Effects of Long-Term Exposure of Pharmaceuticals and Personal Care Products on Algogenic Organic Matter Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Cultivation and AOM Extraction
2.2. Analytical Method
3. Results and Discussion
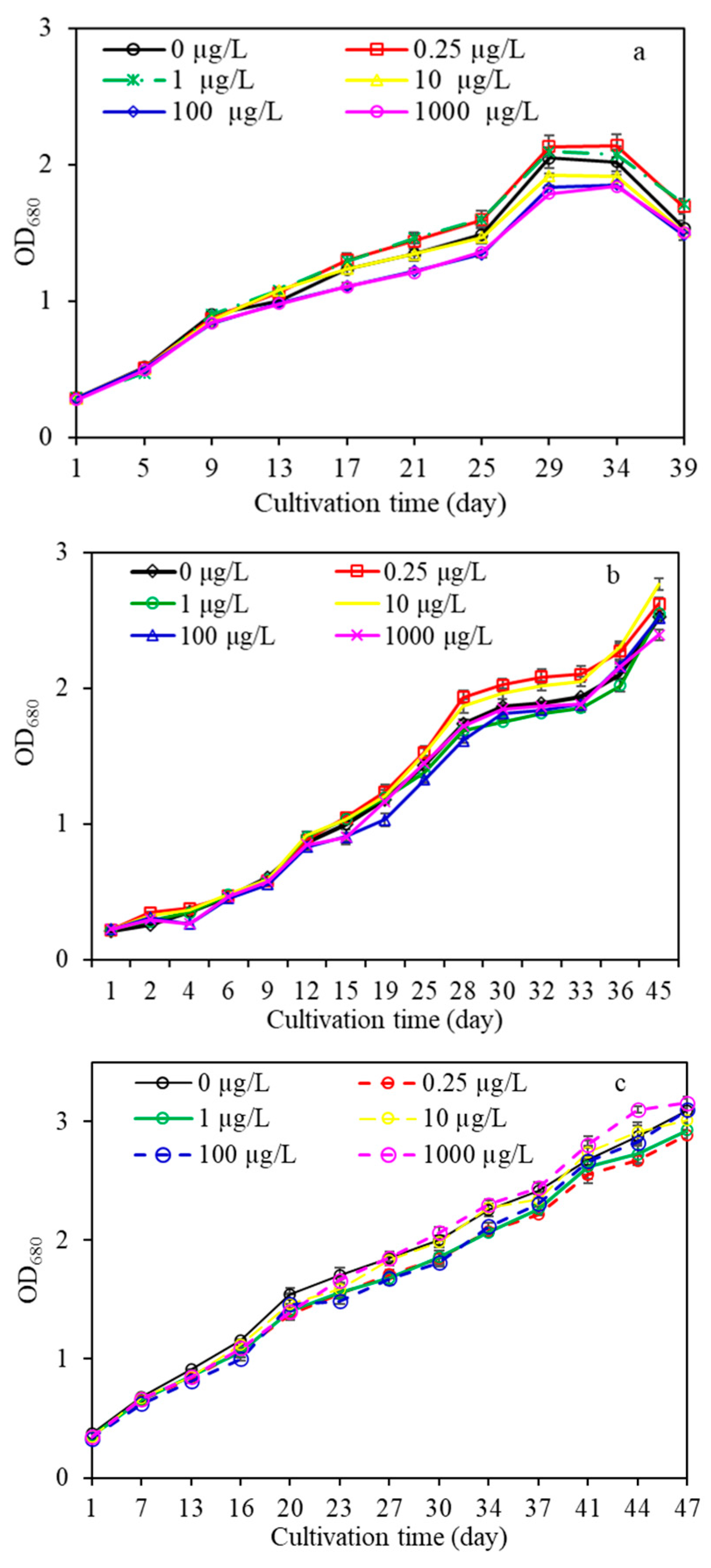
3.1. Variation in Algal Growth during Long-Term Exposure to Various PPCPs
3.2. Variation in AOM Characteristics during Long-Term Exposure to Various PPCPs
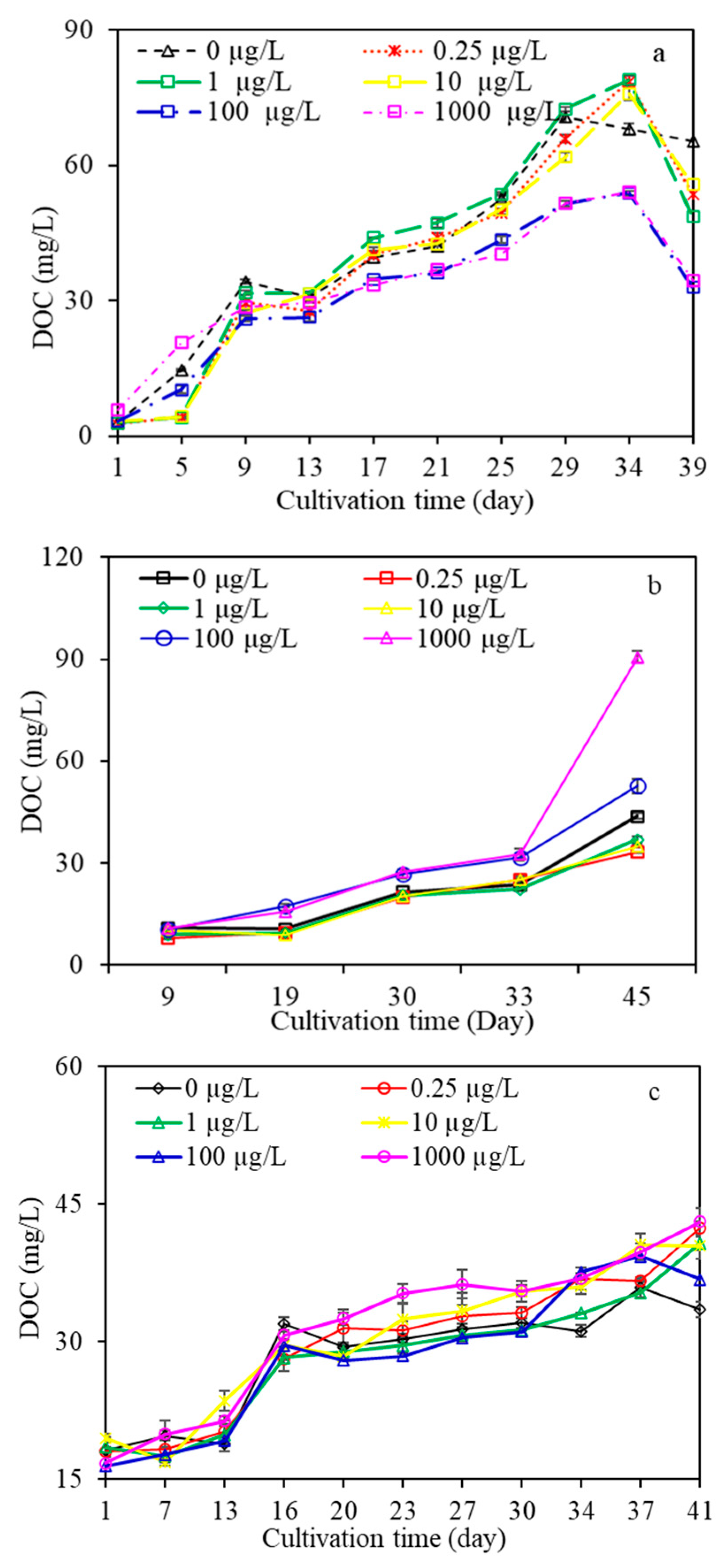
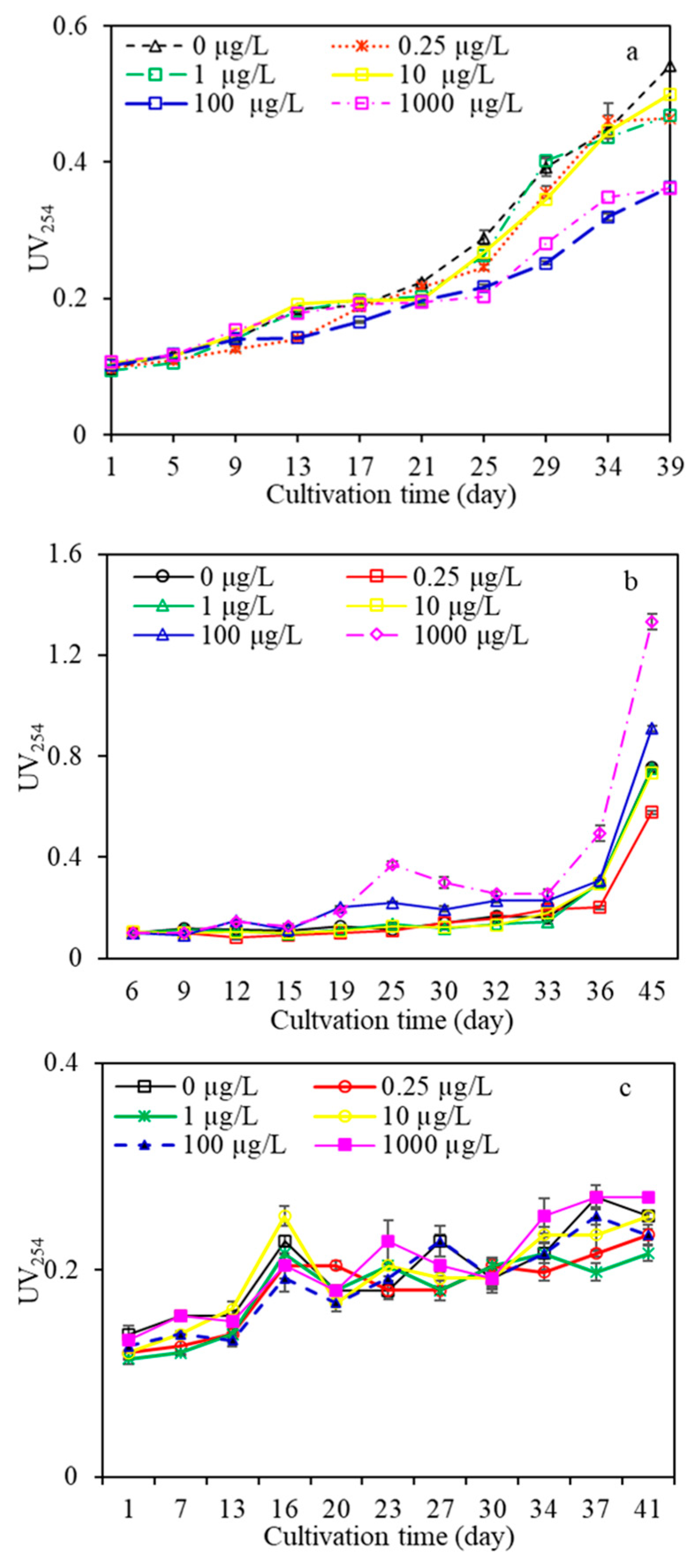
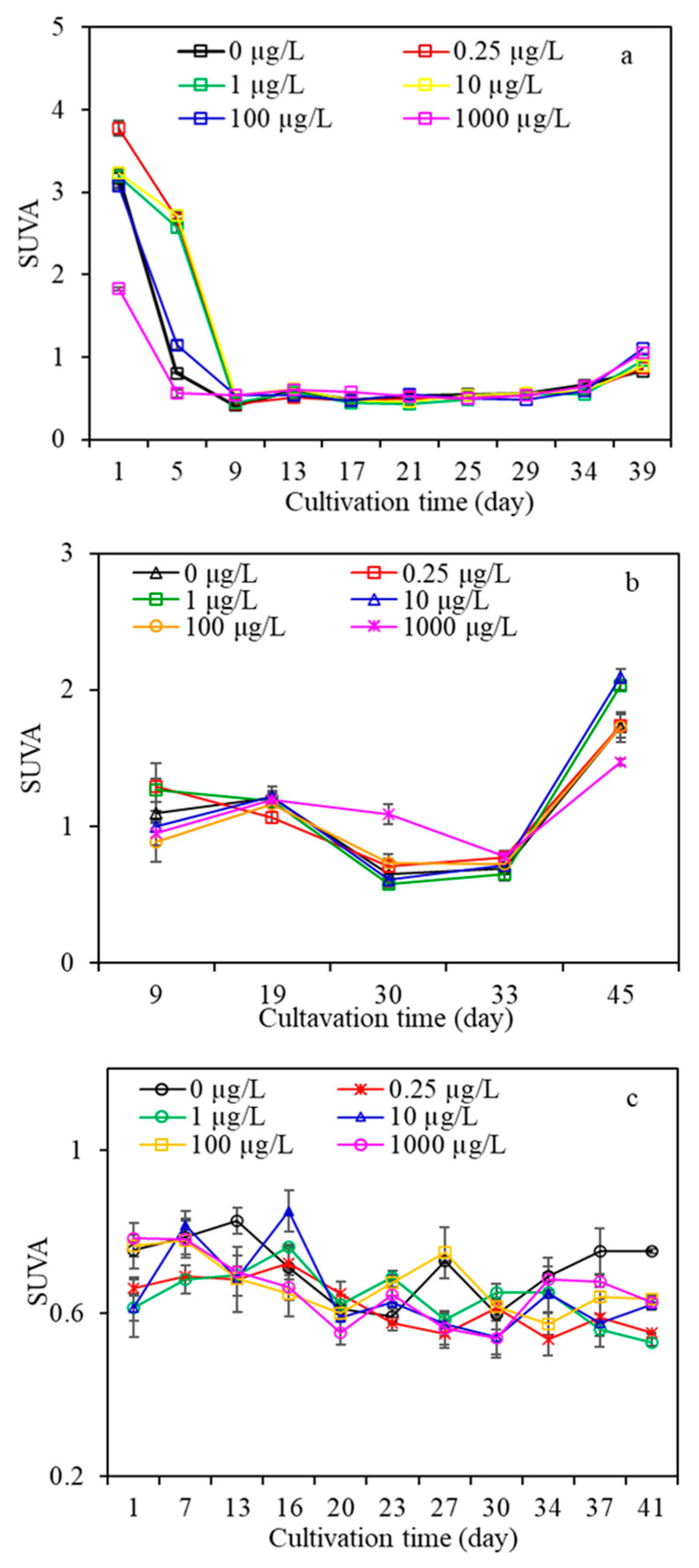
3.2.1. DOC and UV254
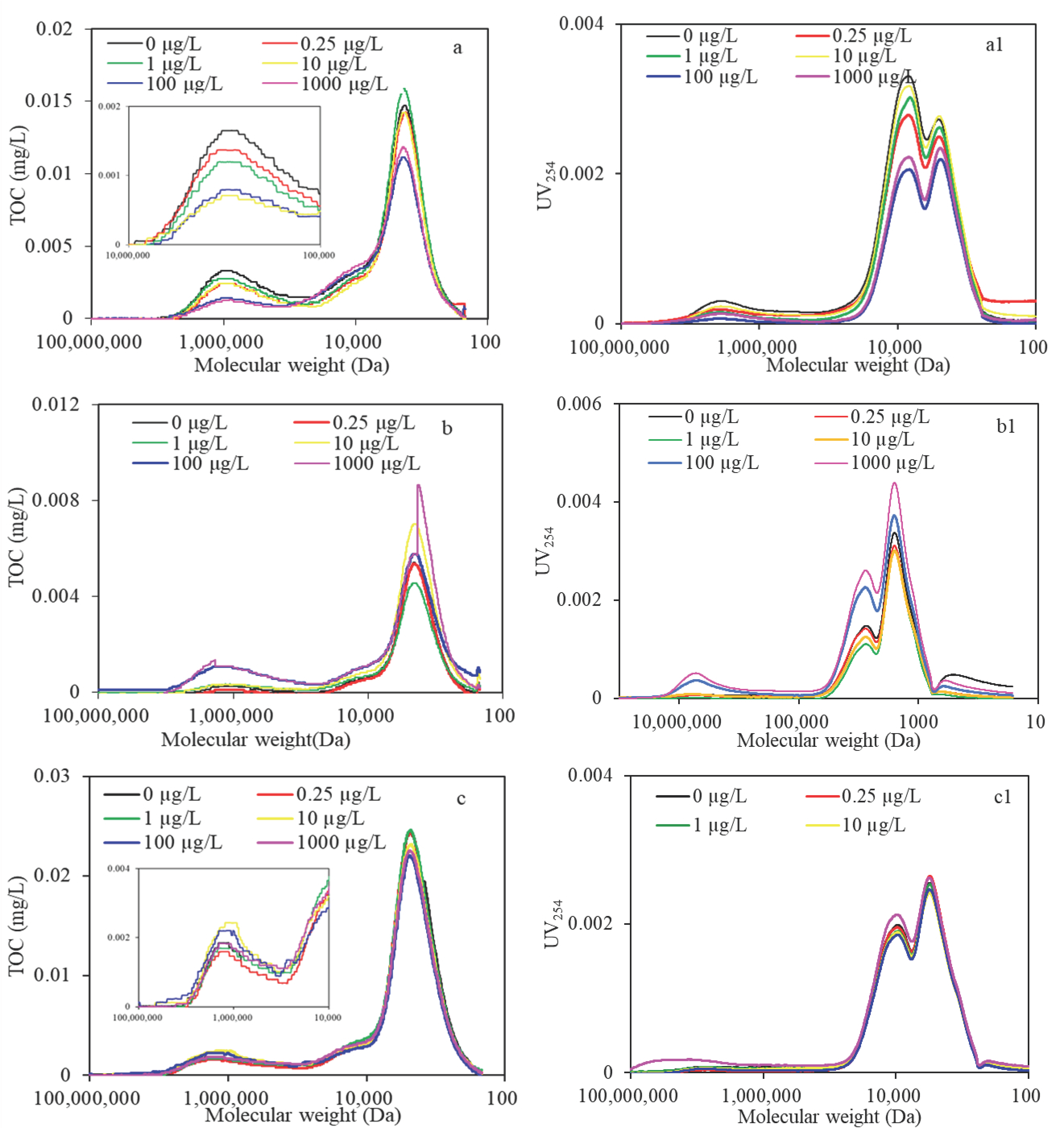
3.2.2. High-Performance Size Exclusion Chromatography
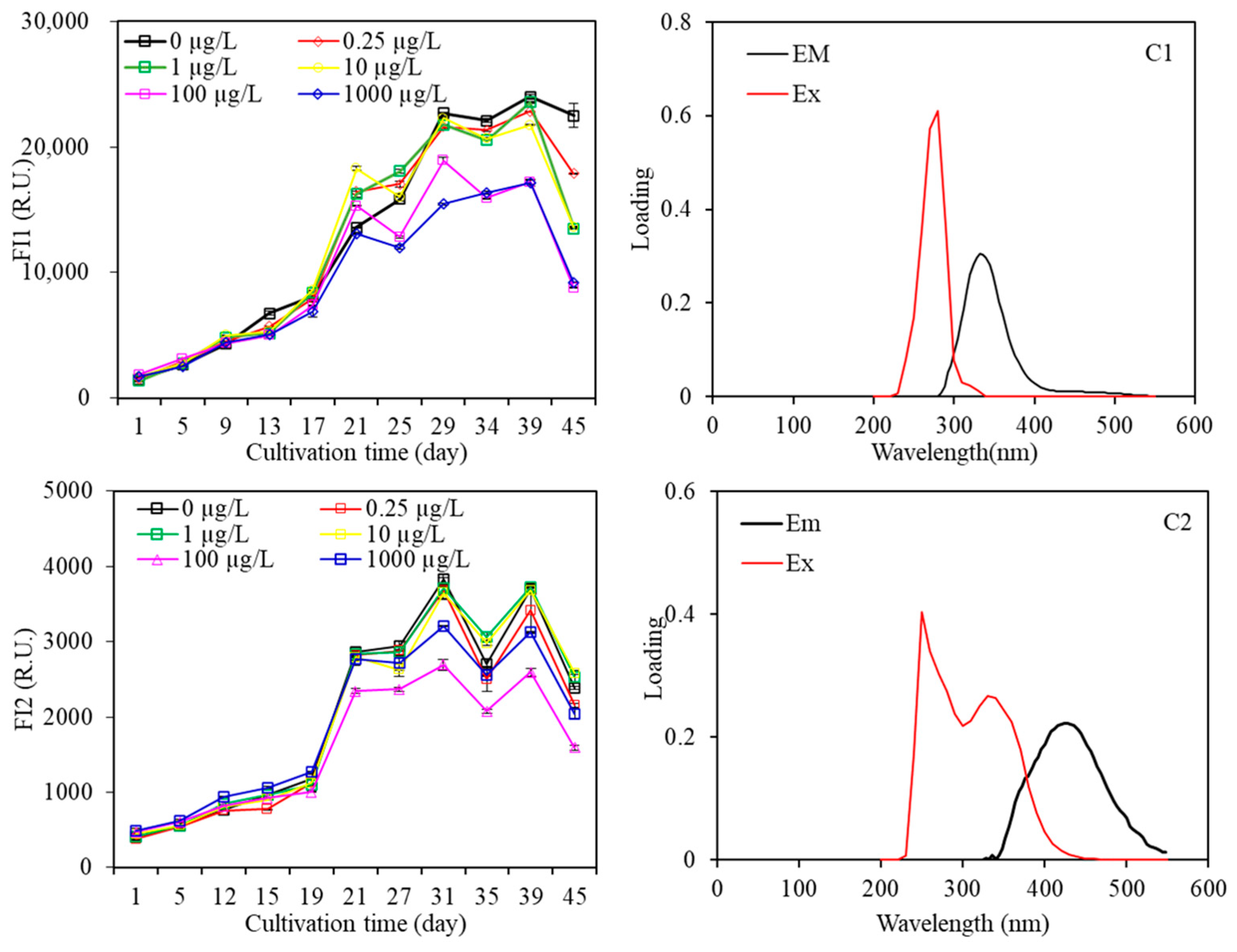
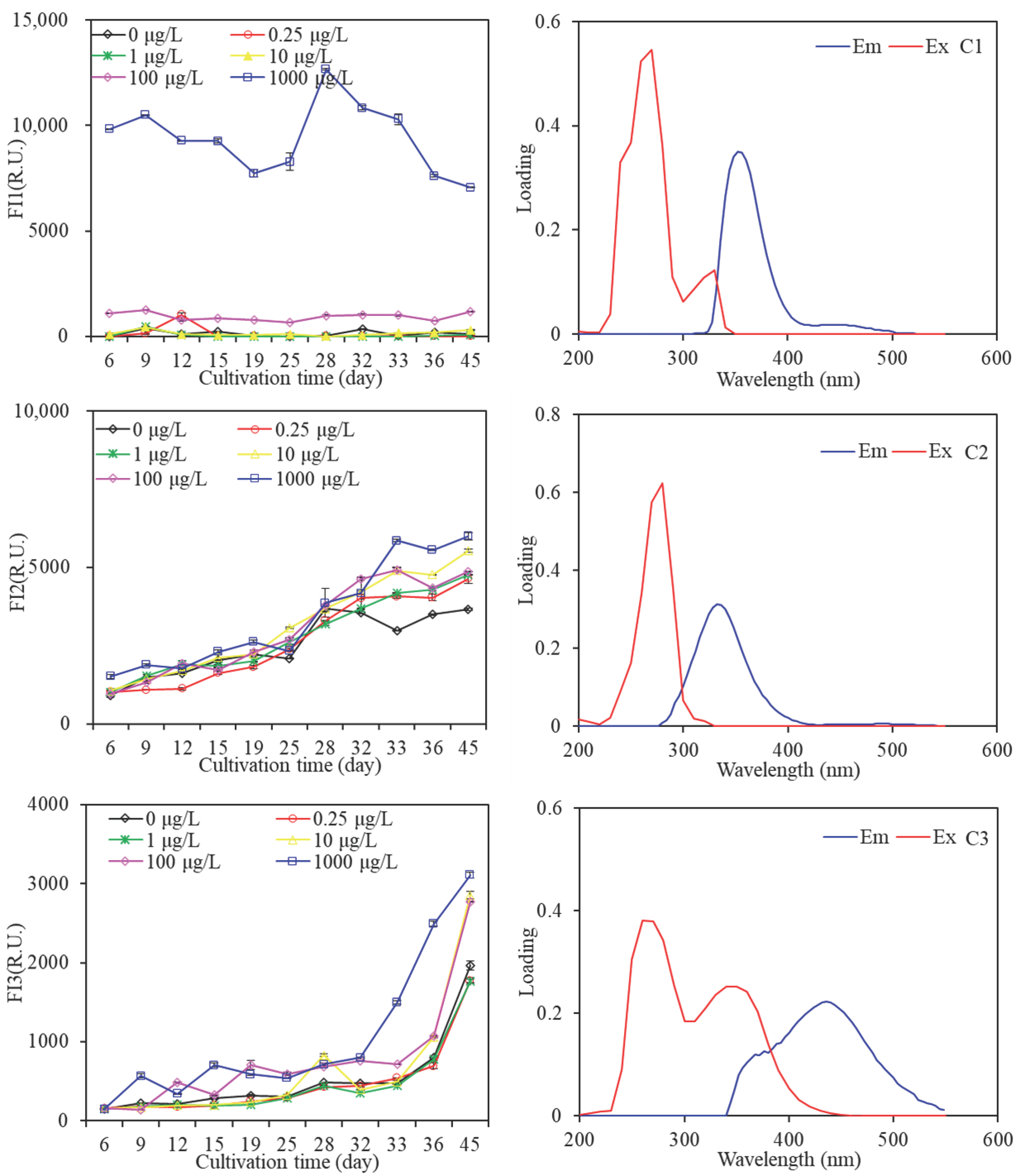
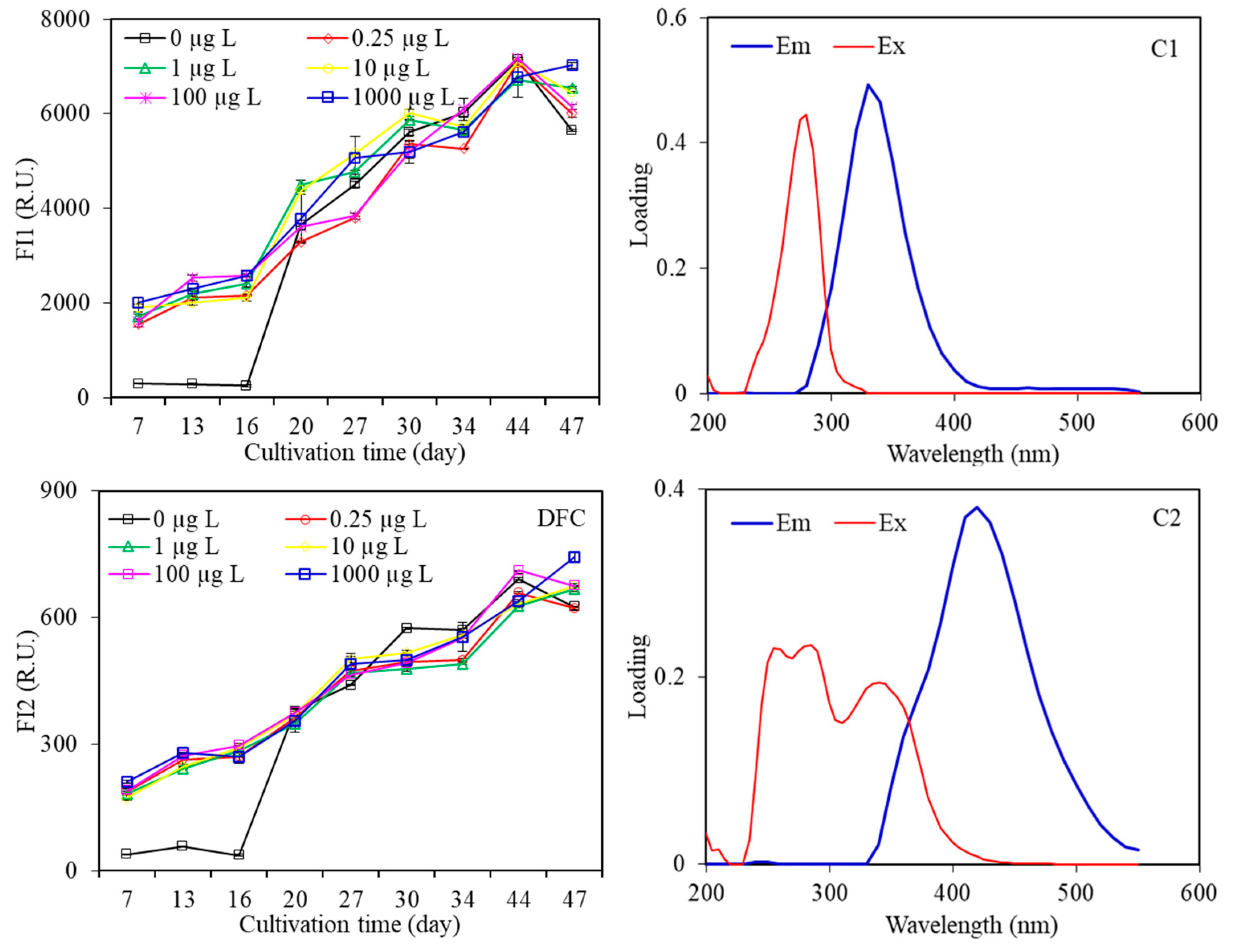
3.2.3. Fluorescence EEM Spectra
3.3. Correlations between PPCPs Concentration and AOM Characteristics
3.4. Implication for Water Treatment
4. Conclusions
- PPCPs in water changed the algal growth and AOM characteristics of of M. aeruginosa. CMZ and NPX in water promoted the growth of algae at low concentrations (<10 µg/L), whereas they were inhibited after long-term exposure to high CMZ and NPX concentrations (>10 µg/L). For the DFC treatment, the results were different, with algal growth promoted under long term exposure to high concentrations (>10 µg/L), yet they were inhibited at low concentrations.
- AOM characteristics suggested that algae under low carbamazepine (<10 µg/L), high naproxen (>10 µg/L), and/or diclofenac at any concentration treatment promoted the release of total organic matter, whereas they were inhibited at high carbamazepine and low naproxen exposure. Moreover, macromolecular organics of AOM were inhibited when algae were exposed to CMZ for a long time at any concentration, and the higher the CMZ concentration was, the more serious macromolecular organics were inhibited. For the NPX and DFC treatments, macro- and medium molecular organics were promoted under high NPX and DFC concentrations (>1 µg/L), yet they were inhibited under low NPX and DFC concentrations (<10 µg/L). This research had significant effects on algal-laden water treatment containing various PPCPs concentrations as well as the risk assessment of PPCPs in water.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Frimmel, F.H. Characterization of natural organic matter as major constituents in aquatic systems. J. Contamin. Hydrol. 1998, 35, 201–216. [Google Scholar] [CrossRef]
- Brzinsiki, K.; Gorczyca, B. An overview of the uses of high performance size exclusion chromatography (HPSEC) in the characterization of natural organic matter (NOM) in potable water and ion-exchange applications. Chemosphere 2019, 217, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.L.; Wang, X.N.; Fang, Z.Y.; Wang, S.; Shan, C.; Wei, S.; Pan, B.C. Unravelling molecular transformation of dissolved effluent organic matter in UV/H2O2, UV/persulfate, and UV/chlorine processes based on FT-ICR-MS analysis. Water Res. 2021, 199, 117158. [Google Scholar] [CrossRef]
- Yang, H.L.; Ye, S.J.; Wang, J.J.; Wang, H.; Wang, Z.W.; Chen, Q.; Wang, W.J.; Xiang, L.; Zeng, G.M.; Tan, X.F. The approaches and prospects for natural organic matter-derived disinfection byproducts control by carbon-based materials in water disinfection progresses. J. Cleaner Product. 2021, 311, 127799. [Google Scholar]
- Li, K.; Qu, F.S.; Liang, H.; Shao, S.L.; Han, Z.S.; Chang, H.Q.; Du, X.; Li, G.B. Performance of mesoporous adsorbent resin and powdered activated carbon in mitigating ultrafiltration membrane fouling caused by algal extracellular organic matter. Desalination 2014, 336, 129–137. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Moon, J.Y.; Lee, Y.C. Microalgal ecotoxicity of nanoparticles: An updated review. Ecotoxicol. Environ. Safety 2020, 201, 110781. [Google Scholar] [CrossRef]
- Zhao, F.C.; Zhang, W.W.; Zhou, X.F. Effect of temperature on extracellular organic matter (EOM) of Chlorella pyrenoidosa and effect of EOM on irreversible membrane fouling. Colloids Surf. B 2015, 136, 431–439. [Google Scholar] [CrossRef]
- Huang, W.W.; Chu, H.Q.; Dong, B.Z. Characteristics of algogenic organic matter generated under different nutrient conditions and subsequent impact on microfiltration membrane fouling. Desalination 2012, 293, 104–111. [Google Scholar] [CrossRef]
- Xu, X.M.; Xu, Y.R.; Xu, N.; Pan, B.Z.; Ni, J.R. Pharmaceuticals and personal care products (PPCPs) in water, sediment and freshwater mollusks of the Dongting Lake downstream the Three Gorges Dam. Chemosphere 2022, 301, 134721. [Google Scholar] [CrossRef]
- Liu, Q.X.; Feng, X.; Chen, N.; Shen, F.; Zhang, H.C.; Wang, S.; Sheng, Z.Y.; Li, J. Occurrence and risk assessment of typical PPCPs and biodegradation pathway of ribavirin in wastewater treatment plants. Environ. Sci. Ecotechnol. 2022, 11, 100184. [Google Scholar] [CrossRef]
- Xie, Z.X.; Wang, X.Y.; Gan, Y.; Cheng, H.M.; Fan, S.S.; Li, X.D.; Tang, J. Ecotoxicological effects of the antidepressant fluoxetine and its removal by the typical freshwater microalgae Chlorella pyrenoidosa. Ecotoxicol. Environ. Safety 2022, 244, 114045. [Google Scholar] [CrossRef] [PubMed]
- Couto, E.; Assemany, P.P.; Carneiro, G.C.A.; Soares, D.C.F. The potential of algae and aquatic macrophytes in the pharmaceutical and personal care products (PPCPs) environmental removal: A review. Chemosphere 2022, 302, 134808. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.Y.; Huang, G.; Zhang, B.Y. Review of aquatic toxicity of pharmaceuticals and personal care products to algae. J. Hazard. Mater. 2021, 410, 124619. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Phung, C.; Grace, M. Pharmaceuticals and personal care products alter growth and function in lentic biofilms. Environ. Chem. 2015, 12, 301–306. [Google Scholar] [CrossRef]
- Zhao, W.T.; Guo, Y.; Lu, S.G.; Yan, P.P.; Sui, Q. Recent advances in pharmaceuticals and personal care products in the surface water and sediments in China. Front. Environ. Sci. Eng. 2016, 10, 29–40. [Google Scholar] [CrossRef]
- Miazek, K.; Brozek-Pluska, B. Effect of PHRs and PCPs on microalgal growth, metabolism and microalgae-based bioremediation processes: A review. Intern. J. Molecul. Sci. 2019, 20, 2492. [Google Scholar] [CrossRef]
- Kołodziejska, M.; Maszkowska, J.; Białk-Bielińska, A.; Steudte, S.; Kumirska, J.; Stepnowski, P.; Stolte, S. Aquatic toxicity of four veterinary drugs commonly applied in fish farming and animal husbandry. Chemosphere 2013, 92, 1253–1259. [Google Scholar] [CrossRef]
- Robinson, A.A.; Belden, J.B.; Lydy, M.J. Toxicity of fluoroquinolone antibiotics to aquatic organisms. Environ. Toxicol. Chem. 2005, 24, 423–430. [Google Scholar] [CrossRef]
- Kondor, A.C.; Molnár, É.; Jakab, G.; Vancsik, A.; Filep, T.; Szeberényi, J.; Szabó, L.; Maász, G.; Pirger, Z.; Weiperth, A.; et al. Pharmaceuticals in water and sediment of small streams under the pressure of urbanization: Concentrations, interactions, and risks. Sci. Total Environ. 2020, 808, 152160. [Google Scholar] [CrossRef]
- Waleng, N.J.; Nomngongo, P.N. Occurrence of pharmaceuticals in the environmental waters: African and Asian perspectives. Environ. Chem. Ecotoxicol. 2022, 4, 50–66. [Google Scholar] [CrossRef]
- Ashfaq, M.; Li, Y.; Rehman, M.S.U.; Zubair, M.; Mustafa, G.; Nazar, M.F.; Yu, C.P.; Sun, Q. Occurrence, spatial variation and risk assessment of pharmaceuticals and personal care products in urban wastewater, canal surface water, and their sediments: A case study of Lahore, Pakistan. Sci. Total Environ. 2019, 688, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Cao, X.Q.; Lu, S.G.; Zhao, W.T.; Qiu, Z.F.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 1–6. [Google Scholar] [CrossRef]
- ErbaPompei, C.M.; Campos, L.C.; Vieira, E.M.; Tucci, A. The impact of micropollutants on native algae and cyanobacteria communities in ecological filters during drinking water treatment. Sci. Total Environ. 2022, 822, 153401. [Google Scholar]
- Mao, Y.F.; Yu, Y.; Ma, Z.X.; Li, H.; Yu, W.W.; Cao, L.; He, Q. Azithromycin induces dual effects on microalgae: Roles of photosynthetic damage and oxidative stress. Ecotoxicol. Environ. Saf. 2021, 222, 112496. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Blain, R.B. The hormesis database: The occurrence of hormetic dose responses in the toxicological literature. Regul. Toxicol. Pharm. 2011, 61, 73–81. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kurade, M.B.; Abou-Shanab, R.A.I.; Ji, M.K.; Choi, J.; Kim, J.O.; Jeon, B.H. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef]
- Ding, T.D.; Lin, K.D.; Yang, B.; Yang, M.T.; Li, J.Y.; Li, W.Y.; Gan, J. Biodegradation of naproxen by freshwater algae Cymbella sp. and Scenedesmus quadricauda and the comparative toxicity. Bioresour. Technol. 2017, 238, 164–173. [Google Scholar] [CrossRef]
- Zhang, W.W.; Chu, H.Q.; Zhou, X.F.; Zhang, Y.L. Study on the effects of diclofenac sodium on the growth of haematococcus pluvialis. Environ. Prevent. Pollut. China 2019, 41, 1146–1150. [Google Scholar]
- Lee, S.J.; Kim, J.H. Differential natural organic matter fouling of ceramic versus polymeric ultrafiltration membranes. Water Res. 2014, 48, 43–51. [Google Scholar] [CrossRef]
- Cheng, W.P.; Chi, F. Influence of eutrophication on the coagulation efficiency in reservoir water. Chemosphere 2003, 53, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Reckhow, D.A.; Singer, P.C.; Malcolm, R.L. Chlorination of humic materials: Byproducts formation and chemical interpretations. Envirn. Sci. Technol. 1990, 24, 1655–1665. [Google Scholar] [CrossRef]
- Li, L.; Gao, N.Y.; Deng, Y.; Yao, J.J.; Zhang, K.J. Characterization of intracellular & extracellular algae organic matters (AOM) of Microcystic aeruginosa and formation of AOM-associated disinfection byproducts and odor & taste compounds. Water Res. 2012, 46, 1233–1240. [Google Scholar] [PubMed]
- Hoyer, O.; Lüsse, B.; Bernhardt, H. Isolation and characterization of extracellular organic matter (EOM) from algae. Z. Wasser-Abwasser-Forsch. 1985, 18, 76–90. [Google Scholar]
- Zhang, X.L.; Fan, L.H.; Roddick, F.A. Understanding the fouling of a ceramic microfiltration membrane caused by algal organic matter released from Microcystis aeruginosa. J. Membr. Sci. 2013, 447, 362–368. [Google Scholar] [CrossRef]
- Cheng, X.X.; Wu, D.J.; Liang, H.; Zhu, X.W.; Tang, X.B.; Gan, Z.D.; Xing, J.J.; Luo, X.S.; Li, G.B. Effect of sulfate radical-based oxidation pretreatments for mitigating ceramic UF membrane fouling caused by algal extracellular organic matter. Water Res. 2018, 145, 39–49. [Google Scholar] [CrossRef]
- Sheng, D.; Bu, L.J.; Zhu, S.M.; Wu, Y.T.; Wang, J.; Li, N.; Zhou, S.Q. Impact of pre-oxidation on the formation of byproducts in algae-laden water disinfection: Insights from fluorescent and molecular weight. J. Environ. Sci. 2022, 117, 21–27. [Google Scholar] [CrossRef]
- Yu, H.R.; Qu, F.S.; Liang, H.; Han, Z.S.; Ma, J.; Shao, S.L.; Chang, H.Q.; Li, G.B. Understanding ultrafiltration membrane fouling by extracellular organic matter of Microcystis aeruginosa using fluorescence excitation-emission matrix coupled with parallel factor analysis. Desalination 2014, 337, 67–75. [Google Scholar] [CrossRef]
- Tang, S.J.; Wang, Z.W.; Wu, Z.C.; Zhou, Q. Role of dissolved organic matters (DOM) in membrane fouling of membrane bioreactors for municipal wastewater treatment. J. Hazard. Mater. 2010, 178, 377–384. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Wang, Z.G.; Wang, S.S.; Yao, D.W.; Chen, X.F. Dynamic Release correlation between extracellular organic components and Microcystins MC-LR based on three-dimensional fluorescence spectroscopy. J. Atmosp. Environ. Opt. China 2020, 15, 285–295. [Google Scholar]
- Yang, J.T.; Yang, S.W.; Jin, W.D.; Liu, L.; Yan, Y.H.; Mao, Q.D. EOM characteristics and release of extracellular amino acids in three typical freshwater algaes. China Environ. Sci. 2017, 37, 1879–1888. [Google Scholar]
- Wang, L.Y.; Wu, F.C.; Zhang, R.Y.; Li, W.; Liao, H.Q. Characterization of dissolved organic matter fractions from Lake Hongfeng, Southwestern China Plateau. J. Environ. Sci. China 2009, 21, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Henderson, R.K.; Baker, A.; Parsons, S.A.; Jefferson, B. Characterisation of algogenic organic matter extracted from cyanobacteria, green algae and diatoms. Water Res. 2008, 42, 3435–3445. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Li, T.; Zhao, Q.Q.; Liu, W.; Liu, J.X.; Song, Y.L.; Chu, H.Q.; Dong, B.Z. UF fouling behavior of allelopathy of extracellular organic matter produced by mixed algae co-cultures. Separ. Purif. Technol. 2021, 261, 118297. [Google Scholar] [CrossRef]
| OD | DOC | UV | BP | HS | LMW | C1 | C2 | C3 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| CMZ | Pearson Correlation | −0.942 ** | −0.870 * | −0.864 * | 0.789 | 0.285 | −0.647 | −0.799 | −0.591 | |
| Sig. | 0.005 | 0.024 | 0.027 | 0.062 | 0.584 | 0.165 | 0.056 | 0.216 | ||
| DFC | Pearson Correlation | 0.807 | 0.770 | 0.595 | 0.379 | −0.130 | −0.727 | 0.653 | 0.905 * | |
| Sig. | 0.052 | 0.074 | 0.213 | 0.459 | 0.807 | 0.102 | 0.160 | 0.013 | ||
| NPX | Pearson Correlation | −0.354 | 0.863 * | 0.914 * | 0.848 * | 0.907 * | 0.843 * | 0.833 * | 0.716 | 0.924 ** |
| Sig. | 0.491 | 0.027 | 0.011 | 0.033 | 0.012 | 0.035 | 0.040 | 0.109 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Lv, W.; Yuan, Q.; Zhou, W.; Li, T.; Dong, B. Effects of Long-Term Exposure of Pharmaceuticals and Personal Care Products on Algogenic Organic Matter Characteristics. Water 2023, 15, 2447. https://doi.org/10.3390/w15132447
Huang W, Lv W, Yuan Q, Zhou W, Li T, Dong B. Effects of Long-Term Exposure of Pharmaceuticals and Personal Care Products on Algogenic Organic Matter Characteristics. Water. 2023; 15(13):2447. https://doi.org/10.3390/w15132447
Chicago/Turabian StyleHuang, Weiwei, Weiwei Lv, Quan Yuan, Wenzong Zhou, Tian Li, and Bingzhi Dong. 2023. "Effects of Long-Term Exposure of Pharmaceuticals and Personal Care Products on Algogenic Organic Matter Characteristics" Water 15, no. 13: 2447. https://doi.org/10.3390/w15132447
APA StyleHuang, W., Lv, W., Yuan, Q., Zhou, W., Li, T., & Dong, B. (2023). Effects of Long-Term Exposure of Pharmaceuticals and Personal Care Products on Algogenic Organic Matter Characteristics. Water, 15(13), 2447. https://doi.org/10.3390/w15132447





