Ascertaining and Optimizing the Water Footprint and Sludge Management Practice in Steel Industries
Abstract
1. Introduction
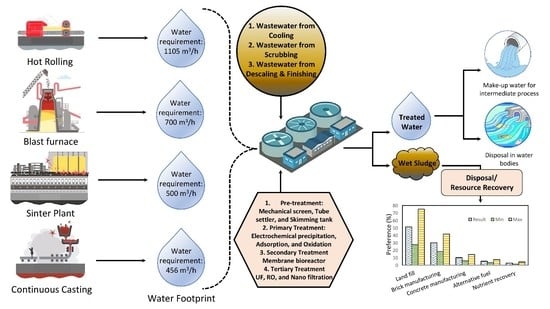
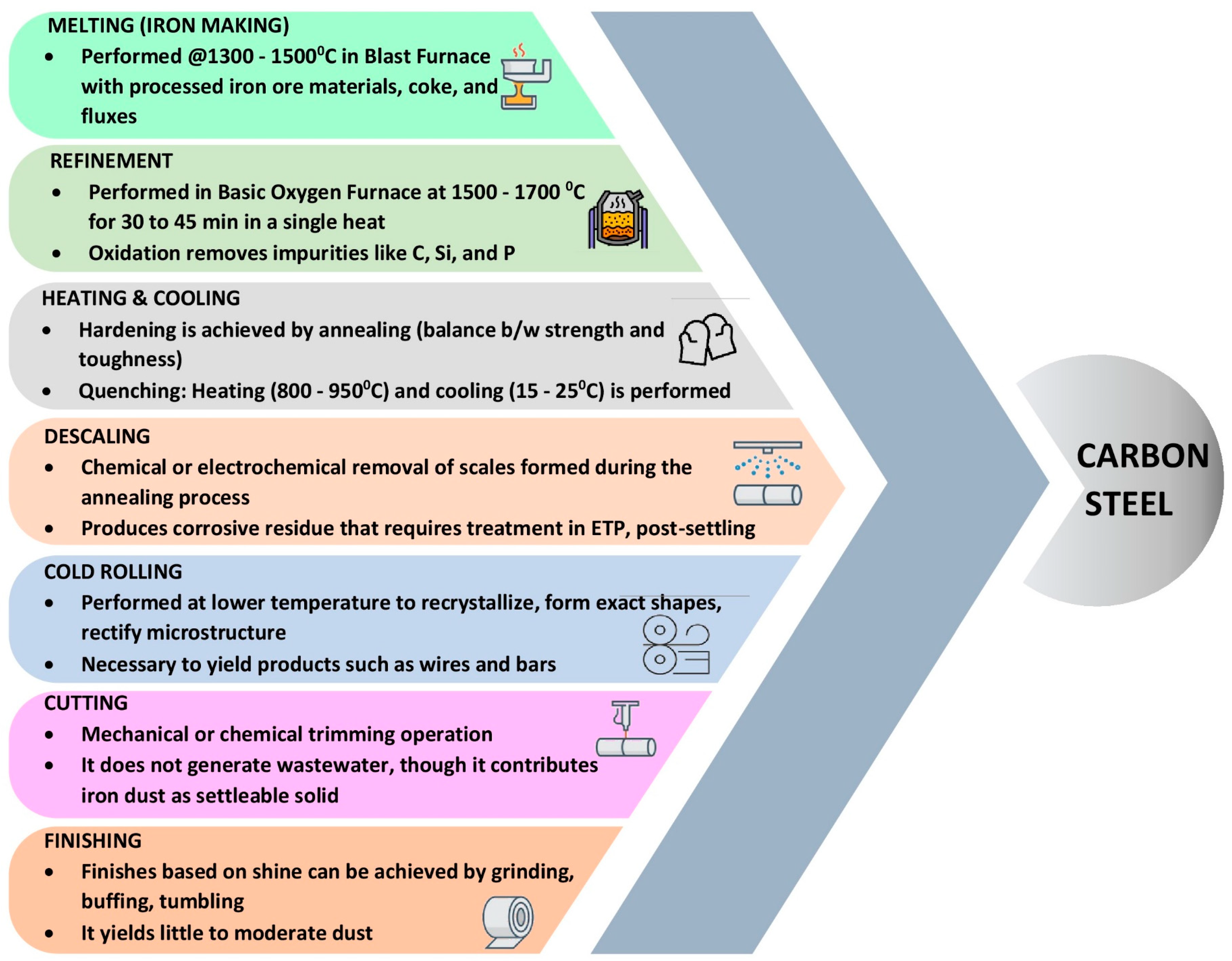
2. The Requirement for Water
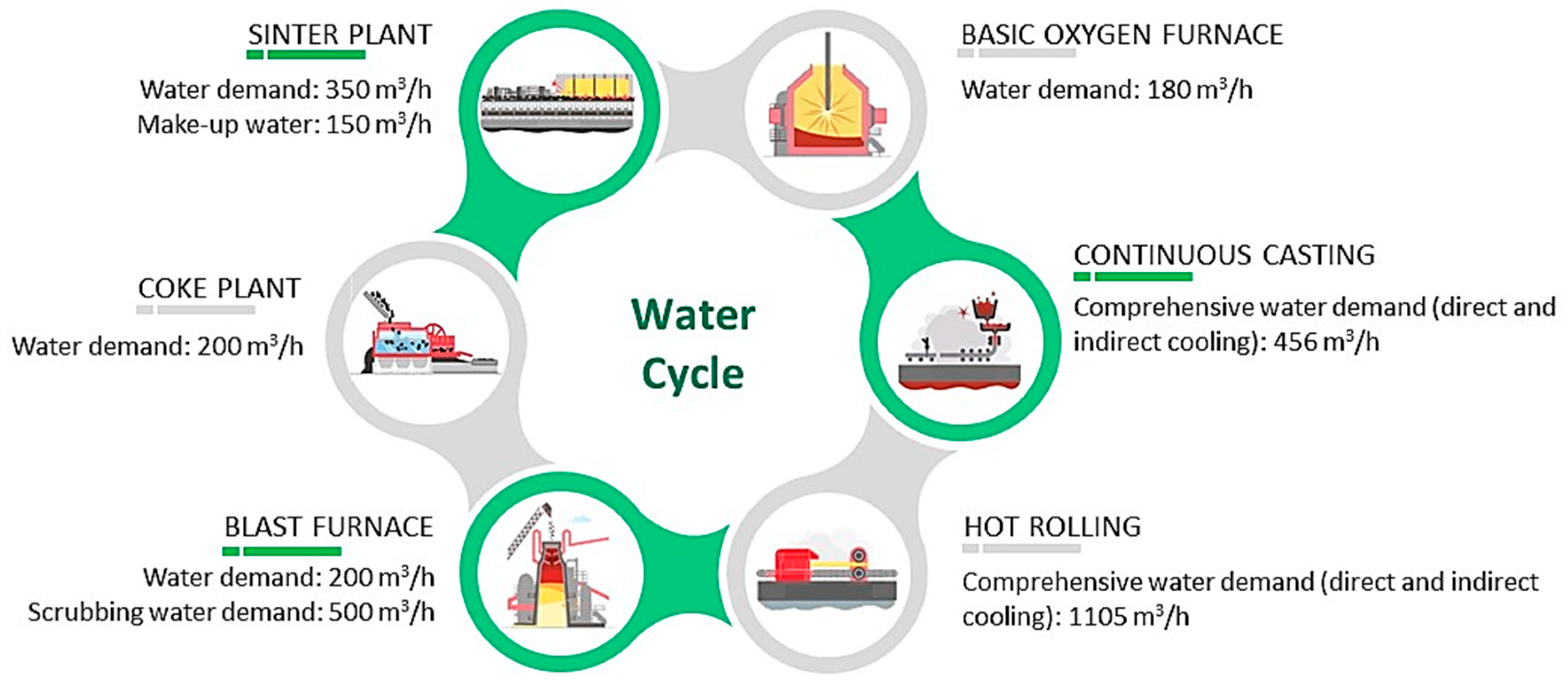
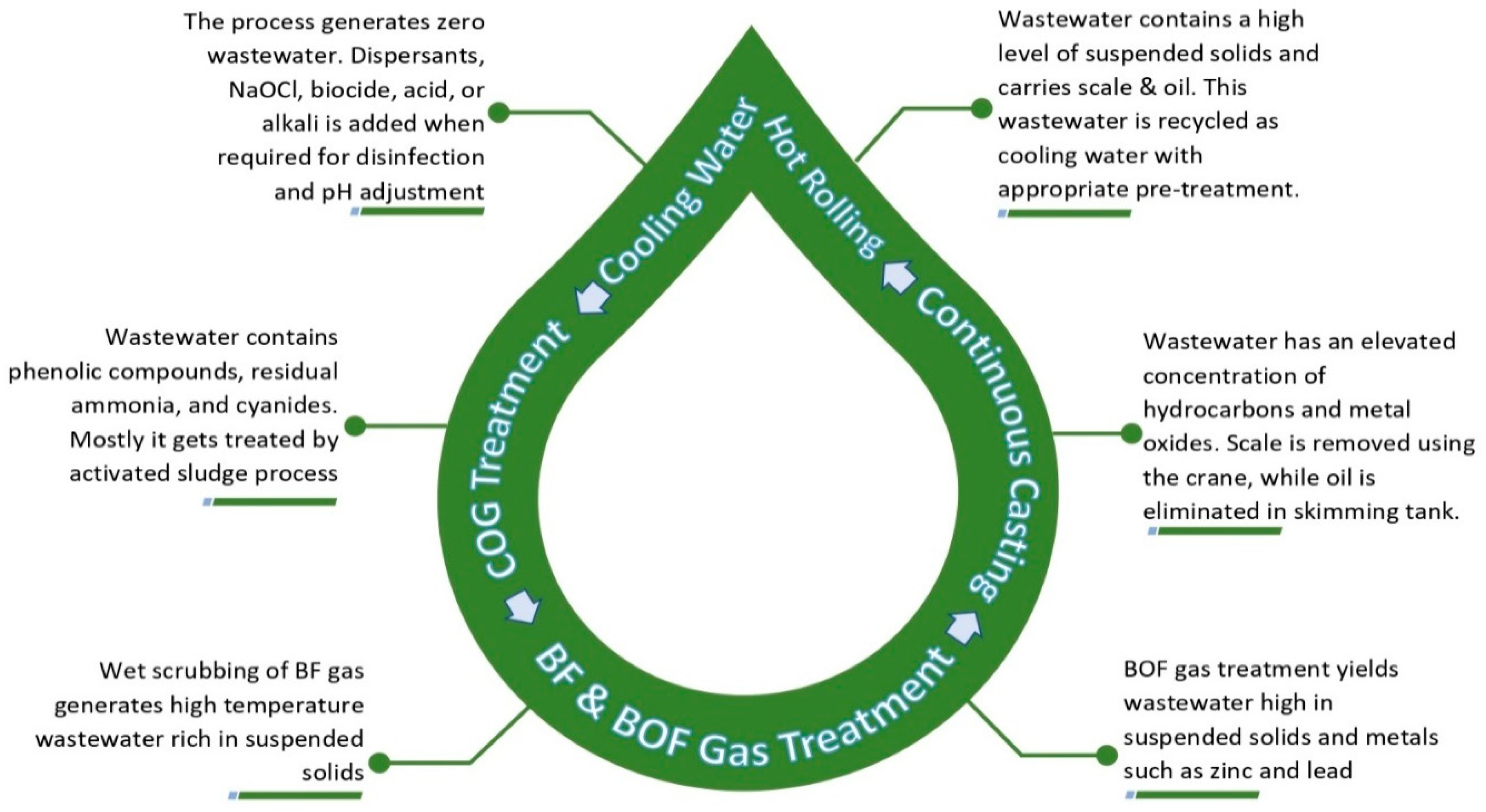
2.1. Water Cycle
2.2. Wastewater Scenario
2.3. Sources and Characteristics
| No. | Plant Name and Location | Steel Production (Tons/Annum) | Water Consumption * | Wastewater Generation (m3/Day) | |
|---|---|---|---|---|---|
| Overall (m3/Day) | For Production (m3/Ton of Product) | ||||
| 1 | Bhilai Steel Plant, Durg, Chhattisgarh | 3,153,000 | 24,215 | 2.772 | 19,616 |
| 2 | JSW steel Ltd., Vijayanagar, Karnataka | 12,000,000 | 27,382 | 0.83 | 23,274 |
| 3 | JSW Steel Ltd., Salem, Tamil Nadu | 1,000,000 | 7230 | 2.62 | 5997 |
| 4 | Bokaro Steel Plant, Bokaro, Jharkhand | 4,000,000 | 38,466 | 3.5 | 30,301 |
| 5 | Rashtriya Ispat Nigam Ltd., Visakhapatnam, Andhra Pradesh | 4,400,000 | 139,506 | 11.47 | 118,580 |
| 6 | Jindal Steel and Power Plant, Raigarh, Chhattisgarh | 3,600,000 | 27,328 | 2.770 | 23,229 |
| 7 | Jindal Steel and Power Plant, Angul, Odisha | 2,700,000 | 11,023 | 1.48 | 8488 |
| 8 | Jindal Steel and Power Plant, Patratu, Jharkhand | 1,600,000 | 6809 | 1.55 | 5372 |
| 9 | Arcelor Mittal Nippon Steel Ltd., Hazira, Gujarat | 1,000,000 | 3230 | 1.16 | 2746 |
| 10 | Durgapur Steel Plant, Durgapur, West Bengal | 2,120,000 | 119,008 | 19.80 | 118,157 |
| 11 | Rourkela Steel Plant, Rourkela, Odisha | 4,200,000 | 43,983 | 3.89 | 37,386 |
| 12 | IISCO, Burnpur, West Bengal | 4,260,000 | 87,065 | 7.38 | 74,005 |
| 13 | Chandrapur Ferro Alloy Steel Plant, Chandrapur, Maharashtra. | 100,000 | 260 | 0.91 | 221 |
| 14 | Salem Steel Plant, Salem, Tamil Nadu | 339,200 | 13,249 | 14.15 | 11,261 |
| 15 | JSW Steel Ltd., Dolvi, Maharashtra | 5,000,000 | 140,774 | 10.11 | 111,657 |
| 16 | TATA Steel Ltd., Ferro Alloy Plant, Bamanipal, Odisha | 61,000 | 3002 | 17.89 | 2553 |
| 17 | TATA Steel Ltd., Kalinganagar, Odisha | 6,000,000 | 36,041 | 2.13 | 30,635 |
| 18 | Ferro Manganese Plant, TATA Steel Ltd., Keonjhar, Odisha | 50,400 | 655 | 4.67 | 560 |
| 19 | TATA steel ltd., Jamshedpur, Jharkhand. | 10,220,000 | 102,220 | 3.27 | 86,887 |
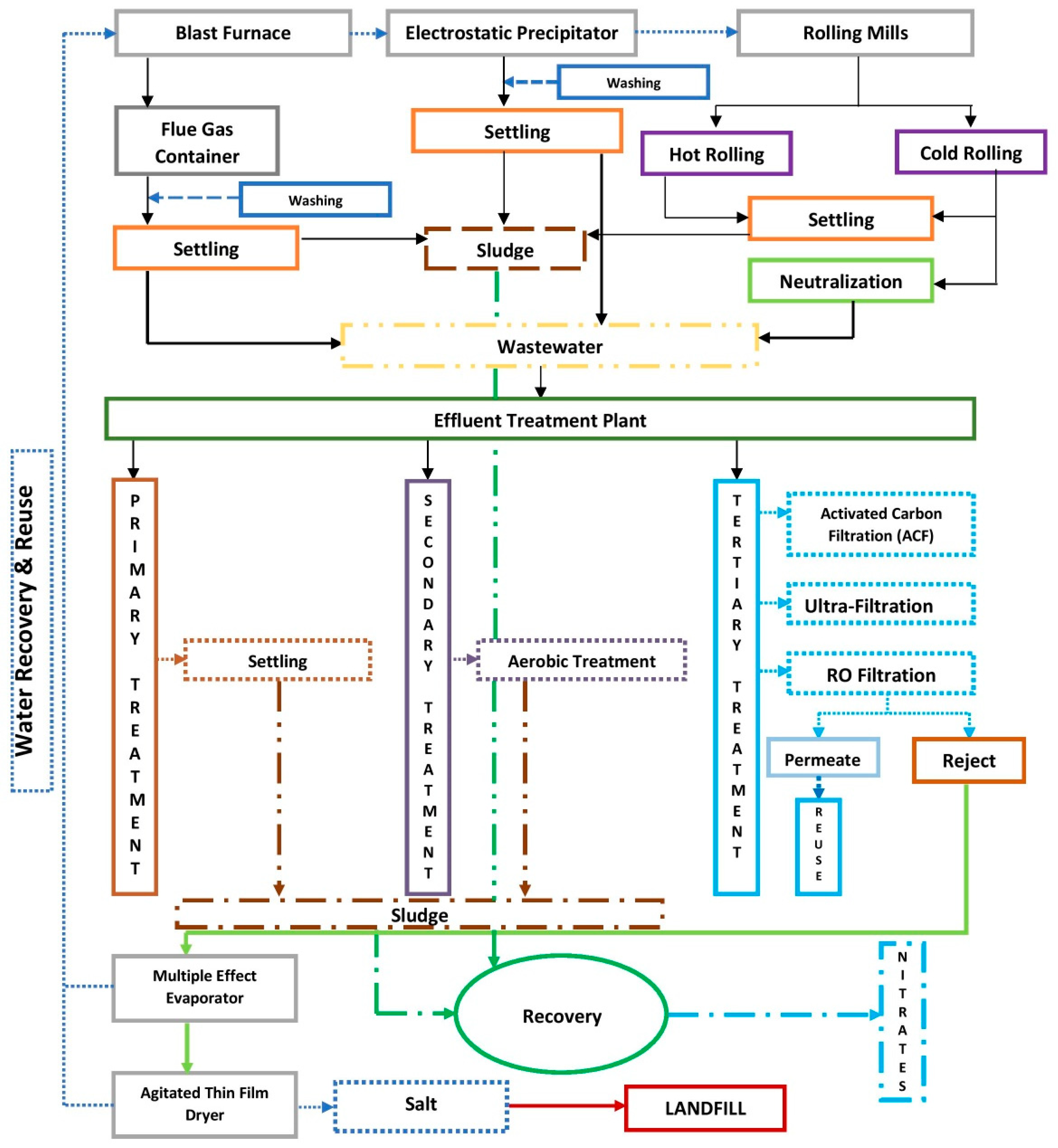
2.4. Treatment of Wastewater
2.5. Effluent Treatment Plant
2.6. Characteristics of ETP Sludge
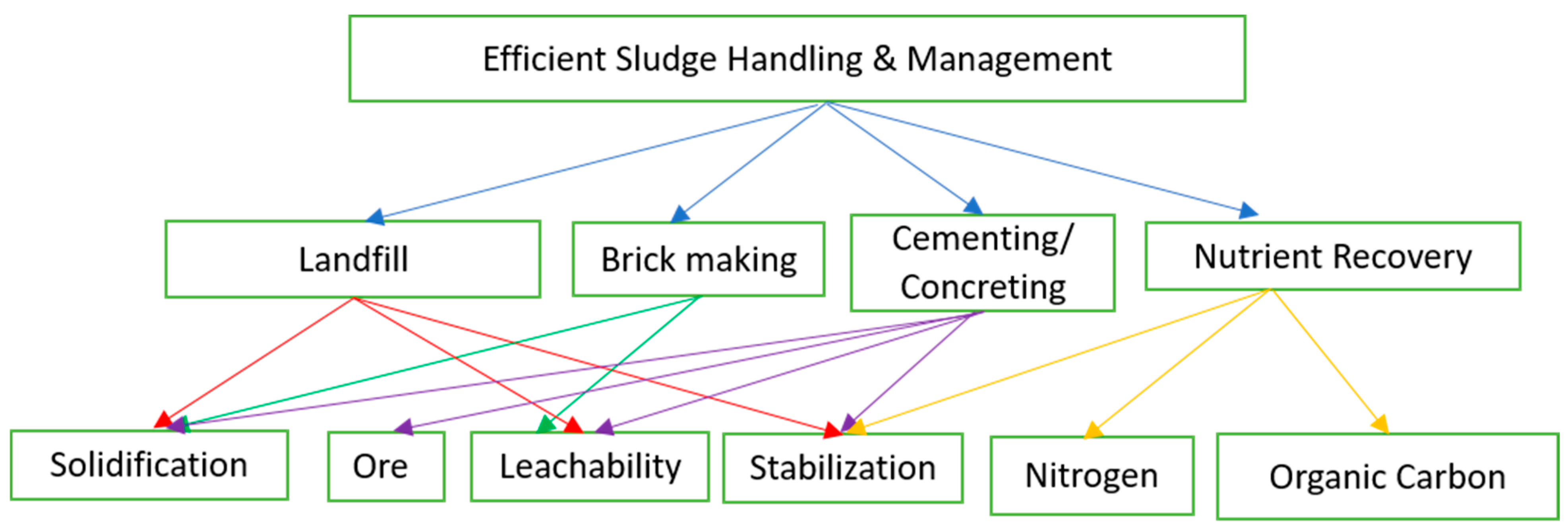
3. Possible Recycling Alternates for ETP Sludge
Sustainable Sludge Management and Hierarchy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Backman, J.; Kyllönen, V.; Helaakoski, H. Methods and Tools of Improving Steel Manufacturing Processes: Current State and Future Methods. IFAC-Pap. Online 2019, 52, 1174–1179. [Google Scholar] [CrossRef]
- Buchmayr, B.; Degner, M.; Palkowski, H. Future Challenges in the Steel Industry and Consequences for Rolling Plant Technologies. BHM 2018, 163, 76–83. [Google Scholar] [CrossRef]
- Silva, F.; Carvalho, A. Evaluating the financial health of the steel industry. In Directorate for Science, Technology and Innovation Steel Committee; Organization for Economic Co-operation and Development: Paris, France, 2015; Available online: http://www.oecd.org/industry/ind/Evaluating-Financial-Health-Steel-Industry.pdf (accessed on 20 June 2019).
- Lambert, P. Sustainability of metals and alloys in construction. In Sustainability of Construction Materials; Khatib, J.M., Ed.; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar] [CrossRef]
- Labiapari, W.S.; Alcântara, C.M.; Costa, H.L.; Mello, J.D.B. Wear debris generation during cold rolling of stainless steels. J. Mater. Process. Technol. 2015, 223, 164–170. [Google Scholar] [CrossRef]
- Hattalli, V.M.; Srivatsa, S.R. Sheet Metal Forming Processes—Recent Technological Advances. Mater. Today Proc. 2018, 5, 2564–2574. [Google Scholar] [CrossRef]
- Colla, V.; Matino, I.; Branca, T.A.; Fornai, B.; Romaniello, L.; Rosito, F. Efficient Use of Water Resources in the Steel Industry. Water 2017, 9, 874. [Google Scholar] [CrossRef]
- Das, P.; Mondal, G.C.; Singh, S.; Singh, A.K.; Prasad, B.; Singh, K.K. Effluent Treatment Technologies in the Iron and Steel Industry—A State of the Art Review. Water Environ. Res. 2018, 90, 395–408. [Google Scholar] [CrossRef]
- Matino, I.; Colla, V.; Romaniello, L.; Rosito, F.; Portulano, L. Simulation techniques for an Efficient Use of Resources: An overview for the steel-making field. In Proceedings of the 2015 World Congress on Sustainable Technologies, London, UK, 14–16 December 2015; pp. 48–54. [Google Scholar] [CrossRef]
- Arjmandi, S.; Tabesh, M.; Esfahani, S.T. Risk Analysis of Water Reuse for Industrial Cooling Water Consumptions. J. Environ. Eng. 2019, 145, 04019067. [Google Scholar] [CrossRef]
- Sun, W.; Xu, X.; Lv, Z.; Mao, H.; Wu, J. Environmental impact assessment of wastewater discharge with multi-pollutants from iron and steel industry. J. Environ. Manag. 2019, 245, 210–215. [Google Scholar] [CrossRef]
- Gao, C.; Gao, W.; Song, K.; Na, H.; Tian, F.; Zhang, S. Comprehensive evaluation on energy-water saving effects in iron and steel industry. Sci. Total Environ. 2019, 670, 346–360. [Google Scholar] [CrossRef]
- Sinha, V.K. Study on the Waste Water Treatment Technology for Steel Industry Recycle and Reuse. Ph.D. Dissertation, Jharkhnad Rai University, Ranchi, India, 2016. [Google Scholar]
- Nezamoleslami, R.; Hosseinian, S.M. Data needed for assessing water footprint of steel production. Data Brief 2020, 30, 105461. [Google Scholar] [CrossRef]
- Henriot, G. Advanced techniques for rolling mill gearing. J. Manuf. Sci. Eng. 1973, 95, 1101–1107. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Ma, Y.; Xu, M.; Qin, S.; Liu, X.; Feng, H.; Hou, L. Purification of pickling wastewater from the steel industry using membrane filters: Performance and membrane fouling. Environ. Eng. Res. 2022, 27, 200486. [Google Scholar] [CrossRef]
- SAIL Annual Report (2005–2021) Steel Authority of India Ltd., New Delhi. Available online: https://sail.co.in/sites/default/files/2021-09/SAILAnnualReport2020-21.pdf (accessed on 20 September 2021).
- Çağin, V.; Yetiş, Ü. Water Reuse Strategies: Iron and Steel Industry Case Study. In Security of Industrial Water Supply and Management; Atimtay, A., Sikdar, S., Eds.; NATO Science for Peace and Security Series C: Environmental Security; Springer: Dordrecht, Switzerland, 2011. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Polo-López, M.I.; Mosteo, R.; Ormad, M.P.; Fernández-Ibáñez, P. Disinfection of real and simulated urban wastewater effluents using a mild solar photo-Fenton. Appl. Catal. B Environ. 2014, 150–151, 619–629. [Google Scholar] [CrossRef]
- Harika, D.; Swetha, D.; Vijay John, T. Role of MBC Plant in Treating Steel Plant Effluent: A Case Study. Int. J. Sci. Technol. Manag. 2015, 4, 183–187. [Google Scholar]
- Kirchmann, H.; Börjesson, G.; Kätterer, T.; Cohen, Y. From agricultural use of sewage sludge to nutrient extraction: A soil science outlook. Ambio 2017, 46, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Shaddel, S.; Bakhtiary-Davijany, H.; Kabbe, C.; Dadgar, F.; Østerhus, S.W. Sustainable Sewage Sludge Management: From Current Practices to Emerging Nutrient Recovery Technologies. Sustainability 2019, 11, 3435. [Google Scholar] [CrossRef]
- Falaciński, P.; Szarek, L. Possible Applications of Hardening Slurries with Fly Ash from Thermal Treatment of Municipal Sewage Sludge in Environmental Protection Structures. Arch. Hydro-Eng. Environ. Mech. 2016, 63, 47–61. [Google Scholar] [CrossRef]
- Yasipourtehrani, S.; Strezov, V.; Evans, T. Investigation of Phosphate Removal Capability of Blast Furnace Slag in Wastewater Treatment. Sci. Rep. 2019, 9, 7498. [Google Scholar] [CrossRef]
- Bubalo, A.; Vouk, D.; Stirmer, N.; Nad, K. Use of Sewage Sludge Ash in the Production of Innovative Bricks—An Example of a Circular Economy. Sustainability 2021, 13, 9330. [Google Scholar] [CrossRef]
- Roychoudhury, A. Seel Plant ETP Sludge Characteristics; ResearchGate: Berlin, Germany, 2022. [Google Scholar] [CrossRef]
- Alleman, J.E.; Berman, N.A. Constructive Sludge Management: Biobrick. J. Environ. Eng. 1984, 110, 301–311. [Google Scholar] [CrossRef]
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and Recycling of By-Products in the Steel Sector: Recent Achievements Paving the Way to Circular Economy and Industrial Symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). PubChem Patent Summary for US-5843204-A, Method for Recycling Iron and Steel Industry Waste. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-5843204-A (accessed on 3 October 2021).
- Liu, B.; Zhang, S.; Pan, D.; Chang, C. Synthesis and Characterization of Micaceous Iron Oxide Pigment from Oily Cold Rolling Mill Sludge. Procedia Environ. Sci. 2016, 31, 653–661. [Google Scholar] [CrossRef]
- Agrawal, R.K.; Pandey, P.K. Productive recycling of basic oxygen furnace sludge in integrated steel plant. J. Sci. Ind. Res. 2005, 64, 702–706. [Google Scholar]
- Mortier, R.; Block, C.; Vandecasteele, C. Water management in the Flemish steel industry: The Arcelor Gent case. Clean Techn. Environ. Policy 2007, 9, 257–263. [Google Scholar] [CrossRef]
- Babatunde, A.O.; Zhao, Y.Q. Constructive Approaches Toward Water Treatment Works Sludge Management: An International Review of Beneficial Reuses. Crit. Rev. Environ. Sci. Technol. 2007, 37, 129–164. [Google Scholar] [CrossRef]
- Xu, S.; Chen, T.; Zhang, M.; Buekens, A.; Yu, Y.; Ling, Y.; Chen, Z.; Li, X. Hot rolling sludge incineration: Suppression of PCDD/Fs by spent anion exchange resins. J. Hazard. Mater. 2018, 343, 149–156. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Fernández, A.M.; Torres, V.M. Hydrometallurgical Processes for the Recovery of Metals from Steel Industry By-Products: A Critical Review. J. Sustain. Metall. 2020, 6, 505–540. [Google Scholar] [CrossRef]
- Fernandez, J.M.M.; Palacios, H.M.; Cabal, J.V.A.; Huerta, G.M.M. Methodology for industrial solid waste management: Implementation to sludge management in Asturias (Spain). Waste Manag. Res. 2014, 32, 1103–1112. [Google Scholar] [CrossRef]
- Lobato, N.C.C.; Villegas, E.A.; Mansur, M.B. Management of solid wastes from steel-making and galvanizing processes: A brief review. Resour. Conserv. Recycl. 2015, 102, 49–57. [Google Scholar] [CrossRef]
- Mishra, A.K.; Banerjee, P.; Mishra, S.; Chattopadhyay, P.; Gupta, R. Suitability of Cold Rolling Mill-Effluent Sludge as a Soil Amendment for Reclamation of Degraded Lands. In Handbook of Environmental Materials Management; Hussain, C., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Andersson, A.; Gullberg, A.; Kullerstedt AAhmed, H.; Sundqvist-Ökvist, L.; Samuelsson, C. Upgrading of Blast Furnace Sludge and Recycling of the Low-Zinc Fraction via Cold-bonded Briquettes. J. Sustain. Metall. 2019, 5, 350–361. [Google Scholar] [CrossRef]
- Falayi, T.; Ntuli, F. Stabilization and solidification of steel industry sludge. In Proceedings of the HNICEM 2017—9th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment and Management (HNICEM 2017—9th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment and Management; Vol. 2018-January), Manila, Philippines, 1–3 December 2017; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Bao, S.; Chen, T. Utilization of Iron Ore Tailings as Raw Material for Portland Cement Clinker Production. Adv. Mater. Sci. Eng. 2016, 2016, 1596047. [Google Scholar] [CrossRef]
- Ghiocel, A.N.; Panaitescu, V.N. Using sewage sludge as an alternative fuel for the cement production process. IOP Conf. Ser. Mater. Sci. Eng. 2018, 400, 022029. [Google Scholar] [CrossRef]

| No. | Parameter | Unit | Method | Result | Remarks |
|---|---|---|---|---|---|
| 1 | Physical State | - | - | Solid | - |
| 2 | Color | - | - | Dark Brown | - |
| 3 | Texture | - | - | Wet Cake | - |
| 4 | Odor | - | - | No | - |
| 5 | Paint Filter Liquid Test | - | USEPA SW-846; 9095A | Pass | No infiltration through PFLT paper |
| 6 | Bulk Density | g/cc | ASTM D 5057-10 | 1.42 | On wet basis |
| 7 | Is there any violent chemical change (in air) (Normally unstable) (Yes/No) | - | - | No | - |
| 8 | Reacts violently with water (Yes/No) | - | - | No | - |
| 9 | Generation of toxic fumes with water/acid/basic (Yes/No) | - | - | No | - |
| 10 | Forms potentially explosive mixture with water (Yes/No) | - | - | No | - |
| 11 | Explosion when subjected to a strong initiating force (Yes/No) | - | - | No | - |
| 12 | Explosion at normal temperature and pressure (Yes/No) | - | - | No | - |
| No. | Parameter | Unit | Method | Result | Remarks |
|---|---|---|---|---|---|
| 1 | pH | - | USEPA SW-846; 9045C | 6.9 | - |
| 2 | Flash Point | °C | USEPA SW-846; 1020A | >200 | Non-flammable |
| 3 | Loss on drying (LOD) at 105 °C | % | APHA 23rd 2540 B | 48.2 | - |
| 4 | Total Solids | % | APHA 23rd 2540 B | 51.8 | - |
| 5 | Volatile Solids | % | APHA 2540 B and E | 2.1 | Non-biodegradable |
| 6 | Calorific Value | cal/g | IS: 1350 (Part-II)-1970 | 368 | On dry basis |
| 7 | Water soluble inorganics (WSI) | % | APHA 2540 B and E | 0.87 | Non water soluble |
| 8 | Water soluble organics (WSO) | % | APHA 2540 B and E | 0.36 | Non water soluble |
| 9 | Reactive Cyanide | mg/Kg | SW-846 9014 B and APHA 4500CN-K | 0.04 | Not detected |
| 10 | Reactive Sulfide | mg/Kg | SW-846 9030B and 9034 | <1.0 | Not detected |
| 11 | Total Chloride as Cl | % | APHA 4500-Cl- B | <1.0 | 10% solution of dried sample |
| 12 | Total Nitrogen as N | % | CHNS analyzer | ND | - |
| 13 | Total Carbon as C | % | CHNS analyzer | ND | - |
| 14 | Total Hydrogen as H | % | CHNS analyzer | ND | - |
| 15 | Total Sulfur as S | % | CHNS analyze | ND | - |
| 16 | Ammonia as N ((Water leaching tests (WLT)) | mg/L | APHA NH3 B, C | 4.12 | - |
| 17 | Total Phenols (WLT) | mg/L | APHA 5530B and D | ND | 10% solution of dried sample |
| 18 | Cyanide in WLT | ppm | APHA 4500CN-K | ND | 10% solution of dried sample |
| 19 | Hexavalent Chromium (WLT) | mg/L | APHA 3500 Cr B | <0.2 | 10% solution of dried sample |
| 20 | Fluoride as F-(WLT) | mg/L | APHA 4500 F-D | <1.0 | 10% solution of dried sample |
| 21 | Nitrate Nitrogen as N | mg/L | APHA 4500 NO3B | 154.89 | 10% solution of dried sample |
| No. | Parameter | Unit | Method | Result | Remark |
|---|---|---|---|---|---|
| 1 | Zinc (Total) | mg/Kg | SW846; 3050 B and 7950/APHA 3120B | 28.46 | 2% solution of dried sample |
| 2 | Zinc (WLT) | mg/L | SW846; 7950/APHA 3120B | <1.0 | 10% solution of dried sample |
| 3 | Copper (Total) | mg/Kg | SW846; 3050 B and 7210/APHA 3120B | 284.9 | 2% solution of dried sample |
| 4 | Copper (WLT) | mg/L | SW846; 7210/APHA 3120B | <0.5 | 10% solution of dried sample |
| 5 | Arsenic as As (Total) | mg/Kg | APHA 3500 As B/APHA 3120B | <1.0 | 2% solution of dried sample |
| 6 | Arsenic as As (WLT) | mg/L | APHA 3500 As B/APHA 3120B | <1.0 | 10% solution of dried sample |
| 7 | Cadmium (Total) | mg/Kg | SW846; 3050 B and 7130/APHA 3120B | <0.1 | 2% solution of dried sample |
| 8 | Cadmium (WLT) | mg/L | SW846; 7130/APHA 3120B | <0.01 | 10% solution of dried sample |
| 9 | Total Chromium (Total) | mg/Kg | SW846; 3050 B and 7190/APHA 3120B | 14,250 | 2% solution of dried sample |
| 11 | Lead (Total) | mg/Kg | SW846; 3050 B and 7420/APHA 3120B | <10 | 2% solution of dried sample |
| 12 | Lead (WLT) | mg/L | USEPA 1998, SW846; 7420/APHA 3120B | <0.1 | 10% solution of dried sample |
| 13 | Nickel (Total) | mg/Kg | SW846; 3050 B and 7520/APHA 3120B | 2689 | 2% solution of dried sample |
| 14 | Nickel (WLT) | mg/L | USEPA 1998, SW846; 7520/APHA 3120B | <1 | 10% solution of dried sample |
| 19 | Mercury as Hg (Total) | mg/Kg | SW846; 7471A/APHA 3120B | <1.0 | 2% solution of dried sample |
| 20 | Mercury as Hg (WLT) | mg/L | SW846; 7470A/APHA 3120B | <0.01 | 10% solution of dried sample |
| No. | Summary of the Study | Key Findings of the Study | Feasibility of the Method | Source |
|---|---|---|---|---|
| 1 |
|
| 100% of sludge can be converted into bio-bricks using the cold pressing process. The process lefts zero residue | [27] |
| 2 |
|
| Entire sludge and ash can be recycled through aggregate, brick, and cement making applications | [25] |
| 3 |
|
| Blast furnace slag is already widely used as a commercially viable material in the production of bricks and concrete. Consequently, it does not seem logical to apply it as a flocculant despite the obstacles involved. | [24] |
| 4 |
|
| Recovering nutrients from sludge can be a challenging process due to its high contamination levels with heavy metals and other pollutants. Nevertheless, utilizing sludge in cement manufacturing has the potential to consume up to 20% of the generated sludge. | [28] |
| 5 |
|
| The reuse of 100% of the sludge in this process is feasible without any reservations. However, it should be noted that constructing a separate kiln with special dimensions is a costly endeavor. | [29] |
| 6 |
|
| The generation of hot rolling mill sludge is trivial and the consumption process is highly sophisticated. It can only be achieved by collecting the specific sludge directly at its source. | [30] |
| 7 |
|
| By eliminating BOF sludge from the wastewater stream and recycling it as a raw material for ironmaking, a reduction of up to 15–20% in the overall sludge burden can be achieved. Hence, this approach can be considered a partial solution when implemented at the source. | [31] |
| 8 |
|
| Mechanical sludge drying is a costly process that comes with its own set of drawbacks. Additionally, the dried sludge does not possess a significant calorific value and largely contributes to ash generation. | [32] |
| 9 |
|
| While reusing the entire sludge as a raw material in ironmaking may have an adverse effect on quality, it is worth noting that the metal-bearing portion of the sludge can be efficiently recycled if properly segregated. | [33] |
| 10 |
|
| Thermal methods for handling wet sludge can be an expensive endeavor and are not sustainable without energy recovery. Moreover, this process generates fly ash and bottom ash, which must be disposed of in secure landfills. | [34] |
| 11 |
|
| Combining sludge with other cementitious elements shows promise as a raw material for concrete production. However, it is not advisable to utilize sludge as a direct substitute for coal due to its lower heating value and associated issues with ash generation. | [23] |
| 12 |
|
| Currently, the economic and technical feasibility of metal recovery from sludge is limited. Additionally, only a small portion of metal dust can be effectively recovered, while the remaining portion necessitates alternative disposal methods. | [35] |
| 13 |
|
| Biological methods are not particularly effective in addressing sludge issues due to the high concentration of inorganic constituents. However, for small-scale applications with appropriate precautions, ceramization can be considered as a viable option. | [36] |
| 14 |
|
| Collecting source-specific sludge is a laborious task, and ceramization only utilizes a small fraction of the sludge. The remaining portion requires parallel recycling or disposal methods to be implemented. | [37] |
| 15 |
|
| The process of sludge stabilization and decontamination is extremely laborious, and attempting to recover nutrients from it afterwards is technically and financially impractical. | [38] |
| 16 |
|
| The metal removal process is complex and requires advanced techniques. However, using only a small percentage (10%) of the sludge as a replacement for raw material in the briquette-making process may not be a practical or meaningful approach. | [39] |
| 17 |
|
| After successfully mitigating the leachability of metals, the resulting product can be utilized for brick making or similar applications. However, the financial feasibility of this approach remains uncertain and requires further evaluation. | [40] |
| 18 |
|
| The addition of steel sludge in cement manufacturing offers advantages. However, the main challenge lies in the dosage limitation of 2% by the weight of raw material, which poses a significant obstacle to its reuse potential. | [41] |
| 19 |
|
| Drying the sludge to the reported extent is an enormous undertaking, which directly impacts its calorific value (CV). Moreover, the substantial generation of ash, as reported, poses a significant financial burden for its disposal. | [42] |
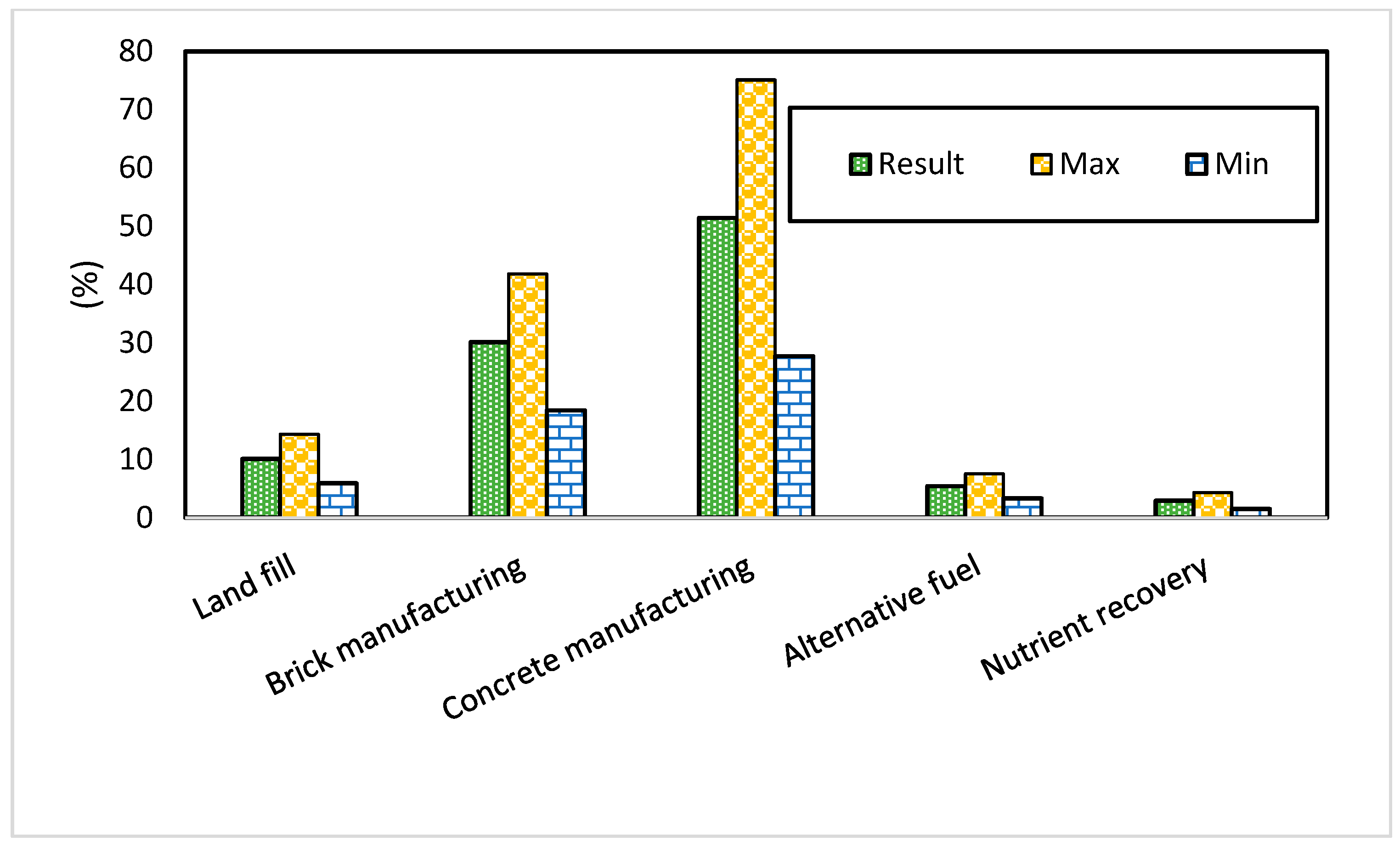
| Category | Priority | Rank | + | − | |
|---|---|---|---|---|---|
| 1 | Land fill | 10.1% | 3 | 4.2% | 4.2% |
| 2 | Brick manufacturing | 30.1% | 2 | 11.7% | 11.7% |
| 3 | Concrete manufacturing | 51.4% | 1 | 23.7% | 23.7% |
| 4 | Alternative fuel | 5.4% | 4 | 2.1% | 2.1% |
| 5 | Nutrient recovery | 2.9% | 5 | 1.4% | 1.4% |
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1 | 1 | 0.20 | 0.14 | 3.00 | 5.00 |
| 2 | 5.00 | 1 | 0.33 | 7.00 | 9.00 |
| 3 | 7.00 | 3.00 | 1 | 7.00 | 9.00 |
| 4 | 0.33 | 0.14 | 0.14 | 1 | 3.00 |
| 5 | 0.20 | 0.11 | 0.11 | 0.33 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, A.R.; Singh, N.; Veeraraghavan, A.; Gupta, A.; Palani, S.G.; Mehdizadeh, M.; Omidi, A.; Al-Taey, D.K.A. Ascertaining and Optimizing the Water Footprint and Sludge Management Practice in Steel Industries. Water 2023, 15, 2177. https://doi.org/10.3390/w15122177
Choudhury AR, Singh N, Veeraraghavan A, Gupta A, Palani SG, Mehdizadeh M, Omidi A, Al-Taey DKA. Ascertaining and Optimizing the Water Footprint and Sludge Management Practice in Steel Industries. Water. 2023; 15(12):2177. https://doi.org/10.3390/w15122177
Chicago/Turabian StyleChoudhury, Atun Roy, Neha Singh, Arutchelvan Veeraraghavan, Ayushi Gupta, Sankar Ganesh Palani, Mohammad Mehdizadeh, Anahita Omidi, and Duraid K. A. Al-Taey. 2023. "Ascertaining and Optimizing the Water Footprint and Sludge Management Practice in Steel Industries" Water 15, no. 12: 2177. https://doi.org/10.3390/w15122177
APA StyleChoudhury, A. R., Singh, N., Veeraraghavan, A., Gupta, A., Palani, S. G., Mehdizadeh, M., Omidi, A., & Al-Taey, D. K. A. (2023). Ascertaining and Optimizing the Water Footprint and Sludge Management Practice in Steel Industries. Water, 15(12), 2177. https://doi.org/10.3390/w15122177