Abstract
Rapeseeds are desirable as feedstock for manufacturing biodiesel due to their high production capacity and oil yield. The standard production of biodiesel from rapeseeds is a lengthy process including multiple time-consuming steps, and the method is not environmentally friendly because of the large energy requirements, the use of high volumes of organic solvents and water, and the need for a catalyst, which must be captured, purified, and recycled. In this study, we report a method to produce biodiesel in a single mechanical step directly from intact rapeseeds without adding a catalyst. The process is more environmentally friendly as there is less need for water than in current industrial processes, and it reduces pollutants from organic solvents, catalysts, and wastewater. Additionally, the remaining biomass shows adsorption characteristics for metals, potentially making it useful in water remediation.
1. Introduction
Biodiesel is a kind of biofuel derived from alcohol and animal fats or vegetable oils. Its renewability, minimal toxicity, and biodegrading ability make it a viable alternative to petroleum. Furthermore, biodiesel is suitable for modern engines with little to no modification, making the introduction of blended diesel/biodiesel fuels or complete biodiesel (B100) fuels straightforward []. It is made from replenishable feedstocks, with rapeseed feedstocks accounting for approximately 70% of global biodiesel production []. The seeds produce up to one ton of oil per hectare, making them the highest-yielding oil crop. Additionally, the resulting biodiesel has a low gel point, which is the temperature at which diesel will no longer flow through fuel lines []. Biodiesels are comprised of mono-alkyl esters of long-chain fatty acids, and in rapeseed oil, approximately 90% of these fatty acids consist of C-18 or longer, leading to a high cetane number, which is a desirable trait in biodiesels to ensure combustion quality [,,]. The exact fatty acid composition varies depending on the rapeseed cultivar, though the general composition consists of oleic, linoleic, linolenic, stearic, and palmitic acids [].
Rapeseed biodiesel production is energy intensive and inefficient on a commercial scale. Raw seeds must be collected in the agricultural phase and then dried and stored prior to extraction, which may be accomplished physically through pressing or chemically through conditioning, solvent extraction, and distillation. This process alone is time-consuming and requires large amounts of energy and resources. Raw oil is the main product, along with rapeseed meal coproduct, obtained from the extraction []. The raw oil is sent through physical and chemical treatments for purification and refinement. The refined oil is transesterified to produce fatty acid methyl esters, or biodiesel, as well as a glycerol byproduct [,]. The reaction is reversible, so typically, an excess of alcohol is necessary to drive the reaction to its products in high yields. Additionally, a catalyst is essential for the reaction. A base such as sodium hydroxide or potassium hydroxide is typically used, as it leads to faster reaction rates and reduced corrosion [,,,]. However, the use of a catalyst also leads to more intense and costly product cleanup and additional processes for recycling the catalyst. Homogenous catalysts, in particular, are popular due to high biodiesel conversion, but they consume large amounts of energy, require intensive catalyst separation, and generate massive amounts of wastewater during separation and cleaning []. The wastewater from biodiesel production contains high concentrations of oil, methanol, and byproduct. Twenty-eight million cubic meters of this wastewater were generated in 2011 alone []. The wastewater has a high pH and high concentrations of oil and phosphorus and, combined with low nitrogen concentrations, is difficult to degrade under natural conditions, as microorganism growth is not favored [,]. Alternative methods for the production of biodiesel are, therefore, desirable to reduce the usage of the catalyst, energy, and solvents.
While several catalyst-free processes for biodiesel production have been reported, most require high pressure, high temperatures, and large excesses of methanol. Examples include Saka and Kusdiana using supercritical methanol, Yamazaki et al. using a semi-batch process, and Joelianingsih et al. using a bubble column reactor [,,]. In the supercritical methanol method, a reaction vessel contains rapeseed oil and methanol in a 1:42 molar ratio, and it is placed in a preheated 350 to 400 °C bath at 45–65 MPa for 240 s. Conversion rates to methyl esters are high at over 95%; however, the energy requirements to obtain this conversion are not ideal. Similar high energies were required for the other methods mentioned above.
It has been shown that mechanical milling provides an alternative approach to the preparation of biodiesel from rapeseed oil []. In this method, thermal energy is replaced by mechanical energy to drive the reaction. Biodiesel studies using mechanical milling are limited; however, Yang et al. found that ball milling soybean oil and methanol in an 18:1 molar ratio with an activated potassium carbonate catalyst led to 100% conversion to biodiesel in 25 min. Energy consumption by the milling process was also determined, and ball milling used a mere 19% of the over 200 kWh/mol required by standard biodiesel production processes []. Using mechanical energy to transesterify the reaction is, therefore, both quicker and more energy efficient and avoids the high pressures and temperatures of previously studied alternative methods.
Previously, mechanophores have been introduced in polymer mechanochemistry. Mechanophores are molecules that are selectively activated with the application of a mechanical force, such as from milling. Mechanical activation can enhance the reaction rate, such as in the first mechanophore discovered by the Moore group in 2005 []. Mechanical force can also drive a reaction to new products through unique reaction pathways. As such, mechanophores have been used for processes including damage detection, controlled release, and catalysis [,,]. For example, Sijbesma’s group determined that mechanochemical scission via sonication of polymeric silver-N-heterocyclic carbene complexes catalyzed the transesterification reaction of benzyl alcohol and vinyl acetate. Sonication led to 65% conversion, whereas conversion was less than 3% without sonication at the same temperature []. We report here a more sustainable and quicker method for the production of biodiesel directly from intact rapeseeds without an added catalyst. This method is rapid, providing 90% conversion of seeds to biodiesel in one to two hours. Current methods require approximately five hours to convert rapeseed to biodiesel []. Additionally, this method requires less energy than normal commercial processes and does not result in the formation of soap, making cleanup much quicker and more energy efficient [].
2. Materials and Methods
2.1. Materials
The methanol used was ACS grade from Thermo Fisher Scientific (Waltham, MA, USA) and was distilled prior to use. Dichloromethane, 99.8+% stabilized with amylene, was from Acros Organics (Geel, Belgium). High erucic acid level Dwarf Essex Rapeseeds (Brassica napus), 98.00%, were from Boonetown Seed Co., Yorktown, IN, USA, and used as received. Methyl stearate and methyl palmitate standards from Alfa Aesar (Haverhill, MA, USA); methyl palmitoleate, methyl behenate, methyl erucate, methyl linoleate, methyl linolenate, and methyl oleate standards from Acros Organics (Geel, Belgium); methyl eicosenate standard from Restek (Bellefonte, PA, USA); and erucic acid standard from Sigma Aldrich (St. Louis, MO, USA) were used. Sussed cold-pressed extra virgin rapeseed oil was used to compare generated oil and biodiesel. Magnesium oxide was from Mallinckrodt (St. Louis, MO, USA), and calcium oxide, 97+%, was from Acros Organics (Geel, Belgium). Lead nitrate and mercury nitrate from Thermo Fisher Scientific (Waltham, MA, USA) were used for adsorption studies.
2.2. Ball Milling Procedure
Samples were prepared according to Table 1, with all reactants and additives placed in a 65 mL stainless steel milling jar. For those trials using a catalyst to compare to trials without, magnesium oxide or calcium oxide was dried and added to the milling jar with no modification. In the first experiment, a Spex 8000 Mixer Mill (SPEX SamplePrep, Metuchen, NJ, USA) was used for milling in 15 min intervals for a total of 60 min before the sample was cooled and removed from the milling jar. The second experiment was prepared and milled as above for 90 min. Subsequent experiments were run for 105, 120, and 135 min total. All experiments were run in triplicate. After the final milling interval, the milling jar was allowed to cool for 30 min before the contents, without steel balls, were transferred to a centrifuge tube and centrifuged at 3500 rpm for 45 min using an LW Scientific E8 Fixed Centrifuge (LW Scientific, Lawrenceville, GA, USA). Three layers formed after centrifugation: a top layer of methanol, a middle layer of biodiesel, and a bottom layer of solid rapeseed biomass. Transfer pipettes were used to remove the top and middle layers after centrifuging. The middle layer was weighed, transferred to a vial, and refrigerated until analysis via gas chromatography and viscosity testing. The percent yield of the biodiesel product was determined via Equation (1) below, in which 0.2787 g of oil per 1 g of rapeseeds was determined by hydraulic pressing an exact amount of rapeseeds with the shells on and obtaining the mass afterward. Gas chromatography analysis of the oil alone did not show methyl esters; therefore, the equation indicates the mass of biodiesel per every 0.2787 g of oil that is capable of being produced through ball milling with methanol. The product was also tested for soap formation by adding an excess volume of water and shaking vigorously. Observing cloudiness or particulate matter would indicate that soaps formed during milling.

Table 1.
Amounts of reactants and additives for each ball milling trial.
2.3. Rapeseed Shell Activation
Rapeseeds were placed in a −41 °C freezer overnight before being ground with mortar and pestle to separate the rapeseed shells from the seeds, as seen in Figure 1. Next, 3.0 g of shells, 3.0 mL of distilled methanol, and 12 3/8-inch stainless steel balls were added to the milling jar, and the contents were milled in 15 min intervals for 4 separate trials of 30 min, 60 min, 90 min, and 135 min to determine the optimal activation time for the catalyst. The best activation settings were then further tested by varying the size and quantity of steel balls used. Once milled, the shells were used in a reflux with store-bought rapeseed oil and methanol, described in Section 2.4 below.

Figure 1.
Illustration of rapeseed shell preparation.
2.4. Reflux
Three separate refluxes were run, the first using store-bought rapeseed oil and methanol only, the second with added mortar and pestle ground rapeseed shells, and the last with pre-milled rapeseed shells. For all trials, 10 mL of oil was added to a round bottom flask, and for those run with shells, 1.5 g of rapeseed shells, manually ground or those run in the ball mill, were added. A water condenser was attached to the round bottom, which was then heated at approximately 65 °C for five hours. After cooling, the solution was transferred to a centrifuge tube, centrifuged, and stored as previously described.
2.5. GC Analysis
A Perkin Elmer Clarus 500 Gas Chromatograph with a flame ionization detector (PerkinElmer, Waltham, MA, USA) was used to analyze all samples. Samples were prepared using 30 µL of the middle layer obtained from ball milling and 495 µL of dichloromethane solvent. Table 2 summarizes the GC method used. Samples were compared to methyl ester standards run using the same method.

Table 2.
Specifications for the GC analysis method.
2.6. Viscosity Testing
A Cannon Fenske Viscometer (Cannon Instrument Company, State College, PA, USA) was used to determine the kinematic viscosity of the middle layer obtained from ball milling. The calibration and calculation of the viscosity constant for the viscometer were completed with deionized water at 60 °C. The time for the rapeseed oil and biodiesel to travel from the top calibration mark to the bottom mark was recorded, and the viscosity was determined by multiplying the time in seconds by the viscosity constant at 60 °C. Tests were performed five times for 60, 90, 105, 120, and 135 min trials.
2.7. Testing of Biomass as a Heavy Metal Adsorbent
From the three layers formed after centrifugation as described for ball milling in Section 2.2 above, the bottom layer of rapeseed biomass was tested as an adsorbent for heavy metals. This was done using stock solutions of lead nitrate and mercury nitrate at 10−2 M. Further, 0.2, 0.5, and 1 g of biomass were weighed and placed in separate vials. Next, 10 mL of a metal ion solution was added to each vial, and the vials were mixed for 180 min, keeping the temperature constant at 22 °C. The solutions were then placed in centrifuge tubes and centrifuged for 30 min, forming two layers: a solid bottom layer of biomass and a top aqueous layer. The aqueous layer was removed via transfer pipette and analyzed using a Thermo Scientific Genesys 180 UV-Visible Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). A calibration curve consisting of 1.0 × 10−2 to 1 × 10−6 M solutions in 1 × 10−1 M decreasing concentrations was made for each metal, using a 1-(2-pyridylazo)-2-naphthol indicator. The maximum wavelength for the lead and mercury solutions were 558 nm and 564 nm, respectively. All solutions were run in triplicate.
3. Results and Discussion
3.1. Ball Milling with a Catalyst
Samples of rapeseeds with shells milled with methanol and calcium oxide contained the methyl esters methyl oleate (C:18:1), methyl linolenate (C:18:2), methyl stearate (C:18:0), and methyl erucate (C:22:1). Longer milling times (105 to 135 min) indicated all four methyl esters, while shorter milling times (60 to 90 min) indicated methyl stearate and methyl erucate only. Samples of rapeseeds with shells milled with methanol and magnesium oxide contained the same methyl esters. Conversion yields of up to 90% were calculated. These results were expected, as they are similar to those found by Yang et al., though notably using intact rapeseeds and a lower ratio of methanol to oil [].
3.2. Ball Milling without a Catalyst
Only methanol and intact rapeseeds were run in the ball mill. When this produced methyl esters, the rapeseeds were deshelled to better determine what could be catalyzing the reaction past the high impact in the ball mill alone. The controls of only rapeseed shells or deshelled rapeseeds did not indicate the formation of any methyl esters. Since the controls did not produce biodiesel, the concern of metal contamination from the milling media potentially catalyzing the reaction rather than the reactants was minimized. Analysis by gas chromatography of biodiesel produced from rapeseeds and shells milled together indicated the formation of methyl palmitoleate (C:16:1), methyl oleate (C:18:1), methyl linolenate (C:18:2), methyl eicosenate (C:20:0), and methyl erucate (C:22:1), as indicated in Table 3. Methyl erucate was present in all samples and is expected for Dwarf Essex rapeseeds, which have a high content of erucic acid. Up to 90% conversion yields were produced, and soap formation was not observed. Similar to results from ball milling with a catalyst, milling times affected the methyl esters formed, with shorter (60 to 90 min) times indicating methyl palmitoleate while longer milling times (135 min) indicate methyl eicosenate. Milling times between these led to varying results, though methyl oleate, linoleate, and eicosenate were frequently observed. The gas chromatogram of biodiesel produced from 105 min of ball milling is shown in Figure 2. The first peak shows DCM, followed by methyl esters. The methyl esters formed were methyl oleate (27.50 min), methyl stearate (28.29 min), and methyl erucate (37.22 min).

Table 3.
Summary of methyl esters found at 60, 90, 105, and 135 min of ball milling. A ✓ indicates the presence of the methyl ester.
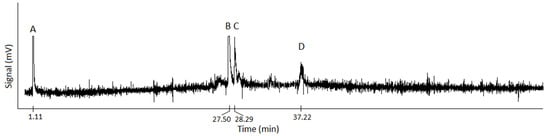
Figure 2.
Gas chromatogram of biodiesel produced from 105 min of ball milling. Peak A is dichloromethane solvent, B is methyl oleate, C is methyl stearate, and D is methyl erucate.
3.3. Reflux with Store-Bought Oil and with Seeds
Commercially purchased rapeseed oil and whole rapeseeds were refluxed separately with methanol to compare the processes. However, no biodiesel was observed after reflux. Reflux was also done with the inner seed alone and the shell alone, yet biodiesel was again not observed. This indicated that the catalyst for the reaction came specifically from the ball milling process.
Rapeseed shell hulls were separated from the seed and ball milled separately prior to adding them to a reflux with commercially purchased oil and methanol as well as a reflux with deshelled seeds and methanol. Both samples indicated the formation of methyl linolenate, methyl oleate, methyl stearate, methyl eicosenate, and methyl erucate, depending on the milling time of the seeds prior to reflux, as seen in Table 4 and Figure 3. Ball milling rapeseeds prior to refluxing with the rapeseed oil produced methyl esters, suggesting that the rapeseed shells contain a mechanophore that is only activated by the collisions within the ball mill, and milling time affects the activation and, therefore, the final product of transesterification. Mechanophores are molecules that respond to mechanical stimuli, such as that from a ball mill. The mechanical force causes structural rearrangement in the molecule, leading to a physical or chemical signal []. Mechanocatalysts do not display chemical reactivity until they are mechanically activated, where they can begin to catalyze a reaction. Those that have been discovered occur from the cleavage of chemical bonds that reveal the active species. Thus far, few mechanocatalysts have been reported, but all of them have utilized metal centers and have been limited to an activity with just one or two reactions [,,,]. Future work will further analyze the rapeseed shells to determine the exact molecule that acts as the catalyst. However, an initial hypothesis is that the molecule has a metal center, as all previously discovered mechanocatalysts have consisted of metal centers, including but not limited to cobalt, copper, iridium, iron, and nickel, and rapeseed has been found to contain trace amounts of metals such as nickel, iron, and copper [,].

Table 4.
Milling times of rapeseed shells and the methyl esters of the resulting transesterification with rapeseed oil.

Figure 3.
Summary of methyl esters present after 30, 60, 90, and 135 min of ball milling rapeseed shells and performing a reflux.
Mechanisms for transesterification via acid and base catalysis have been proposed previously. Though the mechanism for mechanical activation is likely rather different, some similarities, such as tetrahedral intermediate formation, are possible. In base catalysis, a tetrahedral intermediate is formed by an alkoxide ion attacking the carbonyl carbon of the triglyceride. This intermediate then reacts with an alcohol, and the rearrangement produces an ester and a diglyceride, as illustrated in Figure 4 below [].
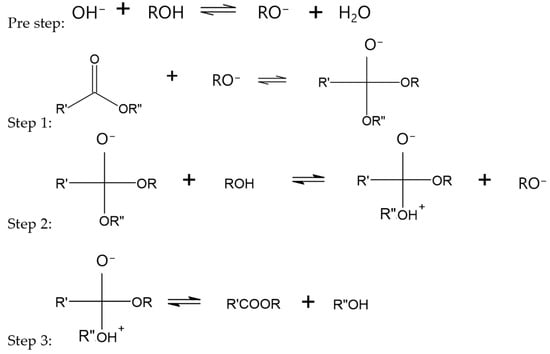
Figure 4.
Base-catalyzed transesterification reaction mechanism where R is the alkyl group of the alcohol, R’ is the carbon chain of the fatty acid, and R” is glyceride.
In acid catalysis, a carbocation is formed from the protonation of the carbonyl group of the ester. A tetrahedral intermediate is also produced in this mechanism from the nucleophilic attack of the alcohol. The intermediate forms a new ester and regenerates the catalyst []. This is demonstrated below in Figure 5. Once the catalyst for our process has been identified, the mechanism will be studied similarly.

Figure 5.
Acid-catalyzed transesterification reaction mechanism where R is the alkyl group of the alcohol, R’ is the carbon chain of the fatty acid, and R” is glyceride.
3.4. Viscosity
The rapeseed oil was found to have a kinematic viscosity of 42 cST, and biodiesel produced from the high-energy ball mill had a kinematic viscosity of 18 cST. ASTM standards require kinematic viscosity to be in the range of 1.9 to 6.0 cST. While the decrease in viscosity from the pure oil to the ball mill samples is indicative of biodiesel being formed, the modification of milling times and volumes still needs to be considered as a means of lowering viscosity further in order to reach ASTM standards.
3.5. Heavy Metal Adsorption
In order to use all of the products of the biodiesel process, heavy metal adsorption by the leftover rapeseed biomass from milling was tested. Extending the usage of biomass prevents a buildup of waste over time and creates a more environmentally sound lifecycle for biodiesel than currently exists in the industry. Studies by Paduraru et al. in 2015 and Teodora et al. in 2022 showed that leftover biomass from typical rapeseed biodiesel can adsorb heavy metals such as lead, mercury, and zinc from water [,]. Similar results were found for our rapeseed biomass leftover from milling. Summarized in Table 5, the samples with the highest concentration of biomass at 1 g to 10 mL of metal nitrate adsorbed the most metal at an average of 94% for lead nitrate and 95% for mercury nitrate across three trials. The samples using 0.5 g of biomass had only slightly lower adsorption efficiencies at averages of 92% and 93% for lead and mercury, respectively. As expected, those tests with the lowest concentration of biomass adsorbed the least: an average of 90% for lead nitrate and 88% for mercury nitrate. The adsorption of additional metals, desorption of all metals, and characterization of the biomass are planned for future studies.

Table 5.
Absorbance, metal concentration, and percent adsorption by the biomass across all trials.
3.6. Energy Consumption
Based on the 2013 study completed by Rajaeifar et al., a typical process for the production of biodiesel from 1 hectare of rapeseed consumes approximately 28,100 MJ, separated into four steps of agricultural crop production: transportation, oil pressing, transesterification, and, finally, biodiesel. The process used in our research removes the oil pressing step entirely, saving approximately 2000 MJ on this step alone []. Furthermore, the energy input of the transesterification step is reduced by the complete removal of any catalyst and greatly reduced volumes of methanol by using a one-to-one ratio instead of an excess. Figure 6 details the energy requirements for our process. The energy required for cleanup is not included in the analysis, but it is significantly lower compared to today’s standard processes, as the removal of a catalyst and soap is not necessary.
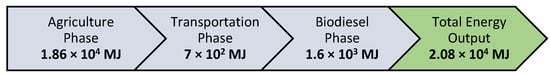
Figure 6.
Flowchart of proposed total energy requirements for our process.
4. Conclusions
We have shown that biodiesel can be produced directly from intact rapeseeds in one step via mechanical milling without adding a catalyst. It was found that the catalyst was present in the shells of the rapeseeds as a mechanophore that is activated by the milling process. This method of producing biodiesel greatly decreases the time, energy, petroleum-based solvents, and required chemicals for catalysis, thus making the produced biodiesel more sustainable and cost-effective than current industry methods. Furthermore, cleanup of soap products is not necessary, greatly reducing wastewater generation. The leftover biomass from the process is also an efficient adsorbent for lead and mercury metals, and the overall process is more energy efficient by approximately 7000 MJ.
Author Contributions
Conceptualization, M.H.; methodology, A.T., M.B. (Morgan Baranek), and T.E.; validation, A.T. and B.B.; formal analysis, A.T. and M.B. (Morgan Baranek); investigation, A.T., M.B. (Morgan Baranek) and T.E.; resources, M.H.; data curation, A.T. and M.B. (Morgan Baranek); writing—original draft preparation, A.T.; writing—review and editing, M.H., M.B. (Melanie Beazley), and B.B.; visualization, M.H.; supervision, B.B. and M.H.; project administration, M.H. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karmakar, B.; Halder, G. Progress and future of biodiesel synthesis: Advancements in oil extraction and conversion technologies. Energy Convers. Manag. 2019, 182, 307–339. [Google Scholar] [CrossRef]
- Kim, D.S.; Hanifzadeh, M.; Kumar, A. Trend of biodiesel feedstock and its impact on biodiesel emission characteristics. Environ. Prog. Sustain. 2018, 37, 7–19. [Google Scholar] [CrossRef]
- Rapier, R. Renewable Diesel. In Biofuels, Solar and Wind as Renewable Energy Systems, 1st ed.; Pimentel, D., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 153–157. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. PECS 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Progress in biodiesel processing. Appl. Energy 2010, 87, 1815–1835. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Erucic Acid in feed and food. EFSA 2016, 14, e04593. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value added uses for crude glycerol—A byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef]
- Otera, J. Transesterification. Chem. Rev. 1993, 93, 1449–1470. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification kinetics of soybean oil 1. JAOCS 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- Noureddini, H.; Zhu, D. Kinetics of transesterification of soybean oil. JAOCS 1997, 74, 1457–1463. [Google Scholar] [CrossRef]
- Mittelbach, M.; Trathnigg, B. Kinetics of alkaline catalyzed methanolysis of sunflower oil. Eur. J. Lipid Sci. Technol. 1990, 92, 145–148. [Google Scholar] [CrossRef]
- Vicente, G.; Martinez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogenous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, V.B.; Stamenkovic, O.S.; Tasic, M.B. The wastewater treatment in the biodiesel production with alkali-catalyzed transesterification. Renew. Sust. Energ. Rev. 2014, 32, 40–60. [Google Scholar] [CrossRef]
- Srirangsan, A.; Ongwandee, M.; Chavalparit, O. Treatment of biodiesel wastewater by electrocoagulation process. Environ. Asia 2009, 2, 15–19. [Google Scholar]
- Kolesarova, N.; Hutnan, M.; Bodik, I.; Spalkova, V. Utilization of Biodiesel By-Products for Biogas Production. J. Biotechnol. Biomed. 2011, 2011, 126798. [Google Scholar] [CrossRef] [PubMed]
- Kusdiana, D.; Saka, S. Kinetics of transesterification in rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel 2001, 80, 693–698. [Google Scholar] [CrossRef]
- Yamazaki, R.; Iwamoto, S.; Nabetani, H.; Osakada, K.; Miyawaki, O.; Sagara, Y. Noncatalytic alcoholysis of oils for biodiesel fuel production by a semi-batch process. JJFE 2007, 8, 11–18. [Google Scholar] [CrossRef]
- Joelianingsih; Maeda, H.; Hagiwara, S.; Nabetani, H.; Sagara, Y.; Soerawidjaya, T.H.; Tambunan, A.H.; Abdullah, K. Biodiesel fuels from palm oil via the non-catalytic transesterification in a bubble column reactor at atmospheric pressure: A kinetic study. Renew. Energy 2008, 33, 1629–1636. [Google Scholar] [CrossRef]
- Todd, J. Mechanochemical synthesis of fuels from sustainable sources utilizing solid catalysts. STARS 2017, 2004–2019, 5566. [Google Scholar]
- Yang, N.; Sheng, X.; Ti, L.; Jia, H.; Ping, Q.; Li, N. Ball-milling as effective and economical process for biodiesel production under Kraft lignin activated carbon stabilized potassium carbonate. Bioresour. Technol. 2023, 369, 128379. [Google Scholar] [CrossRef]
- Berkowski, K.; Potisek, S.; Hickenboth, C.; Moore, J. Ultrashound-induced site-specific cleavage of azo-functionalized poly(ethylene glycol). Macromolecules 2005, 38, 8975–8978. [Google Scholar] [CrossRef]
- Gostl, R.; Sijbesma, R. π-extended anthracenes as sensitive probes for mechanical stress. Chem. Sci. 2016, 7, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zeng, T.; Husic, C.; Robb, M. Mechanically triggered small molecules release from a masked furfuryl carbonate. J. Am. Chem. Soc. 2019, 141, 15018–15023. [Google Scholar] [CrossRef] [PubMed]
- Piermattei, A.; Karthikeyan, S.; Sijbesma, R.P. Activating catalysts with mechanical force. Nat. Chem. 2009, 1, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Rajaeifar, M.A.; Ghobadian, B.; Heidari, M.D.; Fayyazi, E. Energy consumption and greenhouse gas emissions of biodiesel production from rapeseed in Iran. JRSE 2013, 5, 63134. [Google Scholar] [CrossRef]
- Chen, Y.; Mellot, G.; van Luijk, D.; Creton, C.; Sijbesma, R.P. Mechanochemical tools for polymer materials. Chem. Soc. Rev. 2021, 50, 4100–4140. [Google Scholar] [CrossRef]
- Jakobs, R.T.M.; Ma, S.; Sijbesma, R.P. Mechanocatalytic polymerization and cross-linking in a polymeric matrix. ACS Macro Lett. 2013, 2, 613–616. [Google Scholar] [CrossRef]
- Michael, P.; Binder, W.H. A mechanochemically triggered “click” catalyst. Angew Chem. Int. 2015, 54, 13918–13922. [Google Scholar] [CrossRef]
- Wei, K.; Gao, Z.; Liu, H.; Wu, X.; Wang, F.; Xu, H. Mechanical activation of platinum–acetylide complex for olefin hydrosilylation. ACS Macro Lett. 2017, 6, 1146–1150. [Google Scholar] [CrossRef]
- Hara, M.; Komoda, M.; Hasei, H.; Yashima, M.; Ikeda, S.; Takata, T.; Kondo, J.; Domen, K. A study of mechano-catalysts for overall water splitting. J. Phys. Chem. B 2000, 104, 780–785. [Google Scholar] [CrossRef]
- Przybylski, R. Canola/rapeseed oil. In Vegetable Oils in Food Technology: Composition, Properties, and Uses, 2nd ed.; Gunstone, F., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 107–133. [Google Scholar] [CrossRef]
- Meher, C.; Sagar, D.; Naik, S. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energ. Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Paduraru, C.; Tofan, L.; Teodosiu, C.; Bunia, I.; Tudorachi, N.; Toma, O. Biosorption of zinc(II) on rapeseed waste: Equilibrium studies and thermogravimetric investigations. Process Saf. Environ. Prot. 2015, 94, 18–28. [Google Scholar] [CrossRef]
- Arsenie, T.; Cara, I.G.; Popescu, M.C.; Motrescu, I.; Bulgariu, L. Evaluation of the adsorptive performances of rapeseed waste in the removal of toxic metal ions in aqueous media. Water 2022, 14, 4108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).