An Antifouling Redox Sensor with a Flexible Carbon Fiber Electrode for Machine Learning-Based Dissolved Oxygen Prediction in Severely Eutrophic Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Capacitive Electrode
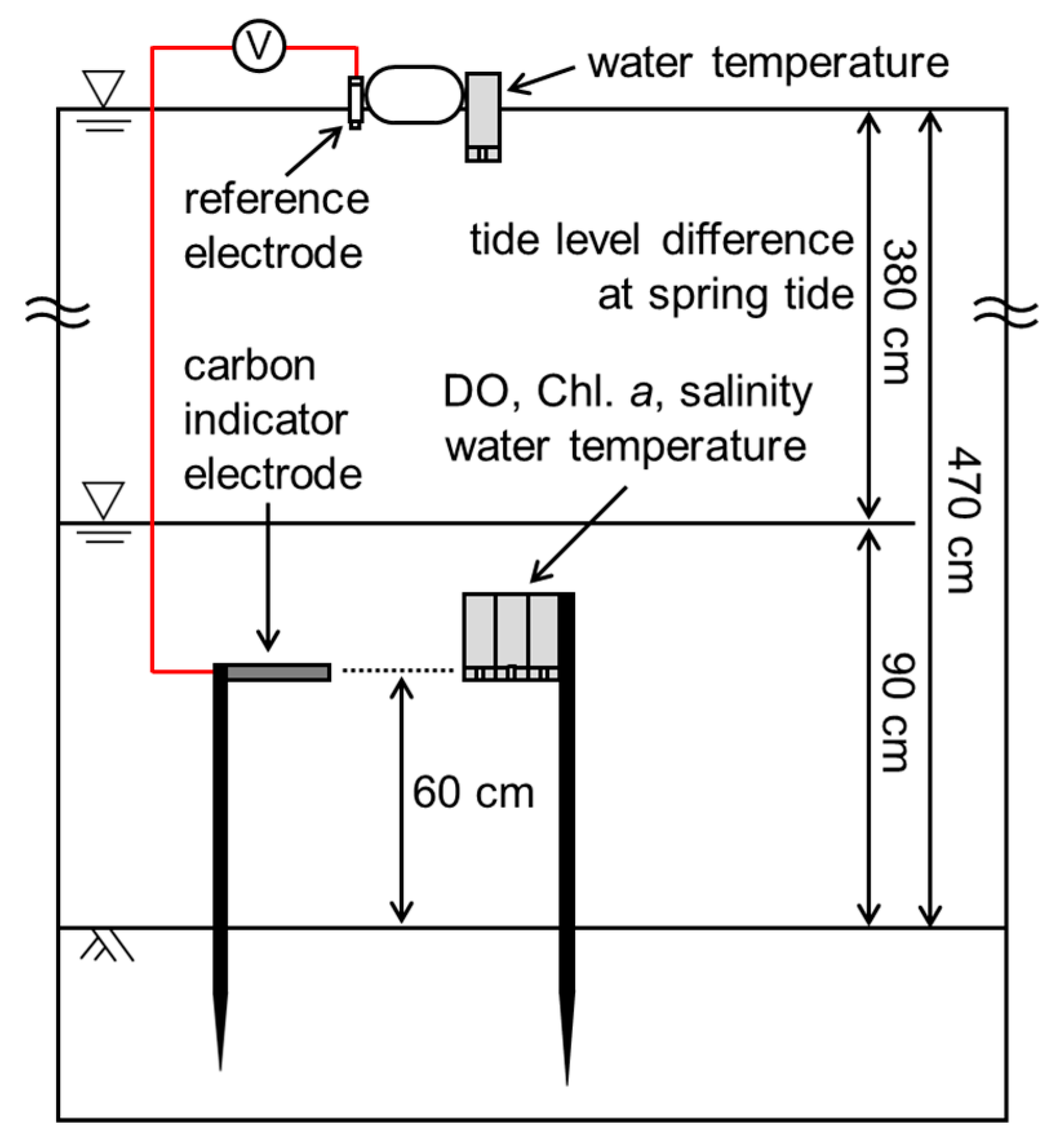
2.2. Field Experiment
2.3. Statistical Assessment
2.4. Data Partitioning
2.5. Long Short-Term Memory Network
2.6. Feature Importance Based on the Random Forest Model
3. Results and Discussion
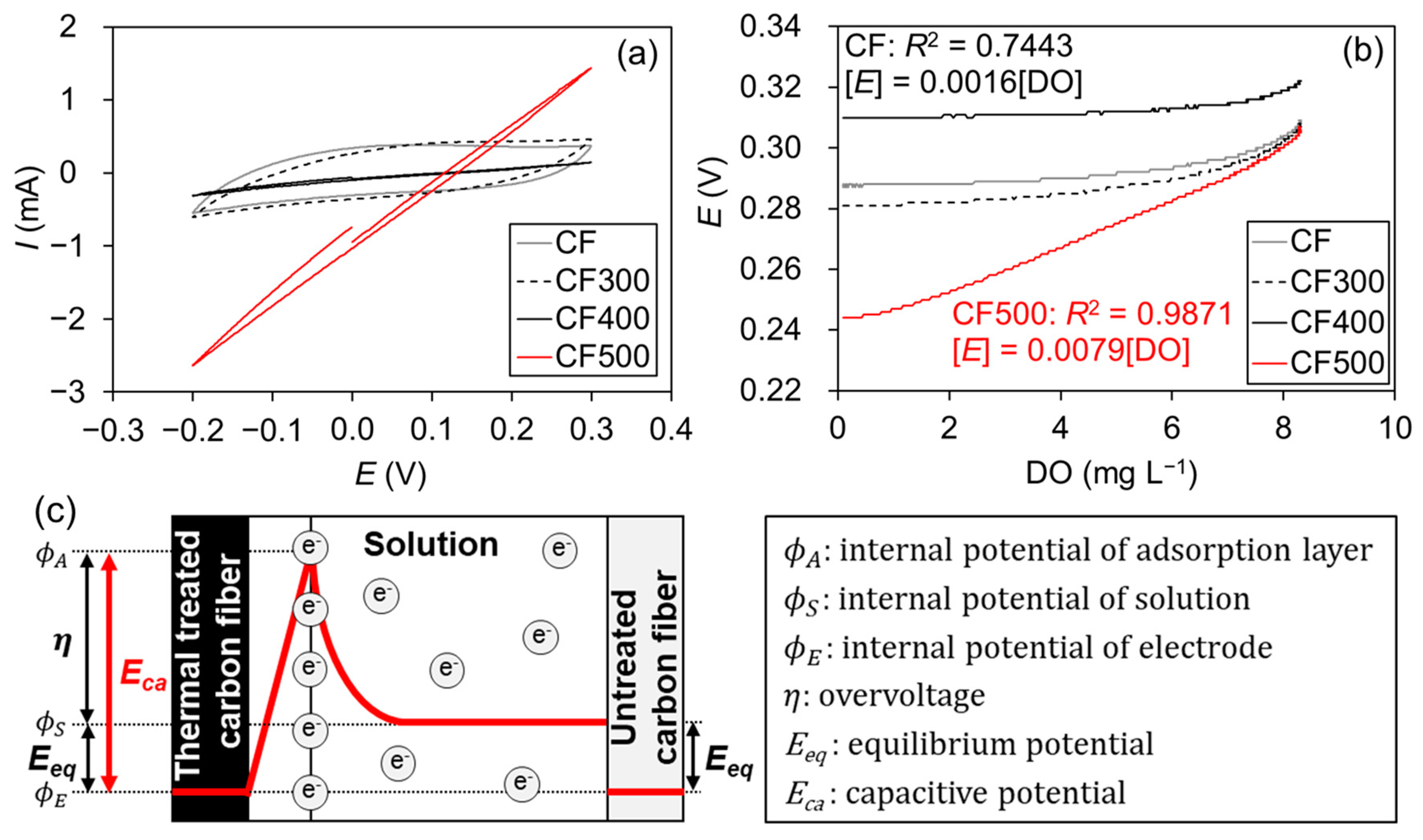
3.1. Reactivity of Flexible Carbon Fiber Electrode
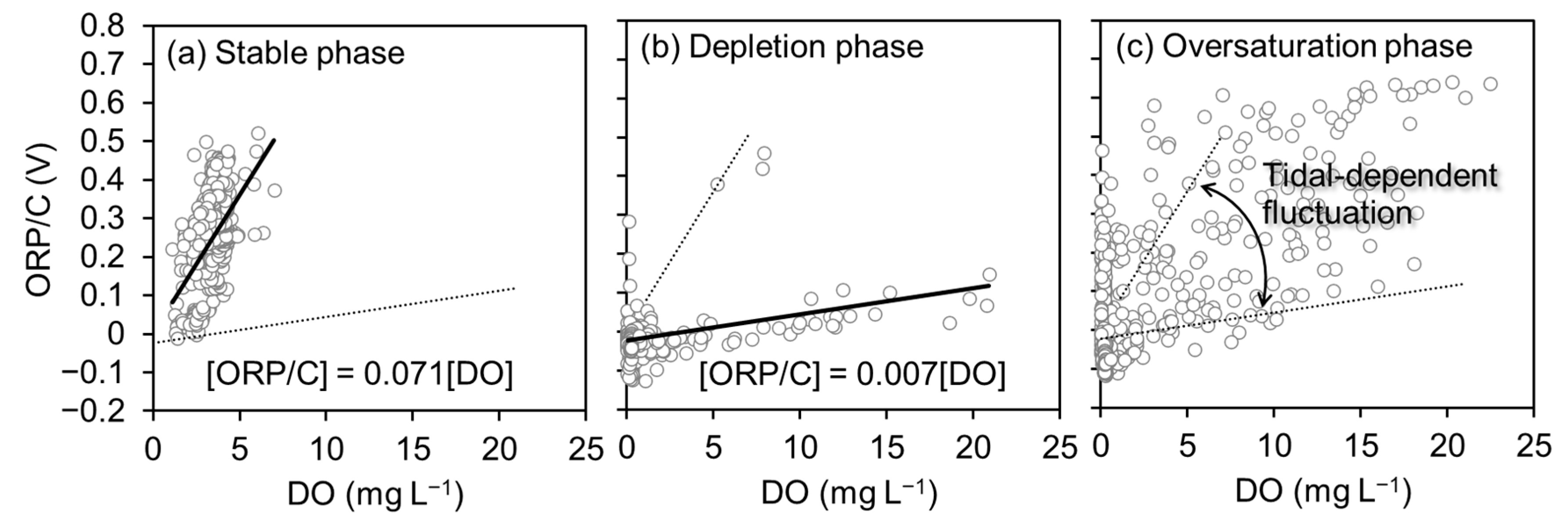
3.2. Correlations between Dissolved Oxygen Concentration and Predictors
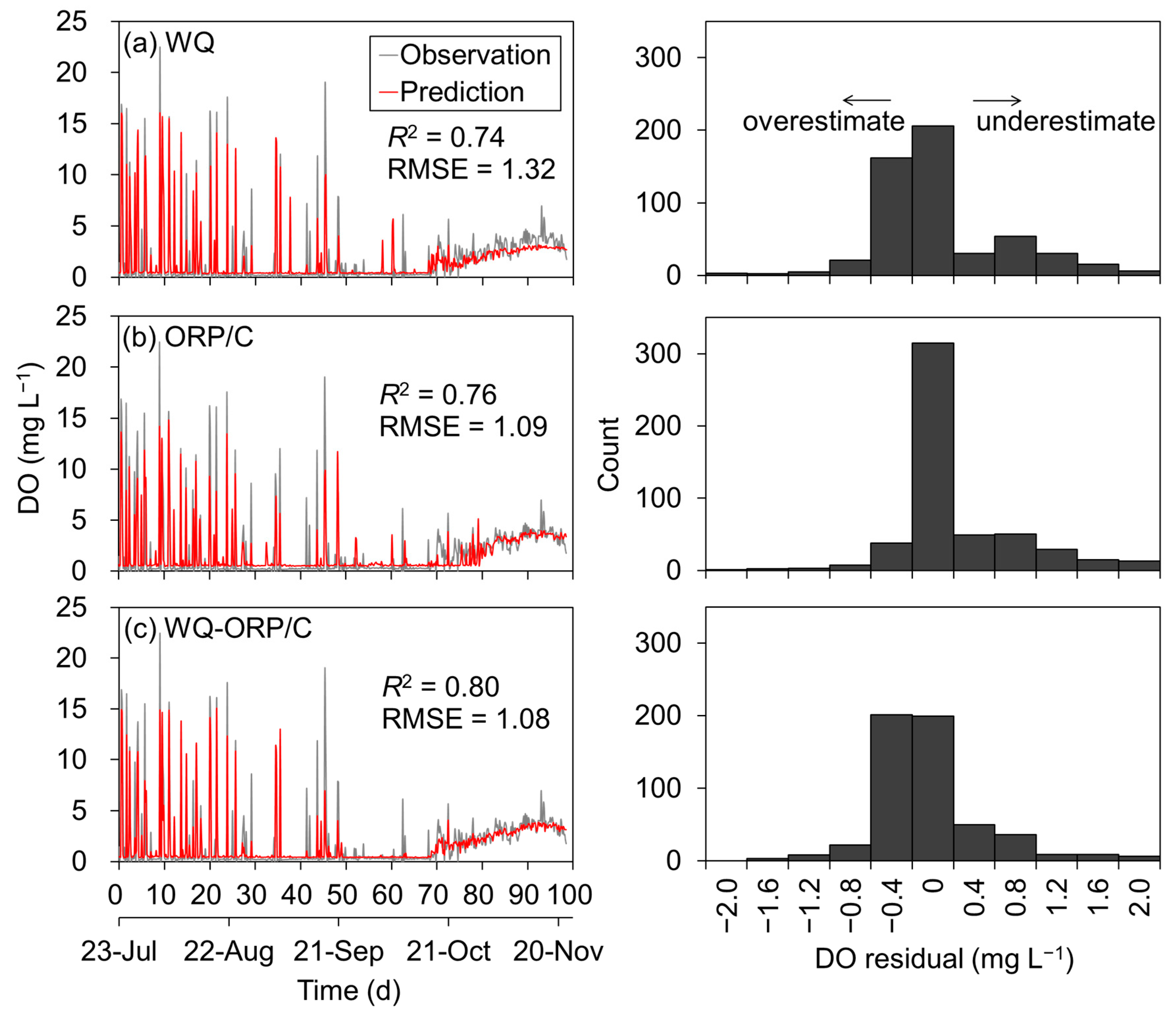
3.3. Prediction of Dissolved Oxygen Concentration
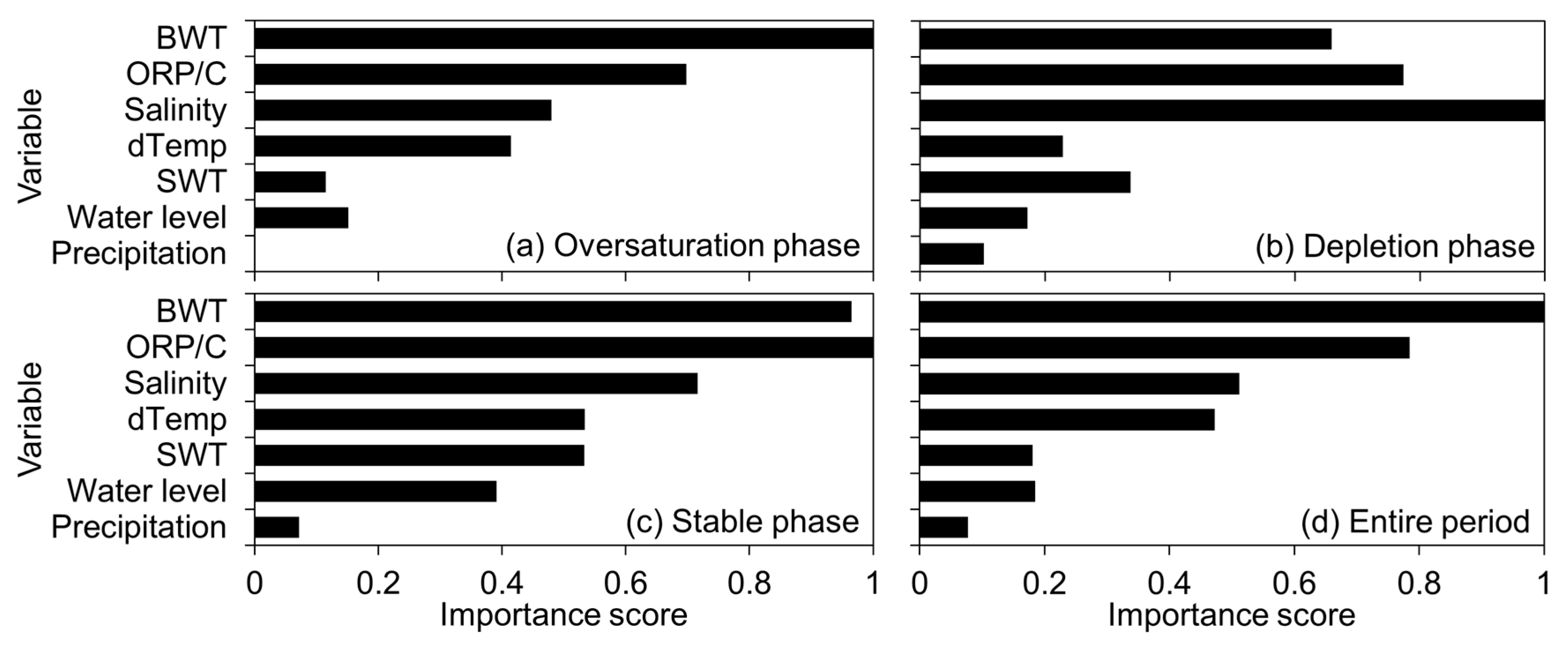
3.4. Dominant Predictors
3.5. Implications and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Fang, H.; Qin, G.; Tan, X.; Huang, Z.; Zeng, F.; Du, H.; Li, S. Concentration estimation of dissolved oxygen in Pearl River Basin using input variable selection and machine learning techniques. Sci. Total Environ. 2020, 731, 139099. [Google Scholar] [CrossRef] [PubMed]
- Taft, J.L.; Taylor, W.R.; Hartwig, E.O.; Loftus, R. Seasonal oxygen depletion in Chesapeake Bay. Estuaries 1980, 3, 242. [Google Scholar] [CrossRef]
- Yu, X.; Shen, J.; Du, J. A machine-learning-based model for water quality in coastal waters, taking dissolved oxygen and hypoxia in Chesapeake Bay as an example. Water Resour. Res. 2020, 56, e2020WR027227. [Google Scholar] [CrossRef]
- Ross, A.C.; Stock, C.A. An assessment of the predictability of column minimum dissolved oxygen concentrations in Chesapeake Bay using a machine learning model. Estuar. Coast. Shelf Sci. 2019, 221, 53–65. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, Q.; Fang, X.; Yu, H.; Li, D. Principal component analysis and long short-term memory neural network for predicting dissolved oxygen in water for aquaculture. Trans. Chin. Soc. Agric. Eng. 2018, 34, 183–191. [Google Scholar] [CrossRef]
- Huan, J.; Li, H.; Li, M.; Chen, B. Prediction of dissolved oxygen in aquaculture based on gradient boosting decision tree and long short-term memory network: A study of Chang Zhou fishery demonstration base. Chin. Comput. Electron. Agric. 2020, 175, 105530. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, T.; Shin, J.; Go, B.; Lee, M.; Lee, J.; Koo, J.; Cho, K.H.; Cha, Y. Forecasting abrupt depletion of dissolved oxygen in urban streams using discontinuously measured hourly time-series data. Water Resour. Res. 2021, 57, e2020WR029188. [Google Scholar] [CrossRef]
- Quinn, N.W.T.; Jacobs, K.; Chen, C.W.; Stringfellow, W.T. Elements of a decision support system for real-time management of dissolved oxygen in the San Joaquin River Deep Water Ship Channel. Environ. Model Softw. 2005, 20, 1495–1504. [Google Scholar] [CrossRef]
- Christwardana, M.; Yoshi, L.A.; Setyonadi, I.; Maulana, M.R.; Fudholi, A. A novel application of simple submersible yeast-based microbial fuel cells as dissolved oxygen sensors in environmental waters. Enzyme Microb. Technol. 2021, 149, 109831. [Google Scholar] [CrossRef]
- Jing, X.; Cao, D.; Liu, Y.; Wang, G.; Yin, J.; Wen, Q.; Gao, Y. The open circuit potential of hydrogen peroxide at noble and glassy carbon electrodes in acidic and basic electrolytes. J. Electroanal. Chem. 2011, 658, 46–51. [Google Scholar] [CrossRef]
- Kim, K.; Nakashita, S.; Yoshimura, K.; Hibino, T. In situ electrochemical remediation of brackish river sediment rich in aromatic organic matter using steel-slag-combined sediment microbial fuel cells. J. Clean. Prod. 2021, 315, 128206. [Google Scholar] [CrossRef]
- Yu, R.-F.; Liaw, S.-L.; Chang, C.-N.; Lu, H.-J.; Cheng, W.-Y. Monitoring and control using on-line ORP on the continuous-flow activated sludge batch reactor system. Water Sci. Technol. 1997, 35, 57–66. [Google Scholar] [CrossRef]
- Kim, K.; Nakagawa, Y.; Takahashi, T.; Yumioka, R.; Hibino, T. High-resolution monitoring of seasonal hypoxia dynamics using a capacitive potentiometric sensor: Capacitance amplifies redox potential. Sci. Total Environ. 2022, 836, 155435. [Google Scholar] [CrossRef]
- Rosenberg, R.; Nilsson, H.C.; Diaz, R.J. Response of benthic fauna and changing sediment redox profiles over a hypoxic gradient. Estuar. Coast. Shelf Sci. 2001, 53, 343–350. [Google Scholar] [CrossRef]
- Kim, K.; Takahashi, T.; Yumioka, R.; Hibino, T. Sediment microbial fuel cells in oxidative sedimentary environments using iron substrate as voltage booster. J. Power Sources 2023, 557, 232510. [Google Scholar] [CrossRef]
- Touch, N.; Hibino, T.; Yamaji, S.; Takata, H. Nutrient salt removal by steel-making slag in sediment microbial fuel cells. Environ. Technol. 2019, 40, 2906–2912. [Google Scholar] [CrossRef]
- Eliasz-Kowalska, M.; Wojtal, A.Z.; Barinova, S. Influence of selected environmental factors on diatom β diversity (Bacillariophyta) and the value of diatom indices and sampling issues. Water 2022, 14, 2315. [Google Scholar] [CrossRef]
- Timmons, M.B.; Ebeling, J.M.; Wheaton, F.W.; Summerfelt, S.T.; Vinci, B.J. Recirculating Aquaculture Systems, 2nd ed.; Cayuga Aqua Ventures: Ithaca, NY, USA, 2002. [Google Scholar]
- Townsend, S.A. The seasonal pattern of dissolved oxygen, and hypolimnetic deoxygenation, in two tropical Australian reservoirs. Lakes Reserv. Res. Manag. 1999, 4, 41–53. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters, 2nd ed.; Wiley: New York, NY, USA, 1981. [Google Scholar]
- Mansfeldt, T. In situ long-term redox potential measurements in a dyked marsh soil. Z. Pflanzenernähr. Bodenkd. 2003, 166, 210–219. [Google Scholar] [CrossRef]
- Eze, E.; Ajmal, T. Dissolved oxygen forecasting in aquaculture: A hybrid model approach. Appl. Sci. 2020, 10, 7079. [Google Scholar] [CrossRef]
- Park, S.; Kim, K. Prediction of DO concentration in Nakdong River estuary through case study based on long short term memory model. J. Korean Soc. Coastal Ocean Eng. 2021, 33, 238–245. [Google Scholar] [CrossRef]
- Li, W.; Wu, H.; Zhu, N.; Jiang, Y.; Tan, J.; Guo, Y. Prediction of dissolved oxygen in a fishery pond based on gated recurrent unit (GRU). Inf. Process. Agric. 2021, 8, 185–193. [Google Scholar] [CrossRef]
- Park, S.; Kim, K. Preliminary study on the reproduction of dissolved oxygen concentration in Jinhae Bay based on deep learning model. J. Korean Soc. Mar. Environ. Saf. 2022, 28, 193–200. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Lin, L.S. Dissolved oxygen concentration predictions for running waters with different land use land cover using a quantile regression forest machine learning technique. J. Hydrol. 2021, 597, 126213. [Google Scholar] [CrossRef]
- Justus, D.; Brennan, J.; Bonner, S.; McGough, A.S. Predicting the computational cost of deep learning models. In Proceedings of the 2018 IEEE International Conference on Big Data, Seattle, WA, USA, 10–13 December 2018; pp. 3873–3882. [Google Scholar] [CrossRef]
- Burge, S.R.; Hristovski, K.D.; Burge, R.G.; Hoffman, D.A.; Saboe, D.; Chao, P.; Taylor, E.; Koenigsberg, S.S. Microbial potentiometric sensor: A new approach to longstanding challenges. Sci. Total Environ. 2020, 742, 140528. [Google Scholar] [CrossRef] [PubMed]



| DO (mg L−1) a | ORP/C (V) b | Water Level (m) | SWT (°C) c | BWT (°C) d | dTemp (°C) e | Salinity (psu) | Precipitation (mm h−1) | |
|---|---|---|---|---|---|---|---|---|
| Mean | 1.8 | −0.2 | 2.7 | 25.8 | 25.6 | 0.2 | 24.2 | 0.1 |
| SD f | 2.9 | 0.1 | 0.9 | 4.2 | 3.1 | 2.0 | 2.4 | 0.8 |
| Min. | 0.0 | −0.4 | 0.6 | 15.2 | 7.2 | −5.9 | 0.1 | 0.0 |
| Median | 0.3 | −0.2 | 2.7 | 26.8 | 26.8 | −0.1 | 24.3 | 0.0 |
| Max. | 22.5 | 0.4 | 4.9 | 35.5 | 33.8 | 11.8 | 29.5 | 16.5 |
| Hyperparameter | Case |
|---|---|
| Prediction time step (h) | 0, 1, 6, 12, 24, 48 |
| Sequence length (h) | 1, 6, 12, 24 |
| Epoch | 300, 500, 1000, 2000 |
| Layer depth | 1, 3, 5 |
| Predictor | Prediction Time Step | Optimal Hyperparameter | ||||
|---|---|---|---|---|---|---|
| Epoch | LD a | SL b | R2 | RMSE | ||
| WQ | 0 | 2000 | 3 | 12 | 0.74 | 1.32 |
| 1 | 2000 | 5 | 12 | 0.71 | 1.43 | |
| 48 | 1000 | 3 | 24 | 0.58 | 1.34 | |
| ORP/C | 0 | 1000 | 5 | 12 | 0.76 | 1.09 |
| 1 | 1000 | 3 | 24 | 0.55 | 1.29 | |
| 48 | 1000 | 5 | 24 | 0.50 | 1.62 | |
| WQ-ORP/C | 0 | 1000 | 5 | 6 | 0.80 | 1.08 |
| 1 | 2000 | 5 | 6 | 0.69 | 1.33 | |
| 48 | 2000 | 5 | 24 | 0.67 | 1.38 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, K.; Hibino, T.; Sakai, Y.; Furukawa, T.; Kim, K. An Antifouling Redox Sensor with a Flexible Carbon Fiber Electrode for Machine Learning-Based Dissolved Oxygen Prediction in Severely Eutrophic Waters. Water 2023, 15, 2467. https://doi.org/10.3390/w15132467
Park S, Kim K, Hibino T, Sakai Y, Furukawa T, Kim K. An Antifouling Redox Sensor with a Flexible Carbon Fiber Electrode for Machine Learning-Based Dissolved Oxygen Prediction in Severely Eutrophic Waters. Water. 2023; 15(13):2467. https://doi.org/10.3390/w15132467
Chicago/Turabian StylePark, Seongsik, Kyunghoi Kim, Tadashi Hibino, Yusuke Sakai, Taito Furukawa, and Kyeongmin Kim. 2023. "An Antifouling Redox Sensor with a Flexible Carbon Fiber Electrode for Machine Learning-Based Dissolved Oxygen Prediction in Severely Eutrophic Waters" Water 15, no. 13: 2467. https://doi.org/10.3390/w15132467
APA StylePark, S., Kim, K., Hibino, T., Sakai, Y., Furukawa, T., & Kim, K. (2023). An Antifouling Redox Sensor with a Flexible Carbon Fiber Electrode for Machine Learning-Based Dissolved Oxygen Prediction in Severely Eutrophic Waters. Water, 15(13), 2467. https://doi.org/10.3390/w15132467






