Movement of Southern European Aquatic Alien Invertebrate Species to the North and South
Abstract
:1. Introduction
- (1)
- analyses of long-term trends in the movement of Mediterranean and Ponto-Caspian aquatic alien invertebrate species;
- (2)
- discussion on the responses of Southern European aquatic alien invertebrate species to changing conditions and management implications.
2. Materials and Methods
3. Results
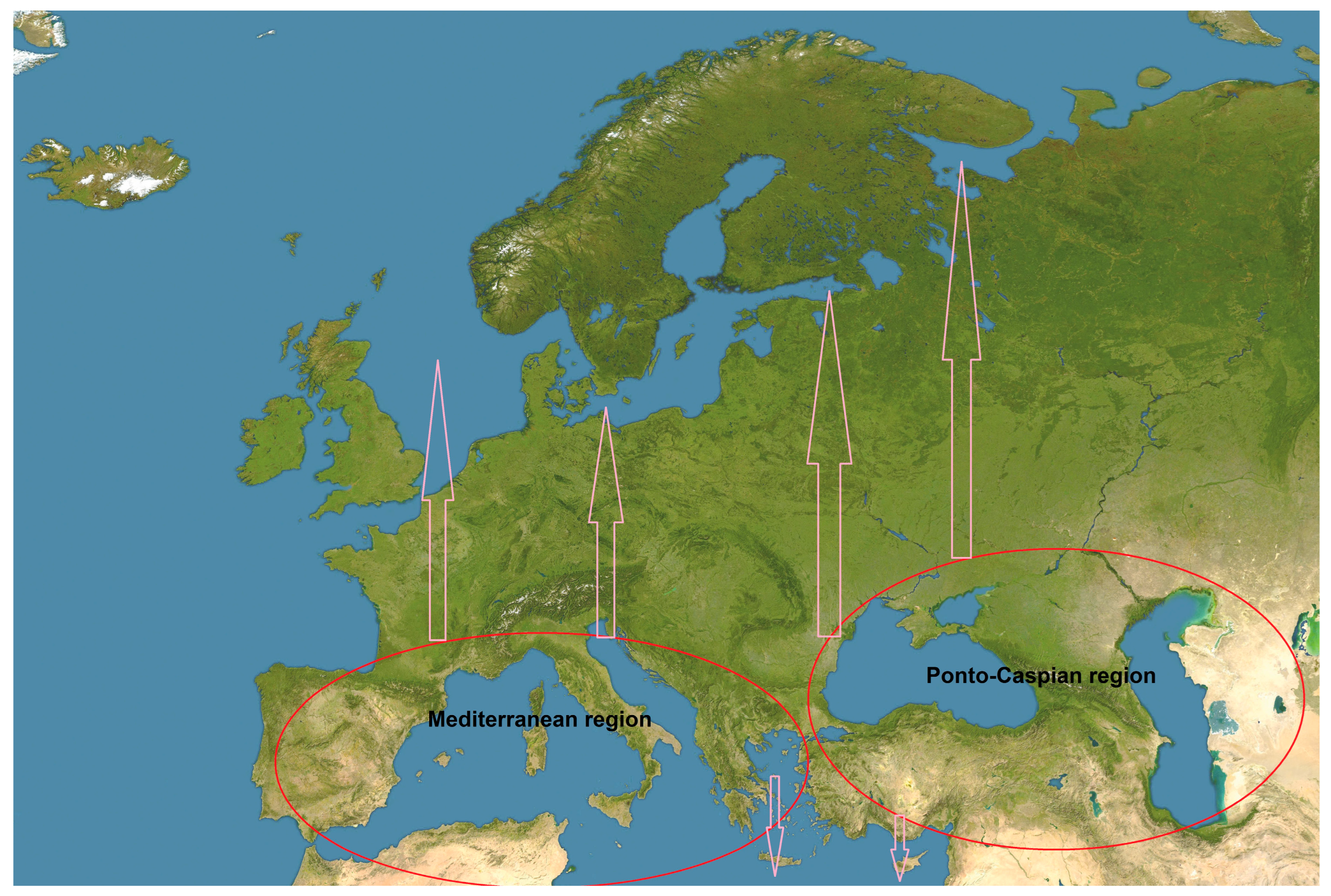
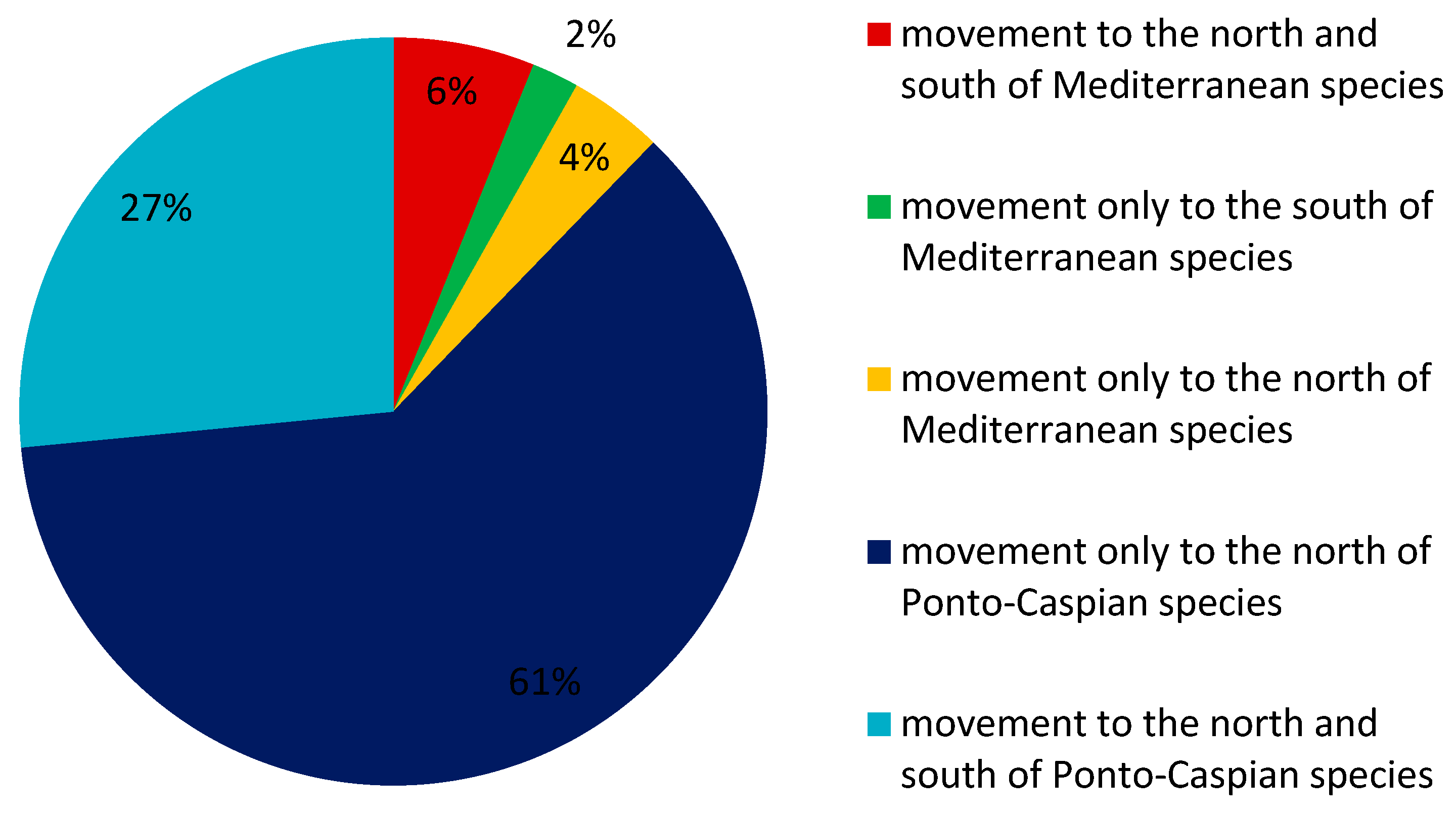
3.1. General Results on the Movement of Southern European Aquatic Alien Invertebrate Species
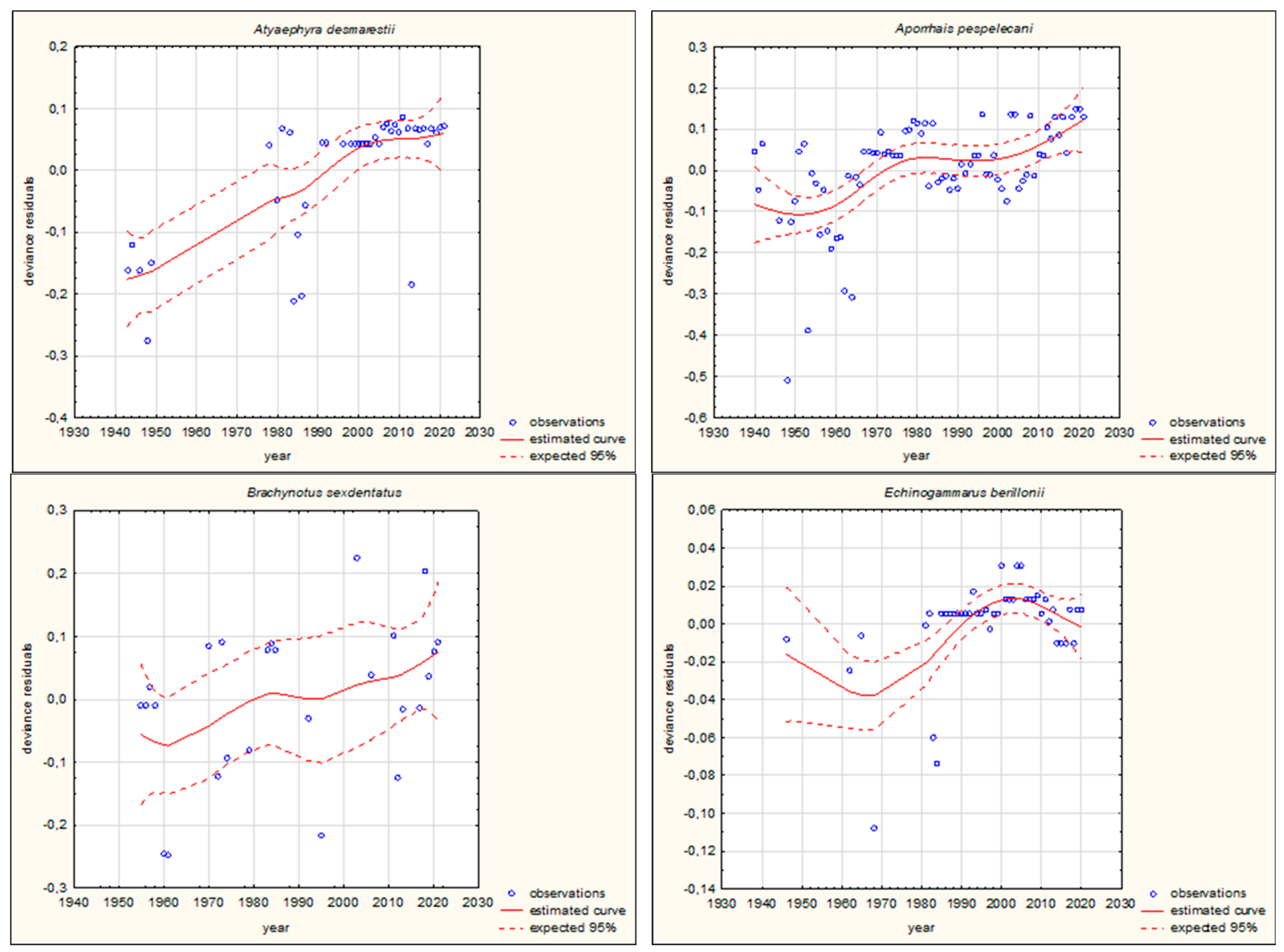
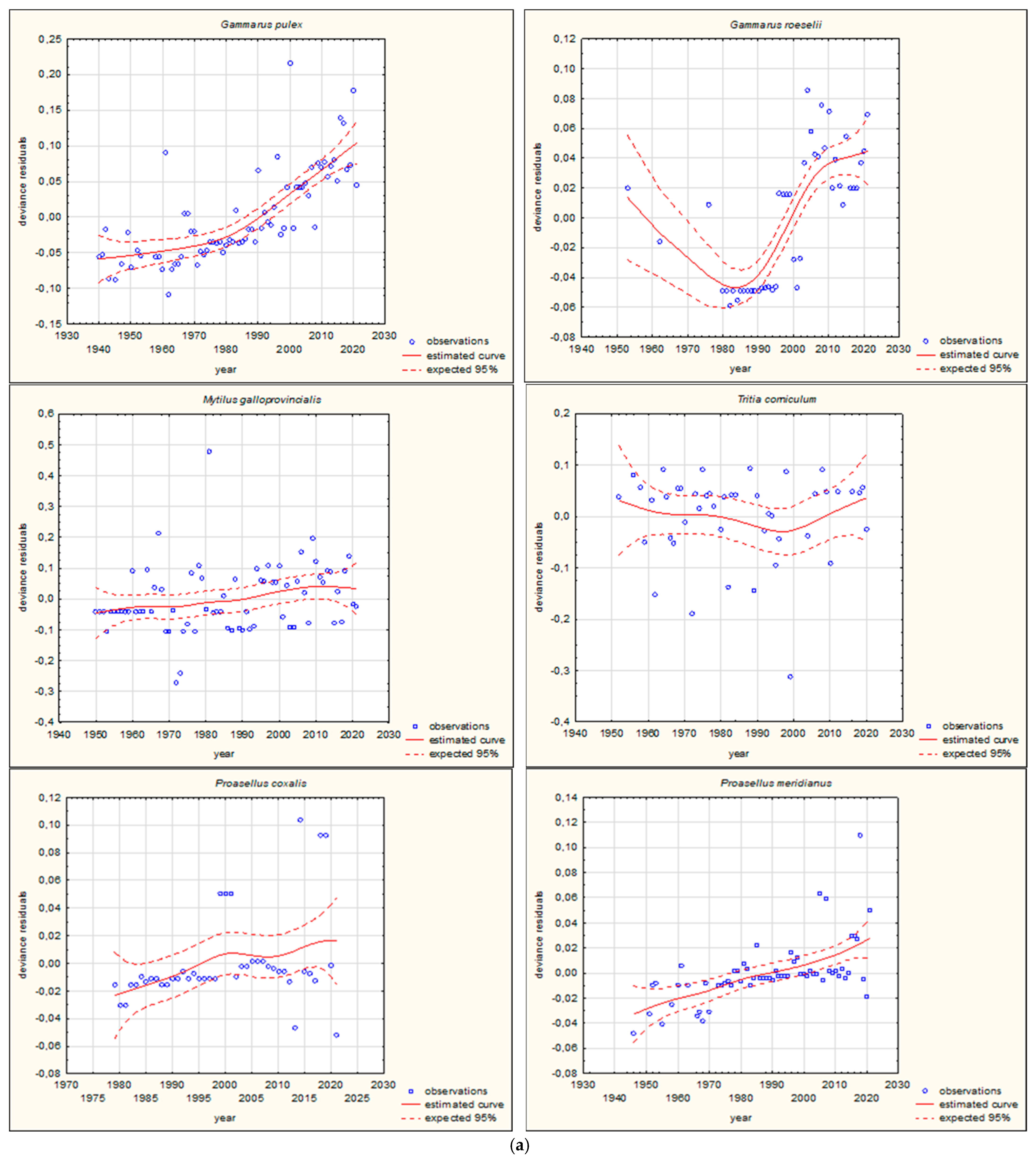
3.2. Long-Term Trends in the Movement of Southern European Aquatic Alien Invertebrate Species
4. Discussion
4.1. Movement of Southern European Aquatic Alien Invertebrates
4.2. Responses of Southern European Aquatic Alien Invertebrates to Changing Conditions
4.3. Management Implications
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeltsch, F.; Bonte, D.; Pe’Er, G.; Reineking, B.; Leimgruber, P.; Balkenhol, N.; Schröder, B.; Buchmann, C.M.; Mueller, T.; Blaum, N.; et al. Integrating movement ecology with biodiversity research—Exploring new avenues to address spatiotemporal biodiversity dynamics. Mov. Ecol. 2013, 1, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, A.M.; Singh, N.J. Linking movement ecology with wildlife management and conservation. Front. Ecol. Evol. 2016, 12, 155. [Google Scholar] [CrossRef] [Green Version]
- Masson-Delmotte, V.; Zhai, P.; Pirani, S.; Connors, C.; Péan, C.; Berger, N.; Caud, Y.; Chen, L.; Goldfarb, M.I.; Gomis, M.I.; et al. Climate Change 2021; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; p. 3949. [Google Scholar]
- Nebel, S. Animal migration. Nat. Educ. Knowl. 2010, 3, 77. [Google Scholar]
- Schifting habitats. Nat. Clim. Chang. 2020, 10, 377. [CrossRef]
- Carlton, J.T.; Geller, J.B. Ecological roulette: The global transport of nonindigenous marine organisms. Science 1993, 261, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bij de Vaate, A.; Jażdżewski, K.; Ketelaars, H.A.M.; Gollasch, S.; Van der Velde, G. Geographical patterns in range extension of Ponto-Caspian macroinvertebrate species in Europe. Can. J. Fish. Aquat. Sci. 2002, 59, 1159–1174. [Google Scholar] [CrossRef] [Green Version]
- Galil, B.; Nehring, S.; Panov, V. Waterways as invasion highways—Impact of climate change and globalization. Biol. Invasions 2007, 193, 59–74. [Google Scholar]
- Kinzelbach, R. The main features of the phylogeny and dispersal of the zebra mussel Dreissena polymorpha. In The Zebra Mussel Dreissena Polymorpha. Ecology, Biological Monitoring and First Application in Water Quality Management; Neumann, D., Jenner, H.A., Eds.; Limnologie Aktuell, Fischer Verlag: Stuttgart, Germany, 1992; Volume 4, pp. 5–17. [Google Scholar]
- Veldkornet, D.A.; Rajkaran, A. Predicting shifts in the geographical distribution of two estuarine plant species from the subtropical and temperate regions of South Africa. Wetlands 2019, 39, 1179–1188. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Thomspon, F.R. Poleward shifts in winter ranges of North American bird. Ecology 2007, 88, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Spies, I.; Gruenthal, K.M.; Drinan, D.P.; Hollowed, A.B.; Stevenson, D.E.; Tarpey, C.M.; Hauser, L. Genetic evidence of a northward range expansion in the eastern Bering Sea stock of Pacific cod. Evol. Appl. 2020, 13, 362–375. [Google Scholar] [CrossRef] [Green Version]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Osland, M.J.; Stevens, P.W.; Lamont, M.M.; Brusca, R.C.; Hart, K.M.; Waddle, J.H.; Langtimm, C.A.; Williams, C.M.; Keim, B.D.; Terando, A.J.; et al. Tropicalization of temperate ecosystems in North America: The northward range expansion of tropical organisms in response to warming temperatures. Glob. Chang. Biol. 2021, 27, 3009–3034. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, J.A.; Wang, Y.J.; Stoks, R. Evolution of cold tolerance and thermal plasticity in life history, behaviour and physiology during a poleward range expansion. J. Anim. Ecol. 2021, 90, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C. Climate-tracking species are not invasive. Nat. Clim. Chang. 2020, 10, 382–384. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Kemp, J.L.; Fidalgo, M.L. Cold-tolerant traits that favour northwards movement and establishment of Mediterranean and Ponto-Caspian alien aquatic invertebrates. Aquat. Sci. 2022, 84, 47. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Tarała, A.; Chabowska, A. Expansion of alien gammarids in the Vistula Lagoon and the Vistula Delta (Poland). Environ. Monit. Assess. 2013, 185, 5165–5175. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Melzer, M.; Majkowski, W. Range extension of Dikerogammarus villosus (Sowinsky, 1894) in Poland (the Baltic Sea basin) and its ability to osmoregulate in different environmental salinities. Oceanol. Hydrobiol. Stud. 2015, 44, 294–304. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Medina-Villar, S. Alien species of Mediterranean origin in the Baltic Sea region—Current state and risk assessment. Environ. Rev. 2020, 28, 339–356. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Stepien, C.; Nuc, Z. Neocosmopolitan distributions of invertebrate aquatic invasive species due to euryhaline geographic history and human-mediated dispersal: Ponto-Caspian versus other geographic origins. Ecol. Process. 2023, 12, 2. [Google Scholar] [CrossRef]
- UNEP. Emissions Gap Report. The Heat Is On—A World of Climate Promises Not Yet Delivered; UNEP: Nairobi, Kenya, 2021; 112p, ISBN 978-92-807-3890-2. Available online: https://www.unep.org/resources/emissions-gap-report-2021 (accessed on 5 March 2023).
- Sunday, J.M.; Bates, A.E.; Duty, N.K. Thermal tolerance and the global redistribution of animals. Nat. Clim. Chang. 2012, 2, 686–690. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Sciences 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Lenoir, J.; Bonebroke, T.C. Land-use change interacts with climate to determine elevational species redistribution. Nat. Commun. 2018, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Morley, J.W.; Selden, R.L.; Latour, R.J.; Frölicher, T.L.; Seagraves, R.J.; Pinsky, M.L. Projecting shifts in thermal habitat for 686 species on the North American continental shelf. PLoS ONE 2018, 13, e0196127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, K.S.; Montenegro, P.F.G.; Santana, G.C.; Vieira, W.L.D.S. Effect of climate change on distribution of species of common horned frogs in South America. PLoS ONE 2018, 13, e0202813. [Google Scholar] [CrossRef] [PubMed]
- Boisvert-Marsh, L.; Blois, S. Unravelling potential northward migration pathways for tree species under climate change. J. Biogeogr. 2021, 48, 1088–1100. [Google Scholar] [CrossRef]
- Lundberg, J.; Moberg, F. Mobile link organisms and ecosystem functioning: Implications for ecosystem resilience and management. Ecosystems 2003, 6, 0087–0098. [Google Scholar] [CrossRef]
- Massol, F.; Gravel, D.; Mouquet, N.; Cadotte, M.W.; Fukami, T.; Leibold, M.A. Linking community and ecosystem dynamics through spatial ecology. Ecol. Lett. 2011, 14, 313–323. [Google Scholar] [CrossRef]
- Verstijnen, Y.J.M.; Lucassen, E.C.H.E.T.; van der Gaag, M.; Wagenvoort, A.J.; Castelijns, H.; Ketelaars, H.A.M.; van der Velde, G.; Smolders, A.J.P. Trophic relationships in Dutch reservoirs recently invaded by Ponto-Caspian species: Insights from fish trends and stable isotope analysis. Aquat. Invasions 2019, 14, 280–298. [Google Scholar] [CrossRef]
- Casties, I.; Clemmesen, C.; Briski, E. Environmental tolerance of three gammarid species with and without invasion record under current and future global warming scenarios. Divers. Distrib. 2018, 25, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Dick, J.T.A.; Platvoet, D. Intraguild predation and species exclusion in amphipods: The interaction of behaviour, physiology and environment. Freshw. Biol. 1996, 36, 375–383. [Google Scholar] [CrossRef]
- Dick, J.T.A.; Platvoet, D. Invading predatory crustacean Dikerogammarus villosus eliminates both native and exotic species. Proc. R. Soc. B 2000, 267, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Borza, P.; Huber, T.; Leitner, P.; Remund, N.; Graf, W. How to coexist with the ‘killer shrimp’ Dikerogammarus villosus? Lessons from other invasive Ponto-Caspian peracarids. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 6. [Google Scholar] [CrossRef] [Green Version]
- Surowiec, J.; Dobrzycka-Krahel, A. New data on the non-indigenous gammarids in the Vistula Delta and the Vistula Lagoon. Oceanologia 2008, 50, 443–447. [Google Scholar]
- MacNeil, C.; Boets, P.; Lock, K.; Goethals, M. Potential effects of the invasive ‘killer shrimp’ (Dikerogammarus villosus) on macroinvertebrate assemblages and biomonitoring indices. Freshw. Biol. 2013, 58, 171–182. [Google Scholar] [CrossRef]
- GRIIS. Global Register of Introduced and Invasive Species. Available online: http://www.griis.org (accessed on 1 October 2021).
- AquaNIS. Information System on Aquatic Non-Indigenous and Cryptogenic Species Database. Available online: http://www.corpi.ku.lt/databases/index.php/aquanis (accessed on 4 March 2023).
- GBIF. Global Biodiversity Information Facility. Available online: https://www.gbif.org (accessed on 4 March 2023).
- Venice System. The Venice System for the Classification of Marine Waters According to Salinity. Limnol. Oceanogr. 1958, 3, 245–352. [Google Scholar]
- Luo, M.; Xu, Z.; Hirsch, T.; Sin Aung, T.; Xu, W.; Ji, L.; Qin, H.; Ma, K. The use of Global Biodiversity Information Facility (GBIF)-mediated data in publications written in Chinese. Glob. Ecol. Conserv. 2021, 25, e01406. [Google Scholar] [CrossRef]
- Pedersen, E.J.; Miller, D.L.; Simpson, G.L.; Ross, N. Hierarchical generalized additive models in ecology: An introduction with mgcv. PeerJ 2019, 7, e6876. [Google Scholar] [CrossRef] [Green Version]
- Murase, H.; Nagashima, H.; Yonezaki, S.; Matsukura, R.; Kitakado, T. Application of a generalized additive model (GAM) to reveal relationships between environmental factors and distributions of pelagic fish and krill: A case study in Sendai Bay, Japan. ICES J. Mar. Sci. 2009, 66, 1417–1424. [Google Scholar] [CrossRef] [Green Version]
- van Strien, A.J.; van Swaay, C.A.M.; Termaat, T. Opportunistic citizen science data of animal species produce reliable estimates of distribution trends if analysed with occupancy models. J. Appl. Ecol. 2013, 50, 1450–1458. [Google Scholar] [CrossRef]
- Ricciardi, A. Chapter 5—Ecology of Invasive Alien Invertebrates. In Thorp and Covich’s Freshwater Invertebrates, 4th Ed; Thorp, J., Rogers, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 83–91. ISBN 9780123850263. [Google Scholar] [CrossRef]
- Quell, F.; Schratzberger, M.; Beauchard, O.; Bruggeman, J.; Webb, T. Biological trait profiles discriminate between native and non-indigenous marine invertebrates. Aquat. Invasions 2021, 16, 571–600. [Google Scholar] [CrossRef]
- Truhlar, A.M.; Aldridge, D.C. Differences in behavioural traits between two potentially invasive amphipods. Dikerogammarus Villosus Gammarus Pulex. Biol. Invasions 2015, 17, 1569–1579. [Google Scholar] [CrossRef]
- Ricciardi, A.; Rasmussen, J.B. Predicting the identity and impact of future biological invaders: A priority for aquatic resource management. Can. J. Fish. Aquat. Sci. 1998, 55, 1759–1765. [Google Scholar] [CrossRef]
- Tricarico, E.; Junqueira, A.O.R.; Dudgeon, D. Alien species in aquatic environments: A selective comparison of coastal and inland waters in tropical and temperate latitudes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 872–891. [Google Scholar] [CrossRef] [Green Version]
- Dobrzycka-Krahel, A.; Tarała, A.; Majkowski, W. Size structure and body condition of Ponto-Caspian gammarids in the Vistula estuary (Poland). Oceanol. Hydrobiol. Stud. 2019, 48, 23–30. [Google Scholar] [CrossRef]
- Martini, S.; Larras, F.; Boyé, A.; Faure, E.; Aberle, N.; Archambault, P.; Bacouillard, L.; Beisner, B.E.; Bittner, L.; Castella, E.; et al. Functional trait-based approaches as a common framework for aquatic ecologists. Limnol. Oceanogr. 2021, 66, 965–994. [Google Scholar] [CrossRef]
- Crocetta, F.; Bitar, G.; Zibrowius, H.; Oliverio, M. Increase in knowledge of the marine gastropod fauna of Lebanon since the 19th century. Bull. Mar. Sci. 2020, 96, 1–22. [Google Scholar] [CrossRef]
- Soto, I.; Cuthbert, R.N.; Ricciardi, A.; Ahmed, D.; Altermatt, F.; Schäfer, R.; Archambaud-Suard, G.; Bonada, N.; Canedo-Arguelles, M.; Csabai, Z.; et al. The faunal Ponto-Caspianization of central and western European waterways. Biol. Invasions 2023, 25, 2613–2629. [Google Scholar] [CrossRef]
- Locklin, J.L.; Corbitt, D.N.; McMahon, R.F. Settlement, density, survival and shell growth of zebra mussels, Dreissena polymorpha, in a recently invaded low latitude, warm water Texas reservoir. Aquat. Invasions 2020, 15, 408–434. [Google Scholar] [CrossRef]
- Lugo, S.C.M.; Baumeister, M.; Nour, O.M.; Wolf, F.; Stumpp, M.; Pansch, C. Warming and temperature variability determine the performance of two invertebrate predators. Sci. Rep. 2020, 10, 6780. [Google Scholar] [CrossRef] [Green Version]
- Peck, L.S.; Clark, M.S.; Morley, S.A.; Massey, A.; Rossetti, H. Animal temperature limits and ecological relevance: Effects of size, activity and rates of change. Funct. Ecol. 2009, 23, 248–256. [Google Scholar] [CrossRef] [Green Version]
- Bates, A.E.; McKelvie, C.M.; Sorte, C.J.B.; Morley, S.A.; Jones, N.A.R.; Mondon, J.A.; Bird, T.J.; Quinn, G. Geographical range, heat tolerance and invasion success in aquatic species. Proc. R. Soc. B 2013, 280, 20131958. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Velde, S.V.D.; Jorissen, E.L.; Neubauer, T.A.; Radan, S.; Pavel, A.B.; Stoica, M.; Van Baak, C.G.; Martínez Gándara, A.; Popa, L.; Stigter, H.D. A conservation palaeobiological approach to assess faunal response of threatened biota under natural and anthropogenic environmental change. Biogeosciences 2019, 16, 2423–2442. [Google Scholar] [CrossRef] [Green Version]
- Gogaladze, A.; Son, M.O.; Lattuada, M.; Anistratenko, V.V.; Syomin, V.L.; Pavel, A.B.; Popa, O.P.; Popa, L.O.; Poorten, J.J.; Biesmeijer, J.C.; et al. Decline of unique Pontocaspian biodiversity in the Black Sea Basin: A review. Ecol. Evol. 2021, 11, 12923–12947. [Google Scholar] [CrossRef]
- Hermoso, V.; Clavero, M.; Blanco-Garrido, F.; Prenda, J. Invasive species and habitat degradation in Iberian streams: An analysis of their role in freshwater fish diversity loss. Ecol. Appl. 2011, 21, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; García Raso, J.E.; Çinar, M.E.; Almogi-Labin, A.; Ates, A.S.; Azzurro, E.; et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine strategy framework directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 2012, 13, 328–352. [Google Scholar] [CrossRef] [Green Version]
- Dobrzycka-Krahel, A.; Graca, B. Effect of salinity on the distribution of Ponto–Caspian gammarids in a non-native area—Environmental and experimental study. Mar. Biol. Res. 2018, 14, 183–190. [Google Scholar] [CrossRef]
- Bacela-Spychalska, K.; van der Velde, G. There is more than one ‘killer shrimp’: Trophic positions and predatory abilities of invasive amphipods of Ponto-Caspian origin. Freshw. Biol. 2013, 58, 730–741. [Google Scholar] [CrossRef]
- Velde, G.; Rajagopal, S.; Kelleher, B.; Musko, I.B.; Bij de Vaate, A. Ecological impact of crustacean invaders: General considerations and examples from the Rhine River. In Biodivers Crisis Crustac; Von Vaupel Klein, J.C., Schram, F.R., Eds.; AA Balkema: Rotterdam, The Netherlands, 2000; pp. 3–33. [Google Scholar]
- Platvoet, D.; van der Velde, G.; Dick, J.T.A.; Li, S.Q. Flexible omnivory in Dikerogammarus villosus (Sowinsky, 1894) (Amphipoda). Crustaceana 2009, 82, 703–720. [Google Scholar] [CrossRef] [Green Version]
- Hanfling, G.; Edwards, F.; Gherardi, F. Invasive alien Crustacea: Dispersal, establishment, impact and control. Biol. Control 2011, 56, 573–595. [Google Scholar] [CrossRef]
- Wijnhoven, S.; van Riel, M.C.; van der Velde, G. Exotic and indigenous freshwater gammarid species: Physiological tolerance to water temperature in relation to ionic content of the water. Aquat. Ecol. 2003, 37, 151–158. [Google Scholar] [CrossRef]
- Rachalewski, M.; Kobak, J.; Szczerkowska-Majchrzak, E.; Bącela-Spychalska, K. Some like it hot: Factors impacting thermal preferences of two Ponto-Caspian amphipods Dikerogammarus villosus (Sovinsky, 1894) and Dikerogammarus haemobaphes (Eichwald, 1841). PeerJ 2018, 6, e4871. [Google Scholar] [CrossRef] [Green Version]
- Velde, G.; Leuven, R.S.; Platelet, D.; Bącela, K.; Huijbregts, M.A.; Hendriks, H.W.; Kruijt, D. Environmental and morphological factors influencing predatory behaviour by invasive non-indigenous gammaridean species. Biol. Invasions 2009, 11, 2043–2054. [Google Scholar] [CrossRef] [Green Version]
- Bovy, H.C.; Barrios-O’Neill, D.; Emmerson, M.C.; Aldridge, D.C.; Dick, J.T. Predicting the predatory impacts of the “demon shrimp” Dikerogammarus haemobaphes, on native and previously introduced species. Biol. Invasions 2015, 17, 597–607. [Google Scholar] [CrossRef]
- Campbell, L.M.; Thacker, R.; Barton, D.; Muir, D.C.G.; Greenwood, D.; Hecky, R.E. Re-engineering the eastern Lake Erie littoral food web: The trophic function of non-indigenous Ponto-Caspian species. J. Great Lakes Res. 2009, 35, 224–231. [Google Scholar] [CrossRef]
- Oreska, M.; Aldridge, D. Estimating the financial costs of freshwater invasive species in Great Britain: A standardized approach to invasive species costing. Biol. Invasions 2011, 13, 305–319. [Google Scholar] [CrossRef]
- Robertson, P.A.; Mill, A.; Novoa, A.; Jeschke, J.M.; Essl, F.; Gallardo, B.; Geist, J.; Jarić, I.; Lambin, X.; Musseau, C.; et al. A proposed unified framework to describe the management of biological invasions. Biol. Invasions 2020, 22, 2633–2645. [Google Scholar] [CrossRef]
- Wood, L.E.; Smith, E.R.C.; Bojko, J.; Stebbing, P. Options for the control of Dikerogammarus villosus (killer shrimp) and other invasive amphipods. Manag. Biol. Invasions 2021, 12, 662–684. [Google Scholar] [CrossRef]
- Davidson, R.; Simard, M.; Kutz, S.J.; Kapel, C.M.; Hamnes, I.S.; Robertson, L.J. Arctic parasitology: Why should we care? Trends Parasitol. 2011, 27, 239–245. [Google Scholar] [CrossRef]
- Desiderato, A.; Beermann, J.; Haddad, M.A.; Fernandes, L.F. Diatom Epibionts on Amphipod Crustaceans: A Possible Vector for Co-introductions? Water 2021, 13, 2227. [Google Scholar] [CrossRef]
- Pysek, P.; Richardson, D.M. Invasive Species, Environmental Change and Management, and Health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef] [Green Version]
- Dobrzycka-Krahel, A.; Medina-Villar, S. A new horizon-scanning tool to identify potential aquatic invasive alien species introduced into the Baltic Sea by shipping. Water 2023, 15, 531. [Google Scholar] [CrossRef]
- Ricciardi, A. Tracking marine alien species by ship movements. PNAS 2016, 113, 5470–5471. [Google Scholar] [CrossRef] [PubMed]
- IMO. International Convention for the Control and Management of Ships Ballast Water and Sediments of IMO. 2004. Available online: http://www.imo.org/home.asp (accessed on 5 March 2023).
- De Wysiecki, A.M.; Cortés, F.; Jaureguizar, A.J.; Barnett, A. Potential global distribution of a temperate marine coastal predator: The role of barriers and dispersal corridors on subpopulation connectivity. Limnol. Oceanogr. 2022, 67, 1805–1819. [Google Scholar] [CrossRef]
- Kotov, A.A.; Karabanov, D.P.; Van Damme, K. Non-Indigenous Cladocera (Crustacea: Branchiopoda): From a Few Notorious Cases to a Potential Global Faunal Mixing in Aquatic Ecosystems. Water 2022, 14, 2806. [Google Scholar] [CrossRef]
- Turbelin, A.J.; Diagne, C.; Hudgins, E.J.; Moodley, D.; Kourantidou, M.; Novoa, A.; Haubrock, P.J.; Berney, C.; Gozlan, R.E.; Francis, R.A.; et al. Introduction pathways of economically costly invasive alien species. Biol Invasions 2022, 24, 2061–2079. [Google Scholar] [CrossRef]
- CABI. CABI Compendium; CAB International: Wallingford, UK, 2023; Available online: https://www.cabidigitallibrary.org/journal/cabicompendium (accessed on 20 May 2023).
- Dobrzycka-Krahel, A.; Bogalecka, M. The Baltic Sea under Anthropopressure—The Sea of Paradoxes. Water 2022, 14, 3772. [Google Scholar] [CrossRef]

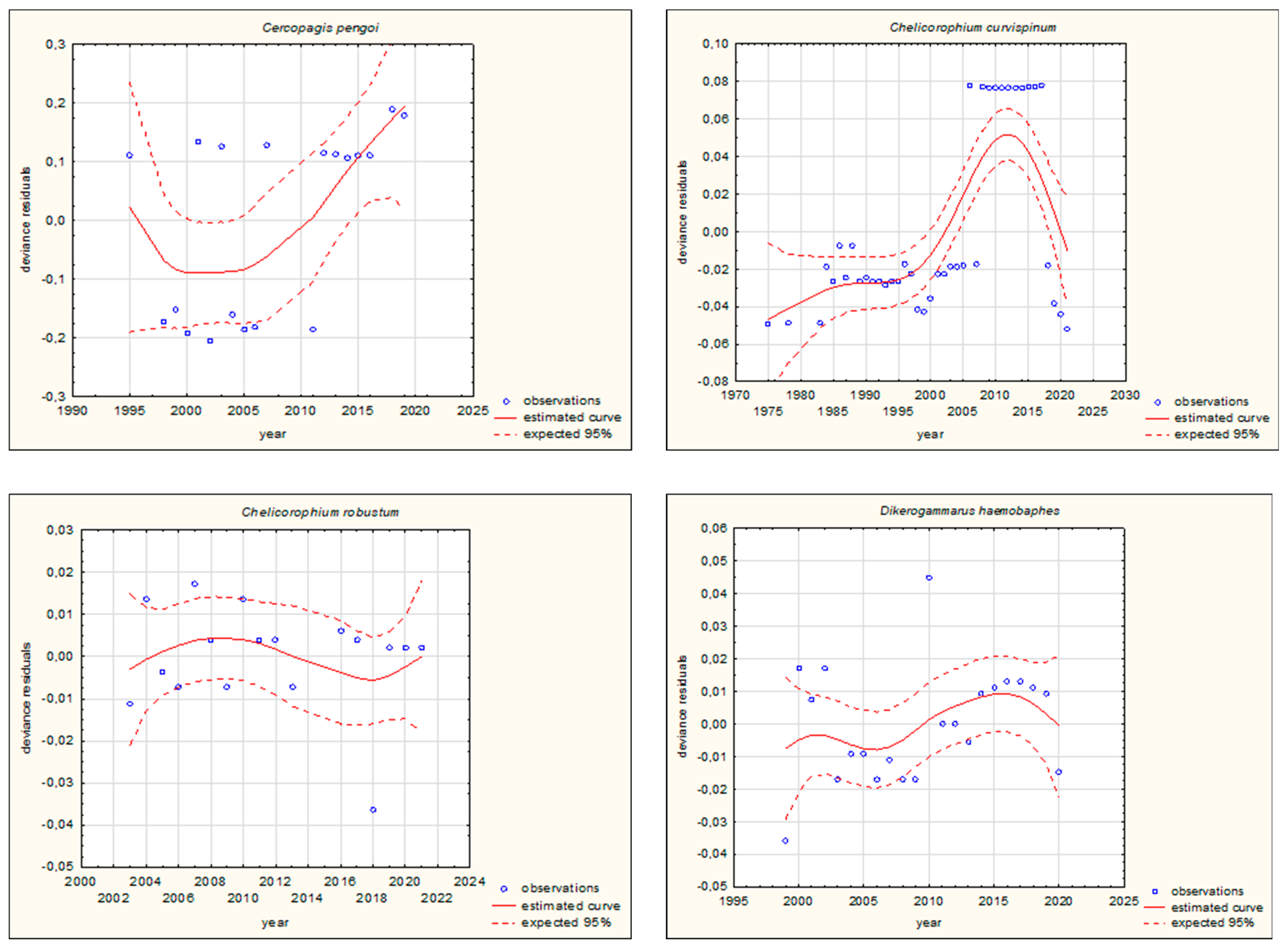
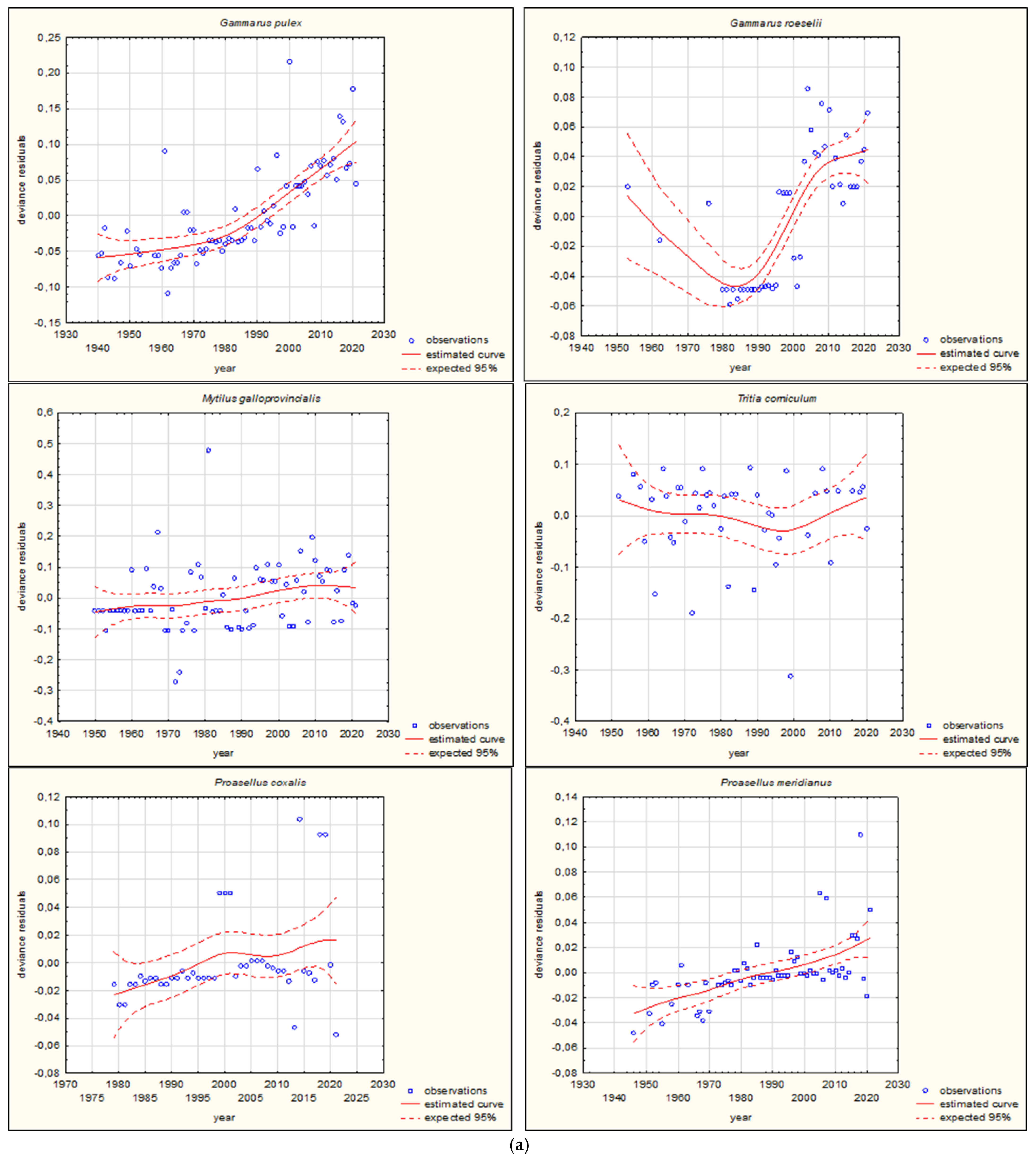
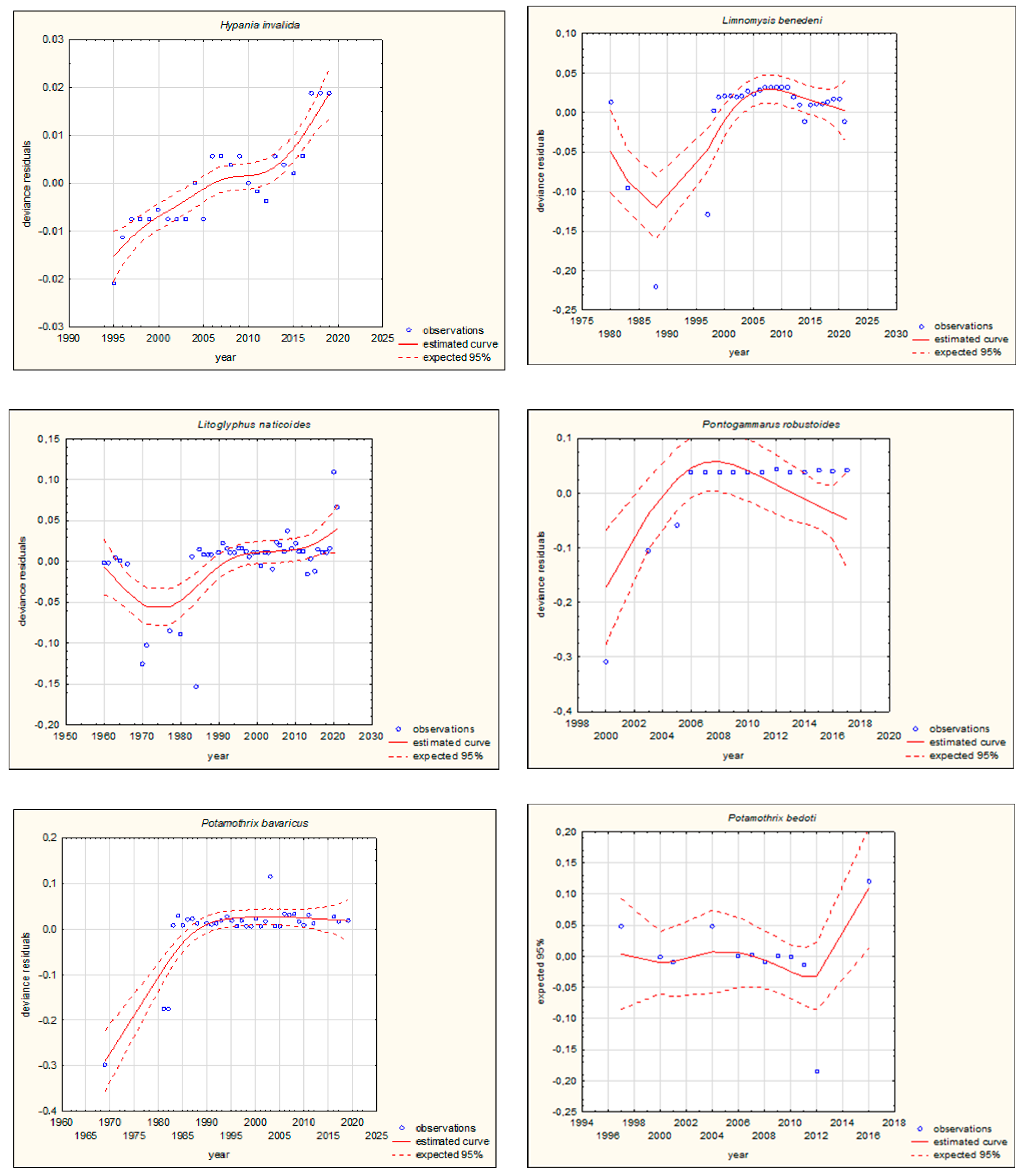

| Species | Group | Movement | Pathway of Introduction | |
|---|---|---|---|---|
| ↑ Northward ↓ Southward | ||||
| 1 | Atyaephyra desmarestii | Crustacea | ↑↓ | Corridor |
| (Millet, 1831) | ||||
| 2 | Brachynotus sexdentatus | Crustacea | ↑↓ | NA |
| (Risso, 1827) | ||||
| 3 | Gammarus pulex | Crustacea | ↑↓ | NA |
| (Linnaeus, 1758) | ||||
| 4 | Gammarus roeselii | Crustacea | ↑ | Corridor |
| (Gervais, 1835) | ||||
| 5 | Echinogammarus berilloni | Crustacea | ↑ | Corridor |
| (Catta, 1878) | ||||
| 6 | Proasellus coxalis | Crustacea | ↑ | Corridor, stowaway, hull, fouling |
| (Dollfus, 1892) | ||||
| 7 | Proasellus meridianus | Crustacea | ↑ | Corridor, stowaway, hull, fouling |
| (Racovitza, 1919) | ||||
| 8 | Aporrhais pespelecani | Mollusca | ↑↓ | NA |
| (Linnaeus, 1758) | ||||
| 9 | Bela menkhorsti | Mollusca | ↓ | NA |
| (van Aartsen, 1988) | ||||
| 10 | Bogia labronica | Mollusa | ↓ | NA |
| (Bogi, 1984) | ||||
| 11 | Mytilus galloprovincialis | Mollusca | ↑↓ | Aquaculture |
| (Lamarck, 1819) | ||||
| 12 | Tritia corniculum | Mollusca | ↑↓ | NA |
| (Olivi, 1792) |
| Species | Group | Movement | Pathway of Introduction | |
|---|---|---|---|---|
| ↑ Northward ↓ Southward | ||||
| 1 | Amathillina cristata | Crustacea | ↑ | NA |
| (G.O.Sars, 1894) | ||||
| 2 | Amathillina pusilla | Crustacea | ↑ | NA |
| (G.O. Sars, 1896) | ||||
| 3 | Cardiophilus marisnigrae | Crustacea | ↑ | NA |
| (Miloslawskaya, 1931) | ||||
| 4 | Caspiocuma campylaspoides | Crustacea | ↑ | NA |
| (G.O. Sars, 1897) | ||||
| 5 | Cercopagis pengoi | Crustacea | ↑ | Canals, shipping-fouling, ballast waters |
| (Ostroumov, 1891) | ||||
| 6 | Chaetogammarus placidus | Crustacea | ↑ | NA |
| (G.O. Sars, 1896) | ||||
| 7 | Chaetogammarus warpachowskyi | Crustacea | ↑ | Deliberate with fish/shellfish |
| (Sars, 1897) | ||||
| 8 | Chelicorophium chelicorne | Crustacea | ↑ | NA |
| (G.O. Sars, 1895) | ||||
| 9 | Chelicorophium curvispinum | Crustacea | ↑ | NA |
| (G.O. Sars, 1895) | ||||
| 10 | Chelicorophium maeoticum | Crustacea | ↑ | NA |
| (Sowinsky, 1898) | ||||
| 11 | Chelicorophium nobile | Crustacea | ↑ | NA |
| (G.O. Sars, 1895) | ||||
| 12 | Chelicorophium mucronatum | Crustacea | ↑ | NA |
| (G.O. Sars, 1895) | ||||
| 13 | Chelicorophium robustum | Crustacea | ↑↓ | NA |
| (G.O. Sars, 1895) | ||||
| 14 | Chelicorophium sowinskyi | Crustacea | ↑ | NA |
| (Martynov, 1924) | ||||
| 15 | Compactogammarus compactus | Crustacea | ↑ | NA |
| (G.O. Sars, 1895) | ||||
| 16 | Cornigerius bicornis | Crustacea | ↑ | NA |
| (Zernov, 1901) | ||||
| 17 | Cornigerius lacustris | Crustacea | ↑ | NA |
| (Spandl, 1923) | ||||
| 18 | Cornigerius maeoticus | Crustacea | ↑ | Canals |
| (Pengo, 1879) | ||||
| 19 | Dikerogammarus bispinosus | Crustacea | ↑ | Corridor, vessels |
| (Martynov, 1925) | ||||
| 20 | Dikerogammarus haemobaphes | Crustacea | ↑↓ | Corridor, vessels |
| (Eichwald, 1841) | ||||
| 21 | Dikerogammarus villosus | Crustacea | ↑↓ | Corridor, vessels |
| (Sowinsky, 1894) | ||||
| 22 | Ectinosoma abrau | Crustacea | ↑ | NA |
| (Krichagin, 1877) | ||||
| 23 | Echinogammarus ischnus | Crustacea | ↑ | Corridor, vessels |
| syn. Chaetogammarus ichnus | ||||
| (Stebbing, 1899) | ||||
| 24 | Euxinia sarsi | Crustacea | ↑ | NA |
| (Sowinsky, 1898) | ||||
| 25 | Echinogammarus trichiatus | Crustacea | ↑↓ | Corridor |
| (Martynov, 1932) | ||||
| 26 | Echinogammarus warpachowskyi | Crustacea | ↑ | NA |
| (G.O. Sars, 1894) | ||||
| 27 | Euxinia weidemanni | Crustacea | ↑ | NA |
| (G.O. Sars, 1896) | ||||
| 28 | Evadne anonyx | Crustacea | ↑ | Canals, shipping |
| (G.O. Sars, 1897) | ||||
| 29 | Hemimysis anomala | Crustacea | ↑↓ | Stowaway, ballast water |
| (G.O. Sars, 1907) | ||||
| 30 | Heterocope caspia | Crustacea | ↑ | NA |
| (Sars G.O., 1897) | ||||
| 31 | Hypaniola kowalewskii | Crustacea | ↑ | Fauna improvement |
| (Grimm and Annenkova, 1927) | ||||
| 32 | Iphigenella acanthopoda | Crustacea | ↑ | NA |
| (G.O. Sars, 1896) | ||||
| 33 | Jaera istri | Crustacea | ↑ | Corridor |
| (Veuille, 1979) | ||||
| 34 | Jaera sarsi | Crustacea | ↑ | Canals |
| (Valkanov, 1936) | ||||
| 35 | Katamysis warpachowskyi | Crustacea | ↑ | Canals |
| (G.O. Sars, 1893) | ||||
| 36 | Kuzmelina kusnezowi | Crustacea | ↑ | NA |
| (Sowinsky, 1894) | ||||
| 37 | Lanceogammarus andrussowi | Crustacea | ↑ | NA |
| (G.O. Sars, 1896) | ||||
| 38 | Limnomysis benedeni | Crustacea | ↑↓ | Corridor |
| (Czerniavsky, 1882) | ||||
| 39 | Niphargoides corpulentus | Crustacea | ↑ | NA |
| (G.O. Sars, 1895) | ||||
| 40 | Niphargogammarus intermedius | Crustacea | ↑ | NA |
| (Carausu, 1943) | ||||
| 41 | Niphargus hrabei | Crustacea | ↑ | NA |
| (S. Karaman, 1932) | ||||
| 42 | Obesogammarus crassus | Crustacea | ↑↓ | Aquaculture |
| (G.O. Sars, 1894) | ||||
| 43 | Obesogammarus obesus | Crustacea | ↑ | Canals, vessels |
| (G.O. Sars, 1894) | ||||
| 44 | Paramysis lacustris | Crustacea | ↑↓ | Fisheries |
| (Czerniavsky, 1882) | ||||
| 45 | Paraniphargoides motasi | Crustacea | ↑↓ | NA |
| (Carausu, 1943) | ||||
| 46 | Pontogammarus robustoides | Crustacea | ↑ | NA |
| (Sars, 1894) | ||||
| 47 | Pontogammarus abbreviatus | Crustacea | ↑ | NA |
| (G.O. Sars, 1894) | ||||
| 48 | Pontogammarus aestuarius | Crustacea | ↑ | NA |
| (Derzhavin, 1924) | ||||
| 49 | Pontogammarus borceae | Crustacea | ↑↓ | NA |
| (Carausu, 1943) | ||||
| 50 | Pontogammarus maeoticus | Crustacea | ↑↓ | NA |
| (Sovinskij, 1894) | ||||
| 51 | Shablogammarus chablensis | Crustacea | ↑ | NA |
| (Carausu, 1943) | ||||
| 52 | Shablogammarus subnudus | Crustacea | ↑ | NA |
| (G.O. Sars, 1896) | ||||
| 53 | Stenogammarus carausui | Crustacea | ↑ | NA |
| (Derzhavin and Pjatakova, 1962) | ||||
| 54 | Stenogammarus compressus | Crustacea | ↑↓ | NA |
| (G.O. Sars, 1894) | ||||
| 55 | Stenogammarus macrurus | Crustacea | ↑ | NA |
| (Sars, 1894) | ||||
| 56 | Stenogammarus similis | Crustacea | ↑↓ | NA |
| (Sars, 1894) | ||||
| 57 | Turcogammarus aralensis | Crustacea | ↑ | NA |
| (Uljanin, 1875) | ||||
| 58 | Uroniphargoides spinicaudatus | Crustacea | ↑ | NA |
| (Carausu, 1943) | ||||
| 59 | Yogmelina limana | Crustacea | ↑ | NA |
| (Karaman and Barnard, 1979) | ||||
| 60 | Abra segmentum | Mollusca | ↑↓ | NA |
| (Récluz, 1843) | ||||
| 61 | Dreissena polymorpha | Mollusca | ↑↓ | NA |
| (Pallas, 1771) | ||||
| 62 | Dreissena rostriformis bugensis | Mollusca | ↑↓ | Corridor, stowaway, contaminant |
| (Andrusov, 1897) | ||||
| 63 | Dreissena rostriformis | Mollusca | ↑ | NA |
| (Deshayes, 1838) | ||||
| 64 | Euxinipyrgula lincta | Mollusca | ↑ | NA |
| (Milaschewitsch, 1908) | ||||
| 65 | Hypanis colorata | Mollusca | ↑ | NA |
| (Eichwald, 1829) | ||||
| 66 | Hypanis pontica | Mollusca | ↑ | NA |
| (Eichwald, 1838) | ||||
| 67 | Hypanis fragilis | Mollusca | ↑ | NA |
| (Milaschevitch, 1908) | ||||
| 68 | Hypanis glabra | Mollusca | ↑ | NA |
| (Ostroumoff, 1905) | ||||
| 69 | Lithoglyphus naticoides | Mollusca | ↑↓ | Corridor |
| (C.Pfeiffer, 1828) | ||||
| 70 | Viviparus acerosus | Mollusca | ↑ | Release, escape |
| (Bourguignat, 1862) | ||||
| 71 | Blackfordia virginica | Cnidaria | ↑↓ | NA |
| (Mayer, 1910) | ||||
| 72 | Cordylophora caspia | Cnidaria | ↑↓ | Stowaway, ballast water, hull, fouling |
| (Pallas, 1771) | ||||
| 73 | Caspiobdella fadejewi | Annelida | ↑ | NA |
| (Epshtein, 1961) | ||||
| 74 | Hypania invalida | Annelida | ↑ | Stowaway, hull, fouling |
| (Grube, 1860) | ||||
| 75 | Hypaniola kowalewskii | Annelida | ↑ | NA |
| (Grimm and Annenkova, 1927) | ||||
| 76 | Isochaetides michaelseni | Annelida | ↑ | NA |
| (Lastockin, 1937) | ||||
| 77 | Potamothrix heuscheri | Annelida | ↑↓ | Corridor |
| (Bretscher, 1900) | ||||
| 78 | Potamothrix vejdovskyi | Annelida | ↑↓ | Corridor, stowaway, ballast water |
| (Hrabe, 1941) | ||||
| 79 | Potamothrix moldaviensis | Annelida | ↑ | NA |
| Vejdovský and Mrázek, 1903 | ||||
| 80 | Potamothrix bavaricus | Annelida | ↑↓ | NA |
| (Oschmann, 1913) | ||||
| 81 | Potamothrix bedoti | Annelida | ↑↓ | NA |
| (Piguet, 1913) | ||||
| 82 | Potamothrix hammoniensis | Annelida | ↑↓ | NA |
| (Michaelsen, 1901) | ||||
| 83 | Psammoryctides moravicus | Annelida | ↑↓ | Corridor, stowaway, ballast water |
| (Hrabe, 1934) | ||||
| 84 | Tubificoides diazi | Annelida | ↑↓ | NA |
| (Brinkhurst and Baker, 1979) | ||||
| 85 | Tubifex newaensis | Annelida | ↑ | NA |
| (Michaelsen, 1903) |
| Species | Taxonomic Position | Statistics of Movement | |||
|---|---|---|---|---|---|
| ↑ Northward | ↓ Southward | ||||
| p-Value | d.f. | p-Value | d.f. | ||
| Gammarus pulex (Linnaeus, 1758) | Crustacea | 0.010010 | 4 | ||
| Gammarus roeselii (Gervais, 1835) | Crustacea | 0.0000001 | 4 | ||
| Echinogammarus berilloni (Catta, 1878) | Crustacea | 0.002880 | 4 | ||
| Aporrhais pespelecani (Linnaeus, 1758) | Mollusca | 0.014661 | 4 | ||
| Mytilus galloprovincialis (Lamarck, 1819) | Mollusca | 0.789822 | 4 | 0.0000001 | 4 |
| Species | Taxonomic Position | Statistics of Movement | |||
|---|---|---|---|---|---|
| ↑ Northward | ↓ Southward | ||||
| p-Value | d.f. | p-Value | d.f. | ||
| Chelicorophium curvispinum (G.O. Sars, 1895) | Crustacea | 0.0000001 | 4 | ||
| Limnomysis benedeni Czerniavsky, 1882 | Crustacea | 0.000002 | 4 | ||
| Dreissena polymorpha (Pallas, 1771) | Mollusca | 0.048831 | 4 | ||
| Dreissena rostriformis bugensis (Andrusov 1897) | Mollusca | 0.005452 | 4 | ||
| Lithoglyphus naticoides (C. Pfeiffer, 1828) | Mollusca | 0.000627 | 4 | ||
| Hypania invalida (Grube, 1860) | Annelida | 0.014347 | 4 | ||
| Potamothrix bavaricus (Oschmann, 1913) | Annelida | 0.0000001 | 4 | ||
| Potamothrix hammoniensis (Michaelsen, 1901) | Annelida | 0.000046 | 4 | ||
| Tubificoides diazi (Brinkhurst and Baker, 1979) | Annelida | 0.000078 | 4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzycka-Krahel, A. Movement of Southern European Aquatic Alien Invertebrate Species to the North and South. Water 2023, 15, 2598. https://doi.org/10.3390/w15142598
Dobrzycka-Krahel A. Movement of Southern European Aquatic Alien Invertebrate Species to the North and South. Water. 2023; 15(14):2598. https://doi.org/10.3390/w15142598
Chicago/Turabian StyleDobrzycka-Krahel, Aldona. 2023. "Movement of Southern European Aquatic Alien Invertebrate Species to the North and South" Water 15, no. 14: 2598. https://doi.org/10.3390/w15142598





