Biodiesel Production Directly from Rapeseeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ball Milling Procedure
2.3. Rapeseed Shell Activation
2.4. Reflux
2.5. GC Analysis
2.6. Viscosity Testing
2.7. Testing of Biomass as a Heavy Metal Adsorbent
3. Results and Discussion
3.1. Ball Milling with a Catalyst
3.2. Ball Milling without a Catalyst
3.3. Reflux with Store-Bought Oil and with Seeds
3.4. Viscosity
3.5. Heavy Metal Adsorption
3.6. Energy Consumption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karmakar, B.; Halder, G. Progress and future of biodiesel synthesis: Advancements in oil extraction and conversion technologies. Energy Convers. Manag. 2019, 182, 307–339. [Google Scholar] [CrossRef]
- Kim, D.S.; Hanifzadeh, M.; Kumar, A. Trend of biodiesel feedstock and its impact on biodiesel emission characteristics. Environ. Prog. Sustain. 2018, 37, 7–19. [Google Scholar] [CrossRef]
- Rapier, R. Renewable Diesel. In Biofuels, Solar and Wind as Renewable Energy Systems, 1st ed.; Pimentel, D., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 153–157. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. PECS 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Progress in biodiesel processing. Appl. Energy 2010, 87, 1815–1835. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Erucic Acid in feed and food. EFSA 2016, 14, e04593. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value added uses for crude glycerol—A byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef] [Green Version]
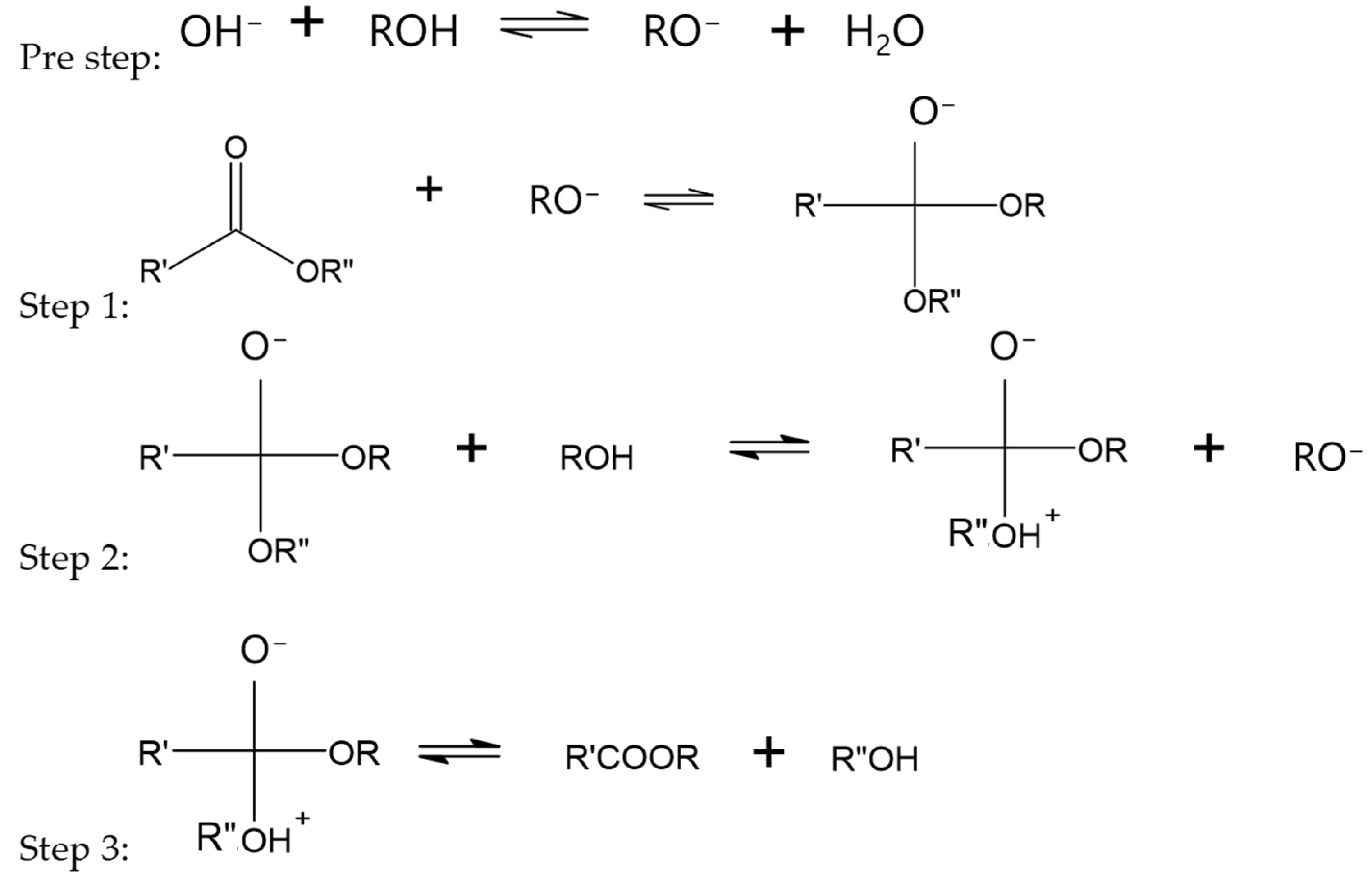
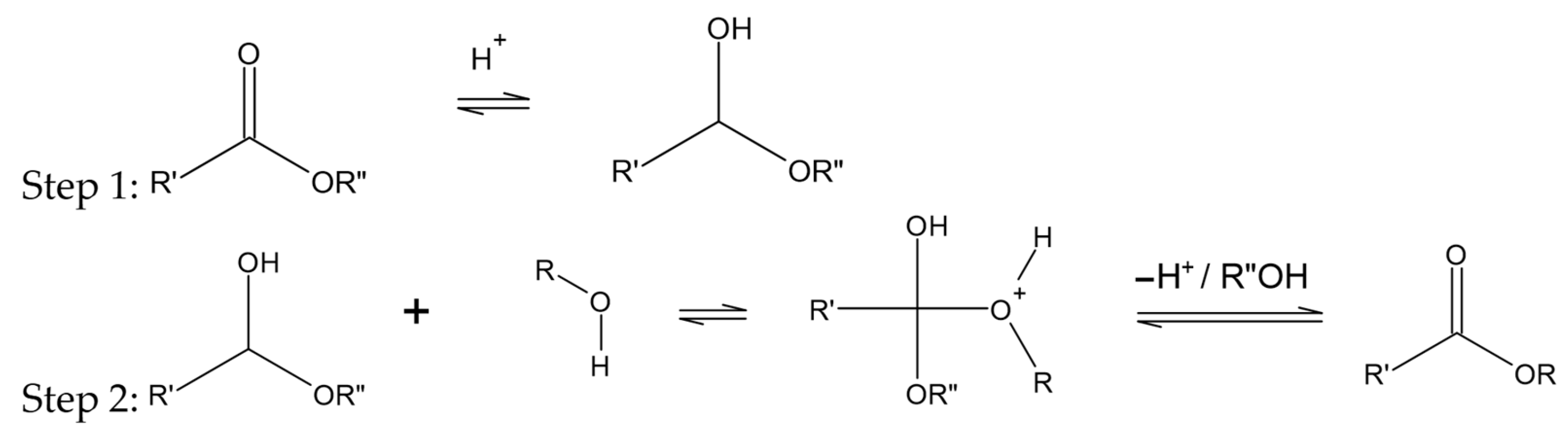
- Otera, J. Transesterification. Chem. Rev. 1993, 93, 1449–1470. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification kinetics of soybean oil 1. JAOCS 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- Noureddini, H.; Zhu, D. Kinetics of transesterification of soybean oil. JAOCS 1997, 74, 1457–1463. [Google Scholar] [CrossRef]
- Mittelbach, M.; Trathnigg, B. Kinetics of alkaline catalyzed methanolysis of sunflower oil. Eur. J. Lipid Sci. Technol. 1990, 92, 145–148. [Google Scholar] [CrossRef]
- Vicente, G.; Martinez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogenous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, V.B.; Stamenkovic, O.S.; Tasic, M.B. The wastewater treatment in the biodiesel production with alkali-catalyzed transesterification. Renew. Sust. Energ. Rev. 2014, 32, 40–60. [Google Scholar] [CrossRef]
- Srirangsan, A.; Ongwandee, M.; Chavalparit, O. Treatment of biodiesel wastewater by electrocoagulation process. Environ. Asia 2009, 2, 15–19. [Google Scholar]
- Kolesarova, N.; Hutnan, M.; Bodik, I.; Spalkova, V. Utilization of Biodiesel By-Products for Biogas Production. J. Biotechnol. Biomed. 2011, 2011, 126798. [Google Scholar] [CrossRef] [PubMed]
- Kusdiana, D.; Saka, S. Kinetics of transesterification in rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel 2001, 80, 693–698. [Google Scholar] [CrossRef]
- Yamazaki, R.; Iwamoto, S.; Nabetani, H.; Osakada, K.; Miyawaki, O.; Sagara, Y. Noncatalytic alcoholysis of oils for biodiesel fuel production by a semi-batch process. JJFE 2007, 8, 11–18. [Google Scholar] [CrossRef]
- Joelianingsih; Maeda, H.; Hagiwara, S.; Nabetani, H.; Sagara, Y.; Soerawidjaya, T.H.; Tambunan, A.H.; Abdullah, K. Biodiesel fuels from palm oil via the non-catalytic transesterification in a bubble column reactor at atmospheric pressure: A kinetic study. Renew. Energy 2008, 33, 1629–1636. [Google Scholar] [CrossRef]
- Todd, J. Mechanochemical synthesis of fuels from sustainable sources utilizing solid catalysts. STARS 2017, 2004–2019, 5566. [Google Scholar]
- Yang, N.; Sheng, X.; Ti, L.; Jia, H.; Ping, Q.; Li, N. Ball-milling as effective and economical process for biodiesel production under Kraft lignin activated carbon stabilized potassium carbonate. Bioresour. Technol. 2023, 369, 128379. [Google Scholar] [CrossRef]
- Berkowski, K.; Potisek, S.; Hickenboth, C.; Moore, J. Ultrashound-induced site-specific cleavage of azo-functionalized poly(ethylene glycol). Macromolecules 2005, 38, 8975–8978. [Google Scholar] [CrossRef]
- Gostl, R.; Sijbesma, R. π-extended anthracenes as sensitive probes for mechanical stress. Chem. Sci. 2016, 7, 370–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Zeng, T.; Husic, C.; Robb, M. Mechanically triggered small molecules release from a masked furfuryl carbonate. J. Am. Chem. Soc. 2019, 141, 15018–15023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piermattei, A.; Karthikeyan, S.; Sijbesma, R.P. Activating catalysts with mechanical force. Nat. Chem. 2009, 1, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Rajaeifar, M.A.; Ghobadian, B.; Heidari, M.D.; Fayyazi, E. Energy consumption and greenhouse gas emissions of biodiesel production from rapeseed in Iran. JRSE 2013, 5, 63134. [Google Scholar] [CrossRef]
- Chen, Y.; Mellot, G.; van Luijk, D.; Creton, C.; Sijbesma, R.P. Mechanochemical tools for polymer materials. Chem. Soc. Rev. 2021, 50, 4100–4140. [Google Scholar] [CrossRef]
- Jakobs, R.T.M.; Ma, S.; Sijbesma, R.P. Mechanocatalytic polymerization and cross-linking in a polymeric matrix. ACS Macro Lett. 2013, 2, 613–616. [Google Scholar] [CrossRef]
- Michael, P.; Binder, W.H. A mechanochemically triggered “click” catalyst. Angew Chem. Int. 2015, 54, 13918–13922. [Google Scholar] [CrossRef]
- Wei, K.; Gao, Z.; Liu, H.; Wu, X.; Wang, F.; Xu, H. Mechanical activation of platinum–acetylide complex for olefin hydrosilylation. ACS Macro Lett. 2017, 6, 1146–1150. [Google Scholar] [CrossRef]
- Hara, M.; Komoda, M.; Hasei, H.; Yashima, M.; Ikeda, S.; Takata, T.; Kondo, J.; Domen, K. A study of mechano-catalysts for overall water splitting. J. Phys. Chem. B 2000, 104, 780–785. [Google Scholar] [CrossRef]
- Przybylski, R. Canola/rapeseed oil. In Vegetable Oils in Food Technology: Composition, Properties, and Uses, 2nd ed.; Gunstone, F., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 107–133. [Google Scholar] [CrossRef]
- Meher, C.; Sagar, D.; Naik, S. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energ. Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Paduraru, C.; Tofan, L.; Teodosiu, C.; Bunia, I.; Tudorachi, N.; Toma, O. Biosorption of zinc(II) on rapeseed waste: Equilibrium studies and thermogravimetric investigations. Process Saf. Environ. Prot. 2015, 94, 18–28. [Google Scholar] [CrossRef]
- Arsenie, T.; Cara, I.G.; Popescu, M.C.; Motrescu, I.; Bulgariu, L. Evaluation of the adsorptive performances of rapeseed waste in the removal of toxic metal ions in aqueous media. Water 2022, 14, 4108. [Google Scholar] [CrossRef]



| Trial | Rapeseeds (g) | Methanol (mL) | Catalyst (g) | 3/8 Inch Stainless Steel Balls |
|---|---|---|---|---|
| Control—Shells | 1.5 g shells | 3.0 | - | 12 |
| Control—No shells | 1.5 g shells without seeds | 3.0 | - | 12 |
| Catalyst—Calcium Oxide | 1.5 g shells | 3.0 | 0.03 | 12 |
| 1.5 g shells without seeds | ||||
| Catalyst—Magnesium Oxide | 1.5 g shells | 3.0 | 0.03 | 12 |
| 1.5 g shells without seeds | ||||
| No Catalyst | 1.5 g shells | 3.0 | - | 12 |
| 1.5 g shells without seeds | ||||
| GC Parameter | Specification |
|---|---|
| Column type | Perkin Elmer Elite 1 Column (30 m long, 0.25 mm id) |
| Flow rate | 1.0 mL/min |
| Injection volume | 1.0 µL |
| Injection temperature | 250 °C |
| Initial column temperature | 100 °C |
| Ramp | 4 °C/min to 240 °C, held at final temperature 30 min |
| Milling Time (min) | Methyl Palmitoleate | Methyl Palmitate | Methyl Linoleate | Methyl Oleate | Methyl Stearate | Methyl Eicosenate | Methyl Erucate | Methyl Behenate |
|---|---|---|---|---|---|---|---|---|
| 60 | ✓ | ✓ | ✓ | |||||
| 90 | ✓ | ✓ | ✓ | ✓ | ||||
| 105 | ✓ | ✓ | ✓ | |||||
| 120 | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| 135 | ✓ | ✓ |
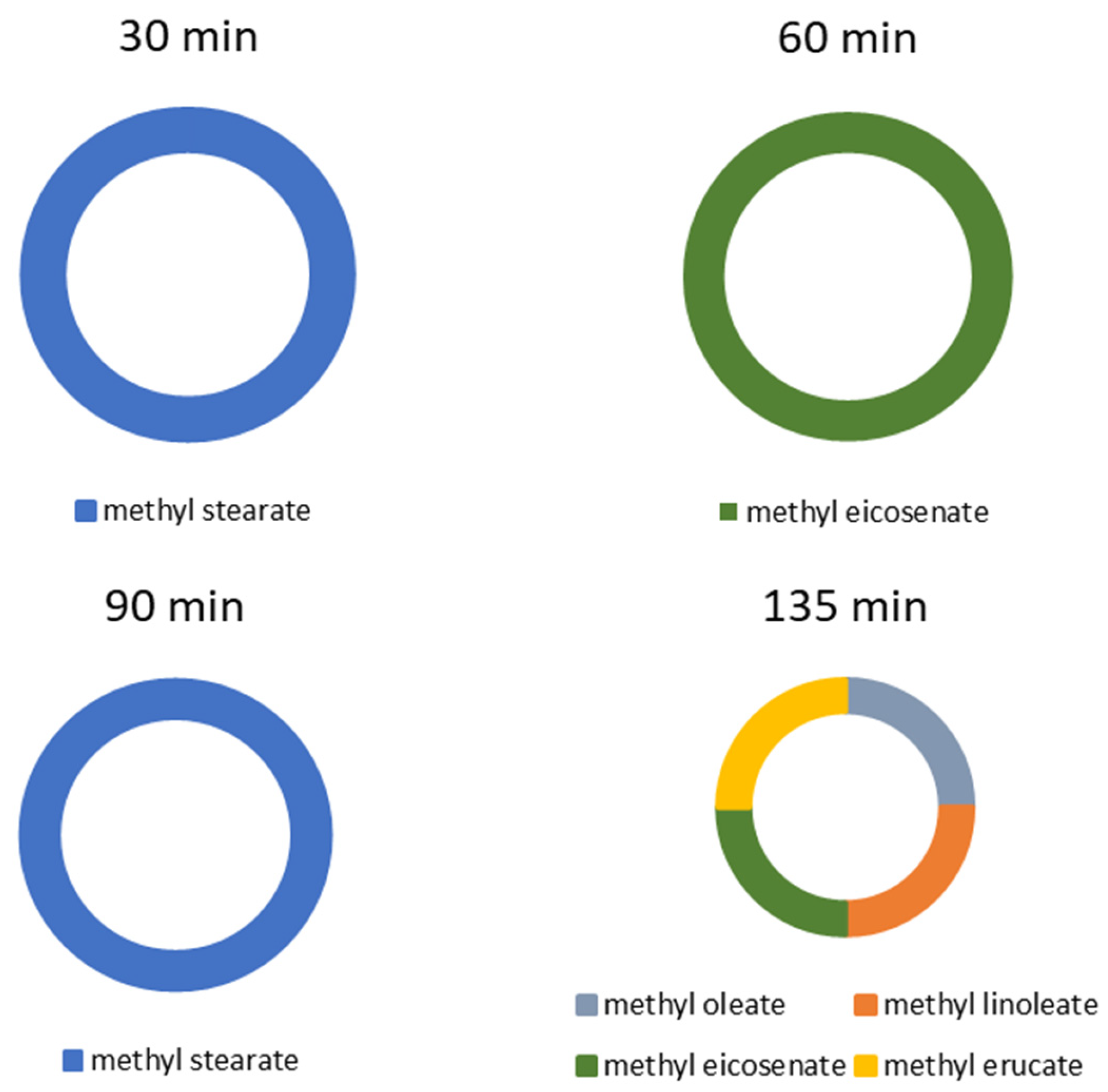
| Milling Times of Shells (min) | Methyl Esters Formed during Reflux |
|---|---|
| 30 | Methyl stearate (C:18:0) |
| 60 | Methyl eicosenate (C:20:0) |
| 90 | Methyl stearate (C:18:0) |
| 135 | Methyl oleate (C:18:1), Methyl linolenate (C:18:2), Methyl eicosenate (C:18:0), Methyl erucate (C:22:1) |
| Lead | ||||
| Biomass (g) | Absorbance | Lead Concentration (M) | Standard Deviation | % Adsorbed by Biomass |
| 0.2037 | 0.509 | 1.08 × 10−3 | 5.7 × 10−5 | 89.2 |
| 0.2059 | 0.432 | 9.46 × 10−4 | 90.5 | |
| 0.2011 | 0.489 | 1.05 × 10−3 | 89.5 | |
| 0.4993 | 0.293 | 7.01 × 10−4 | 6.9 × 10−5 | 93.0 |
| 0.5047 | 0.385 | 8.63 × 10−4 | 91.4 | |
| 0.5043 | 0.315 | 7.40 × 10−4 | 92.6 | |
| 1.0009 | 0.263 | 6.48 × 10−4 | 5.1 × 10−5 | 93.5 |
| 1.0106 | 0.284 | 6.85 × 10−4 | 93.2 | |
| 1.0035 | 0.214 | 5.62 × 10−4 | 94.4 | |
| Mercury | ||||
| Biomass (g) | Absorbance | Mercury Concentration (M) | Standard Deviation | % Adsorbed by Biomass |
| 0.2004 | 1.453 | 1.60 × 10−3 | 2.5 × 10−4 | 84.0 |
| 0.2051 | 0.945 | 1.00 × 10−3 | 90.0 | |
| 0.1998 | 1.076 | 1.16 × 10−3 | 88.4 | |
| 0.5025 | 0.499 | 4.70 × 10−4 | 1.7 × 10−4 | 95.3 |
| 0.5007 | 0.789 | 8.15 × 10−4 | 91.9 | |
| 0.5017 | 0.798 | 8.26 × 10−4 | 91.7 | |
| 1.0148 | 0.377 | 3.25 × 10−4 | 1.7 × 10−4 | 96.8 |
| 1.0092 | 0.459 | 4.22 × 10−4 | 95.8 | |
| 1.0062 | 0.722 | 7.35 × 10−4 | 92.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanner, A.; Baranek, M.; Eastlack, T.; Butts, B.; Beazley, M.; Hampton, M. Biodiesel Production Directly from Rapeseeds. Water 2023, 15, 2595. https://doi.org/10.3390/w15142595
Tanner A, Baranek M, Eastlack T, Butts B, Beazley M, Hampton M. Biodiesel Production Directly from Rapeseeds. Water. 2023; 15(14):2595. https://doi.org/10.3390/w15142595
Chicago/Turabian StyleTanner, Amanda, Morgan Baranek, Taylor Eastlack, Brian Butts, Melanie Beazley, and Michael Hampton. 2023. "Biodiesel Production Directly from Rapeseeds" Water 15, no. 14: 2595. https://doi.org/10.3390/w15142595
APA StyleTanner, A., Baranek, M., Eastlack, T., Butts, B., Beazley, M., & Hampton, M. (2023). Biodiesel Production Directly from Rapeseeds. Water, 15(14), 2595. https://doi.org/10.3390/w15142595







