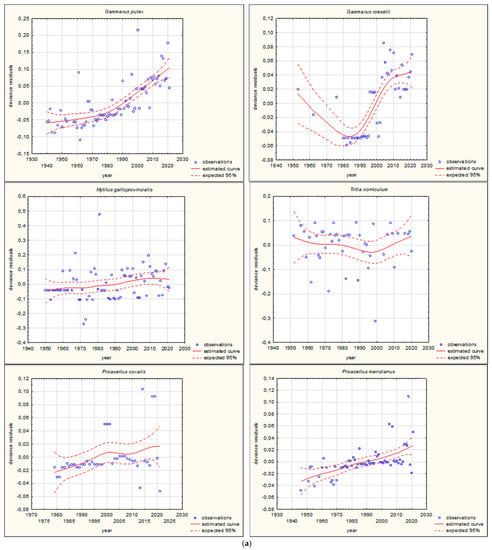
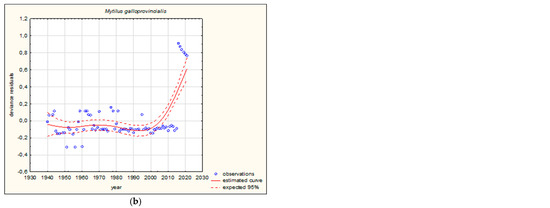
Figure 3.
GAM models presenting curves of movement over time of Mediterranean aquatic alien invertebrates, based on GBIF (Global Biodiversity Information Facility) [38]. (a) Northward movement; (b) Southward movement.
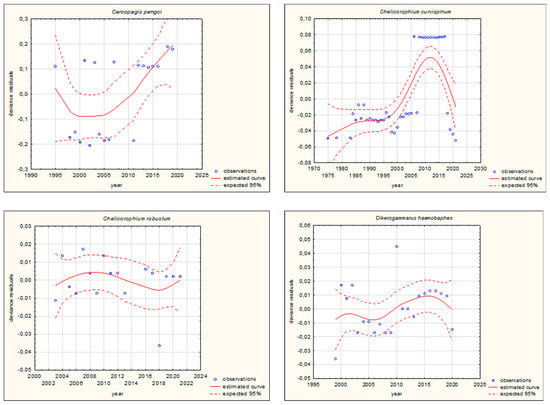
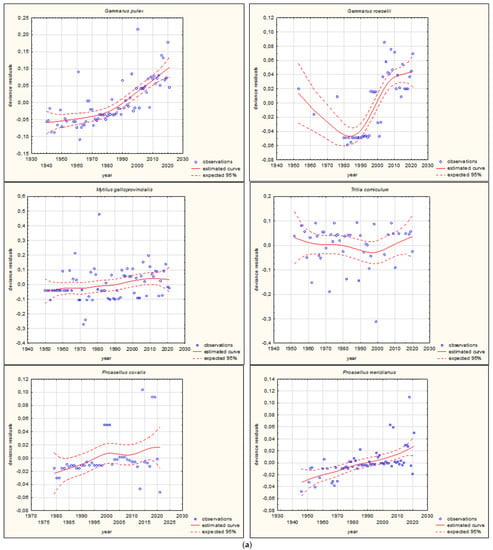
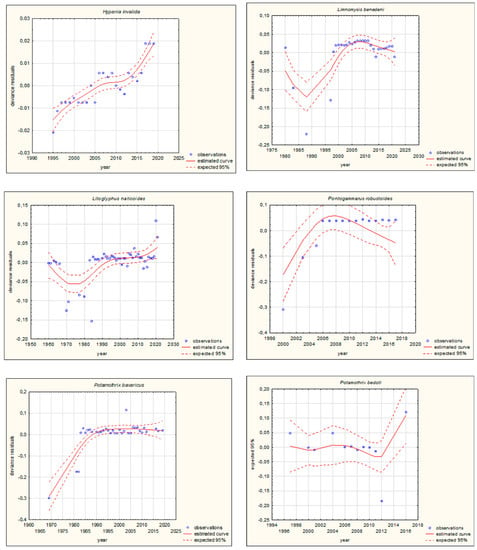
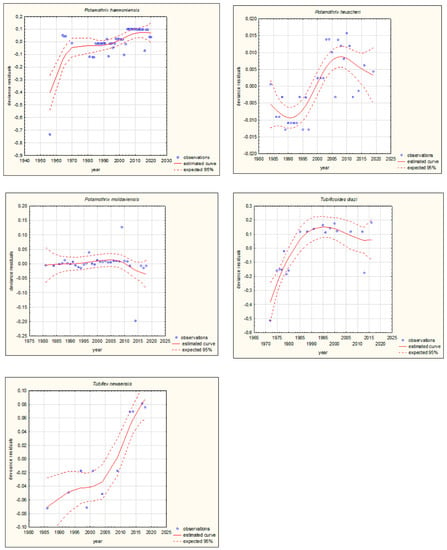
Figure 4.
GAM models presenting curves of movement over time of Ponto-Caspian aquatic alien invertebrates, based on GBIF (Global Biodiversity Information Facility) [38]. Northward movement.
3.2. Long-Term Trends in the Movement of Southern European Aquatic Alien Invertebrate Species
The long-term tendency in the movement of Southern European aquatic alien invertebrate species to higher northern latitudes (observed in the majority—in 98% of species) shows that these species generally moved to cooler areas. But in the case of some species, movement to the south occurred. However, the number of geographical coordinates confirming the movement of species to the south was not sufficient for the preparation of the plot in GAM.
The shapes of the trends of movements over time in GAMs for each species (if sufficient data were available) are illustrated in Figure 3 and Figure 4. Based on the changes in the maximum/minimum latitude over time, the movement of the analysed species to the north, towards higher latitudes, and/or to the south, towards lower latitudes, was confirmed. Interestingly, in the case of some Southern European alien aquatic invertebrates, only one-way movement to the south was recorded, but only in the case of the Mediterranean species Mytilus galloprovincialis was GAM analysis possible. When the deviance residuals were plotted against time (years), clear patterns were verified (Figure 3 and Figure 4). The deviance residuals in the presented models show how well the movement of species is confirmed by the models and present a discrepancy between the observations and the estimated curves. The GAMs confirmed that movement of Southern European aquatic alien species occurs over time (Figure 3 and Figure 4) and indicates that species display non-linear changes in distribution over time. However, a GAM analysis of a multiyear dataset might reveal that the movement in many cases is very low but in others is relatively high. Comparing the shapes of the plots (Figure 3 and Figure 4), the movement patterns of species differ among various species depending on their origin. In the case of Mediterranean aquatic alien species, the shapes are more linear, but in the case of Ponto-Caspian aquatic alien species, they are usually more irregular.
4. Discussion
4.1. Movement of Southern European Aquatic Alien Invertebrates
The study provides the first evidence of long-term movements in the distribution of Southern European aquatic alien invertebrate species using meta-analysis and GAM-based modelling. The study demonstrates that GAM-based modelling could be used to create non-linear, spatial changes in the distribution of species over time. Moreover, GAM-based analyses better illustrate movement patterns than linear models, often used for confirmation of distribution trends [45]. The movement of many Southern European aquatic alien species was confirmed based on available data; for all the rest, there is a knowledge gap. The obtained results were based on the analysed period (from the 1940s to the 2000s) and depended on differences in research/monitoring/reporting efforts. Individual species’ movements vary in their rates of change. Perhaps different shapes of movement could identify different responses of species towards environmental conditions, e.g., temperature, salinity, habitat degradation, etc., as a result of different introduction pathways.
The rate of settling of aquatic alien invertebrate species is influenced by a combination of morphological, behavioural, and ecological features [17,46]. A comprehensive understanding of these traits and interactions is crucial for predicting and managing the spread of aquatic alien species. Morphological adaptations can play a crucial role in the settlement success of alien aquatic invertebrates. Features such as body shape, size, and dispersal mechanisms can affect their ability to colonise new environments. For example, species with efficient dispersal structures such as specialised appendages or buoyancy adaptations may have a higher settlement rate compared to those lacking such traits [17,47]. Behavioural traits of aquatic alien invertebrates can significantly influence their ability to settle in new habitats. For instance, the ability to respond to a wide range of environmental conditions, different resource availability, and competitive interactions with native species can impact the success of settlement [19,36]. Behavioural traits related to, e.g., feeding habit, capacity for behavioural thermoregulation, and aggression can influence the establishment and spread of alien species [17,33,48]. Physiological traits, including quiescence and dormancy (or diapause), which help to overcome adverse conditions, as well as tolerance of a wide range of temperatures, euryeocity, and a high reproduction rate, are important in the settlement success of alien species [17]. Understanding the ecological context in which aquatic alien invertebrates settle is also essential. The availability of suitable resources, including food, shelter, and reproductive sites, can greatly influence their establishment. Furthermore, interactions with native species, both competitive and facilitative, can play a pivotal role in determining the settlement rate of alien species [17,49]. Several case studies provide valuable insights into the relationship between morphological, behavioural, physiological, and ecological features and the settlement rate of alien aquatic invertebrate species. By examining specific examples, such as, e.g., the zebra mussels (D. polymorpha) and the killer shrimp (D. villosus), we can observe how their unique characteristics contribute to the rapid colonisation of new habitats [19,50,51].
By identifying the key morphological, behavioural, physiological, and ecological traits associated with the successful settlement of aquatic alien species, we can enhance early detection and implement targeted control measures to prevent or mitigate their negative impacts on native ecosystems [52].
Moreover, we should understand that aquatic alien invertebrates from Southern Europe gained the opportunity for movement through different pathways. As a consequence of shipping and the creation of navigable canals and waterways enabling connections of the Mediterranean and the Ponto-Caspian regions with other parts of Europe, their spread to the north was possible [7,8], as was movement to the south [53]. The northward movement was confirmed in this study by the majority of Southern non-indigenous aquatic species. This is in line with previous observations: Ponto-Caspianization of central and western European waterways [54]. Interestingly, some Southern European aquatic invertebrates moved to the south, into lower latitudes, where they most probably evolved elevated upper thermal limits relative to the species in northern latitudes, facilitating their establishment in warm water bodies [55] (Figure 2; Table 1, Table 2, Table 3 and Table 4). Generally, it is well known that temperature variability imposes intensified peak stress [56]. However, detailed knowledge of which individuals and species are most likely to survive and why under upper thermal limits is poor, indicating that smaller individuals survived to higher temperatures than large animals and active species survived to higher temperatures than sessile or low-active species when temperatures were raised acutely [57]. Ecological generalists, with higher heat tolerances, are competitive at more extreme and increasing temperatures [58].
A major problem is that changes in the extent and impacts of invasions are occurring with the accumulation of impacts and through synergisms with other components of global change [59].
4.2. Responses of Southern European Aquatic Alien Invertebrates to Changing Conditions
The analysed species were able to move outside their original ranges of distribution. Most likely, increases in regional sea temperatures have triggered a major northward movement of species. Projected climate change in the Mediterranean and Ponto-Caspian areas with higher temperatures and increased periodic drought can be expected to further increase the instability of habitats [60,61,62]. Climate warming will accelerate the movement process and favour species movement from southern to northern latitudes in Europe [62] in search of more favourable thermal conditions compared with those existing in the original areas.
Southern aquatic species may have only a northern direction of movement if climate change is the only reason affecting movement. However, the latest studies report that another reason for species’ movements is most likely the destruction of habitats they previously inhabited. In fact, biodiversity loss in the Mediterranean and Ponto-Caspian regions may be caused by habitat degradation, coastal infrastructural development, and damming of rivers, so biodiversity here is under threat [62,63]. Habitat modifications disturbed previous natural salinity gradients and settings in the Ponto-Caspian area [61]. In many places in the Ponto-Caspian area, native species have been replaced by invasive species, e.g., Mytilopsis leucophaeata (Conrad, 1831), Potamopyrgus antipodarum (Gray, 1843), Rhithropanopeus harrisii (Gould, 1841), and other euryhaline species [62]. Similarly, the Mediterranean region is regarded as a hot spot for invasive alien species that tend to decline native species [64].
4.3. Management Implications
The results of this study may suggest several recommendations for management actions in areas of the introduction of Southern European aquatic invertebrate species. The Convention on Biological Diversity (CBD) prioritises preventing the introduction of alien invasive species and thereby avoiding adverse impacts. Firstly, on-going monitoring activities are recommended to record new species. Many Mediterranean and Ponto-Caspian aquatic invertebrates are easily adaptable to novel environments, e.g., [17,65]. Unfortunately, these species are generalists [65,66,67,68]—they utilise different food sources and have relatively wide thermal tolerance [69,70]. Some of these are tending to decline, compete, and displace native species from their habitats [71,72,73] and to re-engineer the new ecosystems [74]. Further changes in the distribution of Southern European aquatic alien invertebrate species are expected due to climate change and the degradation of habitats in their native areas. Once these species become established, management is difficult and economically costly [75]. It aims for species eradication, complete reproductive removal, containment, and/or population suppression [76]. But new invaders are nearly impossible to fully eradicate [77], so early identification of aquatic alien invaders in new areas should be prioritised because species management is not working well in the marine realm.
Pathogens and parasites are the next aspects connected with species movement. Associated life, e.g., bacteria, viruses, fungi, and other organisms, as well as different epibionts and endobionts, may be consequences of alien species introduction because alien species may carry new organisms [78,79], with unpredictable consequences for humans and other biota.
Therefore, understanding the movement of Southern European aquatic alien invertebrate species enables managers to identify threats and prioritise management actions. An important way to reduce introductions is to manage vectors and pathways [80]. As shipping is the primary pathway for the introduction of aquatic organisms, mainly invertebrates [81,82], it needs such management actions as, e.g., hull cleaning, antifouling, and ballast water exchange [83]. Such actions have the potential to reduce the establishment and spread of aquatic alien species. But not always mentioned interventions are sufficient. Dispersal corridors (water corridors among marine basins) are also considered introduction pathways and require responsibility [84,85,86,87]. Another pathway of introduction of aquatic alien species is cultivation, and some invertebrates, e.g., the commercial species of the mussel Mytilus galloprovincialis (Lamarck, 1819), are introduced in this way. The potential impact of the species once it is established in the new aquatic ecosystem is uncertain because it can outcompete native mussels and because of its high reproductive potential and adaptability to different environments [87]. Prevention measures for such species should include prohibiting cultivation in foreign areas and monitoring unintentional introductions.
Effective biological management demands complex multisectoral and multinational collaboration, and much work remains to be carried out. Success in such ventures holds the key to reducing the influx of alien species.
On the other hand, climate change and habitat degradation facilitate alien species movement [56,88] and should be limited.
5. Conclusions
Prevalent long-term trends in the movement of Southern European aquatic alien invertebrates to the north were confirmed. The movement of Southern European aquatic alien invertebrates to the south was also observed, but not on a sufficiently large scale. Maybe in the future, if the observed trend is continued, Southern European alien species will be common not only in the Northern Hemisphere [21], but also in the Southern one. Due to the potentially adverse impact of many aquatic invasive alien species moving to the north and south, understanding changes in the geographical distribution of species has relevance to management efforts. Understanding the morphological, behavioural, physiological and ecological features associated with the settlement rate of aquatic alien species is vital for developing effective management strategies. Strong human impacts, including shipping, intensification of use of waterways, cultivation, climate change, and habitat degradation, should be limited in the future. For sustainable use of aquatic ecosystems, preventing actions (e.g., hull cleaning, antifouling, ballast water exchange) should be prioritised.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Jeltsch, F.; Bonte, D.; Pe’Er, G.; Reineking, B.; Leimgruber, P.; Balkenhol, N.; Schröder, B.; Buchmann, C.M.; Mueller, T.; Blaum, N.; et al. Integrating movement ecology with biodiversity research—Exploring new avenues to address spatiotemporal biodiversity dynamics. Mov. Ecol. 2013, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Singh, N.J. Linking movement ecology with wildlife management and conservation. Front. Ecol. Evol. 2016, 12, 155. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, S.; Connors, C.; Péan, C.; Berger, N.; Caud, Y.; Chen, L.; Goldfarb, M.I.; Gomis, M.I.; et al. Climate Change 2021; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; p. 3949. [Google Scholar]
- Nebel, S. Animal migration. Nat. Educ. Knowl. 2010, 3, 77. [Google Scholar]
- Schifting habitats. Nat. Clim. Chang. 2020, 10, 377. [CrossRef]
- Carlton, J.T.; Geller, J.B. Ecological roulette: The global transport of nonindigenous marine organisms. Science 1993, 261, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Bij de Vaate, A.; Jażdżewski, K.; Ketelaars, H.A.M.; Gollasch, S.; Van der Velde, G. Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Can. J. Fish. Aquat. Sci. 2002, 59, 1159–1174. [Google Scholar] [CrossRef]
- Galil, B.; Nehring, S.; Panov, V. Waterways as invasion highways—Impact of climate change and globalization. Biol. Invasions 2007, 193, 59–74. [Google Scholar]
- Kinzelbach, R. The main features of the phylogeny and dispersal of the zebra mussel Dreissena polymorpha. In The Zebra Mussel Dreissena Polymorpha. Ecology, Biological Monitoring and First Application in Water Quality Management; Neumann, D., Jenner, H.A., Eds.; Limnologie Aktuell, Fischer Verlag: Stuttgart, Germany, 1992; Volume 4, pp. 5–17. [Google Scholar]
- Veldkornet, D.A.; Rajkaran, A. Predicting shifts in the geographical distribution of two estuarine plant species from the subtropical and temperate regions of South Africa. Wetlands 2019, 39, 1179–1188. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Thomspon, F.R. Poleward shifts in winter ranges of North American bird. Ecology 2007, 88, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Spies, I.; Gruenthal, K.M.; Drinan, D.P.; Hollowed, A.B.; Stevenson, D.E.; Tarpey, C.M.; Hauser, L. Genetic evidence of a northward range expansion in the eastern Bering Sea stock of Pacific cod. Evol. Appl. 2020, 13, 362–375. [Google Scholar] [CrossRef]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Osland, M.J.; Stevens, P.W.; Lamont, M.M.; Brusca, R.C.; Hart, K.M.; Waddle, J.H.; Langtimm, C.A.; Williams, C.M.; Keim, B.D.; Terando, A.J.; et al. Tropicalization of temperate ecosystems in North America: The northward range expansion of tropical organisms in response to warming temperatures. Glob. Chang. Biol. 2021, 27, 3009–3034. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, J.A.; Wang, Y.J.; Stoks, R. Evolution of cold tolerance and thermal plasticity in life history, behaviour and physiology during a poleward range expansion. J. Anim. Ecol. 2021, 90, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C. Climate-tracking species are not invasive. Nat. Clim. Chang. 2020, 10, 382–384. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Kemp, J.L.; Fidalgo, M.L. Cold-tolerant traits that favour northwards movement and establishment of Mediterranean and Ponto-Caspian alien aquatic invertebrates. Aquat. Sci. 2022, 84, 47. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Tarała, A.; Chabowska, A. Expansion of alien gammarids in the Vistula Lagoon and the Vistula Delta (Poland). Environ. Monit. Assess. 2013, 185, 5165–5175. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Melzer, M.; Majkowski, W. Range extension of Dikerogammarus villosus (Sowinsky, 1894) in Poland (the Baltic Sea basin) and its ability to osmoregulate in different environmental salinities. Oceanol. Hydrobiol. Stud. 2015, 44, 294–304. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Medina-Villar, S. Alien species of Mediterranean origin in the Baltic Sea region—Current state and risk assessment. Environ. Rev. 2020, 28, 339–356. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Stepien, C.; Nuc, Z. Neocosmopolitan distributions of invertebrate aquatic invasive species due to euryhaline geographic history and human-mediated dispersal: Ponto-Caspian versus other geographic origins. Ecol. Process. 2023, 12, 2. [Google Scholar] [CrossRef]
- UNEP. Emissions Gap Report. The Heat Is On—A World of Climate Promises Not Yet Delivered; UNEP: Nairobi, Kenya, 2021; 112p, ISBN 978-92-807-3890-2. Available online: https://www.unep.org/resources/emissions-gap-report-2021 (accessed on 5 March 2023).
- Sunday, J.M.; Bates, A.E.; Duty, N.K. Thermal tolerance and the global redistribution of animals. Nat. Clim. Chang. 2012, 2, 686–690. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Sciences 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Lenoir, J.; Bonebroke, T.C. Land-use change interacts with climate to determine elevational species redistribution. Nat. Commun. 2018, 9, 1. [Google Scholar] [CrossRef]
- Morley, J.W.; Selden, R.L.; Latour, R.J.; Frölicher, T.L.; Seagraves, R.J.; Pinsky, M.L. Projecting shifts in thermal habitat for 686 species on the North American continental shelf. PLoS ONE 2018, 13, e0196127. [Google Scholar] [CrossRef] [PubMed]
- Vieira, K.S.; Montenegro, P.F.G.; Santana, G.C.; Vieira, W.L.D.S. Effect of climate change on distribution of species of common horned frogs in South America. PLoS ONE 2018, 13, e0202813. [Google Scholar] [CrossRef] [PubMed]
- Boisvert-Marsh, L.; Blois, S. Unravelling potential northward migration pathways for tree species under climate change. J. Biogeogr. 2021, 48, 1088–1100. [Google Scholar] [CrossRef]
- Lundberg, J.; Moberg, F. Mobile link organisms and ecosystem functioning: Implications for ecosystem resilience and management. Ecosystems 2003, 6, 0087–0098. [Google Scholar] [CrossRef]
- Massol, F.; Gravel, D.; Mouquet, N.; Cadotte, M.W.; Fukami, T.; Leibold, M.A. Linking community and ecosystem dynamics through spatial ecology. Ecol. Lett. 2011, 14, 313–323. [Google Scholar] [CrossRef]
- Verstijnen, Y.J.M.; Lucassen, E.C.H.E.T.; van der Gaag, M.; Wagenvoort, A.J.; Castelijns, H.; Ketelaars, H.A.M.; van der Velde, G.; Smolders, A.J.P. Trophic relationships in Dutch reservoirs recently invaded by Ponto-Caspian species: Insights from fish trends and stable isotope analysis. Aquat. Invasions 2019, 14, 280–298. [Google Scholar] [CrossRef]
- Casties, I.; Clemmesen, C.; Briski, E. Environmental tolerance of three gammarid species with and without invasion record under current and future global warming scenarios. Divers. Distrib. 2018, 25, 603–612. [Google Scholar] [CrossRef]
- Dick, J.T.A.; Platvoet, D. Intraguild predation and species exclusion in amphipods: The interaction of behaviour, physiology and environment. Freshw. Biol. 1996, 36, 375–383. [Google Scholar] [CrossRef]
- Dick, J.T.A.; Platvoet, D. Invading predatory crustacean Dikerogammarus villosus eliminates both native and exotic species. Proc. R. Soc. B 2000, 267, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Borza, P.; Huber, T.; Leitner, P.; Remund, N.; Graf, W. How to coexist with the ‘killer shrimp’ Dikerogammarus villosus? Lessons from other invasive Ponto-Caspian peracarids. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 6. [Google Scholar] [CrossRef]
- Surowiec, J.; Dobrzycka-Krahel, A. New data on the non-indigenous gammarids in the Vistula Delta and the Vistula Lagoon. Oceanologia 2008, 50, 443–447. [Google Scholar]
- MacNeil, C.; Boets, P.; Lock, K.; Goethals, M. Potential effects of the invasive ‘killer shrimp’ (Dikerogammarus villosus) on macroinvertebrate assemblages and biomonitoring indices. Freshw. Biol. 2013, 58, 171–182. [Google Scholar] [CrossRef]
- GRIIS. Global Register of Introduced and Invasive Species. Available online: http://www.griis.org (accessed on 1 October 2021).
- AquaNIS. Information System on Aquatic Non-Indigenous and Cryptogenic Species Database. Available online: http://www.corpi.ku.lt/databases/index.php/aquanis (accessed on 4 March 2023).
- GBIF. Global Biodiversity Information Facility. Available online: https://www.gbif.org (accessed on 4 March 2023).
- Venice System. The Venice System for the Classification of Marine Waters According to Salinity. Limnol. Oceanogr. 1958, 3, 245–352. [Google Scholar]
- Luo, M.; Xu, Z.; Hirsch, T.; Sin Aung, T.; Xu, W.; Ji, L.; Qin, H.; Ma, K. The use of Global Biodiversity Information Facility (GBIF)-mediated data in publications written in Chinese. Glob. Ecol. Conserv. 2021, 25, e01406. [Google Scholar] [CrossRef]
- Pedersen, E.J.; Miller, D.L.; Simpson, G.L.; Ross, N. Hierarchical generalized additive models in ecology: An introduction with mgcv. PeerJ 2019, 7, e6876. [Google Scholar] [CrossRef]
- Murase, H.; Nagashima, H.; Yonezaki, S.; Matsukura, R.; Kitakado, T. Application of a generalized additive model (GAM) to reveal relationships between environmental factors and distributions of pelagic fish and krill: A case study in Sendai Bay, Japan. ICES J. Mar. Sci. 2009, 66, 1417–1424. [Google Scholar] [CrossRef]
- van Strien, A.J.; van Swaay, C.A.M.; Termaat, T. Opportunistic citizen science data of animal species produce reliable estimates of distribution trends if analysed with occupancy models. J. Appl. Ecol. 2013, 50, 1450–1458. [Google Scholar] [CrossRef]
- Ricciardi, A. Chapter 5—Ecology of Invasive Alien Invertebrates. In Thorp and Covich’s Freshwater Invertebrates, 4th Ed; Thorp, J., Rogers, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 83–91. ISBN 9780123850263. [Google Scholar] [CrossRef]
- Quell, F.; Schratzberger, M.; Beauchard, O.; Bruggeman, J.; Webb, T. Biological trait profiles discriminate between native and non-indigenous marine invertebrates. Aquat. Invasions 2021, 16, 571–600. [Google Scholar] [CrossRef]
- Truhlar, A.M.; Aldridge, D.C. Differences in behavioural traits between two potentially invasive amphipods. Dikerogammarus Villosus Gammarus Pulex. Biol. Invasions 2015, 17, 1569–1579. [Google Scholar] [CrossRef]
- Ricciardi, A.; Rasmussen, J.B. Predicting the identity and impact of future biological invaders: A priority for aquatic resource management. Can. J. Fish. Aquat. Sci. 1998, 55, 1759–1765. [Google Scholar] [CrossRef]
- Tricarico, E.; Junqueira, A.O.R.; Dudgeon, D. Alien species in aquatic environments: A selective comparison of coastal and inland waters in tropical and temperate latitudes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 872–891. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Tarała, A.; Majkowski, W. Size structure and body condition of Ponto-Caspian gammarids in the Vistula estuary (Poland). Oceanol. Hydrobiol. Stud. 2019, 48, 23–30. [Google Scholar] [CrossRef]
- Martini, S.; Larras, F.; Boyé, A.; Faure, E.; Aberle, N.; Archambault, P.; Bacouillard, L.; Beisner, B.E.; Bittner, L.; Castella, E.; et al. Functional trait-based approaches as a common framework for aquatic ecologists. Limnol. Oceanogr. 2021, 66, 965–994. [Google Scholar] [CrossRef]
- Crocetta, F.; Bitar, G.; Zibrowius, H.; Oliverio, M. Increase in knowledge of the marine gastropod fauna of Lebanon since the 19th century. Bull. Mar. Sci. 2020, 96, 1–22. [Google Scholar] [CrossRef]
- Soto, I.; Cuthbert, R.N.; Ricciardi, A.; Ahmed, D.; Altermatt, F.; Schäfer, R.; Archambaud-Suard, G.; Bonada, N.; Canedo-Arguelles, M.; Csabai, Z.; et al. The faunal Ponto-Caspianization of central and western European waterways. Biol. Invasions 2023, 25, 2613–2629. [Google Scholar] [CrossRef]
- Locklin, J.L.; Corbitt, D.N.; McMahon, R.F. Settlement, density, survival and shell growth of zebra mussels, Dreissena polymorpha, in a recently invaded low latitude, warm water Texas reservoir. Aquat. Invasions 2020, 15, 408–434. [Google Scholar] [CrossRef]
- Lugo, S.C.M.; Baumeister, M.; Nour, O.M.; Wolf, F.; Stumpp, M.; Pansch, C. Warming and temperature variability determine the performance of two invertebrate predators. Sci. Rep. 2020, 10, 6780. [Google Scholar] [CrossRef]
- Peck, L.S.; Clark, M.S.; Morley, S.A.; Massey, A.; Rossetti, H. Animal temperature limits and ecological relevance: Effects of size, activity and rates of change. Funct. Ecol. 2009, 23, 248–256. [Google Scholar] [CrossRef]
- Bates, A.E.; McKelvie, C.M.; Sorte, C.J.B.; Morley, S.A.; Jones, N.A.R.; Mondon, J.A.; Bird, T.J.; Quinn, G. Geographical range, heat tolerance and invasion success in aquatic species. Proc. R. Soc. B 2013, 280, 20131958. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Velde, S.V.D.; Jorissen, E.L.; Neubauer, T.A.; Radan, S.; Pavel, A.B.; Stoica, M.; Van Baak, C.G.; Martínez Gándara, A.; Popa, L.; Stigter, H.D. A conservation palaeobiological approach to assess faunal response of threatened biota under natural and anthropogenic environmental change. Biogeosciences 2019, 16, 2423–2442. [Google Scholar] [CrossRef]
- Gogaladze, A.; Son, M.O.; Lattuada, M.; Anistratenko, V.V.; Syomin, V.L.; Pavel, A.B.; Popa, O.P.; Popa, L.O.; Poorten, J.J.; Biesmeijer, J.C.; et al. Decline of unique Pontocaspian biodiversity in the Black Sea Basin: A review. Ecol. Evol. 2021, 11, 12923–12947. [Google Scholar] [CrossRef]
- Hermoso, V.; Clavero, M.; Blanco-Garrido, F.; Prenda, J. Invasive species and habitat degradation in Iberian streams: An analysis of their role in freshwater fish diversity loss. Ecol. Appl. 2011, 21, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; García Raso, J.E.; Çinar, M.E.; Almogi-Labin, A.; Ates, A.S.; Azzurro, E.; et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine strategy framework directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 2012, 13, 328–352. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Graca, B. Effect of salinity on the distribution of Ponto–Caspian gammarids in a non-native area—Environmental and experimental study. Mar. Biol. Res. 2018, 14, 183–190. [Google Scholar] [CrossRef]
- Bacela-Spychalska, K.; van der Velde, G. There is more than one ‘killer shrimp’: Trophic positions and predatory abilities of invasive amphipods of Ponto-Caspian origin. Freshw. Biol. 2013, 58, 730–741. [Google Scholar] [CrossRef]
- Velde, G.; Rajagopal, S.; Kelleher, B.; Musko, I.B.; Bij de Vaate, A. Ecological impact of crustacean invaders: General considerations and examples from the Rhine River. In Biodivers Crisis Crustac; Von Vaupel Klein, J.C., Schram, F.R., Eds.; AA Balkema: Rotterdam, The Netherlands, 2000; pp. 3–33. [Google Scholar]
- Platvoet, D.; van der Velde, G.; Dick, J.T.A.; Li, S.Q. Flexible omnivory in Dikerogammarus villosus (Sowinsky, 1894) (Amphipoda). Crustaceana 2009, 82, 703–720. [Google Scholar] [CrossRef]
- Hanfling, G.; Edwards, F.; Gherardi, F. Invasive alien Crustacea: Dispersal, establishment, impact and control. Biol. Control 2011, 56, 573–595. [Google Scholar] [CrossRef]
- Wijnhoven, S.; van Riel, M.C.; van der Velde, G. Exotic and indigenous freshwater gammarid species: Physiological tolerance to water temperature in relation to ionic content of the water. Aquat. Ecol. 2003, 37, 151–158. [Google Scholar] [CrossRef]
- Rachalewski, M.; Kobak, J.; Szczerkowska-Majchrzak, E.; Bącela-Spychalska, K. Some like it hot: Factors impacting thermal preferences of two Ponto-Caspian amphipods Dikerogammarus villosus (Sovinsky, 1894) and Dikerogammarus haemobaphes (Eichwald, 1841). PeerJ 2018, 6, e4871. [Google Scholar] [CrossRef]
- Velde, G.; Leuven, R.S.; Platelet, D.; Bącela, K.; Huijbregts, M.A.; Hendriks, H.W.; Kruijt, D. Environmental and morphological factors influencing predatory behaviour by invasive non-indigenous gammaridean species. Biol. Invasions 2009, 11, 2043–2054. [Google Scholar] [CrossRef]
- Bovy, H.C.; Barrios-O’Neill, D.; Emmerson, M.C.; Aldridge, D.C.; Dick, J.T. Predicting the predatory impacts of the “demon shrimp” Dikerogammarus haemobaphes, on native and previously introduced species. Biol. Invasions 2015, 17, 597–607. [Google Scholar] [CrossRef]
- Campbell, L.M.; Thacker, R.; Barton, D.; Muir, D.C.G.; Greenwood, D.; Hecky, R.E. Re-engineering the eastern Lake Erie littoral food web: The trophic function of non-indigenous Ponto-Caspian species. J. Great Lakes Res. 2009, 35, 224–231. [Google Scholar] [CrossRef]
- Oreska, M.; Aldridge, D. Estimating the financial costs of freshwater invasive species in Great Britain: A standardized approach to invasive species costing. Biol. Invasions 2011, 13, 305–319. [Google Scholar] [CrossRef]
- Robertson, P.A.; Mill, A.; Novoa, A.; Jeschke, J.M.; Essl, F.; Gallardo, B.; Geist, J.; Jarić, I.; Lambin, X.; Musseau, C.; et al. A proposed unified framework to describe the management of biological invasions. Biol. Invasions 2020, 22, 2633–2645. [Google Scholar] [CrossRef]
- Wood, L.E.; Smith, E.R.C.; Bojko, J.; Stebbing, P. Options for the control of Dikerogammarus villosus (killer shrimp) and other invasive amphipods. Manag. Biol. Invasions 2021, 12, 662–684. [Google Scholar] [CrossRef]
- Davidson, R.; Simard, M.; Kutz, S.J.; Kapel, C.M.; Hamnes, I.S.; Robertson, L.J. Arctic parasitology: Why should we care? Trends Parasitol. 2011, 27, 239–245. [Google Scholar] [CrossRef]
- Desiderato, A.; Beermann, J.; Haddad, M.A.; Fernandes, L.F. Diatom Epibionts on Amphipod Crustaceans: A Possible Vector for Co-introductions? Water 2021, 13, 2227. [Google Scholar] [CrossRef]
- Pysek, P.; Richardson, D.M. Invasive Species, Environmental Change and Management, and Health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Medina-Villar, S. A new horizon-scanning tool to identify potential aquatic invasive alien species introduced into the Baltic Sea by shipping. Water 2023, 15, 531. [Google Scholar] [CrossRef]
- Ricciardi, A. Tracking marine alien species by ship movements. PNAS 2016, 113, 5470–5471. [Google Scholar] [CrossRef] [PubMed]
- IMO. International Convention for the Control and Management of Ships Ballast Water and Sediments of IMO. 2004. Available online: http://www.imo.org/home.asp (accessed on 5 March 2023).
- De Wysiecki, A.M.; Cortés, F.; Jaureguizar, A.J.; Barnett, A. Potential global distribution of a temperate marine coastal predator: The role of barriers and dispersal corridors on subpopulation connectivity. Limnol. Oceanogr. 2022, 67, 1805–1819. [Google Scholar] [CrossRef]
- Kotov, A.A.; Karabanov, D.P.; Van Damme, K. Non-Indigenous Cladocera (Crustacea: Branchiopoda): From a Few Notorious Cases to a Potential Global Faunal Mixing in Aquatic Ecosystems. Water 2022, 14, 2806. [Google Scholar] [CrossRef]
- Turbelin, A.J.; Diagne, C.; Hudgins, E.J.; Moodley, D.; Kourantidou, M.; Novoa, A.; Haubrock, P.J.; Berney, C.; Gozlan, R.E.; Francis, R.A.; et al. Introduction pathways of economically costly invasive alien species. Biol Invasions 2022, 24, 2061–2079. [Google Scholar] [CrossRef]
- CABI. CABI Compendium; CAB International: Wallingford, UK, 2023; Available online: https://www.cabidigitallibrary.org/journal/cabicompendium (accessed on 20 May 2023).
- Dobrzycka-Krahel, A.; Bogalecka, M. The Baltic Sea under Anthropopressure—The Sea of Paradoxes. Water 2022, 14, 3772. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).