Abstract
Typically, the shortage of freshwater will limit the social and economic development of island regions. As a non-conventional, high-quality water source, desalinated seawater can be incorporated into the urban water supply system. The genotoxicity of disinfection by-products in drinking water was always viewed as a concern for human health. However, only a few studies were conducted based on this issue of desalinated seawater. In this study, the comet assay was conducted to investigate the genotoxicity caused by organic extracts in the specific drinking water produced in two full-scale desalinated seawater purification plants from the Zhoushan Islands in eastern China. The water samples were collected from four different locations along the treatment train in the plants. The quality of desalinated seawater conformed to the national standards for drinking water in China, except for the higher boron content. The results of the comet assay showed that all the organic extracts from the water samples were able to induce different levels of DNA damage on HL-60 cells (K finished water = 6.635 and 7.698, respectively). Compared with that from the water plants with fresh source water, the genotoxicity of the finished water of the seawater desalination plant was determined to be the lowest. The correlations between desalinated seawater consumption and two important chronic diseases, namely hypertension and coronary heart disease, could not be supported by the current data of epidemiological investigation. These results demonstrate the genotoxicity of the desalinated seawater was, in fact, much lower than the conventional drinking water with fresh source water.
1. Introduction
The scarcity of freshwater sources has long hindered the social and economic progress of island regions. Seawater desalination has emerged as an effective solution to alleviate the water shortage [,], with reverse osmosis (RO)-based technology having been widely adopted worldwide due to its recent technological advancements and cost reductions []. However, this solution has also raised public concerns about the safety of drinking desalinated seawater []. The high concentration of bromine and iodide in seawater can result in the formation of disinfection byproducts (DBPs) with a high cytotoxicity and genotoxicity (i.e., bromine and iodide DBPs) during drinking water production and distribution systems []. Even at low concentrations and long-term exposure, these DBPs can induce tumors/cancers and even cause death []. Nevertheless, the genotoxicity of the desalination processes is an understudied issue, and there is a lack of information on drinking water from desalination.
Physicochemical water quality parameters are generally insufficient to fully evaluate the impacts of chemical mixtures on organisms. Short-term mutagenicity bioassays, such as the Ames test, the SOS/UMU test, the comet test, the micronucleus test, and the chromosome aberration test all offer a more efficient and straightforward means of assessing the genotoxicity of water []. These bioassays represent the primary methods for detecting the genotoxicity of trace organics in drinking water [,], with each reflecting specific genetic endpoints, such as gene mutations, DNA damage, and chromosomal aberrations. Among these endpoints, DNA damage in cells is considered to be particularly useful for understanding the presence of complex compounds in drinking water following in vivo and in vitro exposure.
Single-cell gel electrophoresis (SCGE), which uses eukaryotic cells, is a highly sensitive method for detecting DNA damage []. There are various parameters which are used to measure the DNA damage in SCGE experiments, including the tail length, tail moment, Olive tail moment, and tail DNA % []. However, the use of different interpretation parameters may lead to different DNA damage results, thereby making it crucial to select the appropriate parameters to interpret the SCGE experimental results. In recent years, tail DNA% has gained acceptance as a reliable parameter for measuring DNA damage. This parameter has a linear relationship with the break frequency over a wide range of damage and is relatively unaffected by threshold settings in the software. It also allows for the discrimination of damage over a broad range (from 0 to 100%, respectively) and is a scale-independent parameter, providing a clear indication of comet appearance [,,,]. Therefore, tail DNA% is increasingly being recognized as a valuable parameter for expressing DNA damage in SCGE experiments.
This study used SCGE, with tail DNA% as the parameter to assess changes in the genotoxicity during seawater desalination using RO. The genotoxicity of the desalinated seawater was compared with that of municipal tap water sourced from fresh water. The genotoxicity changes in the seawater desalination process were also compared with those in the freshwater treatment processes to determine the safety of the seawater desalination process in dealing with genotoxicity. Furthermore, the health records of residents in the desalinated seawater service area were examined to gain a better understanding of the potential health risks associated with consuming desalinated seawater.
2. Materials and Methods
2.1. Water Sample Collection
Water samples were collected from two seawater desalination plants, namely the Shengsi plant and the Daishan plant, respectively. These two plants are located on two islands (Shengsi Island and Daishan Island, respectively) in Zhoushan City, China. Shengsi plant supplies desalinated seawater to 104,235 households with its designed daily water supply of 30,000 t, while the Daishan plant serves 35,986 homes with a designed daily water supply of 7000 t, respectively. Both plants use the same seawater desalination process based on RO treatment. From October to December 2020, respectively, 50 L of raw seawater, filtered water, RO effluent, and finished water were collected monthly for water quality analysis (as shown in Figure 1).
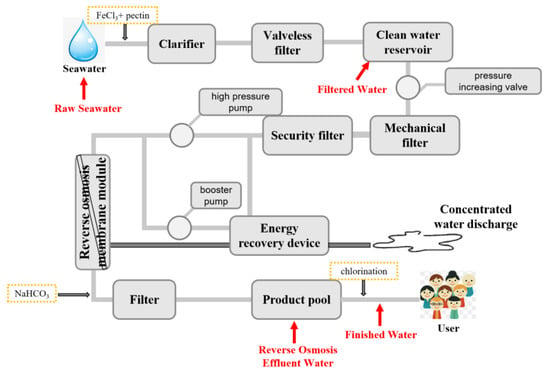
Figure 1.
Seawater desalination process of the Shengsi and Daishan plants. The red arrows indicate the sampling points. The valveless filter in this process uses quartz sand as the media.
2.2. Water Quality Analysis
The water quality parameters measured included turbidity, pH, chemical oxygen demand (CODMn), total dissolved solids (TDS), total hardness, ammonia nitrogen, chloride, sulphate, B, Ca, standard plate count bacteria, total coliforms, and Escherichia coli (E. coli). Turbidity and pH were measured using a portable multi-parameter meter (WTW) during sampling. Other water quality parameters were determined in the laboratory within 24 h of sample collection, following the Chinese standard examination for drinking water (GB/T 5750-2006). The standard permanganate method was used to determine the COD, while TDS was determined by evaporating and drying the water samples at 180 °C, and total hardness was determined by following the standard titrimetric method using EDTA. Ammonia nitrogen was determined using Nessler’s reagent method, and chloride, sulphate, B, and Ca were determined using ion chromatography. Standard plate count bacteria were determined using heterotrophic plate counts (HPCs), total coliforms were determined using the m-Endo medium, and E. coli was determined using the membrane filter technique with NA-MUG medium.
2.3. Genotoxicity Analysis
2.3.1. Extraction of Organic Compounds
Water samples (4 L) were pre-treated with 0.45 μm mixed cellulose ester membranes, and their pH was adjusted to 2.0 ± 0.1 using hydrochloric acid. The organic matter was then enriched using a solid-phase extraction column (HyperSep Retain PEP) that was activated with 10 mL of methanol and 10 mL of deionized water, respectively. The velocity of flow was controlled at 4–5 mL/min. After extracting the water sample, the PEP filter was rinsed with 10 mL of ultrapure water and dried under vacuum for 20 min. The PEP filter column was then eluted with 10 mL of methanol, and the eluate was collected in a brown glass centrifuge tube. The eluate was air-dried using nitrogen and then stored at −20 °C until further experiments were conducted.
2.3.2. Single-Cell Gel Electrophoresis (SCGE)
The water eluate detailed in Section 2.3.1 was dissolved in dimethyl sulfoxide (DMSO) to detect its genotoxicity using SCGE. Human promyelocytic leukemia cells (HL-60) obtained from the Institute of Urban Environment, Chinese Academy of Sciences, were used for this assay. Cells were cultured in IMDM medium enriched with 10% fetal bovine serum and were kept in a 5% CO2 atmosphere.
A log-phase HL-60 cell culture (105~106 cells/mL) was prepared for organics exposure. This cell culture was exposed to water eluates at 0.125, 0.25, 0.5, and 1 L water/mL, respectively. Cells exposed to ethyl methanesulfonate (10 mM) and DMSO were used as the positive and negative controls, respectively. The exposure experiment was completed in a cell incubator for 1.5 h at room temperature, and exposure uniformity was ensured by shaking the system. After exposure, the cell culture was collected, centrifuged (1000 r/min for 5 min), and washed with PBS to obtain a cell suspension for gel electrophoresis.
Gel electrophoresis was performed in triplicate for each water sample according to the method described by Singh et al. []. Briefly, cell solidification was accomplished by spreading 10 µL of a cell suspension of 1.0% low melting point agarose (LMPA) onto a microscope slide that was pre-coated with 0.8% normal melting point agarose (NMPA). The slide was then covered with 100 µL of 1.0% LMPA to prevent nuclear DNA from escaping during cell lysis and electrophoresis. The prepared slide was immersed for 2.0 h at 4 °C in a lysis solution (comprising 2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, and 1% Triton X-100, pH 10) to remove cellular proteins. Then, the slide was submerged in an electrophoretic buffer (1 mM Na2EDTA, 300 mM NaOH, pH > 13) for 30 min at 4 °C to permit DNA unwinding and was then subjected to electrophoresis in the same buffer for 30 min at 30 V and 300 mA. After electrophoresis, the slide was washed with a neutralization buffer (0.4 M Tris, pH 7.5) and then treated with 20 μg/mL ethidium bromide (200 μL per slide) for 20 min at room temperature to stain DNA before their examination. Nuclei damage was visualized under a fluorescence microscope (ECLIPSE 90i, Nikon, Japan) [,], and 269 cells were randomly selected to analyze the tail DNA% using Comet Assay Software Project Lab (Casplab 2004).
2.3.3. Calculation of DNA Damage
The correlation between the water sample volume and the tail DNA% was analyzed using Prism 9. The slope of the correlation equation, which represents the DNA damage per unit volume of the water sample, was used to characterize genotoxicity.
2.4. Assessment of Genotoxicity in Seawater Desalination and between the Water Treatment Plants
The fold change (FC) of the m value (mfinished water/mraw seawater) was calculated to evaluate the impact of these desalination processes on genotoxicity. In terms of data processing, we took the exposure concentration as the horizontal coordinate, the intensity of the mutagenicity effect as the vertical coordinate, and the slope obtained by fitting the line as the “m value”. The higher the slope, the stronger the potential mutagenicity of the water sample. FC values greater than 1 and less than 1 indicate that the desalination process has a negative and positive effect on reducing the genotoxicity, respectively. To further investigate the safety of seawater desalination in reducing genotoxicity, mfinished water, mraw seawater, and the FC values of the two plants in this study were compared with those of other drinking water treatment plants from published data. The literature screening and data extraction methods are described in Text S1.
2.5. Health Risks of Drinking Desalinated Seawater
The health records of residents were surveyed to investigate the potential health risks of drinking desalinated seawater. The residents were divided into three groups according to their drinking water sources: desalinated seawater, freshwater, and blended water. The desalinated seawater group (with a population of 7847 individuals served by the Shengsi plant) has consumed desalinated seawater for more than 12 years. The freshwater group has a population consisting of 8310 individuals who consume the local surface water. Meanwhile, the blending water group (with a population of 8028 individuals) gets its source water from a freshwater reservoir supplemented by desalinated seawater. The populations of the three groups have a similar gender ratio (Figure S1) and age distribution (Figure S2). The prevalence rates of tumors, coronary heart disease, stroke, diabetes, and hypertension in 2020 were surveyed and compared between these three groups.
3. Results and Discussion
3.1. Water Quality Parameters in the Seawater Desalination Process
Table 1 presents the water quality parameters of raw seawater, effluent from each treatment process, and finished water. The pH of the raw seawater was 7.8 and 7.5 for the two plants, respectively, which falls within the range of the natural seawater pH noted in the literature. Other parameters, such as the turbidity, COD, total hardness, ammonia nitrogen, chloride, and sulfate, all met the Chinese national standards for drinking water quality (GB 5749-2006). This suggests that the raw seawater from both plants is of good quality and may not have been contaminated by human activities.

Table 1.
Water quality of each sampling point in the seawater desalination process of two plants.
The low turbidity (<0.5 NTU) of the raw seawater provided suitable water quality conditions for RO since particles can cause the colloidal/particle fouling of RO membranes, leading to a reduction in the permeate flux [,]. Seawater TDS was as high as 51,200 mg/L and 50,400 mg/L for both plants, respectively, but these values decreased significantly after multi-stage filtration and RO.
Compared to the raw seawater, the concentration of calcium ions, magnesium ions, chloride, and sulfate in the finished water of both plants was significantly lower and met the national standards for drinking water quality. However, the extremely low levels of calcium ions (~1 mg/L), magnesium ions (1 mg/L and 2 mg/L, respectively), and sulfate (3 mg/L and 6 mg/L, respectively) in the finished water were below the limits of the hygienic standard of mineralization for drinking water with low mineral levels (GJB1335-92), which highlights potential health risks. Boron was the only parameter that did not meet the national standard (GB5749-2006). The boron concentration in the finished water of the two plants was 0.8 mg/L and 0.6 mg/L, respectively, which exceeds the national standard limit of 0.5 mg/L for boron in drinking water.
These results show that the water quality parameters of the finished water met the required standards. However, the excessive removal of mineral ions, such as calcium and magnesium, as well as the inadequate removal of boron, are common problems which have been associated with seawater desalination using RO as the core technology [,]. Desalination processes are primarily intended to remove dissolved salts and other materials from the seawater and brackish water. In RO, external pressure is applied to the high-solute (concentrated) water, which causes the solvent (water) to migrate through the membrane, leaving the solute (salts and other non-permeating substances) in a more concentrated brine. The desalinated water produced by RO generally has reduced mineralization, which has raised concerns regarding the potential health problems caused by the low salinity of desalinated seawater []. Furthermore, the rejection ratio of the boron-containing anions (mostly as borate) is generally lower than that of most other inorganic compounds based on the current treatment technologies available, making boron removal a worldwide challenge in regions with naturally high boron levels []. This challenge led the WHO to revise the boron guidelines for drinking water from 0.3 mg/L in 1993 (later revised to 0.5 mg/L) to 2.4 mg/L in 2011, respectively [].
3.2. Genotoxicity Change in Desalination
The genotoxicity of the water samples obtained from various desalination processes was determined using SCGE and compared to that of tap water and barreled drinking water (Figure 2). The genotoxicity of barreled drinking water (tail DNA% = 1.19~1.50%) was similar to that of the blank control (DMSO, tail DNA% = 1.12%). In contrast, filtered and finished water samples from these desalination processes showed significantly higher levels of genotoxicity than barreled drinking water and raw seawater, respectively. However, the genotoxicity of the RO effluent was slightly higher (1.40~2.34% for the Shengsi plant and 1.57~2.28% for the Daishan plant, respectively) than the other desalination processes. The low genotoxicity of the RO effluent indicates that the RO treatment effectively removes mutagenic organics from the water. All water samples had lower genotoxicity levels than tap water, suggesting that desalinated seawater has a relatively low genotoxicity. The genotoxicity of all water samples showed a significant dose effect. To account for differences in water intake, the genotoxicity of the water samples per unit volume (m) was calculated and used to compare their health risks. The m value of finished water for both plants was between that of tap water and bottled drinking water. In the seawater desalination process of both plants, the m value decreased after filtration and RO treatment, but increased by 6.23 and 9.97 times after chlorination, respectively. These results suggest that filtration and RO treatment can reduce the genotoxicity of seawater, while chlorination can increase it.
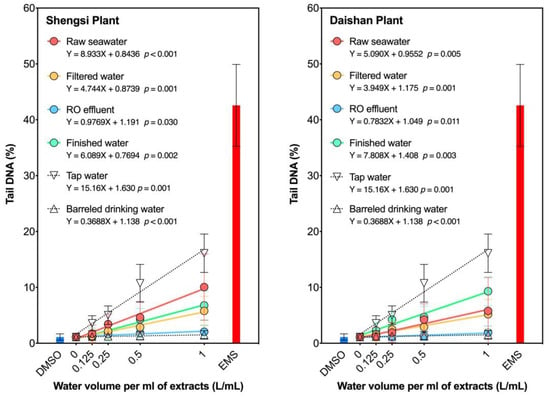
Figure 2.
The genotoxicity of the water samples of different volumes was evaluated by measuring the percentage of tail DNA (tail DNA%). A linear regression equation was used to describe the relationship between the volume of the water sample and its genotoxicity. The slope of the line (m value) was used to quantify the genotoxicity per unit volume of water samples. Tap water was collected from the laboratory of Xiamen University, while barreled drinking water was sourced from a commercially available brand.
Previous studies have reported that RO treatment effectively reduces the genotoxicity of drinking water [], The membranes used in the RO process are highly efficient in rejecting disinfection by-products (DBPs), including trihalomethanes (>60%), haloacetic acids (>90%), haloacetonitriles (>50%), N-nitrosodimethylamine (10–50%), and others [,,,,,,,,]. The high rejection rate of haloacetic acids has been attributed to their pKa values, which are lower than the typical operating pH of the RO processes, leading to their negative charge and consequent electrostatic repulsion from the negatively charged membranes []. Since the concentration of the COD and bacteria in raw seawater is typically low, the disinfection demand and the formation of DBPs are also expected to be low during chlorination. However, in both plants, a significant increase in genotoxicity was observed after chlorination, possibly due to the formation of brominated or iodinated DBPs. Seawater generally contains high levels of bromine (50,000–80,000 mg/L) and iodine (21–60 mg/L) [,], which enhances the formation of these more cytotoxic and genotoxic DBPs as compared to their chlorinated analogues [,].
3.3. Comparison of Desalination with Other Water Treatments in Terms of Genotoxicity
The genotoxicity per unit volume of water samples, represented by the FC of the m value, was calculated from raw seawater to finished water. The FC values for the Shengsi and Daishan plants were 0.682 and 1.534, respectively. These results suggest that the genotoxicity decreased during desalination in the Shengsi plant but increased in the Daishan plant. The FC of m was also calculated for freshwater-source DWTPs based on the published data (as shown in Table 2). Although various types of cells and indicators were used in the SCGE experiments of these studies, making direct comparisons of genotoxicity between these water samples meaningless, the trend of genotoxicity during the water treatments can be compared. The FC value of the source water to finished water ranged from 0.418 to 9.184 in these studies, respectively. Three of the studies that used tail DNA % as the indicator of SCGE had FC values ranging from 0.473 to 2.632, respectively. The results of 13 experiments showed that the genotoxicity of freshwater decreased after treatment by DWTP (FC < 1), while the results of 27 experiments showed that the genotoxicity increased (FC > 1), of which six experiments showed an increase of more than two times. Compared to the freshwater-source drinking water treatment processes, the seawater desalination process conducted in this study did not result in a significant increase in genotoxicity.

Table 2.
Calculated m value and FC value for freshwater drinking water treatment.
3.4. Health Risk Assessment of Drinking Desalinated Water
The prevalence of tumors, coronary heart disease, stroke, diabetes, and hypertension was compared between residents who drank different water sources (Figure 3). The group drinking fresh water had the lowest prevalence of tumors and coronary heart disease, while the desalinated water and blending water groups were more likely to suffer from these two diseases. This trend was consistent across genders and age groups, indicating that the long-term consumption of desalinated seawater may increase the risk of cancer and coronary heart disease. It is worth noting that seawater desalination results in formation of tap water with very low concentrations of essential minerals, such as sodium, potassium, magnesium, and calcium. Consumption of such water can lead to electrolyte abnormalities marked by hyponatremia, hypokalemia, hypomagnesemia, and hypocalcemia, all of which are common features observed in cancer patients []. Epidemiological investigations in Taiwan suggested possible associations between low mineral concentrations in drinking water and an excess risk for esophageal, gastric, rectal, and colon cancers []. However, there is no conclusive evidence in that desalinated water can be used as an independent factor to directly cause cancer [].
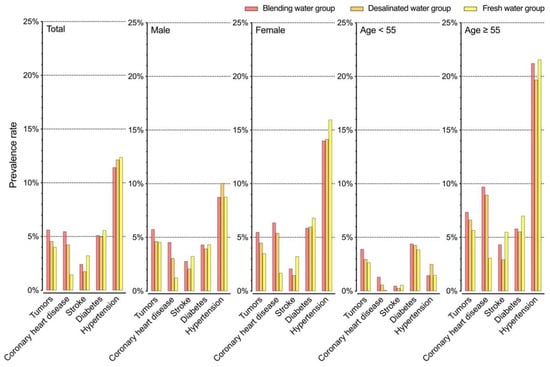
Figure 3.
Prevalence of diseases among local people drinking desalinated water, freshwater, and mixed water.
Table 3 presents the water quality data for point-of-use samples collected from residents in 2019–2020. With the exception of boron levels in the desalinated seawater group exceeding the standard, all other water quality parameters were found to have met the national standards. The desalinated seawater had a lower total hardness, but also had higher chloride and boron contents compared to the fresh water and blending water groups. The blending water and freshwater groups had similar levels across all parameters. However, residents drinking blended water had a higher prevalence of tumors and coronary heart disease, highlighting the health risks associated with blended water consumption. Conventional drinking water treatment processes have a limited effectiveness in removing the hydrophilic and neutral natural organic matter (NOM) fractions, and the dissolved organic carbon (DOC) levels in the treated surface waters are typically higher than those in the seawater reverse osmosis (RO) permeate. Studies have shown that when bromide-rich desalinated seawater is mixed with surface water, more brominated disinfection byproduct (DBP) species are formed in the distribution system, even at low DOC levels (e.g., 1~2 mg C/L) [,,]. Bromine is effectively incorporated into a low specific ultraviolet absorbance at 254 nm (SUVA254), a low molecular weight, and hydrophilic NOM fractions [,,]. Blending the RO permeate with the treated surface waters before post-disinfection can create conditions favoring the formation of significantly more toxic DBPs, even though their total mass concentration may be low, and that the final water quality meets water quality standards.

Table 3.
Drinking water quality at point-of-use of residents.
4. Conclusions
The widespread adoption of seawater desalination technology has significantly alleviated water shortages in island areas. However, the potential health risks associated with drinking desalinated seawater requires further investigation. In this study, we used the single-cell gel electrophoresis assay to evaluate the genotoxicity of seawater during the desalination process and assessed the possible health risks of drinking desalinated seawater. Our findings indicate that except for boron, all the water quality parameters of desalinated seawater met China’s national drinking water standards. Filtration and reverse osmosis treatment significantly reduced the genotoxicity of seawater. The genotoxicity of the reverse osmosis permeate (m = 0.9769 and 0.7832, respectively) was comparable to that of the bottled drinking water (m = 0.3688), but subsequent chlorination increased their genotoxicity by 6.23 and 9.97 times, respectively. The finished water from the two seawater desalination plants demonstrated a lower genotoxicity compared to tap water from freshwater sources. Health records of residents who drank freshwater, desalinated seawater, or mixed water revealed an increased prevalence of tumors (4.01%, 4.56%, and 5.61%, respectively) and coronary heart disease (1.45%, 4.25%, and 5.46%, respectively). Our results suggest that desalinated seawater is relatively safe at the plant, but the increased health risks associated with pipeline delivery or supplemental freshwater sources are concerning.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15132470/s1, Text S1: Literature screening and data extraction methods; Figure S1: Gender ratio of the three groups surveyed; Figure S2: Age distribution of the three groups surveyed.
Author Contributions
Conceptualization, Y.Z., C.Y., K.W. and X.Y., methodology, Y.X., software, Y.X., validation, X.X. and K.W., formal analysis, Y.X., resources, Y.Z., data curation, Y.X., writing—original draft preparation, Y.X., writing—review and editing, K.W. and C.Y., visualization, C.Y., funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of China-Joint Fund Project (U2005206), and the Zhoushan Science and Technology Plan Project (2018C31119).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaldellis, J.; Kondili, E. The water shortage problem in the Aegean archipelago islands: Cost-effective desalination prospects. Desalination 2007, 216, 123–138. [Google Scholar] [CrossRef]
- Feng, H.; Xie, C. Status and prospect of Chinese seawater desalination technology. Chem. Ind. Eng. 2010, 27, 103–109. [Google Scholar]
- Dawoud, M.A. The role of desalination in augmentation of water supply in GCC countries. Desalination 2007, 216, 123–138. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Q.; Qin, Y.; Chen, X.; Yang, X.; Lou, W.; Zhou, M.; He, G.; Lu, K. Knowledge, attitudes and practice of desalinated water among professionals in health and water departments in Shengsi, China: A qualitative study. PLoS ONE 2015, 10, e0118360. [Google Scholar] [CrossRef] [PubMed]
- Agus, E.; Voutchkov, N.; Sedlak, D.L. Disinfection by-products and their potential impact on the quality of water produced by desalination systems: A literature review. Desalination 2009, 237, 214–237. [Google Scholar] [CrossRef]
- Yang, Y.; Komaki, Y.; Kimura, S.; Hu, H.; Wagner, E.; Mariñas, B.; Plewa, M. Toxic impact of bromide and iodide on drinking water disinfected with chlorine or chloramines. Environ. Sci. Technol. 2014, 48, 12362–12369. [Google Scholar] [CrossRef]
- Moawad, H.; El–Rahim, W.M.A.; Khalafallah, W.M.A. Evaluation of biotoxicity of textile dyes using two bioassays. J. Basic Microbiol. Int. J. Biochem. Physiol. Genet. Morphol. Ecol. Microorg. 2003, 43, 218–229. [Google Scholar] [CrossRef]
- Ohe, T.; Watanabe, T.; Wakabayashi, K. Mutagens in surface waters: A review. Mutat. Res./Rev. Mutat. Res. 2004, 567, 109–149. [Google Scholar]
- Zeng, Q.; Zhang, S.; Liao, J.; Miao, D.; Wang, X.; Yang, P.; Yun, L.; Liu, A.; Lu, W. Evaluation of genotoxic effects caused by extracts of chlorinated drinking water using a combination of three different bioassays. J. Hazard. Mater. 2015, 296, 23–29. [Google Scholar] [CrossRef]
- Collins, A.R. The comet assay for DNA damage and repair. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Hartmann, A.; Agurell, E.; Beevers, C.; Brendler-Schwaab, S.; Burlinson, B.; Clay, P.; Collins, A.; Smith, A.; Speit, G.; Thybaud, V.; et al. Recommendations for conducting the in vivo alkaline Comet assay. Mutagenesis 2003, 18, 45–51. [Google Scholar] [CrossRef]
- Collins, A.; Agurell, E.; Beevers, C.; Brendler-Schwaab, S.; Burlinson, B.; Clay, P.; Collins, A.; Smith, A.; Speit, G.; Thybaud, V. Comet assay in human biomonitoring studies: Reliability, validation, and applications. Environ. Mol. Mutagen. 1997, 30, 139–146. [Google Scholar] [CrossRef]
- García, O.; Romero, I.; González, J.E.; Moreno, D.L.; Cuétara, E.; Rivero, Y.; Gutiérrez, A.; Pérez, C.L.; Álvarez, A.; Carnesolta, D. Visual estimation of the percentage of DNA in the tail in the comet assay: Evaluation of different approaches in an intercomparison exercise. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 720, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.F.; Chen, C.O.; Yeum, K.; Taylor, A.; Blumberg, J.B.; Liu, Y. Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190. [Google Scholar] [PubMed]
- Potts, D.; Ahlert, R.C.; Wang, S.S. A critical review of fouling of reverse osmosis membranes. Desalination 1981, 36, 235–264. [Google Scholar] [CrossRef]
- Pandey, S.R.; Jegatheesan, V.; Baskaran, K.; Shu, L. Fouling in reverse osmosis (RO) membrane in water recovery from secondary effluent: A review. Rev. Environ. Sci. Bio/Technol. 2012, 11, 125–145. [Google Scholar] [CrossRef]
- Yermiyahu, U.; Tal, A.; Ben-Gal, A.; Bar-Tal, A.; Tarchitzky, J.; Lahav, O. Rethinking desalinated water quality and agriculture. Science 2007, 318, 920–921. [Google Scholar] [CrossRef]
- Wang, X.N.; Liu, Y.; Pan, X.H.; Han, J.X.; Hao, J. Parameters for seawater reverse osmosis product water: A review. Expo. Health 2017, 9, 157–168. [Google Scholar] [CrossRef]
- Hilal, N.; Kim, G.; Somerfield, C. Boron removal from saline water: A comprehensive review. Desalination 2011, 273, 23–35. [Google Scholar] [CrossRef]
- Kim, D.; Amy, G.L.; Karanfil, T. Disinfection by-product formation during seawater desalination: A review. Water Res. 2015, 81, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Drewes, J.E.; Bellona, C.; Amy, G.; Kim, T.U.; Adam, M.; Heberer, T. Rejection of emerging organic micropollutants in nanofiltration–reverse osmosis membrane applications. Water Environ. Res. 2005, 77, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Chalatip, R.; Chawalit, R.; Nopawan, R. Removal of haloacetic acids by nanofiltration. J. Environ. Sci. 2009, 21, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Amy, G.; Drewes, J.E.; Heberer, T.; Kim, T.U.; Watanabe, Y. Rejection of organic micropollutants (disinfection by-products, endocrine disrupting compounds, and pharmaceutically active compounds) by NF/RO membranes. J. Membr. Sci. 2003, 227, 113–121. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry. Environ. Pollut. 2003, 122, 435–445. [Google Scholar] [CrossRef]
- Waniek, A.; Bodzek, M.; Konieczny, K. Trihalomethane removal from water using membrane processes. Pol. J. Environ. Stud. 2002, 11, 171–178. [Google Scholar]
- Agus, E.; Sedlak, D.L. Formation and fate of chlorination by-products in reverse osmosis desalination systems. Water Res. 2010, 44, 1616–1626. [Google Scholar] [CrossRef]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Roux, A.; Poussade, Y.; Drewes, J.E.; Nghiem, L.D. N-nitrosamine rejection by nanofiltration and reverse osmosis membranes: The importance of membrane characteristics. Desalination 2013, 316, 67–75. [Google Scholar] [CrossRef]
- Farré, M.; Keller, J.; Holling, N.; Poussade, Y.; Gernjak, W. Occurrence of N-nitrosodimethylamine precursors in wastewater treatment plant effluent and their fate during ultrafiltration-reverse osmosis membrane treatment. Water Sci. Technol. 2011, 63, 605–612. [Google Scholar] [CrossRef]
- Steinle-Darling, E.; Zedda, M.; Plumlee, M.H.; Ridgway, H.F.; Reinhard, M. Evaluating the impacts of membrane type, coating, fouling, chemical properties and water chemistry on reverse osmosis rejection of seven nitrosoalklyamines, including NDMA. Water Res. 2007, 41, 3959–3967. [Google Scholar] [CrossRef]
- Verliefde, A.R.; Cornelissen, E.R.; Heijman, S.G.J.; Verberk, J.Q.J.C.; Amy, G.L.; Van der Bruggen, B.; Van Dijk, J.C. The role of electrostatic interactions on the rejection of organic solutes in aqueous solutions with nanofiltration. J. Membr. Sci. 2008, 322, 52–66. [Google Scholar] [CrossRef]
- Duranceau, S. Determination of the total iodide content in desalinated seawater permeate. Desalination 2010, 261, 251–254. [Google Scholar] [CrossRef]
- Magara, Y.; Aizawa, T.; Kunikane, S.; Itoh, M.; Kohki, M.; Kawasaki, M.; Takeuti, H. The behavior of inorganic constituents and disinfection by products in reverse osmosis water desalination process. Water Sci. Technol. 1996, 34, 141–148. [Google Scholar] [CrossRef]
- Richardson, S.D.; Fasano, F.; Ellington, J.J.; Crumley, F.G.; Buettner, K.M.; Evans, J.J.; Blount, B.C.; Silva, L.K.; Waite, T.J.; Luther, G.W. Occurrence and mammalian cell toxicity of iodinated disinfection byproducts in drinking water. Environ. Sci. Technol. 2008, 42, 8330–8338. [Google Scholar] [CrossRef]
- Plewa, M.J.; Kargalioglu, Y.; Vankerk, D.; Minear, R.A.; Wagner, E.D. Mammalian cell cytotoxicity and genotoxicity analysis of drinking water disinfection by-products. Environ. Mol. Mutagen. 2002, 40, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Tian, H.J.; Heng, Z.C.; Li, N.; La, B.Z.M. The Study on the Effects of Organic Extracts From Source Water and Drinking Water on DNA Damage in V79 Cells. J. Environ. Health 1999, 16, 10–12. [Google Scholar]
- Qiu, Z.; Shu, W.; Tian, H. An analysis of the organic extracts from source water and drinking water in city C and the effects on DNA damage in primary hepatocytes in rats. J. Third Mil. Med. Univ. 2003, 5, 423–426. [Google Scholar]
- Zhang, Q.; Heng, Z.; Chen, Z. Study on Effects of Organic Extracts from Drinking Water on DNA Damage in Primary Hepatocytes. Ind. Health Occup. Dis.-Beijing 2004, 30, 216–218. [Google Scholar]
- Zheng, N.X.; Wang, C.L.; You-Qiong, X.U. Genetic toxicity of organic extractions from drinking water in Fuzhou city. Chin. J. Public Health 2011, 27, 129–130. [Google Scholar]
- Wu, N.; Li, X.; Tao, H. Removal of Organic Mutagen in Tap Water. J. Environ. Health 1992, 12, wpr-545978. [Google Scholar]
- Tang, G.; Jie, W.; Yin, J.; Yan, Q.; Gong, Y.; Jiao, Z.; Cao, M.E.; Huang, S.; Zhu, G.; Ran, L. Establishment and application of evaluation method for genotoxicity of drinking water. J. Southeast Univ. 2018, 48, 170–173. [Google Scholar]
- Zhang, L.; Zhai, H. DNA damage in L-02 cells exposed to organic extracts of chlorinated drinking water. J. Environ. Health 2010, 27, 14–16. [Google Scholar]
- Zhang, Q.; Chen, Z.; Gan, Z.; Heng, Z.; Li, X. Mutagenicity of Organic Extract From Drinking Water in a Certain City. J. Environ. Health 2005, 4, 289–291. [Google Scholar]
- Sun, L.; Qu, M.; Li, Z.; Chen, H.; Li, Y.; Kong, Z. Toxicity evaluation of drinking water in human peripheral blood lymphocytes using the comet assay. ACTA Sci. Circumstantiae 2005, 25, 324–328. [Google Scholar]
- Zhang, Q.B.; Gan, Z.L.; Chen, Z.Q. Study of Organic Extracts from Water Samples on DNA Damage of Primary Hepatocytes Cells with Comet Assay. J. Prev. Med. Inf. 2003, 2, 116–117. [Google Scholar]
- Zhang, R.; Hairong, D.U.; Cao, X. Screening DNA damage of organic extract in water sample of environment by eukaryotic cell screening system. Hebei Med. J. 2008, 5, 581–583. [Google Scholar]
- Nriagu, J.; Darroudi, F.; Shomar, B. Health effects of desalinated water: Role of electrolyte disturbance in cancer development. Environ. Res. 2016, 150, 191–204. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chiu, H.F.; Cheng, M.F.; Tsai, S.S.; Hung, C.F.; Lin, M.C. Esophageal cancer mortality and total hardness levels in Taiwan’s drinking water. Environ. Res. 1999, 81, 302–308. [Google Scholar] [CrossRef]
- Hua, G.; Reckhow, D.A.; Kim, J. Effect of bromide and iodide ions on the formation and speciation of disinfection byproducts during chlorination. Environ. Sci. Technol. 2006, 40, 3050–3056. [Google Scholar] [CrossRef]
- Kitis, M.; Karanfil, T.; Wigton, A.; Kilduff, J.E. Probing reactivity of dissolved organic matter for disinfection by-product formation using XAD-8 resin adsorption and ultrafiltration fractionation. Water Res. 2002, 36, 3834–3848. [Google Scholar] [CrossRef]
- Liang, L.; Singer, P.C. Factors influencing the formation and relative distribution of haloacetic acids and trihalomethanes in drinking water. Environ. Sci. Technol. 2003, 37, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).