Phosphorus Recovery from Sewage Sludge as Struvite
Abstract
1. Introduction
2. Materials and Methods
2.1. Wet Oxidation Process
2.2. Struvite Precipitation Experiments
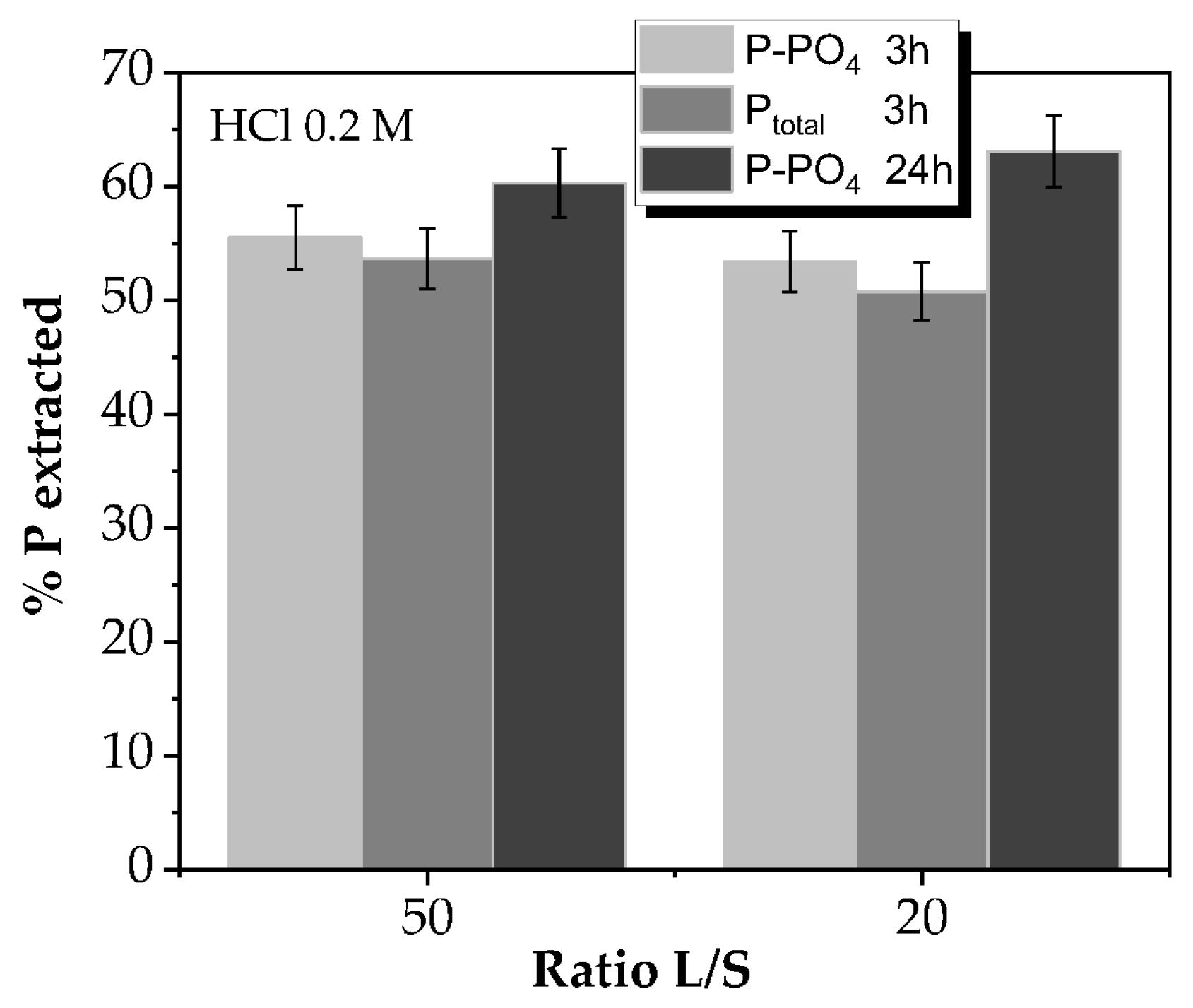
2.3. Solid Leaching Experiments
- NaHCO3 fraction. A total of 20 mL of NaHCO3, 0.5 M to determine the P fraction weakly bound.
- NaOH fraction. A total of 20 mL of NaOH, 0.1 M to determine Al and Fe bound P.
- HCl diluted fraction. A total of 20 mL of HCl, 1.0 M to define Ca bound P.
- HCl concentrated and hot fraction. A total of 12 mL of HCl, 10 M in a water bath at 80 °C to extract stable P. This fraction needs a large amount of energy to be recovered.
3. Results and Discussion
3.1. Characterisation of Sewage Sludge
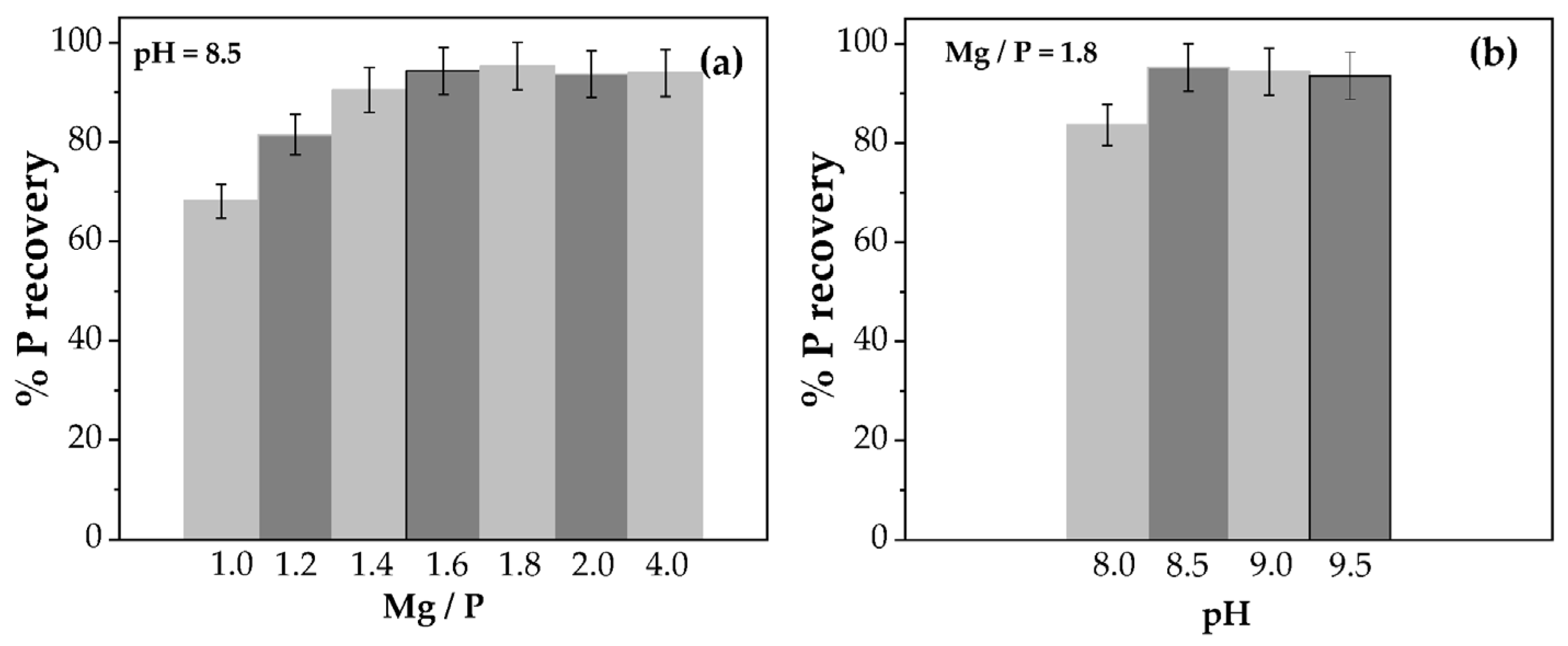
3.2. Struvite Precipitation Experiments
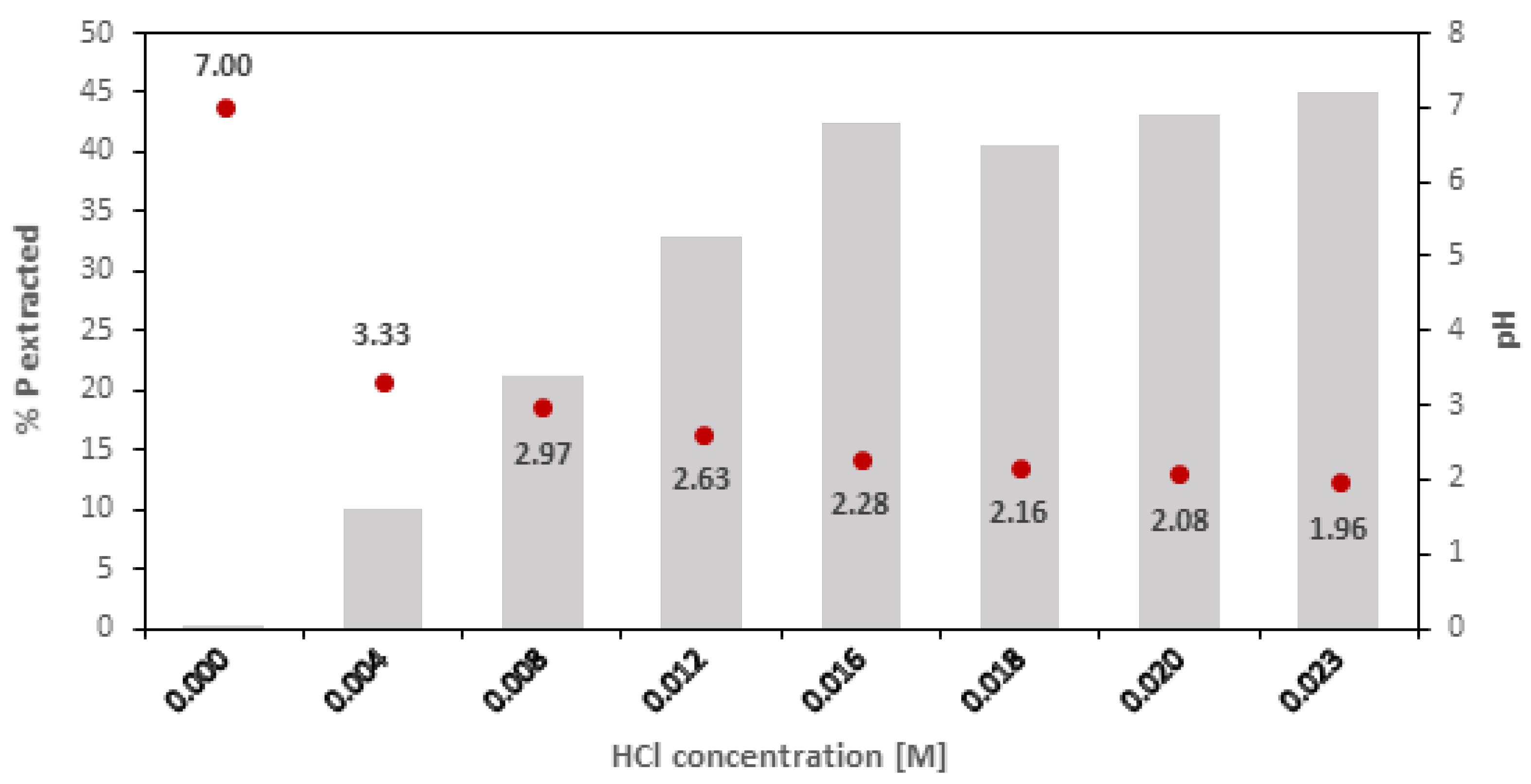
3.3. Preliminary Experiments
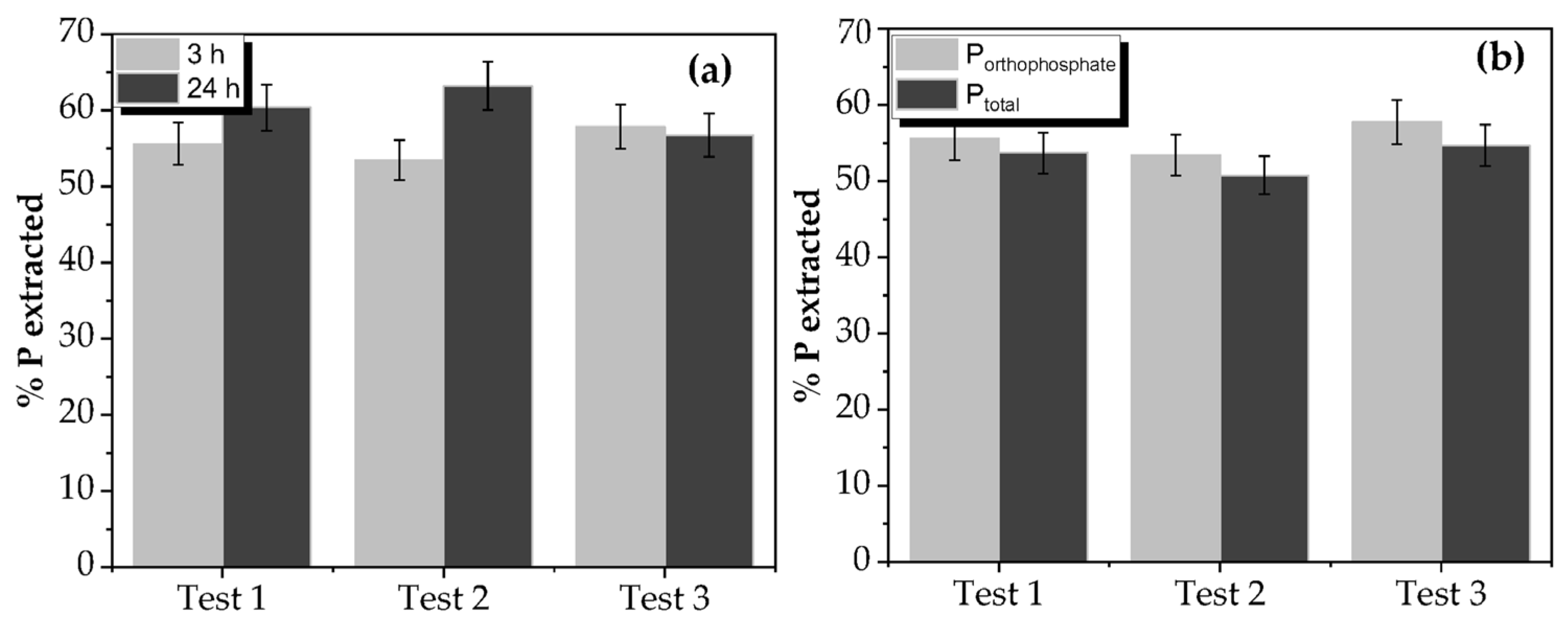
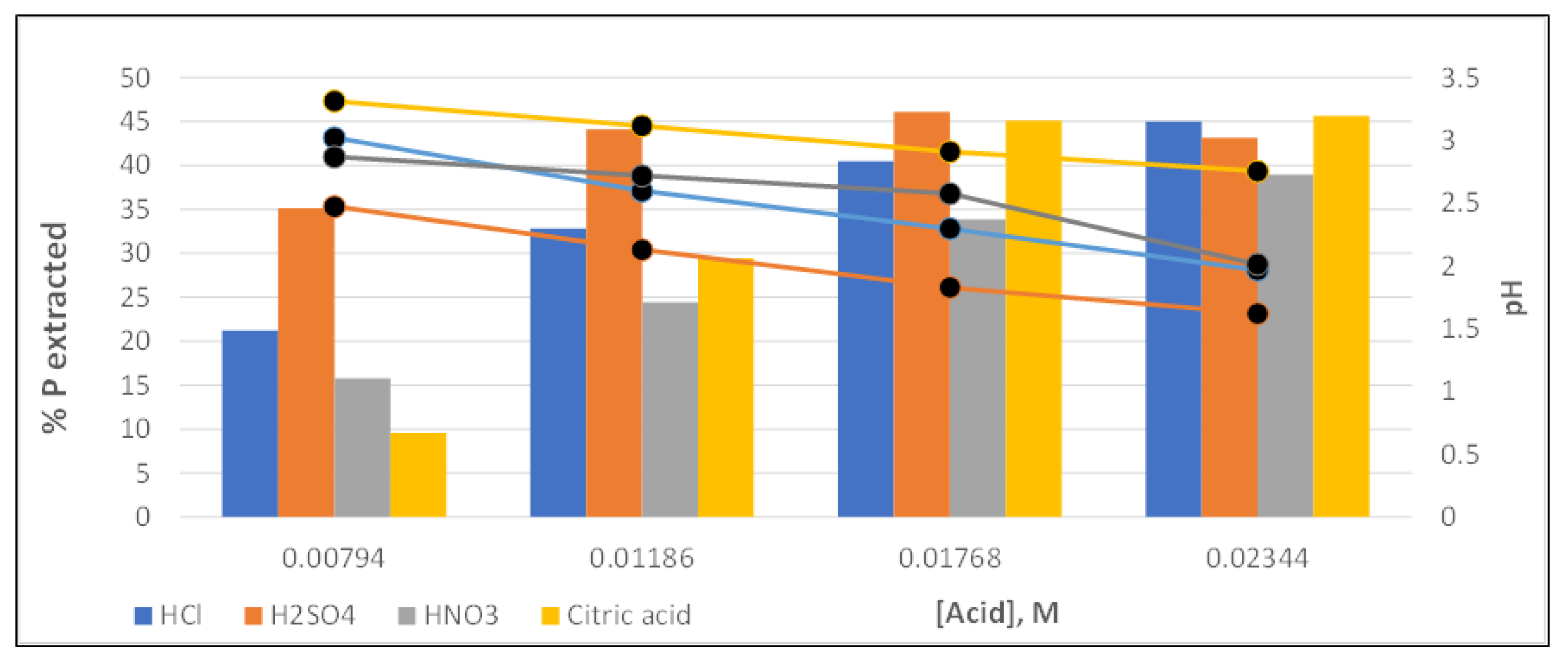
3.4. Solid Phosphorous Extraction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foussard, J.-N.; Debellefontaine, H.; Besomes-Vailhe, J. Efficient elimination of organic liquid wastes: Wet Air Oxidation. J. Environ. Eng. 1989, 115, 367–385. [Google Scholar] [CrossRef]
- Wurm, J.; Poschmann, R.; Thielert, H.; von Morstein, O.; Repke, J.U. Kinetics of ammonium thiosulfate wet air oxidation under high-temperature conditions. Chem. Eng. Technol. 2022, 45, 1588–1597. [Google Scholar] [CrossRef]
- Benitez, F.J.; García, J.; Acero, J.L.; Real, F.J.; Roldan, G. Non-catalytic and catalytic wet air oxidation of pharmaceuticals in ultra-pure and natural waters. Process Saf. Environ. Prot. 2011, 89, 334–341. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, P.; Álvarez-Torrellas, S.; Larriba, M.; Gil, M.V.; Garrido-Zoido, J.M.; García, J. Efficient removal of antibiotic ciprofloxacin by catalytic wet air oxidation using sewage sludge-based catalysts. Degradation mechanism by DFT studies. J. Environ. Chem. Eng. 2023, 11, 109344. [Google Scholar] [CrossRef]
- Hii, K.; Baroutian, S.; Parthasarathy, R.; Gapes, D.J.; Eshtiaghi, N. A review of wet air oxidation and Thermal Hydrolysis technologies in sludge treatment. Bioresour. Technol. 2014, 155, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Vallet, A.; Ovejero, G.; Rodríguez, A.; Peres, J.A.; García, J. Ni/MgAlO regeneration for catalytic wet air oxidation of an azo-dye in trickle-bed reaction. J. Hazard. Mater. 2013, 244, 46–53. [Google Scholar] [CrossRef]
- Gomes, H.T.; Serp, P.; Kalck, P.; Figueiredo, J.L.; Faria, J.L. Carbon supported platinum catalysts for catalytic wet air oxidation of refractory carboxylic acids. Top. Catal. 2005, 33, 59–68. [Google Scholar] [CrossRef]
- Segura, Y.; del Álamo, A.C.; Munoz, M.; Álvarez-Torrellas, S.; García, J.; Casas, J.A.; De Pedro, Z.M.; Martínez, F. A comparative study among catalytic wet air oxidation, Fenton, and Photo-Fenton technologies for the on-site treatment of hospital wastewater. J. Environ. Manag. 2021, 290, 112624. [Google Scholar] [CrossRef]
- Barca, C.; Martino, M.; Hennebert, P.; Roche, N. Kinetics and capacity of phosphorus extraction from solid residues obtained from wet air oxidation of sewage sludge. Waste Manag. 2019, 89, 275–283. [Google Scholar] [CrossRef]
- Slavik, E.; Galessi, R.; Rapisardi, A.; Salvetti, R.; Bonzagni, P.; Bertanza, G.; Menoni, L.; Orhon, D.; Sözen, S. Wet oxidation as an advanced and sustainable technology for sludge treatment and management: Results from research activities and industrial-scale experiences. Dry Technol. 2015, 33, 1309–1317. [Google Scholar] [CrossRef]
- Urrea, J.L.; Collado, S.; Oulego, P.; Diaz, M. Wet oxidation of the structural sludge fractions. J. Clean Prod. 2017, 168, 1163–1170. [Google Scholar] [CrossRef]
- Besagni, G. The effect of operating and design parameters on bubble column performance: The LOPROX case study. Chin. J. Chem. Eng. 2022, 40, 48–52. [Google Scholar] [CrossRef]
- Liu, X.M.; Wan, J.; Zhu, C.B.; Zhou, Z.; Zhang, F.; Zhang, Z.B. Energy optimisation of wet air oxidation reactors with sub-millimetre bubble intensification. Chem. Eng. Sci. 2022, 260, 117864. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, H.; Li, D.B. Maximise methane recovery from sludge anaerobic digestion by combining an optimal wet air oxidation process. Renew. Energy 2021, 179, 359–369. [Google Scholar] [CrossRef]
- De Boer, M.A.; Wolzak, L.; Slootweg, J.C. Phosphorus: Reserves, Production, and Applications; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Blackwell, M.; Darch, T.; Haslam, R. Phosphorus use efficiency and fertilizers: Future improvement opportunities. Front. Agric. Sci. Eng. 2019, 6, 332–340. [Google Scholar] [CrossRef]
- Panagos, P.; Koningner, J.; Ballabio, C.; Liakos, L.; Muntwyler, A.; Borrelli, P.; Lugato, E. Improving the phosphorus budget of European agricultural soils. Sci. Total Environ. 2022, 853, 158706. [Google Scholar] [CrossRef]
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The future distribution and production of global phosphate rock reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- El Bamiki, R.; Raji, O.; Ouabid, M.; Elghali, A.; Yazami, O.K.; Bodinier, J.L. Phosphate rocks: A review of sedimentary and igneous occurrences in Morocco. Minerals 2021, 11, 1137. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; El Boukhari, M.E.; Mphatso, C.; Zeroual, Y.; Lyamlouli, K. Conversion of waste into organo-mineral fertilizers: Current technological trends and prospects. Rev. Environ. Sci. Biotechnol. 2022, 21, 425–446. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus recovery from wastewater by struvite crystallization: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Zhang, T.; He, X.Y.; Deng, Y.X.; Tsang, D.C.W.; Jiang, R.F.; Becker, G.C.; Kruse, A. Phosphorus recovered from digestate by hydrothermal processes with struvite crystallization and its potential as a fertilizer. Sci. Total Environ. 2019, 698, 134240. [Google Scholar] [CrossRef]
- Brye, K.R.; Omidire, N.S.; English, L.; Parajuli, R.; Kekedy-Nagy, L.; Sultana, R.; Popp, J.; Thoma, G.; Roberts, T.L.; Greenlee, L.F. Assessment of struvite as an alternative sources of fertilizer-phosphorus for flood-irrigated rice. Sustainability 2022, 14, 9621. [Google Scholar] [CrossRef]
- Butusov, M.; Jernelöv, A. Phosphorus: An Element That Could Have Been Called Lucifer; Springer: Berlin/Heidelberg, Germany, 2013; p. 101. [Google Scholar] [CrossRef]
- Cañas, J.; García, J.; Hermana, B.; Águeda, V.I.; Álvarez-Torrellas, S. Revision of the most harmful organic compounds present in sewage and sludge. In Emerging Pollutants in Sewage Sludge and Soils; Núñez-Delgado, A., Arias-Estévez, M., Eds.; The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2022; p. 114. [Google Scholar] [CrossRef]
- Saoudi, M.A.; Dabert, P.; Vedrenne, F.; Daumer, M.L. Mechanisms governing the dissolution of phosphorus and iron in sewage sludge by the bioacidification process and its correlation with iron phosphate speciation. Chemosphere 2022, 307, 135704. [Google Scholar] [CrossRef]
- Schaum, C. Phosphorus: Polluter and resource of the future—Removal and recovery from wastewater. Water Intell 2018, 17, 450–457. [Google Scholar] [CrossRef]
- Korchef, A.; Naffouti, S.; Souid, I. Recovery of high concentrations of phosphorus and ammonium through struvite crystallization by CO2 repelling. Cryst. Res. Technol. 2022, 57, 2200123. [Google Scholar] [CrossRef]
- Zin, M.M.T.; Kim, D.J. Simultaneous phosphorus and nitrogen recovery from sewage sludge ash and food wastewater as struvite by Mg-biochar. J. Hazard. Mater. 2021, 403, 123704. [Google Scholar] [CrossRef]
- Munir, M.T.; Li, B.; Mardon, I.; Young, B.R.; Baroutian, S. Integrating wet oxidation and struvite precipitation for sewage sludge treatment and phosphorus recovery. J. Clean. Prod. 2019, 232, 1043–1052. [Google Scholar] [CrossRef]
- Ovsyannikova, E.; Kruse, A.; Becker, G.C. Valorization of byproducts from hydrothermal liquefaction of sewage sludge and manure: The development of a struvite-producing unit for nutrient recovery. Energy Fuels 2021, 35, 9408–9423. [Google Scholar] [CrossRef]
- Hao, X.D.; Wang, C.C.; Lan, L.; Van Loosdrecht, M.C.M. Struvite formation, analytical methods and effects of pH and Ca2+. Water Sci. Technol. 2008, 58, 1687–1692. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Impact of calcium on struvite crystal size, shape and purity. J. Cryst. Growth 2005, 283, 514–522. [Google Scholar] [CrossRef]
- Daneshgar, S.; Cecconet, D.; Capsoni, D.; Capodaglio, A.G. Side-stream phosphorus recovery in activated sludge processes. Water 2022, 14, 1861. [Google Scholar] [CrossRef]
- Wang, Y.C.; Kuntke, P.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N.; Lei, Y. Electrochemically mediated precipitation of phosphate minerals for phosphorus removal and recovery: Progress and perspective. Water Res. 2022, 209, 117891. [Google Scholar] [CrossRef]
- Cieślik, B.; Konieczka, P. A review of phosphorus recovery methods at various wastewater treatment and sewage sludge management steps. The concept of no solid waste generation and analytical methods. J. Clean. Prod. 2017, 142, 1728–1740. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhai, Y.B.; Li, S.H.; Liu, X.M.; Wang, B.; Liu, X.P.; Fan, Y.W.; Shi, H.R.; Li, C.T.; Zhu, Y. Thermal treatment of sewage sludge: A comparative review of the conversion principle, recovery methods and bioavailability-predicting of phosphorus. Chemosphere 2022, 291, 133053. [Google Scholar] [CrossRef] [PubMed]
- Husek, M.; Mosko, J.; Pohorely, M. Sewage sludge treatment methods and P-recovery possibilities: Current state-of-the-art. J. Environ. Manag. 2022, 315, 115090. [Google Scholar] [CrossRef]
- Donatello, S.; Cheeseman, C.R. Recycling and recovery routes for incinerated sewage sludge ash (ISSA): A review. Waste Manag. 2013, 33, 2328–2340. [Google Scholar] [CrossRef]
- Liang, S.; Yang, L.; Chen, H.; Yu, W.; Tao, S.; Yuan, S.; Xiao, K.; Hu, J.; Hou, H.; Liu, H.; et al. Phosphorus recovery from incinerated sewage sludge ash (ISSA) and reutilization of residues for sludge pretreated by different conditioners. Resour. Conserv. Recycl. 2021, 169, 105524. [Google Scholar] [CrossRef]
- Hong, H.; Chen, Y.; Liu, M.; Xu, L. Phosphorus extraction from sludge incinerated bottom ash with hydrochloric acid at low liquid-solid ratio. Environ. Eng. Sci. 2021, 39, 125–134. [Google Scholar] [CrossRef]
- Eaton, A.D.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Fernandez-Delgado, M.; Del Amo-Mateos, E.; Garcia-Cubero, M.T.; Coca, M.; Lucas, S. Phosphorus recovery from organic waste for its agronomic valorization: Technical and economic evaluation. J. Chem. Technol. Biotechnol. 2021, 97, 167–178. [Google Scholar] [CrossRef]
- Abeysiriwardana-Arachchige, I.S.A.; Samarasingha, N.; Rosalez, R.; Munasinghe-Arachchige, S.P.; Delanka-Pedige, H.M.K.; Brewer, C.E.; Nirmalakhandan, N. Maximizing phosphorus recovery as biofertilizer in an algal wastewater treatment system. Resour. Conserv. Recycl. 2021, 170, 105552. [Google Scholar] [CrossRef]
- European Parliament. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilizing Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation. 2019. Available online: http://data.europa.eu/eli/reg/2019/1009/oj (accessed on 23 June 2023).

| Parameters | Inlet Sludge | Effluent Outlet | Conversion (%) |
|---|---|---|---|
| COD (g O2/L) | 17.90 ± 3.5 | 3.11 ± 0.05 | 82.71 |
| TS (g/kg) | 27.80 ± 2.4 | 7.05 ± 1.5 | 74.64 |
| TVS (g/kg) | 12.10 ± 2.4 | 0.60 ± 0.1 | 95.04 |
| TFS (g/kg) | 9.20 ± 2.4 | 6.70 ± 1.5 | 27.17 |
| Parameters | Liquid Effluent |
|---|---|
| COD (g O2/L) | 3.1 ± 0.05 |
| Total, P (mg P/L) | 93.1 ± 4 |
| P-PO43− (mg P/L) | 86.1 ± 5 |
| Total, N (mg N/L) | 975 ± 22 |
| N-NH4+ (mg N/L) | 848 ± 17 |
| Mg+ (mg Mg/L) | 2.5 ± 1 |
| Ca2+ (mg Ca/L) | 7.1 ± 1 |
| Total, P (mg P gsolid−1) | 68.20% (in solid effluent) |
| Elemental analysis | Compounds (%) |
| C H N | 0.88 ± 1 0.92 ± 1 0.69 ± 1 |
| XRF analysis | Oxides (%) a |
| Si | 14.01 |
| Fe | 12.79 |
| Al | 7.39 |
| Ca | 5.84 |
| Mg | 2.40 |
| K | 1.80 |
| ∑ (Ti, Na, Zn, Cu, Mn, Ga, Pb, Ba, Sr, Co, Cr, Zr, Ni, Rb, Sn) | 1.39 |
| CL | 51.89 |
| pH | Molar Ratio Mg/P | %P Purity | %N Purity | % Mg Purity | % C | Compound | Concentration (µg/gstruvite) |
|---|---|---|---|---|---|---|---|
| 8.0 | 1.2 | 92.7 | 91.1 | 65.7 | 0.12 | Si | 19,255 ± 1759 |
| 8.0 | 1.4 | 92.7 | 91.1 | 72.7 | 0.17 | Al | 1687 ± 185 |
| 8.0 | 1.6 | 91.9 | 91.2 | 73.7 | 0.14 | Fe | 563.5 ± 53 |
| 8.0 | 1.8 | 92.7 | 91.2 | 73.7 | 0.10 | As | 123.5 ± 12 |
| 8.5 | 1.2 | 91.9 | 90.0 | 73.7 | 0.17 | S | 72.0 ± 7 |
| 8.5 | 1.4 | 91.9 | 89.8 | 73.7 | 0.18 | Mn | 46.5 ± 5 |
| 8.5 | 1.6 | 91.9 | 89.7 | 72.7 | 0.17 | Zn | 29.0 ± 3 |
| 8.5 | 1.8 | 92.3 | 90.0 | 72.7 | 0.22 | Cu | 13.6 ± 1.4 |
| 8.5 | 2.0 | 91.1 | 88.4 | 72.7 | 0.15 | Cr | 12.2 ± 1.2 |
| 8.5 | 4.0 | 79.2 | 76.7 | 70.5 | - | Cd | <10 Below the limit of detection (LOD). |
| 9.0 | 1.2 | 90.3 | 89.7 | 72.7 | 0.20 | Mo | |
| 9.0 | 1.4 | 89.5 | 89.1 | 72.7 | 0.20 | Ni | |
| 9.0 | 1.6 | 88.0 | 87.6 | 71.7 | 0.26 | Pb | |
| 9.0 | 1.8 | 87.2 | 87.0 | 69.7 | 0.28 | Hg | |
| 9.5 | 1.2 | 88.0 | 89.3 | 68.7 | 0.33 | ||
| 9.5 | 1.4 | 87.2 | 87.4 | 68.7 | 0.33 | ||
| 9.5 | 1.6 | 88.0 | 86.9 | 70.7 | 0.35 | ||
| 9.5 | 1.8 | 88.0 | 87.6 | 66.7 | 0.35 | ||
| Steps | Struvite Extraction (%) |
|---|---|
| Not extracted | 5 |
| NaHCO3 fraction | 1 |
| NaOH fraction | 11 |
| HCl diluted fraction | 53 |
| HCl concentrated and hot fraction | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañas, J.; Álvarez-Torrellas, S.; Hermana, B.; García, J. Phosphorus Recovery from Sewage Sludge as Struvite. Water 2023, 15, 2382. https://doi.org/10.3390/w15132382
Cañas J, Álvarez-Torrellas S, Hermana B, García J. Phosphorus Recovery from Sewage Sludge as Struvite. Water. 2023; 15(13):2382. https://doi.org/10.3390/w15132382
Chicago/Turabian StyleCañas, Javier, Silvia Álvarez-Torrellas, Blanca Hermana, and Juan García. 2023. "Phosphorus Recovery from Sewage Sludge as Struvite" Water 15, no. 13: 2382. https://doi.org/10.3390/w15132382
APA StyleCañas, J., Álvarez-Torrellas, S., Hermana, B., & García, J. (2023). Phosphorus Recovery from Sewage Sludge as Struvite. Water, 15(13), 2382. https://doi.org/10.3390/w15132382









