Abstract
Sodium hypochlorite (NaClO) solution is wildly used to remove membrane fouling-derived organic materials and restore membrane flux, which can result in the formation of halogenated by-products. To reduce the halogenated by-products, a combined cleaning process with NaClO and peroxides including hydrogen peroxide (H2O2), peroxydisulfate (PDS), and peroxymonosulfate (PMS) were applied in offline mode to remove the organic fouling. It was found that all the combined cleaning processes could effectively restore the membrane flux. Compared with the process of NaClO cleaning followed by peroxide cleaning (NaClO–peroxide), fewer halogenated by-products were generated in the NaClO post-combined cleaning process (peroxide–NaClO), and the PDS–NaClO cleaning process exhibited the best performance in controlling by-products. Overall, most by-product generation showed a positive correlation with reaction time and temperature.
1. Introduction
As an efficient physical separation technology without the addition of chemicals, ultrafiltration membrane technology has been broadly applied in the fields of advanced purification of drinking water and wastewater [1]. However, membrane fouling remains a persistent problem during the filtration process due to the attachment of particulates, colloids, and micro-biological organisms on membrane surface and pores, which can lead to energy consumption and longer filtration time [2]. Membrane fouling has been widely recognized as a major obstacle to the further application of ultrafiltration technology [3]. To overcome this issue, various strategies have been developed, including membrane material modification, pretreatment of feed water, and optimization of the operation process [4,5,6]. The chemical structure, surface charge, hydrophilicity, and pore size of membrane materials significantly affect the filtration efficiency and fouling generation. Therefore, much of the research on membrane materials modification has focused on the development of new membrane materials with high flux and excellent anti-fouling performance, which can effectively control the generation of membrane fouling [7]. Pretreatment of feed water is intended to alter the properties of feed water through coagulation, adsorption, or oxidation prior to the filtration process, which can alleviate the generation of membrane fouling [8,9]. The optimization of the operation process focused on the change in operating parameters such as membrane flux (or transmembrane pressure), cleaning frequency, and filtration mode (constant pressure or constant current), which can reduce membrane fouling [10,11,12].
Compared to other strategies, membrane cleaning is an effective method for removing foulants from membrane surface and pores, resulting in membrane flux recovery and the reuse of membrane [13]. Membrane cleaning can be divided into physical cleaning and chemical cleaning [14,15]. The cleaning processes can also be classified into online and offline cleaning depending on whether the membrane components need to be transferred from the filtration system [16,17]. Sodium hypochlorite (NaClO) is an extensive choice in both online and offline cleaning processes due to its effectiveness in removing organic and biological fouling [18]. For online cleaning, enhanced back-flush with water containing NaOCl at concentrations ranging from several mg/L to several dozen mg/L can be used to remove foulants [17,19]. Alternatively, fouled membranes can be soaked in NaOCl solution at concentrations ranging from several dozen mg/L to several thousand mg/L for offline cleaning [20]. However, previous studies reported that NaClO cleaning in offline mode can lead to the generation of toxic halogenated by-products such as trichloromethanes (THMs), haloacetic acids (HAAs), chloral hydrate (CH), haloacetonitriles (HANs), and haloketones (HKs) during the cleaning of membrane fouled by organic and biological matters [21]. Cai et al. also proved that the exposure of activated sludge to NaClO in the MBR online chemical cleaning process could result in the release of a substantial amount of DOM, and a series of halogenated by-products such as haloacetic acids, haloquinones, halophenols, and halopyrroles were detected in the cleaning solution [22]. Some of the toxic halogenated by-products generated would inevitably be released into = natural water bodies through the discharge of cleaning solution in offline mode and permeate in online mode, resulting in potential environmental risks to human health and the natural environment.
Acids, bases, oxidants, surfactants, and chelating agents are the commonly used chemicals in the membrane cleaning process. The combination of different agents is also used to remove different types of membrane fouling, which may reduce the formation of halogenated by-products during NaClO cleaning [22]. For instance, Woo et al. used the combination of oxalic acid (H2C2O4) and NaClO to clean the ultrafiltration membranes that were applied as pretreatment for seawater desalination. They found that a cleaning in series of H2C2O4–NaClO–H2C2O4 showed optimal cleaning efficiency with a recovery efficiency of 96.8%, 95.8%, 98.3%, and 99.9% after first, second, third, and fourth cleanings, respectively [23]. Tian et al. reported that individual sodium hydroxide (NaOH) and ethanol cleaning had limited performance in removing irreversible resistance caused by river water. However, consecutive use of NaOH and ethanol had a synergistic effect on the removal of membrane fouling [24]. Alongside NaClO, hydrogen peroxide (H2O2) is also a common chemical agent in membrane cleaning process [25]. In recent years, peroxydisulfate (PDS), and peroxymonosulfate (PMS) have also been wildly used in drinking water and wastewater treatment, as well as membrane cleaing [26,27,28,29]. Due to the absence of a halogen atom, the application of these peroxides could avoid the generation of halogenated by-products. Wang et al. applied Fe(II)/PMS process for online chemical cleaning of membrane bioreactor (MBR), which was reported to be a promising alternative for NaClO cleaning [30]. He et al. found a novel H2O2–MnO2 system for the efficient cleaning of fouled ultrafiltration membranes, which was attributed to simultaneous generation of reactive free radicals and oxygen [31]. However, the cost of these processes would be much higher than NaClO cleaning due to the low cost of NaClO. Previous studies have also reported that these peroxides can react with organic matter and NaClO, limiting the by-products during the reaction between organic matter and NaClO [32]. Thus, the combination of peroxide and NaClO would also be a good choice for membrane cleaning. However, very limited research has been conducted in this area.
In the present study, H2O2, PDS, and PMS were selected as representative peroxides to combine with NaClO for membrane cleaning in offline mode. To simulate membrane fouling, a synthetic feed water consisting of a mixture of humic acid (HA), bovine serum albumin (BSA), and sodium alginate (SA) was prepared. The effects of different cleaning sequences, including peroxide–NaClO (peroxide cleaning followed by NaClO cleaning) and NaClO–peroxide (NaClO cleaning followed by peroxide cleaning), on membrane flux recovery and generation of halogenated toxic by-products were investigated. Subsequently, the impact of various cleaning parameters on the generation of by-products was analyzed. The results of this study would provide insights into the development of combined cleaning which could make a balance in membrane cleaning and halogenated by-products generation control and be helpful for parameters optimization in practical combined cleaning process.
2. Materials and Methods
2.1. Chemical Agents
Ultrapure water used in this study was produced by a water purifier (MicroPure UV, Thermo Fisher Scientific, Waltham, MA, USA). Chemicals including PDS (Na2S2O8), H2O2, NaClO, NaOH, and sulfuric acid (H2SO4) were of analytical grade at least and purchased from Sinopharm Chemical Reagent Co., Ningbo, China. PMS (KHSO5·0.5KHSO4·0.5K2SO4), HA, BSA, and SA were obtained from Aladdin Co., Ltd. (Shanghai, China). The stock solutions of HA, BSA, and SA were prepared by dissolving corresponding solid powder in ultrapure water according to the method described by Cheng et al. [33]. The concentrations of HA, BSA, and SA in water samples for the filtration process were set at 10 mg/L. Before the experiment, the PDS and PMS stock solutions were prepared and determined by the oxidative coloration of N,N-diethyl-p-phenylene diamine (DPD, Aladdin Co., Shanghai, China) at an absorption of 510 nm [34]. The H2O2 solution was calibrated by colorimetric methods with DPD at an absorption of 551 nm [35]. Chlorine stock solution was prepared by diluting NaClO in ultrapure water and standardized periodically using the DPD/FAS titration method [32]. The concentrations of PDS, PMS, H2O2, and NaClO in the cleaning solutions were set at 1 mM. Standards of haloaldehydes (HAs), HKs, HANs, and trichloronitromethane (TCNM) were obtained from AccuStandard (New Haven, CT, USA), while THMs, trichloroethylene (TCE), monochloroacetic acid (MCAA), dichloroacetic acid (DCAA), and trichloroacetic acid (TCAA) were purchased from Supelco (Bellefonte, PA, USA), and the abbreviations of the selected halogenated by-products are shown in Table 1.

Table 1.
Abbreviations of the selected halogenated by-products.
2.2. Experimental Procedures
A schematic diagram of the experimental setup is displayed in Figure S1. A UF system, consisting of a dead-end filtration cell (Mosu Tech, Shanghai, China), a nitrogen gas cylinder coupled with reducing valves for stable pressure supply, and an electronic balance (Shunyu, JA21002, Shanghai, China) connected to a computer for the automatic recording, was used for the filtration process. Polyethersulfone flat UF membranes (Mosu Tech, Shanghai, China) with an effective membrane area of 38.5 cm2 and a molecular weight cut-off (MWCO) of 50 kDa were employed in this work [8]. Prior to use, the membranes were soaked in ultrapure water over 12 h to remove the preservative [4]. For each experiment, a UF membrane was placed at the bottom of the UF cup, and the initial flux was measured by 100 mL ultrapure water at a pressure of 80 kPa. The fouling process for the first cycle was conducted with the filtration of 250 mL feed water sample under the same pressure.
After filtration, the fouled membranes were immersed into different cleaning solutions (250 mL) for chemical cleaning. The chemical cleaning was conducted in two ways: (1) NaClO–peroxide and (2) peroxide–NaClO. The selected peroxides are H2O2, PDS, and PMS. As an example, for H2O2–NaClO cleaning, the fouled membrane was first immersed in H2O2 solution and then transferred to the NaClO solution for cleaning. The initial pH of all cleaning solutions was maintained at 7.0. After cleaning for a certain period, the cleaned membrane was rinsed by ultrapure water and then filtrated with feed water sample again for the second cycle. Two portions of 20 mL samples were withdrawn from NaClO solution and quenched with excess ascorbic acid for the next halogenated by-products analysis.
2.3. Analytical Methods
The cleaning efficiency was evaluated in the form of flux recovery. The membrane flux recovery could be calculated by Equation (1) [36]:
where is the pure water flux (L/m2 h, LMH) of membrane before fouling, represents the average permeate flux (LMH) at the end of first filtration process, and represents the average permeate flux (LMH) during the filtration of ultrapure water after chemical cleaning.
Gas chromatography (GC-2014 C, Shimadzu, Japan) was applied to analyze volatile chlorinated by-products and HAAs. According to USEPA methods 551.1 and 552.3, GC was coupled with an electron capture detector (ECD) and a ZB-5column (30 m × 0.25 mm, ID 0.25 µm) [37,38]. To analyze volatile chlorinated by-products, the temperature of injector and ECD were set to 200 °C and 290 °C, respectively. The temperature program of the oven began at 35 °C for 7 min and was then ramped to 200 °C at a rate of 40 °C/min and held for 2 min. For the measurement of HAAs, including MCAA, DCAA, and TCAA, the temperature of injector and ECD were set at 210 °C and 280 °C, respectively. The temperature program of oven began at 35 °C for 7 min, increased to 80 °C at a rate of 8 °C/min and held for 10 min, was ramped to 200 °C at a rate of 20 °C/min, and then held for 1 min. The detection limit of each halogenated by-product by the USEPA method was determined and shown in Table S1 [21,39]. Student’s t-test was employed to analyze the significance of by-product generation in different cleaning processes [40].
3. Results and Discussion
3.1. Removal of Membrane Fouling by the Combination of Peroxide and NaClO
In this study, the efficiency of different cleaning methods for fouling removal was compared using a combination of peroxide and NaClO. The results are displayed in Figure 1. During the experiment, the concentrations of NaClO and peroxide were 1 mM, and the fouled membrane was immersed in the peroxide solution for 1 h, followed by another 1 h for NaClO cleaning. It is observed that when the concentrations of HA, BSA, and SA in the simulated natural water were 10 mg/L, the membrane fouling was significant, causing the membrane flux at the end of filtration to decrease to around 30% of its initial value. While the membrane flux was found to have recovered to 98%, 100%, 105%, and 106% of its initial value after the cleaning by PDS–NaClO, PMS–NaClO, H2O2–NaClO, and NaClO alone, respectively. The membrane flux recovery were calculated to be 97%, 100%, 107%, and 109%, respectively. This suggests that the combined processes of PDS–NaClO and PMS–NaClO are effective in removing pollutants from the fouled membrane. Previous research has indicated that the fouling of membranes caused by HA–BSA–SA primarily leads to cake layer filtration, which can be recovered to approximately 80% of the initial flux through hydraulic backwashing [8]. Due to the strong oxidizing properties, NaClO can disrupt the organic matter’s structure and facilitate the detachment of organic matter from the membrane surface and pores. Consequently, in this study, various NaClO-based cleaning combinations show high efficiency to restore the membrane flux, and there is no significant disparity in the membrane flux recovery among the different combination methods. However, the membrane flux after cleaning was slightly higher than its original value after H2O2–NaClO cleaning and NaClO cleaning alone. Prior studies have reported consistent results, indicating that the membrane flux after NaClO or HCl cleaning exhibits an increase compared to the initial flux. This observation may be attributed to an enhancement of membrane hydrophilicity and an enlargement of membrane pore size [41,42]. During the second filtration cycle, the flux of membrane cleaned by PDS–NaClO, PMS–NaClO, H2O2–NaClO, and NaClO alone decreased by 69%, 71%, 71%, and 77%, respectively. These results indicated that PDS–NaClO, PMS–NaClO, and H2O2–NaClO cleaning methods had little impact on membrane fouling, while NaClO cleaning alone could exacerbate fouling during secondary use. Therefore, PDS–NaClO and PMS–NaClO exhibited better performance for membrane cleaning among the four peroxide–NaClO cleaning methods.
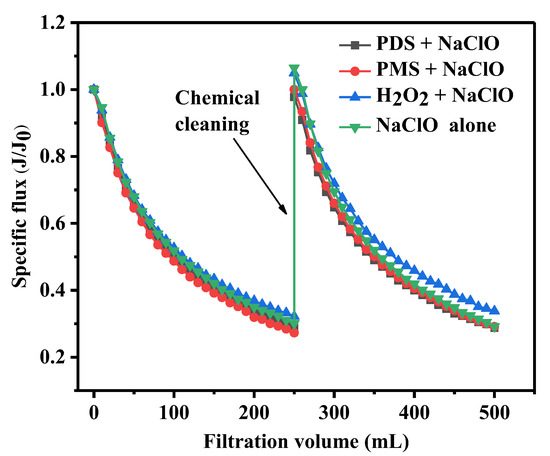
Figure 1.
Effect of peroxide–NaClO combination cleaning on membrane fouling removal.
As displayed in Figure 2, the efficiency of fouling removal by various NaClO–peroxide cleaning methods was compared. The four cleaning methods were found to be effective in restoring membrane flux, with NaClO–PDS, NaClO–PMS, NaClO–H2O2, and NaClO alone recovering 100%, 98%, 113%, and 106% of the initial flux, respectively. The membrane flux recovery were calculated to be 100%, 97%, 119%, and 109%, respectively. The membrane flux after H2O2–NaClO cleaning and NaClO cleaning alone is higher than that of a virgin membrane, suggesting that the combined NaClO–H2O2 would damage the UF membrane materials. Combining the results from Figure 1 and Figure 2, it is evident that the removal of membrane fouling by NaClO–H2O2 cleaning is superior to that of H2O2–NaClO cleaning. Furthermore, higher flux recovery could be found in NaClO–H2O2 compared to other combined cleaning process, which might be due to the production of HO• with higher oxidation capacity from H2O2 decomposition and the interaction between NaClO and H2O2. During the second filtration cycle, the flux of the membrane cleaned by NaClO–PDS, NaClO–PMS, NaClO–H2O2, and NaClO alone decreased by 70%, 68%, 79%, and 77%, respectively. This indicates that the combined cleaning processes of NaClO–PDS and NaClO–PMS had little effect on membrane fouling behavior during the following filtration process, whereas the membrane anti-fouling performance was reduced after NaClO–H2O2 cleaning. Hence, among the three NaClO–peroxide combined cleaning processes, NaClO–PDS and NaClO–PMS exhibited better cleaning performance with little influence on membrane properties.
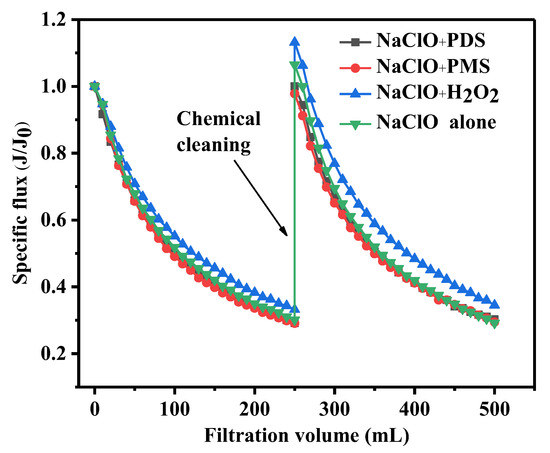
Figure 2.
Effect of NaClO–peroxide combination cleaning on membrane fouling removal.
3.2. Formation of Halogenated By-Product in Different Combined Processes
Figure 3 presents a comparison of halogenated by-product generation during peroxide–NaClO cleaning. The identified by-products include trichloromethane (TCM), trichloroacetaldehyde (CH), dichloroacetonitrile (DCAN), dichloroacetone (1,1-DCP), TCNM, trichloroacetone (TCP), and HAAs [21]. Table 2 displays the results of the significance analysis between combined cleaning processes and NaClO cleaning alone, and combined cleaning of fouled membranes affected the formation of by-products significantly with p < 0.05 for most of by-products. The highest formation of by-products could be found in the NaClO-alone cleaning process, with concentrations of TCM, CH, DCAN, TCP, MCAA, DCAA, and TCAA being 44.4, 6.5, 11.2, 4.7, 3.1, 62.9, and 137.3 μg/L, respectively. Combined peroxide–NaClO cleaning processes significantly reduced the generation of various halogenated by-products, and the lowest generation could be found in the PDS–NaClO cleaning process. The total carbonaceous (C-) and nitrogenous (N-) by-product concentrations generated in the PDS–NaClO cleaning process were 22.7 and 1.0 μg/L, respectively, which were much lower than those during NaClO alone (259.2 μg/L and 12.1 μg/L). Combining the results of membrane flux recovery shown in Figure 1, the PDS–NaClO cleaning process showed the best performance on membrane flux recovery and by-product generation control.
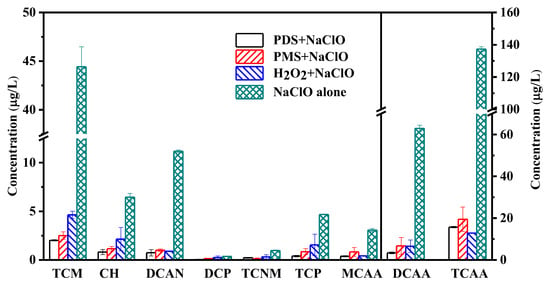
Figure 3.
Comparison of halogenated by-products formation during the combined cleaning of peroxides and NaClO.

Table 2.
Significance analysis of halogenated by-products formation variation during the cleaning by combined process and NaClO alone.
Compared to NaClO alone, a lower generation of by-products in the combined cleaning processes can be attributed to several factors. First, the peroxide could oxidize pollutants on the membrane surface, thereby removing pollutants and reducing by-product precursors before subsequent NaClO cleaning [4,8]. Second, the use of PDS, PMS, and H2O2 treatment could shorten the NaClO cleaning time [43]. Third, peroxide oxidation could alter the characteristics of organic foulants on the membrane surface, which affects by-product generation during subsequent NaClO cleaning [44]. Xie et al. previously investigated the impact of PDS and H2O2 pre-oxidation on the generation of disinfection by-product during subsequent chlorination of natural organic matter (NOM) [32], revealing that PDS pre-oxidation led to a slight decrease in by-product generation, which was likely due to the weak oxidation capacity of PDS resulting in a partial change in NOM properties. The PDS dosage (1 mM) and oxidation time (1 h) used in this study were much higher than Xie et al.’s research (30 μM and 10 min). Therefore, it can be speculated that the oxidation of mixed foulants (HA, BSA, and SA) on the membrane surface by PDS can partly inhibit the generation of by-products in the subsequent NaClO cleaning process [45]. However, Xie et al. also found that H2O2 pretreatment significantly increased halogenated by-product generation during the subsequent chlorination due to the production of HO• from H2O2 decomposition, which could cause a hydrolysis reaction with aromatic functional groups in NOM and promote the reaction between NOM and NaClO. Meanwhile, singlet oxygen (1O2) could be generated by the reaction between H2O2 and NaClO [46]. On the one hand, 1O2 can promote the electron densities of functional groups in NOM through the addition reaction, which can enhance the reaction reactivity between NOM and NaClO [47]; on the other hand, 1O2 can oxidize NOM to produce halogenated by-product precursors such as aldehydes, ketones, and carboxylic acids, which promote the formation of some by-products [48,49]. In this study, the reaction of residual H2O2 on the membrane surface with NaClO during the NaClO–H2O2 cleaning process could contribute to the formation of by-products. Furthermore, PMS was found to enhance the reactivity between chlorine and organic compounds, potentially promoting the formation of halogenated by-products during NaClO-PMS cleaning [32]. Therefore, the pre-oxidation of foulant and the catalytic chlorination of foulant by PMS may promote the formation of by-products.
Figure 4 shows the generation of by-products in the combined cleaning process of NaClO–peroxide and the NaClO alone cleaning process. The significance analysis indicates that the changes of most by-products produced in NaClO–peroxide cleaning and NaClO-alone cleaning were significant, except for TCP and MCAA. Compared to NaClO cleaning alone, the concentrations of TCM, CH, DCAN, DCAA, and TCAA in NaClO–peroxide cleaning decreased by 31%, 27%, 29%, 11%, and 12%, respectively. The reduced by-products in the NaClO–peroxide cleaning would be attributed to the short reaction time between NaClO and foulants. As shown in Figure 2, NaClO–PDS and NaClO–PMS cleaning can effectively remove foulants without affecting the membrane properties. However, when comparing Figure 3 and Figure 4, peroxide–NaClO cleaning produced fewer by-products than NaClO–peroxide cleaning. Therefore, peroxide–NaClO cleaning is more effective in controlling by-product formation, and PDS–NaClO shows the best performance among the selected peroxide–NaClO cleaning processes.
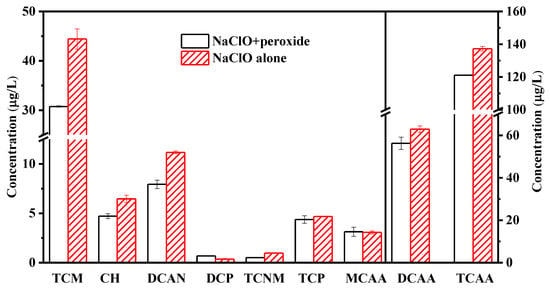
Figure 4.
Comparison of halogenated by-products formation during the combined cleaning and NaClO alone.
3.3. Effect of Cleaning Time and Temperature on Halogenated By-Products Generation
The effect of cleaning time on the production of halogenated by-products in the combined cleaning process was studied under conditions of 10 mg/L of organic matter (HA, BSA, or SA) in the feed water, an initial pH of 7.0, and a temperature of 25 °C. As presented in Figure 5, a significant increase in DCAA and TCAA was found when cleaning time was extended from 0.5 to 1 h. By further increasing the cleaning time to 4 h, the concentrations of TCM, CH, DCAA, and TCAA increased by 161%, 741%, 137%, and 88%, respectively, indicating that the increase in cleaning times can enhance the formation of halogenated by-product. Although extended cleaning time can improve the removal of foulant from the membrane surface, the extended reaction time for the NaClO cleaning process will also promote the reaction between foulants and NaClO, thus enhancing the generation of halogenated by-products [50]. Therefore, the long-term contact between the fouled membrane and NaClO should be reduced while ensuring the membrane flux recovery in practice, which can be achieved by prolonging the cleaning time of PDS and reducing the cleaning time of NaClO.
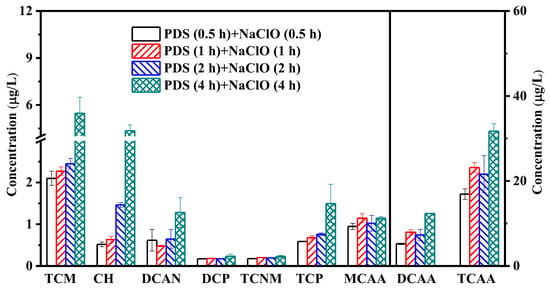
Figure 5.
Effect of cleaning time on the formation of halogenated by-products during the combined cleaning of PDS and NaClO.
Figure 6 shows the effect of reaction temperature on the generation of halogenated by-products in the combined PDS–NaClO cleaning process. During the experiments, the concentration of HA, BSA, and SA in the feed water was 10 mg/L, the initial pH was 7.0, and the cleaning time for PDS and NaClO was 1 h. The significance analysis revealed that increasing temperature from 15 to 25 °C promoted the production of TCM, 1,1-DCP, and TCAA. Moreover, the formation of CH, dichloroacetonitrile (DCAN), DCAA, and TCAA increased from 0.5, 0.2, 2.9, and 11.7 μg/L to 1.1, 0.4, 4.4, and 15.4 μg/L, respectively, while raising the temperature from 15 °C to 35 °C, indicating that higher temperature promoted the generation of halogenated by-products in the combined cleaning process. The increase in reaction temperature can enhance proton transport efficiency, promote foulant oxidation and modification, and further improve the removal of foulants from the membrane surface, leading to a reduction in by-product precursors during the subsequent NaClO cleaning [51,52]. On the other hand, it can accelerate the chemical reaction rate between NaClO and organic matter, thereby promoting the formation of halogenated by-products [53,54]. As a result, the slight increase in by-product generation in this study may be due to multiple factors. A correlation analysis was performed to examine the relationship between the concentrations of different by-products, cleaning times, and temperatures. The results of the analysis are presented in Tables S2 and S3. It can be observed that except for MCAA, there is a significant positive correlation between the concentrations of the remaining eight by-products and the cleaning time. The correlation coefficients (R2) for these correlations exceed 0.8, indicating a strong relationship. Additionally, Table S3 indicates a negative correlation between TCNM concentration and temperature, with an R2 value of 0.87. Conversely, the concentration of the remaining eight by-products shows a positive correlation with cleaning temperature. Notably, the R2 values between TCM, CH, DCAN, TCNM, TCP, and temperature all exceed 0.8. These findings demonstrate that the concentration of most by-products exhibit a positive correlation with both cleaning time and temperature.
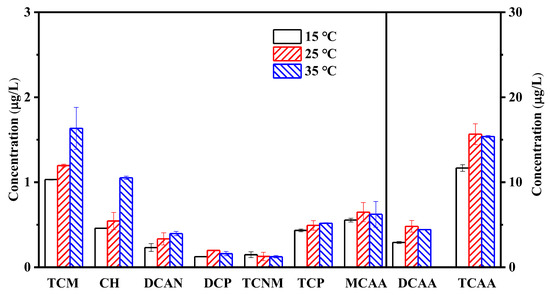
Figure 6.
Effect of reaction temperature on the formation of halogenated by-products during the combined cleaning of PDS and NaClO.
4. Conclusions
In this study, various combinations of peroxide and NaClO were applied to remove membrane fouling, and the generation of halogenated by-products during the cleaning process was explored. The main findings of the study are as follows:
- (1)
- The composite pollutant composed of HA, BSA, and SA caused serious membrane fouling during the filtration process. Combined cleaning processes of peroxide and NaClO effectively removed pollutants and restored membrane flux, with the flux for the cleaned membrane being similar to the initial membrane flux.
- (2)
- Halogenated by-products, including TCM, CH, DCAN, 1,1-DCP, TCNM, TCP, and HAAs, were formed during the cleaning of the membrane by NaClO. Compared with the combined NaClO–peroxide cleaning process, the combined peroxide–NaClO cleaning process resulted in a lower generation of halogenated by-products, and PDS–NaClO cleaning showed the best performance for the control of halogenated by-products.
- (3)
- In the PDS–NaClO cleaning process, the increase in reaction time from 1 h to 4 h mainly promoted the concentration of TCM, CH, DCAA, and TCAA, while TCM, CH, DCAN, DCAA, and TCAA had a relatively high production with the increase in reaction temperature from 15 °C to 35 °C. Most by-products exhibit a positive correlation with both cleaning time and temperature.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15132498/s1, Figure S1: Schematic diagram of UF system experimental set-up; Table S1: Sensitivities of the determination methods for various halogenated by-products.; Table S2: Correlation analysis between different by-product generation and cleaning time; Table S3: Correlation analysis between different by-product generation and cleaning temperature.
Author Contributions
Experimental design, P.X. and J.D.; methodology, J.D. and Y.W.; investigation, Y.W., J.D. and Y.Z.; writing-review and revision, J.D., Y.W., X.H. and P.X.; visualization, X.H. and S.W.; formal analysis, Y.W.; acquisition of funding, S.W., P.X. and X.H. All authors have read and agreed to the published version of the manuscript.
Funding
The article was funded by the Natural Science Foundation of China (Nos. 52070081), National Key Research and Development Program of China (2022YFC3203500) and the Key Projects of Power Construction Corporation of China (DJ-ZDXM-2019-30, DJ-ZDXM-2020-36, DJ-ZDXM-2021-45).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chang, H.; Zhu, Y.; Yu, H.; Qu, F.; Zhou, Z.; Li, X.; Yang, Y.; Tang, X.; Liang, H. Long-term operation of ultrafiltration membrane in full-scale drinking water treatment plants in China: Characteristics of membrane performance. Desalination 2022, 543, 116122. [Google Scholar] [CrossRef]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.-L.; Han, Z.-S.; Li, G.-B. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Tian, J.-Y.; Ernst, M.; Cui, F.; Jekel, M. Correlations of relevant membrane foulants with UF membrane fouling in different waters. Water Res. 2013, 47, 1218–1228. [Google Scholar] [CrossRef]
- Wan, Y.; Xie, P.; Wang, Z.; Ding, J.; Wang, J.; Wang, S.; Wiesner, M.R. Comparative study on the pretreatment of algae-laden water by UV/persulfate, UV/chlorine, and UV/H2O2: Variation of characteristics and alleviation of ultrafiltration membrane fouling. Water Res. 2019, 158, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Goh, P.; Lau, W.; Othman, M.; Ismail, A. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Ly, Q.V.; Nghiem, L.D.; Cho, J.; Hur, J. Insights into the roles of recently developed coagulants as pretreatment to remove effluent organic matter for membrane fouling mitigation. J. Membr. Sci. 2018, 564, 643–652. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Fouling control in reverse osmosis membranes through modification with conductive carbon nanostructures. Desalination 2019, 470, 114118. [Google Scholar] [CrossRef]
- Wang, Z.; Wan, Y.; Xie, P.; Zhou, A.; Ding, J.; Wang, J.; Zhang, L.; Wang, S.; Zhang, T.C. Ultraviolet/persulfate (UV/PS) pretreatment of typical natural organic matter (NOM): Variation of characteristics and control of membrane fouling. Chemosphere 2019, 214, 136–147. [Google Scholar] [CrossRef]
- Deng, L.; Ngo, H.-H.; Guo, W.; Zhang, H. Pre-coagulation coupled with sponge-membrane filtration for organic matter removal and membrane fouling control during drinking water treatment. Water Res. 2019, 157, 155–166. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Ennaceri, H.; Fischer, K.; Schulze, A.; Moheimani, N.R. Membrane fouling control for sustainable microalgal biodiesel production: A review. Renew. Sustain. Energy Rev. 2022, 161, 112335. [Google Scholar] [CrossRef]
- Liu, H.; Gu, J.; Wang, S.; Zhang, M.; Liu, Y. Performance, membrane fouling control and cost analysis of an integrated anaerobic fixed-film MBR and reverse osmosis process for municipal wastewater reclamation to NEWater-like product water. J. Membr. Sci. 2020, 593, 117442. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Luo, J.; Wan, Y. A novel paradigm of photocatalytic cleaning for membrane fouling removal. J. Membr. Sci. 2022, 641, 119859. [Google Scholar] [CrossRef]
- Aktij, S.A.; Taghipour, A.; Rahimpour, A.; Mollahosseini, A.; Tiraferri, A. A critical review on ultrasonic-assisted fouling control and cleaning of fouled membranes. Ultrasonics 2020, 108, 106228. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Zhu, X.; Herzberg, M.; Walker, S.; Jassby, D. Impact of physical and chemical cleaning agents on specific biofilm components and the implications for membrane biofouling management. Ind. Eng. Chem. Res. 2018, 57, 3359–3370. [Google Scholar] [CrossRef]
- Liu, J.; He, K.; Zhang, J.; Li, C.; Zhang, Z. Coupling ferrate pretreatment and in-situ ozonation/ceramic membrane filtration for wastewater reclamation: Water quality and membrane fouling. J. Membr. Sci. 2019, 590, 117310. [Google Scholar] [CrossRef]
- Ham, S.-Y.; Kim, H.-S.; Jang, Y.; Ryoo, H.-S.; Lee, J.-H.; Park, J.-H.; Park, H.-D. Synergistic control of membrane biofouling using linoleic acid and sodium hypochlorite. Chemosphere 2021, 268, 128802. [Google Scholar] [CrossRef]
- Yue, X.; Koh YK, K.; Ng, H.Y. Membrane fouling mitigation by NaClO-assisted backwash in anaerobic ceramic membrane bioreactors for the treatment of domestic wastewater. Bioresour. Technol. 2018, 268, 622–632. [Google Scholar] [CrossRef]
- Jiang, C.-K.; Tang, X.; Tan, H.; Feng, F.; Xu, Z.-M.; Mahmood, Q.; Zeng, W.; Min, X.-B.; Tang, C.-J. Effect of scrubbing by NaClO backwashing on membrane fouling in anammox MBR. Sci. Total Environ. 2019, 670, 149–157. [Google Scholar] [CrossRef]
- Rong, C.; Wang, T.; Luo, Z.; Hu, Y.; Kong, Z.; Qin, Y.; Hanaoka, T.; Ito, M.; Kobayashi, M.; Li, Y.-Y. Pilot plant demonstration of temperature impacts on the methanogenic performance and membrane fouling control of the anaerobic membrane bioreactor in treating real municipal wastewater. Bioresour. Technol. 2022, 354, 127167. [Google Scholar] [CrossRef]
- Ding, J.; Wang, S.; Xie, P.; Zou, Y.; Wan, Y.; Chen, Y.; Wiesner, M.R. Chemical cleaning of algae-fouled ultrafiltration (UF) membrane by sodium hypochlorite (NaClO): Characterization of membrane and formation of halogenated by-products. J. Membr. Sci. 2020, 598, 117662. [Google Scholar] [CrossRef]
- Cai, W.; Liu, J.; Zhang, X.; Ng, W.J.; Liu, Y. Generation of dissolved organic matter and byproducts from activated sludge during contact with sodium hypochlorite and its implications to on-line chemical cleaning in MBR. Water Res. 2016, 104, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.C.; Lee, J.J.; Tijing, L.D.; Shon, H.K.; Yao, M.; Kim, H.-S. Characteristics of membrane fouling by consecutive chemical cleaning in pressurized ultrafiltration as pre-treatment of seawater desalination. Desalination 2015, 369, 51–61. [Google Scholar] [CrossRef]
- Tian, J.-Y.; Chen, Z.-L.; Yang, Y.-L.; Liang, H.; Nan, J.; Li, G.-B. Consecutive chemical cleaning of fouled PVC membrane using NaOH and ethanol during ultrafiltration of river water. Water Res. 2010, 44, 59–68. [Google Scholar] [CrossRef]
- Ibrar, I.; Yadav, S.; Ganbat, N.; Samal, A.K.; Altaee, A.; Zhou, J.L.; Nguyen, T.V. Feasibility of H2O2 cleaning for forward osmosis membrane treating landfill leachate. J. Environ. Manag. 2021, 294, 113024. [Google Scholar] [CrossRef]
- Cao, Y.; Qiu, W.; Zhao, Y.; Li, J.; Jiang, J.; Yang, Y.; Pang, S.-Y.; Liu, G. The degradation of chloramphenicol by O3/PMS and the impact of O3-based AOPs pre-oxidation on dichloroacetamide generation in post-chlorination. Chem. Eng. J. 2020, 401, 126146. [Google Scholar] [CrossRef]
- Luo, J.; Liu, T.; Zhang, D.; Yin, K.; Wang, D.; Zhang, W.; Liu, C.; Yang, C.; Wei, Y.; Wang, L. The individual and Co-exposure degradation of benzophenone derivatives by UV/H2O2 and UV/PDS in different water matrices. Water Res. 2019, 159, 102–110. [Google Scholar] [CrossRef]
- Ding, J.; Xiao, H.; Huang, X.; Zou, Y.; Ye, Z.; Wang, S.; Xie, P.; Chen, Y.; Ma, J. Application of heat-activated peroxydisulfate process for the chemical cleaning of fouled ultrafiltration membranes. Chin. Chem. Lett. 2023, 108316, in press. [Google Scholar] [CrossRef]
- Ding, J.; Nie, H.; Wang, S.; Chen, Y.; Wan, Y.; Wang, J.; Xiao, H.; Yue, S.; Ma, J.; Xie, P. Transformation of acetaminophen in solution containing both peroxymonosulfate and chlorine: Performance, mechanism, and disinfection by-product formation. Water Res. 2021, 189, 116605. [Google Scholar] [CrossRef]
- Wang, S.; Chew, J.W.; Liu, Y. An environmentally sustainable approach for online chemical cleaning of MBR with activated peroxymonosulfate. J. Membr. Sci. 2020, 600, 117872. [Google Scholar] [CrossRef]
- He, X.; Li, B.; Wang, P.; Ma, J. Novel H2O2–MnO2 system for efficient physico-chemical cleaning of fouled ultrafiltration membranes by simultaneous generation of reactive free radicals and oxygen. Water Res. 2019, 167, 115111. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Ma, J.; Liu, W.; Zou, J.; Yue, S. Impact of UV/persulfate pretreatment on the formation of disinfection byproducts during subsequent chlorination of natural organic matter. Chem. Eng. J. 2015, 269, 203–211. [Google Scholar] [CrossRef]
- Cheng, X.; Wu, D.; Liang, H.; Zhu, X.; Tang, X.; Gan, Z.; Xing, J.; Luo, X.; Li, G. Effect of sulfate radical-based oxidation pretreatments for mitigating ceramic UF membrane fouling caused by algal extracellular organic matter. Water Res. 2018, 145, 39–49. [Google Scholar] [CrossRef]
- Gokulakrishnan, S.; Mohammed, A.; Prakash, H. Determination of persulphates using N, N-diethyl-p-phenylenediamine as colorimetric reagent: Oxidative coloration and degradation of the reagent without bactericidal effect in water. Chem. Eng. J. 2016, 286, 223–231. [Google Scholar] [CrossRef]
- Bader, H.; Sturzenegger, V.; Hoigne, J. Photometric method for the determination of low concentrations of hydrogen peroxide by the peroxidase catalyzed oxidation of N, N-diethyl-p-phenylenediamine (DPD). Water Res. 1988, 22, 1109–1115. [Google Scholar] [CrossRef]
- Long, X.; Meng, Q.; Zhang, G. Application of biosurfactant rhamnolipid for cleaning of UF membranes. J. Memb. Sci. 2014, 457, 113–119. [Google Scholar] [CrossRef]
- Domino, M.; Pepich, B.; Munch, D.; Fair, P.; Xie, Y. Method 552.3 Determination of Haloacetic Acids and Dalapon in Drinking Water by Liquid-Liquid Microextraction, Derivatization, and Gas Chromatography with Electron Capture Detection. Environmental Protection Agency (EPA). Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=901V0400.txt (accessed on 3 July 2023).
- Munch, D.J.; Hautman, D.P. Method 551.1: Determination of Chlorination Disinfection Byproducts, Chlorinated Solvents, and Halogenated Pesticides/Herbicides in Drinking Water by Liquid-Liquid Extraction and Gas Chromatography with Electron-Capture Detection: Methods for the Determination of Organic Compounds in Drinking Water. 1995. Available online: https://www.epa.gov/sites/default/files/2015-06/documents/epa-551.1.pdf (accessed on 3 July 2023).
- Wang, Z.; Ding, J.; Xie, P.; Chen, Y.; Wan, Y.; Wang, S. Formation of halogenated by-products during chemical cleaning of humic acid-fouled UF membrane by sodium hypochlorite solution. Chem. Eng. J. 2018, 332, 76–84. [Google Scholar] [CrossRef]
- Crebelli, R.; Conti, L.; Monarca, S.; Feretti, D.; Zerbini, I.; Zani, C.; Veschetti, E.; Cutilli, D.; Ottaviani, M. Genotoxicity of the disinfection by-products resulting from peracetic acid-or hypochlorite-disinfected sewage wastewater. Water Res. 2005, 39, 1105–1113. [Google Scholar] [CrossRef]
- Madaeni, S.; Samieirad, S. Chemical cleaning of reverse osmosis membrane fouled by wastewater. Desalination 2010, 257, 80–86. [Google Scholar] [CrossRef]
- Ang, W.S.; Lee, S.; Elimelech, M. Chemical and physical aspects of cleaning of organic-fouled reverse osmosis membranes. J. Membr. Sci. 2006, 272, 198–210. [Google Scholar] [CrossRef]
- Ding, S.; Deng, Y.; Bond, T.; Fang, C.; Cao, Z.; Chu, W. Disinfection byproduct formation during drinking water treatment and distribution: A review of unintended effects of engineering agents and materials. Water Res. 2019, 160, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ling, L.; Dionysiou, D.D.; Wang, Y.; Huang, J.; Guo, K.; Li, X.; Fang, J. Chlorate formation mechanism in the presence of sulfate radical, chloride, bromide and natural organic matter. Environ. Sci. Technol. 2018, 52, 6317–6325. [Google Scholar] [CrossRef] [PubMed]
- Gallard, H.; Von Gunten, U. Chlorination of natural organic matter: Kinetics of chlorination and of THM formation. Water Res. 2002, 36, 65–74. [Google Scholar] [CrossRef]
- To, T.-L.; Fadul, M.J.; Shu, X. Singlet oxygen triplet energy transfer-based imaging technology for mapping protein–protein proximity in intact cells. Nat. Commun. 2014, 5, 4072. [Google Scholar] [CrossRef]
- Brame, J.; Long, M.; Li, Q.; Alvarez, P. Trading oxidation power for efficiency: Differential inhibition of photo-generated hydroxyl radicals versus singlet oxygen. Water Res. 2014, 60, 259–266. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Singlet-oxygen generation in alkaline periodate solution. Environ. Sci. Technol. 2015, 49, 14392–14400. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Mitch, W.A. Halonitroalkanes, halonitriles, haloamides, and N-nitrosamines: A critical review of nitrogenous disinfection byproduct formation pathways. Environ. Sci. Technol. 2012, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Yang, M.; Liu, J.; Li, W.; Graham, N.J.; Li, X.; Yang, B. Three-step effluent chlorination increases disinfection efficiency and reduces DBP formation and toxicity. Chemosphere 2017, 168, 1302–1308. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Ioannidi, A.A.; Mantzavinos, D.; Frontistis, Z. Heat-activated persulfate for the degradation of micropollutants in water: A comprehensive review and future perspectives. J. Environ. Manag. 2022, 318, 115568. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Yang, H.-W.; Wang, X.-M.; Fu, J.; Xie, Y.F. Formation of disinfection by-products: Effect of temperature and kinetic modeling. Chemosphere 2013, 90, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Schmalz, C.; Zhou, J.; Zwiener, C.; Chang, V.W.-C.; Ge, L.; Wan, M.P. An insight of disinfection by-product (DBP) formation by alternative disinfectants for swimming pool disinfection under tropical conditions. Water Res. 2016, 101, 535–546. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).