Abstract
Heavy metal contamination of agricultural soils is potentially concerning when growing crops for human consumption. Industrial hemp (Cannabis sativa L.) has been reported to tolerate the presence of heavy metals such as cadmium (Cd) in the soil. Therefore, the objectives of this study were to evaluate Cd uptake and translocation in two day-length sensitive (DLS) and two day-neutral (DN) hemp varieties grown for the medicinal market and to determine the impact of Cd exposure on cannabinoid concentrations in flowers. A hydroponic experiment was conducted by exposing plants to 0 mg·L−1 Cd and 2.5 mg·L−1 Cd in the nutrient solution. Cadmium concentrations ranged from 16.1 to 2274.2 mg·kg−1 in roots, though all four varieties accumulated significant concentrations of Cd in aboveground tissues, with translocation factors ranging from 6.5 to 193. Whole-plant bioconcentration factors ranged from 20 to 1051 mg·kg−1. Cannabinoid concentrations were negatively impacted by Cd exposure in DN varieties but were unaffected in DLS varieties. Biomass was reduced by Cd exposure demonstrating that these varieties might not be suitable for growth on contaminated soil or for phytoremediation. There is potential for Cd accumulation in flowers, showing the need for heavy metal testing of C. sativa consumer products.
1. Introduction
For centuries, humans have cultivated hemp (Cannabis sativa L.) for its fiber, seed, therapeutic, and psychoactive properties. During the domestication process, wild Cannabis populations have been subject to selection, giving rise to the multiple varieties that exist today [1]. The term industrial hemp is commonly used to refer to C. sativa plants [2] with total tetrahydrocannabinol (THC) concentrations below 0.3%. Plants with total THC concentrations above 0.3% are classified as marijuana and subject to federal prohibition in the United States (U.S.) [3]. Current industrial hemp breeding efforts target fiber, seed, or cannabinoid production, based on the end user. Hemp varieties with high cannabidiol (CBD) concentrations are often selected for medicinal and therapeutic uses. There is also a focus on developing varieties that are day-neutral (DN) or minimally sensitive to photoperiod in order to expand production opportunities [4].
Hemp is generally considered a qualitative short-day plant that flowers in response to decreasing photoperiods. Hemp selections that flower in response to photoperiod are known as day length sensitive (DLS). After emergence, hemp undergoes a photoperiod-dependent vegetative phase maintained by exposure to approximately 14–18 h or more of light daily [5,6]. When hemp is planted during periods of short days (<13 h light), it may flower prematurely. Premature flowering, prior to complete vegetative development, can result in yield reductions [7,8]. In contrast, some hemp varieties exhibit DN flowering tendencies known colloquially as “auto-flower” hemp. These DN varieties are relatively insensitive to photoperiod for flower induction. The DN trait is speculated to arise from Cannabis ruderalis (C. sativa ssp. ruderalis) and may have originated from hemp located in high latitudes where photoperiods can be long and growing seasons are typically short or regions with relatively short and constant photoperiods [5,9,10]. Advantages of DN hemp varieties include the ability to flower in regions that have little variation in photoperiod throughout the year (tropics) or during times of the year when photoperiods may be inadequate to grow DLS varieties. However, many DN types of hemp have been reported to be particularly sensitive to environmental stressors such as high temperatures and may have lower yields than comparable DLS varieties [11].
In addition to uses for fiber, seed, and medicinal purposes, hemp has also been proposed as a candidate for use in phytoremediation, which utilizes plants to remove contaminants, such as heavy metals or other chemicals from soils [12,13]. Accumulator plant species can uptake heavy metals from soils, even at low external concentrations, and concentrate them in plant tissues [14]. By growing accumulator plants in contaminated soil, it is possible to realize in situ decontamination, an economically viable approach that preserves physicochemical soil characteristics, while removing contaminants [15]. The morphophysiological characteristics of hemp, such as high biomass production, deep roots, and short life cycle make it a potential candidate for phytoremediation [16,17,18,19,20].
Heavy metal contamination of agricultural soils is a concern when growing crops for food or medicinal purposes, due to potential harm to human and animal health [21,22,23]. Cadmium (Cd) contamination in the environment has been linked to anthropogenic activities, such as mining and smelting. Further, Cd can be introduced to soils via contaminated manure, sewage sludge, and phosphate fertilizers [24]. Cadmium is known to cause health issues when ingested in amounts greater than the provisional tolerable monthly intake (PTMI) of 25 μg·kg−1 of body weight [25]. In previous studies utilizing naturally and artificially contaminated soil and substrate containing from 0 to up to 200 mg·kg−1 Cd, hemp varieties grown for fiber production accumulated Cd in aboveground tissues at levels that could be harmful to human health [26,27,28,29]. For instance, the hemp fiber variety Silistrinski grown in naturally contaminated soil containing 12.2 mg·kg−1 Cd accumulated 1.22 mg·kg−1 Cd in its flowers [27].
There are multiple indicators that can be used to determine the accumulation potential of a plant species. Bioconcentration factor (BCF) is the ratio between the metal concentration in plant tissues and the initial metal concentration in the soil or growing solution [20,30,31,32,33]. This indicator has also been used interchangeably with terms such as accumulation factor (AF) [28], biological absorption coefficient (BAC) or index of bioaccumulation (IBA) [23]. A separate indicator of accumulation potential is the translocation factor (TF), which is the ratio between the metal concentration in the above ground biomass and the metal concentration in the roots [31,32]. Additionally, plant growth parameters can be assessed to determine the tolerance index (TI), calculated as the ratio between growth in contaminated and non-contaminated soils [20,30]. There is significant variability in BCF among plant species and chemical elements. It has been proposed that plants with BCF >100 mg·kg−1 Cd on a dry weight (DW) basis in its leaves could be referred to as hyperaccumulators [14]. Conversely, [12] suggested that true hyperaccumulators are able to accumulate higher concentrations of metals in leaves than in roots (TF > 1).
Few studies have evaluated heavy metal accumulation in hemp flowers, with most research utilizing fiber hemp varieties to determine heavy metal uptake for phytoremediation purposes [16,17,19,34]. Due to the harmful effects of Cd and other heavy metals on human health, the U.S. hemp industry has attempted to implement standards regarding maximum allowable levels of metals in C. sativa consumer products, which vary by state [35]. As hemp flowers are increasingly grown for the medicinal market, determining Cd distribution among plant organs, as well as bioconcentration and root-to-shoot translocation factors, are of importance. We hypothesize that there are distinctions in Cd accumulation and distribution among plant tissues in hemp varieties with different growth and flowering habits. Therefore, the objectives of this study were to evaluate nutrient partitioning and Cd uptake, translocation, and accumulation in DLS and DN hemp varieties, and to determine the impact of Cd exposure to cannabinoids in plant flowers.
2. Materials and Methods
2.1. Experimental Setting
The experiment was conducted in a greenhouse in Watkinsville, GA, USA (lat. 33°5′ N, long. 83°3′ W) from January 2022 to April 2022. Feminized seed from two DLS hemp varieties, T1 and Von (Sunbelt Hemp Source, Moultrie, GA, USA), and two DN varieties Apricot Auto (Blue Forest Farms, New York, NY, USA) and Auto CBD Alpha Explorer (Alpha Explorer) (Phylos Bioscience, Portland, OR, USA) were sown into engineered foam cubes (3.33 cm L × 2.54 cm W × 3.81 cm D; Oasis Grower Solutions, Kent, OH, USA) for germination. Foam cubes were placed in plastic trays over a germination mat set at 24 °C exposed to a mist irrigation system, which applied water twice daily for 1 min each. Supplemental lighting (approximately 100 µmol·m−2·s−1) was used during germination. Seedlings were maintained under these conditions for 4 weeks, after which they were placed into plastic netted containers (4.7 cm W × 5.1 cm D) and transferred to 37.9 L plastic containers (Rubbermaid Inc. Wooster, OH, USA) filled with 28 L of well water. The well water was analyzed for nutrient concentrations periodically throughout the experiment (Table 1). Four seedlings per replicate were placed equidistantly (24.3 cm apart) in holes drilled in the container lid. Welded wire mesh frames were attached to each lid to support plants. A 15.2 cm aquarium air stone attached to an air pump (Active Aqua; Hydrofarm, Petaluma, CA, USA) was placed inside the container to aerate the nutrient solution throughout the experiment. Container volume was maintained by adding well water every 2–3 days. At transplant, a nutrient solution was added to the plastic containers using a half-strength Hoagland’s solution [36] (Table 1).

Table 1.
Mineral nutrient concentrations in the nutrient solution used in this study.
Plants were grown for 3 weeks in the base nutrient solution after which nutrient solutions were replaced completely and Cd treatments were added using CdSO4·8H2O, to achieve 0 (control) and 2.5 mg·L−1 Cd. Cadmium concentrations were chosen based on the results of previous studies [34,35], which evaluated hemp exposure to Cd in hydroponic systems. The experimental treatments were arranged in a randomized complete block design with four hemp varieties exposed to two levels of Cd with four replicates each. Nutrient solutions were maintained to a constant volume by adding water every 2–3 days and nutrients were replaced every 3 weeks for the remainder of the experiment. The electrical conductivity (EC) and pH of the solutions were measured weekly. Solution pH was adjusted to 5.5 when necessary (pH down; General Hydroponics, Santa Rosa, CA, USA). Supplemental light (approximately 100 µmol·m−2·s−1) was used to provide 18/6 light/dark hours for 4 weeks of vegetative growth after transplanting seedlings into containers. Supplemental lights were turned off to allow for flower induction in the DLS varieties for the remaining 7 weeks of production (average day length 12 h 38 min). The DN varieties (Apricot Auto and Auto CBD Alpha Explorer) exhibited visually detectable flower development 1 week prior to the induction of flowering in the DLS varieties (Von and T1). Therefore, the DN varieties were harvested 1 week prior to the DLS varieties to ensure that plants flowered for the same length of time.
Nutrient solutions were sampled at the beginning and end of each 3-week cycle using 20 mL scintillation vials (HDPE; Thermo-Fisher Scientific™, Waltham, MS, USA), and stored at −4 °C until the analysis of mineral nutrient concentrations (Table S1). Temperature and relative humidity (RH) of the greenhouse were monitored at canopy height hourly (VP4; Meter Group Inc., Pullman WA, USA) and averaged 19.1 ± 3.1 °C and 74 ± 0.1% RH for the experiment. Photosynthetic active radiation was also monitored hourly throughout the experiment (QSO-S; Meter Group Inc.) and plants were exposed to an average daily light integral (DLI) average of 21.6 ± 9.0 mol·m−2·d−1.
2.2. Mineral Analysis
Samples of fresh root, stem, leaf, and flower tissues were collected for Cd analysis at harvest. Composite samples (50 g fresh material) were taken from each of the four plants in a replicate (container). Roots were triple-washed with deionized water after removal. Ten of the youngest fully expanded leaves were collected from the top one-third of each plant (main stem and lateral branches) and rinsed with deionized water. Stem samples were collected from the bottom two-thirds of the main stem. Flower material was sampled from the top of the main stem and the top one-third of plants. Samples were placed in a forced air oven set at 55 °C for 72 h until a constant weight was achieved. Dried plant material was then ground in a Wiley mill (Thomas Scientific, Swedesboro, NJ, USA) and passed through a 20-mesh screen. Samples were digested using EPA Method 3052 [37]. In brief, 0.5 g samples were placed in fluorocarbon polymer microwave vessels, 10 mL of concentrated nitric acid were added to each vessel which was then sealed. The microwave digester (Mars 6 Microwave; CEM Corp., Matthews, NC, USA) was heated to 200 °C for 30 min and digested (solutions) were then transferred quantitatively into volumetric flasks and brought to 100 mL volume with deionized water prior to analysis.
Samples of the hydroponic solutions were filtered using a 0.45 µM PTFE membrane (Thermo-Fisher Scientific™ Choice™ Polypropylene Syringe Filters) and acidified using 2% (v/v) high purity nitric acid (HNO3) (Certified ACS Plus, Fisher Scientific, Pittsburgh, PA, USA) prior to analysis. Hydroponic solutions and plant tissues were analyzed for multiple elements (P, K, S, Ca, Mg, Fe, Mn, Al, B, Cu, Zn, Ni, and Cd) following EPA Method 200.8 [38] by Inductively Coupled Plasma–Optical Emission Spectroscopy (Spectro Arcos FHS16; Spectro Ametek USA, Wilmington, MA, USA). The instrument parameter settings and wavelengths used are displayed in the supplementary material (Tables S3 and S4, and Figure S1). The instrument reporting limit for Cd was <0.005 mg·L−1. Results were expressed as mg·L−1. Calibration standards utilized in this analysis were from a certified source (Inorganic Ventures, Christiansburg, VA, lot number: N2-MEB667614). Independent laboratory performance checks were also run with acceptable deviations for recoveries set at 100 ± 5.0%.
The BCF was calculated by dividing the Cd concentration in plant tissues by the initial Cd concentration in the nutrient solution [20,30,31,32,33]. The TF (%) was calculated by dividing the sum of Cd concentration in leaves, flowers, and stems by the Cd concentration in roots, and multiplying by 100 [31,32]. Cadmium uptake rates (µmol·plant−1·d−1) were calculated by the following equation (adapted from [30]):
((([Cdinitial − Cdfinal])/number of plants)/treatment days)/root biomass)
2.3. Plant Growth and Biomass Yield
Plant height was determined by measuring the distance from the base of the stem to the tip of the apically dominant flower at harvest [11].
To quantify leaf, flower, stem, and root biomass, four whole plants per replicate were air-dried at ambient temperatures inside the greenhouse for 2 weeks and then separated into roots, stems, and leaf and flower biomass. Dry leaf and flower materials were manually pulled from plants following industry standards used for hemp biomass intended in cannabinoid extraction. Subsamples were taken from the air-dried materials and further dried in a forced air oven set at 55 °C for 48 h until a constant weight was achieved. The dry weights of the whole plant samples were then normalized based on subsample moisture content [11].
2.4. Cannabinoid Analysis
Approximately 25 g of fresh flower tissue sampled from inflorescences located on the top one-third of the plants were sampled during weeks 6 (DN varieties) and 7 (DLS varieties) of flowering (49 and 56 days after treatment (DAT), respectively) and dried separately from other samples as follows. Flower material was placed on a perforated aluminum baking sheet and dried to approximately 15% moisture content in a walk-in cooler with a temperature set point of 13 °C and 55% relative humidity for 14 days. The appropriate relative humidity was maintained using a dehumidifier. The dried material was hand trimmed to remove leaves, sealed in a metalized resealable food bag (Uline, Braselton, GA, USA) and stored at −4 °C for cannabinoid analysis. The acidic and neutral forms of the cannabinoids, THC and CBD, were determined in dried flower material by a commercial laboratory using high-performance liquid chromatography and a diode array detector set to 230 nm (SJ Labs and Analytics, Macon, GA, USA). The limit of detection for THC and CBD was 0.02%. Total cannabinoid concentrations were calculated by the following formula: total cannabinoid = neutral + (acidic form × 0.877). The percentage of dry matter for all samples was recorded and the results were reported on a dry weight basis.
2.5. Statistical Analysis
Statistical analysis was conducted using JMP© Pro 15 (SAS, Cary, NC, USA). Data were subjected to a one-way ANOVA procedure with Student’s t-test (p < 0.05) or Tukey’s Honest Significant Difference test (p < 0.05) conducted for mean separation when appropriate. Tissue Cd and cannabinoid concentrations were log-transformed to ensure equal variance prior to statistical analysis. Non-transformed data are presented.
3. Results and Discussion
3.1. Plant Height and Biomass Yield
Plant heights were significantly reduced by Cd exposure in DN varieties, Apricot Auto and Alpha Explorer. Plants exposed to 2.5 mg·L−1 Cd were shorter than plants in the control (0 mg·L−1 Cd) treatment (Figure 1). Plant heights of the two DLS varieties, Von and T1, were not significantly affected by Cd treatment. Nevertheless, the average decline in plant height of plants exposed to Cd was 20.4% in the DLS varieties and 35.6% in the DN varieties. Exposure to Cd also significantly reduced flower and leaf, stem, and root biomass in the DN varieties, Apricot Auto and Alpha Explorer (Table 2). In the DLS variety Von, the dry weight of flower and leaf tissues significantly decreased in the 2.5 mg·L−1 Cd treatment compared to the control, while dry weights of stem and root tissues were unaffected by Cd. The dry weights of the flower and leaf, and root tissue were significantly reduced by exposure to Cd in the DLS variety T1. Whole plant biomass in all four varieties was significantly reduced by Cd treatments, with an average reduction of 74.4% in the DN varieties and 50.2% in the DLS varieties.
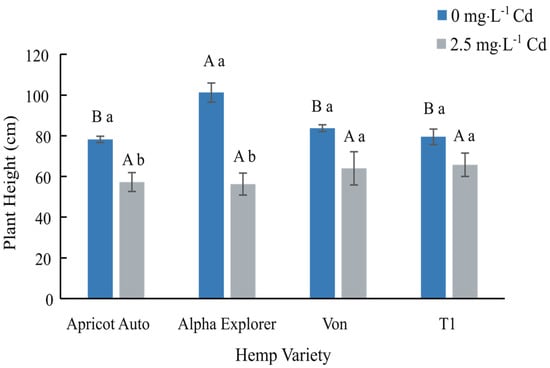
Figure 1.
Average plant height at harvest ± SE of four hemp (Cannabis sativa L.) varieties. Values correspond to averages of four replicates with four plants each. Bars associated with the same uppercase letter(s) indicate no significant differences among hemp varieties at p ≤ 0.05 according to Tukey’s HSD test. Bars associated with the same lowercase letter indicate no significant differences between control and treated plants for a given hemp variety at p ≤ 0.05 according to Student’s t-test.

Table 2.
Dry weight of flowers + leaves, stems, roots, and whole plant at harvest ± SE of four hemp (Cannabis sativa L.) varieties exposed to 0 (control) and 2.5 (treated) mg·L−1 Cd in a nutrient solution.
The DLS varieties, Von and T1, generated significantly greater root biomass relative to the DN varieties when not exposed to Cd. In Cd-treated plants, root biomass was significantly greater in Von compared to the two DN varieties. Root biomass in T1 was not significantly different from any other variety when exposed to Cd. Flower and leaf tissue yields were highest in DLS variety T1 in both control and Cd-exposed treatments. Exposure to Cd resulted in a significant decrease in flower and leaf biomass of 75% and 78%, in Apricot Auto and Alpha Explorer DN varieties, respectively. Exposure to Cd significantly reduced flower and leaf biomass in the DLS varieties Von and T1 by 52% and 41%, respectively. Whole-plant, stem, and root biomass was also significantly reduced to a greater extent in DN varieties compared to the DLS varieties. This suggests that the DLS varieties used in the present study may be more tolerant to Cd exposure at 2.5 mg·L−1 than the DN varieties evaluated. These results agree with previous studies that have reported a decrease in shoot biomass of hemp plants exposed to Cd [26,34,35,39].
3.2. Cd Concentration in Hemp Tissues
Cd concentrations in hemp tissues were affected by plant variety and Cd treatment (Table 3). In DN varieties, Apricot Auto and Alpha Explorer, and the DLS variety Von, Cd concentrations were highest in roots. In contrast, the DLS variety, T1 had similar Cd concentrations in roots, leaf, and stem tissue upon exposure to Cd. These results are consistent with previous literature, which reported that roots were the preferred tissue for Cd accumulation in hemp [27,34,35,40,41] and that Cd accumulation increased with increasing Cd concentrations in the growing media [39]. For instance, Cd concentrations in the roots of C. sativa fiber variety Santhica 27 exposed to 20 μM Cd (2.25 mg·L−1 Cd) for one week averaged 2687 mg·kg−1 dw, while Cd concentrations in stems and leaves averaged 1243 mg·kg−1 dw and 717 mg·kg−1 dw, respectively [34]. Additionally, the C. sativa medicinal variety Purple Tiger exposed to 2.5 mg·L−1 Cd for 68 days had average Cd concentrations of 1982.6 mg·kg−1 dw in roots, 13.2 mg·kg−1 dw in leaves, 5.1 mg·kg−1 dw in stems, and 7.6 mg·kg−1 dw in flowers [35].

Table 3.
Average cadmium (Cd) concentration at harvest in plant tissues of four hemp (Cannabis sativa L.) varieties exposed to 0 (control) and 2.5 (treated) mg·L−1 Cd in a nutrient solution on a dry weight (dw) basis.
All varieties had increased concentrations of Cd in all plant tissues exposed to Cd in the nutrient solution, except in the floral tissues of T1. Cadmium-treated DN varieties, Apricot Auto and Alpha Explorer, had increased Cd concentrations in flower and stem tissues, and Alpha Explorer had significantly greater Cd concentrations in leaf tissue compared to the DLS varieties. These results suggest that Cd concentration in plant tissues is negatively correlated to biomass accumulation, as the DN varieties had a higher reduction in flower and leaf biomass when exposed to 2.5 mg·L−1 Cd, compared to the DLS varieties. It should be noted that detectable Cd levels were observed in the control tissues. We expected all the control tissues to be below detection and can only speculate on the source of the Cd. It could have been slight cross contamination due to the aeration system that connected all the tanks. Another possibility is the Cd was already in the plant tissues before the treatments were applied. We analyzed the propagation foam cubes and found some Cd in the material (0.25 mg·L−1), which potentially could have slightly contaminated the tanks.
Whole-plant BCF ranged from 1051.8 in Alpha Explorer to 20.9 in T1 (Table 4 and Figure S2). In Alpha Explorer, whole-plant, root, and leaf BCF were significantly greater than the other three varieties evaluated. The BCF in stems and flowers was significantly higher in the DN varieties, Alpha Explorer and Apricot Auto, when compared to the DLS varieties, Von and T1. In Alpha Explorer, Apricot Auto, and Von varieties, BCF values were the highest in roots, while in T1 plants BCF was the highest in stems. For all varieties, the BCF in floral tissue was significantly less than that of roots, except for T1, which accumulated most Cd in stem tissues instead of roots. Previous studies reported that BCF values were consistently higher in roots when compared to above-ground biomass [39,41]. Furthermore, in a review of the capacity of different varieties of C. sativa to accumulate heavy metals, root BCF values in plants exposed to different Cd treatments ranged from 0.08 to 30.99 [17]. Most prior studies utilized contaminated soil or a soil-like substrate, which makes it challenging to determine the exact concentration of Cd that was available to plants. Nevertheless, our results support the previous literature demonstrating that plant genetics play a role in the Cd tolerance and accumulation potential of hemp plants.

Table 4.
Translocation factor (TF) and bioconcentration factor (BCF) in whole plants, roots, leaves, stems, and flowers of the hemp (Cannabis sativa L.) varieties, Apricot Auto, Alpha Explorer, Von, and T1 exposed to 2.5 mg·L−1 Cd in a nutrient solution.
The TF was calculated as the ratio between Cd concentration in aboveground tissue (stems, leaves, and flowers) and Cd concentration in roots. The variety, T1, had the highest TF compared to the other three varieties (Table 4). Our results suggest T1 had the highest translocation factor due to increased Cd accumulation in stems, and relatively low accumulation in roots compared to other varieties. Therefore, this DLS variety may favor Cd sequestration in aboveground parts of the plant while the other three varieties accumulated more Cd in the roots. The tolerance index, which was calculated as the ratio between biomass accumulation (whole plant) in the 2.5 mg·L−1 treatment and biomass accumulation in the control treatment, was not significantly different among hemp varieties (data not shown).
The cadmium uptake rate was significantly greater in Alpha Explorer compared to the other three hemp varieties (Figure 2). Both BCF and Cd uptake rates were the highest in Alpha Explorer, suggesting that this variety was the most efficient in taking up Cd from the solution, accumulating it primarily in the roots but also translocating it to the shoots. However, Alpha Explorer had significantly lower whole-plant biomass than the DLS varieties, Von and T1, and biomass is considered a more accurate indicator of Cd toxicity than plant height [39]. In a typical field production scenario, DN varieties would generally have a shorter life cycle than the DLS varieties and significant differences in growing degree-day requirements for maturity in DN and DLS hemp varieties have been reported [11]. Although all plants in the present study were harvested at the same time, the increased maturation rate of the DN varieties likely led to increased rates of Cd uptake. Reduced Cd uptake rates in Von and T1 varieties could potentially allow these plants to better cope with Cd exposure.
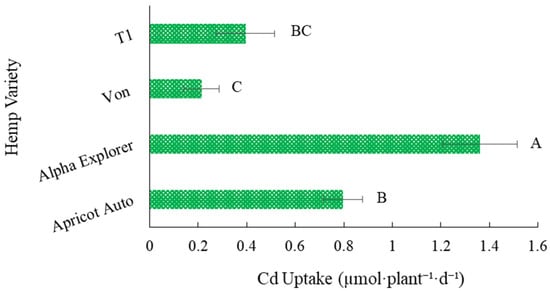
Figure 2.
Average Cd uptake rates ± SE in four hemp (Cannabis sativa L.) varieties exposed to 2.5 mg·L−1 Cd. Bars associated with the same letter(s) indicate no significant differences among hemp varieties at p ≤ 0.05 according to Tukey’s HSD test.
Although the TF was the highest in T1, the other three hemp varieties had significant TF values within or above the range of approximately 2.5 to 12% that has been reported in previous studies [39,41], suggesting that hemp may tolerate Cd stress (Table 4). Furthermore, the TF of all hemp varieties was above 1% and, therefore, these varieties could be classified as high-efficiency plants for metal translocation from roots to above-ground organs [42]. Although BCF was above 100 mg·kg−1 DW in leaves, which is the minimal value for hyperaccumulator plants [14], whole plant biomass was reduced by Cd exposure. Therefore, these hemp varieties might not be candidates to be considered Cd hyperaccumulators as previously indicated [34]. Nevertheless, our data suggest that hemp characteristics related to metal uptake and distribution within plant tissues can fluctuate by variety.
3.3. Nutrient Partitioning
The concentrations of the macronutrients N, P, K, and Ca were affected by both plant variety and Cd treatment (Table S2). However, no clear trends for any macronutrients were observed in response to Cd or among varieties. Previous data on nutrient distribution under metal stress is contradictory. For instance, the high-THC C. sativa variety “NB100” growing in uncontaminated substrate supplied with a commercial fertilizer (65, 17, 90 ppm N, P, and K, respectively) had higher N, P, and K concentrations in flower tissue, when compared to inflorescence leaves, fan leaves, and stems (roots were not analyzed), while Ca concentration was higher in fan leaves [43]. Conversely, there was a significant reduction in N, P, K, and Ca content in edible parts of tomato and lettuce grown in spiked soil containing 2.5 mg·kg−1 Cd, when compared to plants grown in non-contaminated soil [44]. Furthermore, chickpea plants (Cicer arietinum L.) exposed to Cd in nutrient solution (approximately 68 mg·L−1 Cd) showed a significant decrease in root and shoot Ca concentrations when compared to plants growing in an uncontaminated nutrient solution [45]. Nitrogen, P, and K concentrations in the shoots of Pfaffia glomerata were reported to increase with increasing Cd concentrations in the growing media [46]. These macronutrients are involved in the synthesis of Cd-detoxifying chelator molecules, such as glutathione and phytochelatins, and in the increase in activity of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidade (APX) [46,47].
3.4. Total THC and CBD in Hemp Flower
Total CBD concentrations were significantly lower in Apricot Auto and Alpha Explorer plants exposed to 2.5 mg·L−1 Cd compared to the 0 mg·L−1 Cd treatment, while total CBD concentrations in T1 and Von plants were not significantly affected by Cd exposure (Table 5). Similarly, total THC was significantly lower (below the detection limit) in Apricot Auto and Alpha Explorer plants exposed to 2.5 mg·L−1 Cd, compared to the control. Interestingly, THC concentrations in the DLS varieties, Von and T1, were not significantly affected by Cd exposure. Both DN varieties exhibited a significant reduction in total THC and CBD when exposed to Cd, while the DLS varieties did not. In addition, DN varieties had significantly greater Cd uptake rates (Figure 2) and BCF (Figure S2) than DLS plants. Further, both DN varieties had significantly greater Cd accumulation in the floral tissue compared to the DLS varieties (Table 3). This suggests that the increased Cd uptake and accumulation in the floral tissue in Apricot Auto and Alpha Explorer may have led to a decrease in cannabinoid synthesis. A previous study [28] reported no differences in THC content in leaves of the DLS fiber hemp variety “Fibranova” grown in substrate contaminated with 25 and 100 μg·g−1 Cd. Previous results suggest that plant genetics might play a role in cannabinoid synthesis under metal stress [28,48].

Table 5.
Average total CBD and THC ± SE in the hemp (Cannabis sativa L.) varieties Apricot Auto, Alpha Explorer, Von, and T1 exposed to 0 (control) and 2.5 (treated) mg·L−1 Cd in a nutrient solution.
While total THC concentrations in all hemp varieties in the control treatment reached concentrations above the legal threshold (0.3%), this is not uncommon in both DLS and DN high CBD hemp varieties [11,49] as age, genetics, and environmental factors may impact cannabinoid synthesis [50]. These results indicate that the impact of Cd stress on CBD and THC synthesis is variety-dependent, and exposure to 2.5 mg·L−1 Cd may affect cannabinoid synthesis in some varieties of C. sativa.
4. Conclusions
The impact of Cd on plant growth as well as BCF, uptake rate, and TF were affected by variety. Whole plant biomass yield in all four varieties was significantly reduced by the Cd treatment, suggesting that the hemps studied here may not be classified as hyperaccumulator as they may accumulate Cd with other ions in a nutrient solution until it becomes toxic. While Cd concentration was significantly higher in roots, all four varieties were efficient in translocating Cd from roots to shoots, with Cd concentrations in flowers ranging from 0.2 to 51 mg·kg−1 Cd DW in T1 and Alpha Explorer varieties, respectively. Flower and leaf biomass were significantly reduced in all four varieties in response to Cd. Further, the DN varieties, Alpha Explorer, and Apricot Auto, had a significant decrease in total THC and CBD concentrations in plants exposed to Cd when compared to plants in the control treatment, while the DLS varieties did not. Additional studies are warranted to determine if there are different Cd tolerance mechanisms in DN compared to DLS hemp varieties. All four hemp varieties analyzed in this study are suitable for the medicinal market, given that heavy metal testing is conducted throughout production and on finished consumer products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15122176/s1, Table S1: Mean concentrations of cadmium (Cd) in nutrient solutions measured at the beginning and end of each cycle and daily uptake. Values are averages ± SE of four replications per treatment; Table S2: Average concentrations of nitrogen (N), phosphorus (P), potassium (K), and calcium (Ca) among different tissues in the hemp (Cannabis sativa L.) varieties, Apricot Auto, Alpha Explorer, Von, and T1 on a dry weight (dw) basis exposed to 0 (control) and 2.5 (treated) mg·L−1 Cd in a nutrient solution; Table S3. ICP-OES parameters; Table S4. ICP-OES wavelengths used; Figure S1. Calibration curve of cadmium on ICP-OES; Figure S2. Bioconcentration factor (BCF) for Cd ± SE in plant tissues of four hemp (Cannabis sativa L.) varieties exposed to 2.5 mg·L−1 Cd.
Author Contributions
Conceptualization, A.O.M. and T.W.C.; methodology, A.O.M. and T.W.C.; software, A.O.M. and T.W.C.; validation, T.W.C. and J.T.L.; formal analysis, A.O.M. and T.W.C.; investigation, A.O.M. and T.W.C.; resources, T.W.C. and J.T.L.; data curation, A.O.M. and T.W.C.; writing—original draft preparation, A.O.M. and T.W.C.; writing—review and editing, T.W.C. and J.T.L.; visualization, A.O.M. and T.W.C.; supervision, T.W.C.; project administration, T.W.C.; funding acquisition, T.W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in the article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clarke, R.C.; Merlin, M.D. Cannabis Evolution and Ethnobotany; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 2013; pp. 29–57. [Google Scholar]
- Cherney, J.; Small, E. Industrial Hemp in North America: Production, Politics and Potential. Agronomy 2016, 6, 58. [Google Scholar] [CrossRef]
- US Department of Agriculture. Establishment of A Domestic Hemp Production Program; Federal Register: Washington, DC, USA, 2019; Volume 84, pp. 58522–58564.
- Clarke, R.C.; Merlin, M.D. Cannabis Domestication, Breeding History, Present-day Genetic Diversity, and Future Prospects. Crit. Rev. Plant Sci. 2016, 35, 293–327. [Google Scholar] [CrossRef]
- Potter, D.J. Cannabis Horticulture. In Handbook of Cannabis; Pertwee, R.G., Ed.; Oxford University Press: Cary, NC, USA, 2014; pp. 65–88. [Google Scholar]
- Ranalli, P. Advances in Hemp Research; The Haworth Press, Inc.: Binghamton, NY, USA, 1999. [Google Scholar]
- Amaducci, S.; Colauzzi, M.; Zatta, A.; Venturi, G. Flowering dynamics in monoecious and dioecious hemp genotypes. J. Ind. Hemp. 2008, 13, 5–19. [Google Scholar] [CrossRef]
- Hall, J.; Bhattarai, S.P.; Midmore, D.J. Review of flowering control in industrial hemp. J. Nat. Fibers. 2012, 9, 23–36. [Google Scholar] [CrossRef]
- Rosenthal, E. The Big Book of Buds: More Marijuana Varieties from the World’s Great Seed Breeders; Quick Trading Co.: Oakland, CA, USA, 2010; Volume 4. [Google Scholar]
- Small, E. Cannabis: A Complete Guide; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-6163-5. [Google Scholar]
- Coolong, T.; Cassity-Duffey, K.; Joy, N. Role of planting date on yield and cannabinoid content of day-neutral and photoperiod-sensitive hemp in Georgia, USA. Horttechnology 2023, 33, 138–145. [Google Scholar] [CrossRef]
- Chaney, R.L.; Baklanov, I.A. Phytoremediation and Phytomining. In Phytoremediation; Advances in Botanical Research; Cuypers, A., Vangronsveld, J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 189–221. [Google Scholar]
- Nesler, A.; Furini, A. Phytoremediation: The utilization of Plants to Reclaim Polluted Sites. In Plants and Heavy Metals; Furini, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 75–86. ISBN 978-94-007-4440-0. [Google Scholar]
- Greger, M. Metal Availability and Concentration in Plants. In Heavy Metal Stress in Plants; Prasad, M.N.V., Hagemeyer, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–27. [Google Scholar]
- Kirkham, M.B. Cadmium in Plants on Polluted Soils: Effects of Soil Factors, Hyperaccumulation, and Amendments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- De Vos, B.; De Souza, M.F.; Michels, E.; Meers, E. Industrial Hemp (Cannabis sativa L.) Field Cultivation in a Phytoattenuation Strategy and Valorization Potential of The Fibers for Textile Production. Environ. Sci. Pollut. Res. 2023, 30, 41665–41681. [Google Scholar] [CrossRef] [PubMed]
- Golia, E.E.; Bethanis, J.; Ntinopoulos, N.; Kaffe, G.-G.; Komnou, A.A.; Vasilou, C. Investigating the Potential of Heavy Metal Accumulation from Hemp: The Use of Industrial Hemp (Cannabis sativa L.) for Phytoremediation of Heavily and Moderated Polluted Soils. Sustain. Chem. Pharm. 2023, 31, 100961. [Google Scholar] [CrossRef]
- Placido, D.F.; Lee, C.C. Potential of Industrial Hemp for Phytoremediation of Heavy Metals. Plants 2022, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Rheay, H.T.; Omondi, E.C.; Brewer, C.E. Potential of hemp (Cannabis sativa L.) for paired phytoremediation and bioenergy production. GCB Bioenergy 2021, 13, 525–536. [Google Scholar] [CrossRef]
- Shi, G.; Cai, Q. Zinc Tolerance and Accumulation in Eight Oil Crops. J. Plant Nutr. 2010, 33, 982–997. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Furini, A. An Overview of Heavy Metal Challenge in Plants: From Roots to Shoots. Metallomics 2013, 5, 1117–1132. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, Toxicity, and its Interactions with Selenium Fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 73–122. [Google Scholar]
- Sebastian, A.; Nangia, A.; Prasad, M.N.V.; Rattanapolsan, L.; Nakbanpote, W. Cadmium Toxicity and Tolerance in Micro- and Phytobiomes. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 19–46. ISBN 978-0-12-814864-8. [Google Scholar]
- Codex Alimentarius. General Standard for Contaminants and Toxins in Food and Feed [CXS 193-1995]; FAO: Rome, Italy, 2019; pp. 47–48. Available online: https://www.fao.org/fao-who-codexalimentarius (accessed on 12 February 2023).
- Ahmad, A.; Hadi, F.; Ali, N. Effective Phytoextraction of cadmium (Cd) with increasing concentration of total phenolics and free proline in Cannabis sativa (L) plant under various treatments of fertilizers, plant growth regulators and sodium salt. Int. J. Phytoremediat. 2015, 17, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Angelova, V.; Ivanova, R.; Delibaltova, V.; Ivanov, K. Bio-accumulation and Distribution of Heavy Metals in Fibre Crops (flax, cotton and hemp). Ind. Crops Prod. 2004, 19, 197–205. [Google Scholar] [CrossRef]
- Citterio, S.; Santagostino, A.; Fumagalli, P.; Prato, N.; Ranalli, P.; Sgorbati, S. Heavy metal Tolerance and Accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil 2003, 256, 243–252. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Kumar, V.; Rani, A.; Jain, R. Cannabis sativa: A Plant Suitable for Phytoremediation and Bioenergy Production. In Phytoremediation Potential of Bioenergy Plants; Baudh, K., Singh, B., Korstad, J., Eds.; Springer: Singapore, 2017; pp. 269–285. [Google Scholar]
- Ali, N.A.; Bernal, M.P.; Ater, M. Tolerance and Bioaccumulation of Copper in Phragmites australis and Zea mays. Plant Soil 2002, 239, 103–111. [Google Scholar] [CrossRef]
- Liu, W.X.; Liu, J.W.; Wu, M.Z.; Li, Y.; Zhao, Y.; Li, S.R. Accumulation and Translocation of Toxic Heavy Metals in Winter Wheat (Triticum aestivum L.) Growing in Agricultural Soil of Zhengzhou, China. Bull. Environ. Contam. Toxicol. 2009, 82, 343–347. [Google Scholar] [CrossRef]
- Pachura, P.; Ociepa-Kubicka, A.; Skowron-Grabowska, B. Assessment of the Availability of Heavy Metals to Plants Based on the Translocation Index and the Bioaccumulation Factor. Desalin. Water Treat. 2015, 57, 1469–1477. [Google Scholar] [CrossRef]
- Zayed, A.; Gowthaman, S.; Norman, T. Phytoaccumulation of Trace Elements by Wetland Plants: I. Duckweed. J. Environ. Qual. 1998, 27, 715–721. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.F.; Blanquet, M.; Guerriero, G.; Lutts, S. Silicon reduces cadmium absorption and increases root-to-shoot translocation without impacting growth in young plants of hemp (Cannabis sativa L.) on a short-term basis. Environ. Sci. Pollut. Res. 2021, 28, 37963–37977. [Google Scholar] [CrossRef]
- Marabesi, A.O.; Nambeesan, S.U.; van Iersel, M.W.; Lessl, J.T.; Coolong, T.W. Cadmium exposure is associated with increased transcript abundance of multiple heavy metal associated transporter genes in roots of hemp (Cannabis sativa L.). Front. Plant Sci. 2023, 14, 1183249. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants without Soil. Calif. Agric. Exp. Sta. Circ. 1950, 347, 32. [Google Scholar]
- USEPA Method 3052. Microwave assisted acid digestion of siliceous and organically based matrices. In Test methods for evaluating solid waste, 3rd ed.; US Environmental Protection Agency: Washington, DC, USA, 1995. [Google Scholar]
- Creed, J.T.; Brockhoff, C.A.; Martin, T.D. US-EPA Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma Mass Spectrometry; Revision 5.4 EMMC Version; Environmental Monitoring Systems Laboratory Office of Research and Development, U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1994.
- Shi, G.; Cai, Q. Cadmium Tolerance and Accumulation in Eight Potential Energy Crops. Biotechnol. Adv. 2009, 27, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Ćaćić, M.; Perčin, A.; Zgorelec, Ž.; Kisić, I. Evaluation of heavy metals accumulation potential of hemp (Cannabis sativa L.). J. Cent. Eur. Agric. 2019, 20, 700–711. [Google Scholar] [CrossRef]
- Shi, G.; Liu, C.; Cui, M.; Ma, Y.; Cai, Q. Cadmium Tolerance and Bioaccumulation of 18 Hemp Accessions. Appl. Biochem. Biotechnol. 2012, 168, 163–173. [Google Scholar] [CrossRef]
- Majid, S.; Khwakaram, A.; Ghafoor, M.R.; Ahmed, Z. Bioaccumulation, Enrichment and Translocation Factors of some Heavy Metals in Typha Angustifolia and Phragmites Australis Species Growing along Qalyasan Stream in Sulaimani City/IKR. J. Zankoy Sulaimani 2014, 16, 93–109. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and Humic Acid Supplementation on the Chemical Profile of Medical Cannabis (Cannabis sativa L). Front. Plant Sci. 2019, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, S.; Alam, M.; Khan, M.A.; Aamir, M.; Qamar, Z.; Ur Rehman, Z.; Perveen, S. Toxic Metal Interactions Affect the Bioaccumulation and Dietary Intake of Macro- and Micro-Nutrients. Chemosphere 2016, 146, 121–128. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Abd Allah, E.F.; Hashem, A.; Sarwat, M.; Anjum, N.A.; Gucel, S. Calcium and Potassium Supplementation Enhanced Growth, Osmolyte Secondary Metabolite Production, and Enzymatic Antioxidant Machinery in Cadmium-Exposed Chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 513. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Marques, T.C.L.L.S.E.M.; Soares, A.M. Cadmium Effects on Mineral Nutrition of the Cd-hyperaccumulator Pfaffia glomerata. Biologia 2013, 68, 223–230. [Google Scholar] [CrossRef]
- Sarwar, N.; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of Mineral Nutrition in Minimizing Cadmium Accumulation by Plants. J. Sci. Food Agricul. 2010, 90, 925–937. [Google Scholar] [CrossRef]
- Husain, R.; Weeden, H.; Bogush, D.; Deguchi, M.; Soliman, M.; Potlakayala, S.; Katam, R.; Goldman, S.; Rudrabhatla, S. Enhanced tolerance of industrial hemp (Cannabis sativa L.) plants on abandoned mine land soil leads to overexpression of cannabinoids. PLoS ONE 2019, 14, e0221570. [Google Scholar] [CrossRef]
- Yang, R.; Berthold, E.C.; McCurdy, C.R.; da Silva Benevenute, S.; Brym, Z.T.; Freeman, J.H. Development of Cannabinoids in Flowers of Industrial Hemp (Cannabis sativa L.): A Pilot Study. J. Agric. Food Chem. 2020, 68, 6058–6064. [Google Scholar] [CrossRef] [PubMed]
- Trancoso, I.; de Souza, G.A.R.; dos Santos, P.R.; dos Santos, K.D.; de Miranda, R.M.D.S.N.; da Silva, A.L.P.M.; Santos, D.Z.; García-Tejero, I.F.; Campostrini, E. Cannabis sativa L.: Crop Management and Abiotic Factors that Affect Phytocannabinoid Production. Agronomy 2022, 12, 1492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).