Abstract
Urban riverine networks are hotspots of CO2 and CH4 emissions, due to river impoundment and pollution. The river–lake connection is considered to be an important way to improve the ecological environment of urban rivers; however, its impact on CO2 and CH4 emissions from urban rivers and regulatory mechanisms are still unclear. Rivers and lakes have been studied separately by lots of traditional studies. In this study, we investigated the concentration and emission of CO2 and CH4 from March 2021 to December 2021 in an interconnected river–lake system in Central China. We found that the urban river–lake system was a hotspot of CO2 and CH4 emissions. CO2 and CH4 emissions from urban rivers were much higher than those from the lakes, which are 2.7 times and 11.9 times that of lakes, respectively. The correlation analysis indicated that the spatial variation of CO2 and CH4 emissions was determined by nutrient content. The abundant nutrients promoted microbial growth and consumed dissolved oxygen (DO), thus resulting in high emissions of CO2 and CH4 in the isolated urban rivers (UR). The average CO2 and CH4 emissions of urban rivers are 991.56 and 14.82 mmol m−2 d−1, respectively. The river–lake connection decreased the nutrients of urban rivers connected to lakes (LUR). The moderate nutrients wreaked in situ respiration, exhibiting moderate CO2 emission in the LUR. The average CO2 emission of LUR is 543.49 mmol m−2 d−1. The river–lake connection increased the DO concentrations in the LUR, inhibited methanogenesis, and enhanced CH4 oxidation, reducing CH4 emission from LUR sharply. The average CH4 emission of LUR is 1.26 mmol m−2 d−1. A correlation analysis showed that the seasonal variations of CO2 and CH4 emissions were controlled by DO and T. Hence, the highest emissions of CO2 were observed in the spring and the lowest in the winter, and the CO2 emissions in spring were 10.7 times that in winter. The highest emissions of CH4 were observed in the summer and the lowest in the winter, and the CH4 emissions in summer were 6.6 times those in winter. The connection of urban rivers and lakes changes the environmental factors, thereby varying the production and emission of greenhouse gases. This study advanced the knowledge of the greenhouse gas emission response to the river–lake connection, providing the theoretical basis for greenhouse gas emission reduction from urban rivers.
1. Introduction
Carbon dioxide (CO2) and methane (CH4) are two important greenhouse gases. Recent monitoring by the World Meteorological Organization shows that the concentrations of CH4 and CO2 in the global atmosphere have reached 1869 ± 2 parts per billion and 408.0 ± 0.1 parts per million, a steady increase of 259% and 147%, respectively, since the Industrial Revolution [1]. Inland waters, including rivers, reservoirs, and lakes, are essential to the global carbon cycle and are a significant source of atmospheric greenhouse gases [2,3,4]. Global inland waters absorb about 5.1 PgC yr−1 from the global terrestrial landscape, and at the same time, they emit about 3.9 PgC yr−1 of carbon dioxide [5] and 0.13 PgC yr−1 of methane (CH4) [6]. Annual CO2 emissions from lakes and reservoirs are about 244–503 Tg CO2 [7], accounting for about 21–29% of global CO2 emissions from rivers and streams [8]. CH4 emissions from rivers and streams were as high as 26.8 Tg CH4 yr−1 [6]. It is estimated that about 54.1 ± 41.0 TgC enters the atmosphere from CO2 from rivers in China, 6.8 ± 8.8 TgC from lakes, and 1.1 ± 1.6 TgC from reservoirs. Rivers account for 87.3% of China’s inland water CO2 emissions [9]. China launched the carbon neutrality goal in 2020, promising to achieve carbon neutrality by 2060. Therefore, rivers, which account for the majority of carbon emissions, are becoming the focus of carbon emissions research.
With the rapid development of urbanization, urban water networks are increasingly affected by human activities. A large number of studies have found that the higher the level of urbanization, the more greenhouse gas (GHGs) emissions of urban water networks are exceptionally high, at even several times or even dozens of times that of other types of rivers [10,11,12,13,14]. In the Chaohu Lake Basin, the greenhouse gas emissions of different landscape types were studied, and it was found that the CO2 and CH4 of urban rivers were two times and seven times that of other varieties [12]. Some other studies found that GHG emissions increased significantly once having flowed through the urban area [15,16]. These studies have suggested that the magnitude and spatiotemporal variations of GHG emissions are affected by weather conditions (e.g., water temperature and wind speed), nutrient level (e.g., nitrogen, phosphorus, and carbon), hydrological conditions (e.g., flow velocity and water depth) and biological activities [17,18]. Impacts by human activities include increased pollution levels of urban water networks, significant inputs of organic matter, and changes in hydrogeomorphology [19]. For example, the water level of urban rivers is low due to water shortage, and the diffusion path of CH4 is short, which can reduce the oxidation of CH4 and increase emissions [20]. Moreover, urban rivers are shallow, and the surface runoff formed by rainfall will bring sediment into urban rivers, causing the sediment layer of urban rivers to gradually thicken, which will promote CO2 and CH4 emissions [21,22]. Low water depth of urban rivers also results in low water hydrostatic pressure, leading to GHG emissions through ebullition [23]. A study of urban rivers in Shanghai found that the escape of CH4 through ebullition has no time change and accounts for 99% of CH4 emissions [24]. A large amount of nutrient input will cause microorganisms to decompose organic matter to produce a large amount of CO2 and, at the same time, consume oxygen to form an anaerobic environment in urban rivers [25], creating favorable conditions for methanogens to produce CH4. However, the enrichment of nutrients in rivers and lakes will also promote the growth of environmental algae, thereby increasing primary production, taking CO2 in rivers and lakes, and reducing CO2 emissions from rivers and lakes [26,27,28].
Shallow lakes are inland water that contributes GHG emissions due to their large area and eutrophication [29,30]. Compared with deep lakes, shallow lakes are more vulnerable to eutrophication due to the low buffer capacity for nutrient input [31]. A number of studies reported that eutrophic status plays a critical role in impacting the magnitude of GHG emissions from lakes [7,32,33]. It was found that eutrophication can both increase GHG emissions due to stimulating mineralization and decrease GHG emissions due to the enhancement of primary production [34]. However, Zhou et al. (2023) reviewed that CO2 emission from lakes (mean ± SD:20.0 ± 26.0 mmol m−2 d−1) is notably lower than those from rivers (mean ± SD:179.7 ± 149.5 mmol m−2 d−1) of China due to urbanization [9]. Zheng et al. (2022) reported that the mean value of CH4 emission from lakes is about half of that from rivers globally [35]. The reason for the relatively lower GHG emissions from lakes is the lower nutrient levels in lakes. Lakes connecting to rivers could play the role of converter and water accumulator [36]. Thus, the river–lake connection is considered to be an important way to improve the ecological environment of urban rivers [37]. Since 2010, more than 100 cities in China have constructed interconnected river–lake projects. However, lakes and rivers have traditionally been studied as separate systems for GHG emissions, and a knowledge gap still exists in the understanding of spatiotemporal variations of GHG emissions in an urban interconnected river–lake system. This study was conducted in an interconnected river–lake system in Wuhan, Central China. The objectives were (1) to estimate the spatial and seasonal variations and correlated environmental factors associated with the emissions of CO2 and CH4, and (2) to reveal the impact of river–lake connection on CO2 and CH4 emission in urban rivers.
2. Materials and Methods
2.1. Study Area
The urban river–lake system is located in Wuhan City of Hubei Province, Central China (Figure 1). Wuhan is located in the middle reaches of the Yangtze River, the longest river in China. The study area is characterized by a typical subtropical monsoon climate, and the annual average temperature is 15.8–17.5 °C. The annual precipitation is 1150–1450 mm, and the rainfall is mainly from April to September, accounting for about 70% of the annual rainfall. The complex river–lake system in Wuhan, consisting of 166 lakes and 2 big rivers (Yangtze River and Han River), accounts for a quarter of the total urban area. Lake Tangxun is the largest urban lake in the world, with an area of 47.6 km2. This study area is an area with abundant surface water resources. However, with extensive economic development and population growth, river and lakes in Wuhan suffered heavy pollution [38].
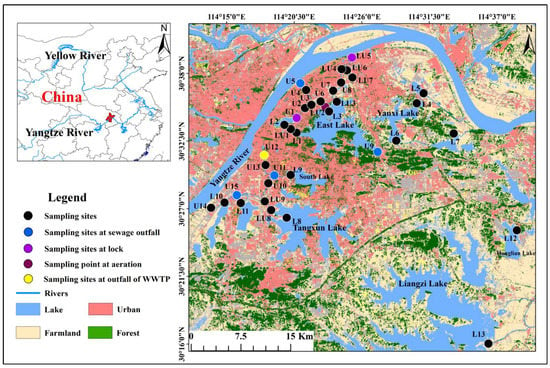
Figure 1.
Geographic locations and sampling sites of the interconnection river–lake system in Wuhan City, Central China. U1-U15 are UR sites, LU1-LU9 are LUR sites, and L1-L13 are lake sites.
There are many lakes distributed in the urban area on the south bank of the Yangtze River, and the major source of water to these lakes is precipitation, groundwater recharge, and domestic sewage. To improve the water quality and quantity of urban rivers, the rivers and lakes are connected by channels, thereby forming a complex plain urban water system. However, although the rivers in the city are physically connected with the lakes by channels, the actual connectivity between the rivers and lakes is quite different due to water level, sluice, and dams. Some urban rivers are still isolated from the lakes, so the urban water system was divided into isolated urban rivers (UR), urban rivers connected to lakes (LUR), and lakes.
2.2. Experimental Samples and Analysis Methods
Samples were collected every three months from 2021 to 2022. A total of 37 sampling points were set up, and 13, 15, and 9 samples were set for lakes, UR, and LUR, respectively. The water temperature (T), dissolved oxygen (DO), pH, and electrical conductivity (EC) were measured with a portable water quality parameter sensor (Hach Company, CO Loveland, USA). Water samples of 200 mL were filtered using 0.45 μm cellulose acetate membranes to measure the dissolved inorganic nitrogen (TDN) and dissolved organic carbon (DOC). A total of 50 mL of water sample was filtered through a needle filter with a 0.45 μm pore size, and then 1–2 drops of 1 mol L−1 hydrochloric acid were added to fix the NH4+. NH4+-N, NO3−-N, and NO2−-N were determined by a flow analyzer (San++, Skalar, Netherlands). TDN and DOC were determined using a total organic carbon/total nitrogen analyzer (multi N/C 2100, Jena, Germany). A depth of surface water of 0–20 cm was collected from rivers and lakes. A 100 mL syringe pre-loaded with saturated HgCl2 was used to collect 50 mL of water samples to make a 50 mL headspace, and the gas in the headspace was transferred to the airbag after CO2 and CH4 were in the water at equilibrium with the air. The gas sample in the airbag was measured within 24 h. The concentration of CO2 and CH4 in the equilibrated gas was measured using gas chromatograph (7890 B, Agilent Technologies, Santa Clara, CA, USA), and the instrument detection limits were 0.1 ppm. An amount of 100 mL surface water was collected for chlorophyll a (Chl-a); Chl-a was extracted from the water samples using a 90% buffered acetone solution, and the concentration was determined using an ultraviolet (UV) spectrophotometer (Shimadzu, UV-2600 PC). We collected dissolved inorganic carbon (DIC) samples using 250 mL bottles and then added 1–2 drops of saturated HgCl2 solution. δ13C-DIC values were analyzed by a GasBench II System interfaced to a Delta V Plus IRMS (Thermo Scientific, Bremen, Germany). The method detection limit and long-term standard deviation for δ13C-DIC as CO2 were approximately 150 nmol and 0.1‰.
2.3. Experimental Methodology
The surface water CO2 and CH4 concentrations in rivers and lakes (Csample) were calculated using Henry’s law [39] to obtain the CO2 and CH4 concentrations in the samples. Calculate the CO2 and CH4 concentrations (μmol L−1) by Formulas (1)~(4).
Ca is the concentration of CO2 and CH4 in the liquid phase in the headspace equilibrium state (μmol L−1), Cg is the concentration of CO2 and CH4 in the gas phase (μmol L−1), and KHCC is a constant. KHCC is unknown in this formula, and we need the following procedure:
KH is the Henry coefficient, and T is the temperature when T = 298.15 K:
when T = 298.15 K, the KH values of CO2 and CH4 are, respectively, 3.6 × 10−2 and 1.4 × 10−3 [40,41], from which Ca be calculated, and then the following formula is used:
Va is the liquid volume (L) in the headspace equilibrium state, Vg is the gas volume (L) in the headspace equilibrium state, Vsample is the sample volume before equilibrium (L), and Csample is the concentration of CO2 and CH4 in the sample (μmol L−1).
When the CO2 and CH4 concentrations in rivers and lakes are calculated, we also need to calculate the flux (F), which is calculated using the water–air interface diffusion model [42], and the calculation formula is as follows:
K is the gas transfer velocity at the water–air interface, and Cw is the concentration of CO2 and CH4 in the surface water of rivers and lakes (μmol L−1). Ceq is the concentration of CO2 and CH4 in the river and lake at the sampling point when the concentration of CO2 and CH4 in the surface water of the river and lake reaches the equilibrium with the attention in the atmosphere (μmol L−1). In this study, K is calculated using the calculation method of Wanninkhof et al. [43]. The formula is as follows:
n is the coefficient and takes 0.5, Sc is the Schmidt number of the gas, T is the temperature of the sampling point, and K600 is the diffusion rate of the gas when Sc is equal to 600. K600 is controlled by physical factors related to water flow and is calculated from models of water flow and wind speed [44,45,46]. In this study, we choose the wind speed model [46], and the calculation formula is as follows:
u10 is the average wind speed at 10 m from the sampling point (m s−1). The wind speed at the sampling point adopts the average wind speed reported by the Meteorological Bureau on the day. The nonparametric Kruskal–Wallis method (K-W test) was used to test whether there were statistical differences in the spatial and temporal CO2 and CH4 concentrations in rivers and lakes. Kolmogorov–Smirnov was used to test the normality of environmental factors, gas concentration, and flux, and the test results did not conform to normality. Therefore, the Spearman correlation was used to analyze the correlation level between CO2 and CH4 fluxes and environmental factors, using IBM SPSS 26.0 and R 4.1.0 software. The spatial and temporal distribution characteristics of CO2 and CH4 concentrations and fluxes were drawn using Origin 2021 software.
3. Results
3.1. Spatiotemporal Variation of Environmental Factors
The interconnected river–lake system exhibited significant spatial and temporal variations of environmental factors (Table 1). Except for water temperature, all other environmental factors have significant differences among the rivers and lakes. The mean value of DO (9.4 ± 1.8 mg L−1) concentration and pH (8.2 ± 0.5) was similar to the lakes, but was much higher than those of values in the UR. The mean value of EC (361.8 ± 54.0 μs cm−1) and TN (2.1 ± 1.3 mg L−1) was similar to the lakes, but was much lower than that in the UR. The mean concentration of DOC and Chl-a in the LUR was lower than that in the UR and lakes. The abundances of bacteria in the LUR were the lowest, and those values in the UR were the highest. Except for the pH values and TDN, there were significant differences in the mean value of T, DO, EC, Chl-a, and DOC. The DO concentrations were higher in the spring and summer than those values in the autumn and winter. The mean value of EC was the highest in the winter and the lowest in the summer. The highest concentrations of Chl-a and DOC were observed in the autumn, and the lowest were observed in the winter. The highest abundances of bacteria were observed in the spring, with no significant differences in the other seasons. Because of the hydrological connection, the hydrodynamic conditions of the connected river channels are improved, which can promote the dissolution of oxygen in the air in the water body and enable DO to enter the deep water columns, thereby increasing the DO of the river. It will also cause the flow velocity of connected rivers to increase and the carbon residence time to decrease, which is not conducive to algae reproduction; the carbon dioxide produced will also decrease, and the pH will increase accordingly.

Table 1.
Statistical characteristics of riverine and lake physicochemical indicators.
3.2. Spatiotemporal Variation of CO2 Concentrations and Emission Fluxes
The CO2 concentrations ranged from 55.9 to 2246.1 μmol L−1, with an overall mean value of 539.1 ± 512.5 μmol L−1 (Figure 2). All these values were higher than those of the atmosphere, resulting in the urban river–lake system being the emission source of CO2 in the atmosphere. The concentrations of dissolved CO2 varied spatially and temporally. The CO2 concentration was highest in the spring, followed by the summer, and the lowest in the autumn and winter without significant differences in the UR. In the LUR and lakes, mean values of CO2 concentration were higher in the spring, and there was no difference among the other seasons. Spatially, concentrations of CO2 in the LUR were lower than those in the UR, and higher than those in the lakes.

Figure 2.
Seasonal and spatial variations of CO2 concentrations and emission fluxes in the interconnection urban river–lake system.
The emission fluxes of CO2 ranged from 48.2 to 4351.0 mmol m−2 d−1, with a mean value of 614.7 ± 727.4 mmol m−2 d−1. In the UR, the highest CO2 emissions were observed in the summer, followed by the spring, which is contrary to the seasonal dynamics trend in CO2 concentrations. However, the seasonal variation pattern was consistent with that of CO2 concentrations; that is, the highest CO2 emission was observed in the spring and the lowest in the winter. In addition, CO2 emissions in the LUR were lower than those in the UR, and higher than those in the lakes, which was consistent with the dynamics pattern of CO2 concentrations. The spatial difference of CO2 is mainly caused by the difference in nutrient load, which is greatly affected by the pollution emission from human activities. The main factors affecting the time difference of CO2 are the temperature and the time change in algae, because the main way of CO2 consumption in the study area is the primary production of algae.
3.3. Spatiotemporal Variability in CH4 Concentrations and Fluxes
The temporal and spatial variation characteristics of CH4 concentrations and emissions are presented in Figure 3. The CH4 concentrations varied from 0.02 to 78.6 μmol L−1, with a mean value of 5.5 ± 11.5 μmol L−1. All these concentrations indicated the saturation of CH4, leading to the emission source of CH4. The mean value of CH4 concentrations was the highest in spring and the lowest in winter. All the values in the UR were much higher than those values in the LUR and lakes, while no significant difference existed between the LUR and lakes.
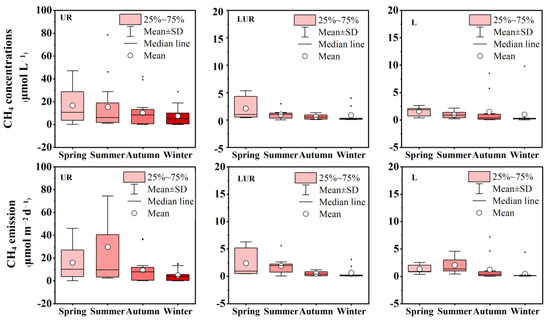
Figure 3.
Seasonal and spatial variations of CH4 concentrations and emission fluxes in the interconnection urban river–lake system.
CH4 emission ranged from 0.01 to 158.1 μmol L−1, with a mean value of 6.37 ± 17.2 μmol L−1. According to the dynamics pattern of CH4 concentrations, CH4 emissions in the UR were the highest, while no significant difference existed between the LUR and lakes. Seasonal variations of CH4 emissions were also estimated, with the highest values in the summer and the lowest values in the winter, exhibiting different dynamics patterns with CH4 concentrations. DO and temperature mainly affect the temporal and spatial changes in CH4. Anaerobic conditions are conducive to the production of CH4 and inhibit the oxidation of CH4. The level of temperature will affect the activity of methanogens and thus affect the production of CH4.
3.4. δ13. C-DIC Signatures of Dissolved Inorganic Carbon
The δ13C-DIC values of dissolved inorganic carbon in the river–lake system are shown in Figure 4. The δ13C-DIC varied from −13.88 to −2.37‰, with a mean value of −9.26 ± 2.7 ‰. The lower values of δ13C-DIC were observed, and in the UR, the higher values were observed in the lakes. The variation range of δ13C-DIC was relatively large in the LUR. The δ13C-DIC had a clear seasonal variation pattern in the LUR and lakes, that is, gradually increasing from summer to winter. In particular, although the lowest value of δ13C-DIC was still observed in the summer, there is no significant difference between autumn and winter. The δ13C-DIC values were close to the endmember of the atmosphere in the winter, and those values were close to the endmember value of organic matter degradation. Therefore, the low δ13C-DIC value in summer is due to a large amount of decomposition of organic matter, and the reason for the high value in winter is that the decomposition intensity of organic matter decreases, the proportion of CO2 produced decreases, and the atmospheric source increases, which promotes the increase in the δ13C-DIC value.

Figure 4.
Seasonal and spatial variations of δ13C-DIC values in the interconnection urban river–lake system.
3.5. Correlation between CO2 and CH4 Emissions and Environmental Factors
The emissions of CO2 and CH4 are affected by various environmental factors. Spearman correlation analysis was used to estimate the correlation between CO2 and CH4 emissions and environmental factors (Figure 5). The Spearman correlation analysis showed that CO2 emission was positively correlated with EC and TDN (p < 0.05) and negatively correlated with DO, pH, and Chl-a in all seasons. CO2 emission was positively correlated with DOC and abundance of 16S in the spring, but not significantly correlated with DOC in the other seasons. Spatially, T was positively correlated with CO2 emission in all the rivers and lakes. CO2 emission was negative with DO and pH in the UR. There is no significant correlation observed between CO2 emission and other factors in the LUR. DO concentration was negatively correlated with CO2 emission in the lakes. The decomposition of organic matter to produce CO2 requires the consumption of oxygen, and the more CO2 is produced, the more oxygen is consumed. The primary production of algae consumes CO2, so the higher the concentration of chlorophyll a, the greater the CO2 consumption. Therefore, CO2 emissions are negatively correlated with DO and Chl-a.
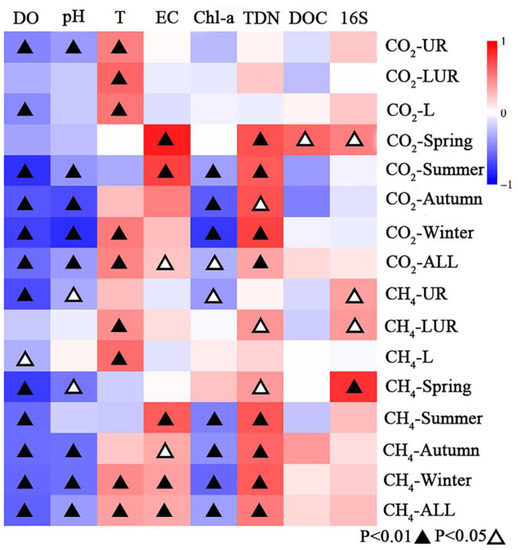
Figure 5.
Heat map of Spearman correlation coefficients between CO2 and CH4 emission and environmental factors.
Chl-a and δ13C-DIC are the most important indicators that reveal the processes of CO2 production and consumption. Therefore, a more detailed correlation analysis between these factors and CO2 emission was performed in this study (Figure 6 and Figure 7). For the UR, CO2 emission was negatively correlated with Chl-a in the autumn. For the LUR, CO2 emission was negatively correlated with Chl-a in the winter. For the lakes, CO2 emission was negatively correlated with Chl-a in the winter, and positively correlated in the summer. The most significant correlation between CO2 emission and Chl-a was observed in the lakes. Dissolved CO2 was negatively correlated with δ13C-DIC in all the seasons, rivers, and lakes (Figure 7). The CO2 produced by the decomposition of organic matter decreases in winter, and then the primary production of algae consumes CO2, and the impact on CO2 emissions is amplified. Therefore, winter CO2 emissions had the strongest correlation with Ch-a. Due to the high nutrient load, the CO2 produced by the decomposition of organic matter in UR is much higher than that in LUR and lakes, so algae consume CO2 and have less impact on UR’s CO2 emissions than in LUR and lakes.
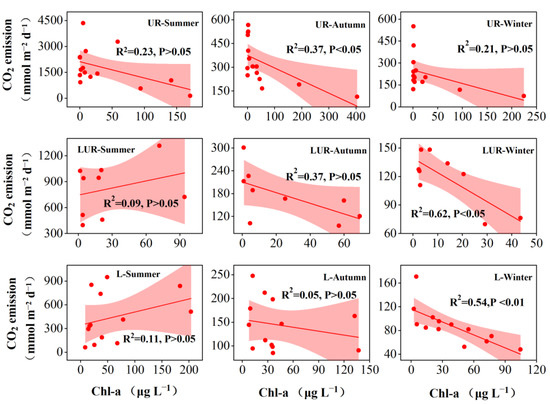
Figure 6.
Seasonal and spatial variations of correlation between CO2 emission and Chl-a.

Figure 7.
Seasonal and spatial variations of correlation between CO2 emission and δ13C-DIC.
The Spearman correlation analysis showed that CH4 emission was positively correlated with T, EC, and TDN, and negatively correlated with DO, pH, and Chl-a among all the data (Figure 5). The correlation between CH4 emission and environmental factors in different seasons was consistent with all the data. Spatially, a negative correlation was observed between CH4 emission and DO, pH, and Chl-a, but no significant correlation was observed with other factors in the UR. TN and T were positively correlated with CH4 emission in the UR. CH4 emission was correlated with T and DO in the lakes. CH4 is produced under anaerobic conditions, because the production of CO2 consumes oxygen, reduces the concentration of DO in the river, and creates favorable conditions for the production of more CH4, so there is a negative correlation. Chl-a is also negatively correlated with CH4, because the more algae there are, the more oxygen will be produced in the surface water due to primary production, and the high DO concentration in the surface water will increase the oxidation of CH4 in the emission process.
4. Discussion
4.1. Effect of River–Lake Connection on Environmental Factors
Hydrological connectivity could affect the diffusion and distribution of environmental factors in inland waters [47]. Environmental factors exhibited significant differences between the UR and LUR, suggesting that the factors are affected by the river–lake connection. The Kruskal–Wallis test showed that DO concentrations were much higher in the LUR than those in the UR, which is consistent with previous studies [48,49,50]. Yue et al. (2015) found that the DO concentration in connected wetlands was higher than that in isolated wetlands. The vertical mixing is uniform with good hydrological connectivity, which is conducive to the transfer of DO from surface water to deep water columns, resulting in higher DO in the rivers. However, a water body with poor hydrodynamic conditions is more prone to vertical stratification, which inhibits the vertical transfer of DO and leads to the hypoxia conditions of the rivers [51]. Stratification of rivers is conducive to the sedimentation of suspended particles, causing the accumulation of nutrients such as carbon and nitrogen in disconnected rivers and lakes [52]. Therefore, TDN and DOC concentrations were significantly lower in the LUR than those in the UR. The river–lake connection increased the pH values in the LUR, due to the absence of production of large amounts of CO2. EC values reflect the total content of anions and cations in the water body [53]. The EC values of the LUR were influenced by the connection of rivers and lakes, which was very close to the EC values of lakes, but much lower than those values in the UR. In addition to the above physical factors, Chl-a and the abundance of 16S are also affected by the river–lake connection. High flow velocities and the short residence time of carbon under good hydrological connectivity are not conducive to the growth of algae and bacteria [54]. Thus, the concentration of Chl-a and 16S are supposed to be higher in lakes and reservoirs than those in rivers. In this study, the concentration of Chl-a in the isolated urban river (UR) had a similar level with the lakes, due to the poor hydrological connectivity of the UR. The relative lower concentrations of Chl-a were observed in the LUR (Table 1).
4.2. Control Processes on CO2 and CH4 Emissions
CO2 emissions from river and lake surfaces are mainly controlled by the balance of respiration and primary production [55,56]. Previous studies suggested that external biodegradation from terrestrial organic carbon and in situ biodegradation of organic carbon are the main sources of aqueous CO2 [54]. However, the contribution of external biodegradation-sourced CO2 is limited in urban rivers, while the in situ biodegradation of organic carbon is likely more relevant to aqueous CO2 [57]. This is supported by the significant negative correlation between CO2 emission and DO. In addition, the inflow of treated and untreated sewage has been considered a source that cannot be ignored in urban rivers [14,31]. CO2 concentrations in the final effluent of water treatment plants (WWTPs) can reach ~30 mmol L−1 [58]. This is supported by the mean value of δ13C-DIC in the UR (−10.7 ± 1.7) and LUR (−9.2 ± 2.6‰), which were very close to that found in WWTP effluent in Brazil (−12.2‰) [59] and in China (−12‰) [60]. The absorption of CO2 by algae photosynthesis is the main process of CO2 consumption in rivers and lakes [32,56]. This is supported by the significant negative correlation between CO2 emission and Chl-a. The mean Chl-a concentrations of this study were much higher than those in other rivers and lakes, and the significant negative correlation between CO2 emission and Chl-a both indicated that the impact of primary production on CO2 emission should be considered in this study [27,54,61]. Xu et al. (2018) reported that DIC in a river–lake continuum originated from 13C-depleted sources with a mean δ13C-DIC value of −18.5‰ [62]. However, the inflow of sewage and algae photosynthesis could be what enriched δ13C-DIC values in this study. CH4 emission depends on anaerobic methanogenesis, aerobic methanotrophy, and CH4 oxidation [63]. Methanogenesis is sensitive to DO concentrations, and high CH4 emission always been observed in anaerobic rivers and lakes [64,65,66]. The lower DO associated with the higher CH4 emission showed that methanogenesis-sourced CH4 contributed to CH4 emission in the UR. CH4 oxidation is also sensitive to DO, and the significant negative correlation between CH4 emission and DO indicates that CH4 oxidation dominated the decrease in the CH4 emission in the LUR and lakes.
4.3. Control Factors on CO2 Emissions
Environmental factors control the production and consumption process of CO2 to regulate the spatiotemporal variations of CO2 emission [67]. According to the correlation analysis, DO and T were the best predictors of CO2 emission in the river–lake system, as suggested by the strongest correlation between CO2 emission and DO and T (Figure 5). CO2 is the end product of respiration, strongly affected by temperature [6,68]. Water temperature also plays a vital role in gas solubility and gas transfer velocity (k) at the water–air interface [46]. An increasing water temperature can decrease gas solubility and increase the value of k. Thus, a significant positive correlation was observed between CO2 emission and water temperature. However, a negative correlation has been reported in many other studies [14,69,70]. An increasing water temperature can enhance algae growth and photosynthesis, decreasing CO2 concentration and emission in the summer [71]. Although the CO2 concentrations in the summer were lower than those in the spring, the CO2 emissions are still the highest in the summer due to the larger value of k, similar to the findings of Tang et al. (2021) [48]. CO2 is produced by aerobic respiration with the consumption of DO, promoting the development of an anaerobic environment [72]. Furthermore, an anaerobic environment accelerates the degradation of complex organic compounds to soluble small molecular organic compounds, increasing DOC concentration and further promoting CO2 production. Therefore, a significantly negative correlation was observed between DO and CO2 emission, in line with previous studies [48,73]. The high production of CO2 by respiration could produce H+ and HCO3-, decreasing pH values [74]. This may explain the negative correlation between pH and CO2 emission that has been reported by most relevant studies and also by this study [48].
Shallow lakes and urban rivers with high Chl-a concentrations can uptake CO2 from organic carbon respiration and even from the atmosphere, supported by the high Chl-a, DO, and pH [28,34]. This may explain the negative correlation between Chl-a and CO2 emission. Factors that reflect nutrients, such as TDN and EC, are important factors affecting CO2 emission [75]. The strongest correlations between CO2 emission and Chl-a were observed in the winter. Within this season, the correlation between CO2 emission and Chl-a was only observed in the lakes, indicating that CO2 uptake in the winter had the most significant effect on the reduction in CO2 emission (Figure 6). Nutrients can both promote microbial growth and mineralization, and stimulate algae growth and photosynthesis [76,77]. CO2 emission was negatively correlated with TDN and EC, indicating that the increase in nutrients had a greater promoting effect on microbial respiration than algae photosynthesis. This is supported by the positive correlation between CO2 emission and 16S. According to these correlations between CO2 emission and environmental factors, seasonal and spatial variation can be explained. Seasonally, the highest CO2 emissions were observed in the summer, due to the intense respiration with low DO and high T. The lowest CO2 emissions were observed in the winter, due to the inhibition of respiration by high DO and low T [78]. Spatially, the highest CO2 emissions were observed in the UR, due to low DO and high nutrient levels. The most depleted δ13C in DIC was observed in the summer, and the UR verified this intense respiration. The moderate CO2 emissions were observed in the LUR, due to the high DO and moderate nutrient levels. The moderate values of δ13C-DIC verified the moderate respiration, compare with the UR and lakes. The lowest CO2 emissions were observed in the lakes, due to the high DO and low nutrient levels.
4.4. Control Factors on CH4 Emissions
Similarly to CO2 emission, seasonal variation of CH4 emission was also controlled by DO and T, due to methanogens being strongly dependent on T and DO [79,80]. CH4 concentrations were the highest in the spring, but CH4 emissions were the highest in the summer due to the highest T in the summer, in line with the study in the Chongqing River [9]. However, some studies reported exceptionally higher CH4 emissions in winter due to the CH4 accumulation under ice [75]. The lower DO reflected the intense anaerobic methanogens in the spring and summer, resulting in higher CH4 emissions. Spatial variations of CH4 emission were influenced by DO, pH, EC, TDN, Chl-a, and 16S. Previous studies showed that methanogens are sensitive to pH, and it is most beneficial within the range of 6 to 8 [27,70]. The CH4 production would decrease outside this pH range decreases. pH values ranged from 6.9 to 10.2; hence, CH4 emission exhibited a negative correlation with pH, and the higher CH4 emission was observed in the UR with the lower values of pH. Nutrient levels also influence the production of CH4. Alshboul et al. reported that the concentration of CH4 in the drainage area with high nutrient levels was 40 times that of the level upstream [81]. Positive correlations were found between CH4 emission and EC and TDN. Previous studies reported that competition for DO exists between nitrification of NH4+ and CH4 oxidation, accumulating CH4 in rivers and lakes [82]. The positive correlation between CH4 emission and NH4+-N confirmed this speculation. Hence, the highest CH4 emissions were found in the UR, also due to the higher nutrient level. However, although the TDN and EC in the LUR were higher than those in the lakes, its CH4 emissions were not higher than those of the lakes, suggesting that the CH4 emissions in the LUR were controlled by DO. Algae photosynthesis releases O2, inhibits methanogenesis, promotes methane oxidation, and reduces the CH4 concentration in water bodies [28,83]. Hence, significant negative correlations were found between CH4 emission and Chl-a.
5. Conclusions
We investigated CO2 and CH4 emissions and environmental factors in the urban interconnected river–lake system in March 2021, July 2021, October 2021, and December 2021. This study presented the seasonal and spatial variations in the dissolved concentrations and emissions of CO2 and CH4. Our results indicated that the urban interconnected river–lake system is a hotspot of CO2 and CH4 emission, and the connection of river and lake decreases CO2 and CH4 emission of the urban rivers substantially. The CO2 emissions of LUR and UR are 543.49 and 991.56 mmol m−2 d−1, and their CH4 emissions are 1.26 and 14.82 mmol m−2 d−1, respectively. We found that in situ aerobic and anaerobic respiration and sewage were the sources of CO2 and CH4 emissions in this river–lake system. The abundant nutrients promoted microbial growth, consuming DO and thus resulting in high emissions of CO2 and CH4 in the UR. The nutrients also enhanced the algae growth, taking CO2, resulting in the lowest emission of CO2 and CH4 in the lakes. The CO2 emission from the lake is 365.14 mmol m−2 d−1, and the CH4 emission is 1.25 mmol m−2 d−1. The moderate nutrients wreaked in situ respiration, exhibiting moderate CO2 emission in the LUR. Good hydrology connectivity made short hydrology residence time, inhibiting the growth of algae that decreased the CO2 uptake in the LUR. The DO concentration of LUR is 1.7 times higher than that of urban rivers, because the connection of rivers and lakes increases the DO concentration of LUR, making the CH4 emission of LUR lower than that of urban rivers. Correlation analysis showed that the seasonal variation of CO2 and CH4 emissions was controlled by DO and T. Hence, the highest emissions of CO2 were observed in the spring and the lowest in the winter, and the CO2 emissions in spring were 10.7 times that in winter. The highest emissions of CH4 were observed in the summer, and the lowest in the winter, and the CH4 emissions in summer were 6.6 times that in winter. In summary, the connection of urban rivers and lakes changes the environmental factors, thereby varying the production and emission of greenhouse gases. However, hydrological connectivity has complex spatiotemporal variations. Further research should be conducted to improve the knowledge of the correlation between hydrological connectivity and greenhouse gas emission, providing the theoretical basis for greenhouse gas emission reduction in urban rivers.
There are large uncertainties in our calculations of carbon dioxide and methane emissions. Because we use wind speed and temperature as key variables, and do not consider factors such as river velocity and channel width, further research is needed to accurately estimate CO2 and CH4 emissions. In this study, only CH4 emissions diffused through the water–air interface were calculated, which were not included in the boiling volume. However, during the sampling process, we found that air bubbles were constantly emerging from the sediments in heavily polluted river sections, and the amount of CH4 emissions by urban rivers may be seriously underestimated. Therefore, the amount of CH4 released by air bubbles should be measured in the future.
Author Contributions
Conceptualization, S.L. and X.L.; methodology, C.W., S.L. and X.L.; software, C.W. and Y.X.; validation, C.W.; formal analysis, C.W.; investigation, C.W., Y.X. and X.L.; data curation, C.W. and X.L.; writing—original draft preparation, C.W.; writing—review and editing, C.W. and X.L.; visualization, C.W., Y.X. and X.L.; supervision, X.L.; project administration, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Graduate Innovation Foundation of Wuhan Institute of Technology (CX2021448).
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the kind help of our work team in fieldwork and sample analysis. We also thank anonymous reviewers and editors for their insightful comments.
Conflicts of Interest
No conflict of interest exist in the submission of this manuscript, and the manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and is not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
References
- WMO. WMO greenhouse gas bulletin: The state of greenhouse gases in the atmosphere using global observations through 2018. In Proceedings of the Global Atmosphere Watch, World Meteorological Organization, Geneva, Switzerland, 25 November 2019. [Google Scholar]
- Li, Q.; Han, Y.; Liu, X.; Ansari, U.; Cheng, Y.; Yan, C. Hydrate as a by-product in CO2 leakage during the long-term sub-seabed sequestration and its role in preventing further leakage. Environ. Sci. Pollut. Res. 2022, 29, 77737–77754. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, F.; Wang, Y.; Zhou, C.; Chen, J.; Forson, K.; Miao, R.; Su, Y.; Zhang, J. Effect of reservoir characteristics and chemicals on filtration property of water-based drilling fluid in unconventional reservoir and mechanism disclosure. Environ. Sci. Pollut. Res. 2023, 30, 55034–55043. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, J. Factors affecting the lower limit of the safe mud weight window for drilling operation in hydrate-bearing sediments in the Northern South China Sea. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 82. [Google Scholar] [CrossRef]
- Drake, T.W.; Raymond, P.A.; Spencer, R.G. Terrestrial carbon inputs to inland waters: A current synthesis of estimates and uncertainty. Limnol. Oceanogr. Lett. 2017, 3, 132–142. [Google Scholar] [CrossRef]
- Stanley, E.H.; Casson, N.J.; Christel, S.T.; Crawford, J.T.; Loken, L.C.; Oliver, S.K. The ecology of methane in streams and rivers: Patterns, controls, and global significance. Ecol. Monogr. 2016, 86, 146–171. [Google Scholar] [CrossRef]
- DelSontro, T.; Beaulieu, J.J.; Downing, J.A. Greenhouse gas emissions from lakes and impoundments: Upscaling in the face of global change. Limnol. Oceanogr. Lett. 2018, 3, 64–75. [Google Scholar] [CrossRef]
- Gomez-Gener, L.; Rocher-Ros, G.; Battin, T.; Cohen, M.J.; Dalmagro, H.J.; Dinsmore, K.J.; Drake, T.W.; Duvert, C.; Enrich-Prast, A.; Horgby, Å. Global carbon dioxide efflux from rivers enhanced by high nocturnal emissions. Nat. Geosci. 2021, 14, 289–294. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zhou, L.; Zhang, Y.; Qin, B.; Spencer, R.G.; Brookes, J.D.; Jeppesen, E.; Weyhenmeyer, G.A.; Wu, F. Urbanization in developing countries overrides catchment productivity in fueling inland water CO2 emissions. Glob. Chang. Biol. 2022, 29, 16475. [Google Scholar]
- Wang, R.; Zhang, H.; Zhang, W.; Zheng, X.; Han, S. An urban polluted river as a significant hotspot for water–atmosphere exchange of CH4 and N2O. Environ. Pollut. 2020, 264, 114770. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Chen, H.; Yuan, X.; Peng, C.; Yue, J.; Zhang, Q.; Zhou, L. CH4 concentrations and fluxes in a subtropical metropolitan river network: Watershed urbanization impacts and environmental controls. Sci. Total Environ. 2018, 622, 1079–1089. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Xiao, Q.; Li, X. Urban rivers are hotspots of riverine greenhouse gas (N2O, CH4, CO2) emissions in the mixed-landscape chaohu lake basin. Water Res. 2021, 189, 116624. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xia, X.; Liu, S.; Zhang, L.; Zhang, Q. Intense methane ebullition from urban inland waters and its significant contribution to greenhouse gas emissions. Water Res. 2021, 189, 116654. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, D.; Zhou, J.; Meng, W.; Li, C.; Sun, Z.; Guo, X.; Wang, Z. Greenhouse gases emission from the sewage draining rivers. Sci. Total Environ. 2018, 612, 1454–1462. [Google Scholar] [CrossRef]
- Yang, L.; Yan, W.; Ma, P.; Wang, J. Seasonal and diurnal variations in N2O concentrations and fluxes from three eutrophic rivers in Southeast China. J. Geogr. Sci. 2011, 21, 820–832. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, Y.; Tang, X.; Zhang, Y.; Jeppesen, E. Biodegradable dissolved organic carbon shapes bacterial community structures and co-occurrence patterns in large eutrophic Lake Taihu. J. Environ. Sci. 2021, 107, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Panneer Selvam, B.; Natchimuthu, S.; Arunachalam, L.; Bastviken, D. Methane and carbon dioxide emissions from inland waters in I ndia–implications for large scale greenhouse gas balances. Glob. Chang. Biol. 2014, 20, 3397–3407. [Google Scholar] [CrossRef]
- Yang, H.; Andersen, T.; Dörsch, P.; Tominaga, K.; Thrane, J.-E.; Hessen, D.O. Greenhouse gas metabolism in Nordic boreal lakes. Biogeochemistry 2015, 126, 211–225. [Google Scholar] [CrossRef]
- Walsh, C.J.; Roy, A.H.; Feminella, J.W.; Cottingham, P.D.; Groffman, P.M.; Morgan, R.P. The urban stream syndrome: Current knowledge and the search for a cure. J. N. Am. Benthol. Soc. 2005, 24, 706–723. [Google Scholar] [CrossRef]
- Li, M.; Peng, C.; Zhu, Q.; Zhou, X.; Zhang, K. The significant contribution of lake depth in regulating global lake diffusive methane emissions. Water Res. 2020, 172, 115465. [Google Scholar] [CrossRef]
- Khaleghi, M.; Varvani, J. Sediment rating curve parameters relationship with watershed characteristics in the semiarid river watersheds. Arab. J. Sci. Eng. 2018, 43, 3725–3737. [Google Scholar] [CrossRef]
- Varvani, J.; Khaleghi, M.R.; Gholami, V. Investigation of the relationship between sediment graph and hydrograph of flood events (case study: Gharachay River Tributaries, Arak, Iran). Water Resour. 2019, 46, 883–893. [Google Scholar] [CrossRef]
- DelSontro, T.; Kunz, M.J.; Kempter, T.; Wuest, A.; Wehrli, B.; Senn, D.B. Spatial heterogeneity of methane ebullition in a large tropical reservoir. Environ. Sci. Technol. 2011, 45, 9866–9873. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, D.; Ding, Y.; Yu, Z.; Liu, L.; Li, Y.; Yang, D.; Gao, Y.; Tian, H.; Cai, R. Ebullition controls on CH4 emissions in an urban, eutrophic river: A potential time-scale bias in determining the aquatic CH4 flux. Environ. Sci. Technol. 2021, 55, 7287–7298. [Google Scholar] [CrossRef] [PubMed]
- Teurlincx, S.; Kuiper, J.J.; Hoevenaar, E.C.; Lurling, M.; Brederveld, R.J.; Veraart, A.J.; Janssen, A.B.; Mooij, W.M.; De, S.D.; Lisette, N. Towards restoring urban waters: Understanding the main pressures. Curr. Opin. Environ. Sustain. 2019, 36, 49–58. [Google Scholar] [CrossRef]
- Engel, F.; Drakare, S.; Weyhenmeyer, G.A. Environmental conditions for phytoplankton influenced carbon dynamics in boreal lakes. Aquat. Sci. 2019, 81, 35. [Google Scholar] [CrossRef]
- Sun, H.; Lu, X.; Yu, R.; Yang, J.; Liu, X.; Cao, Z.; Zhang, Z.; Li, M.; Geng, Y. Eutrophication decreased CO2 but increased CH4 emissions from lake: A case study of a shallow Lake Ulansuhai. Water Res. 2021, 201, 117363. [Google Scholar] [CrossRef]
- Balmer, M.B.; Downing, J.A. Carbon dioxide concentrations in eutrophic lakes: Undersaturation implies atmospheric uptake. Inland Waters 2011, 1, 125–132. [Google Scholar] [CrossRef]
- Bastviken, D.; Tranvik, L.J.; Downing, J.A.; Crill, P.M.; Enrich-Prast, A. Freshwater methane emissions offset the continental carbon sink. Science 2011, 331, 50. [Google Scholar] [CrossRef]
- Verpoorter, C.; Kutser, T.; Seekell, D.A.; Tranvik, L.J. A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Lett. 2014, 41, 6396–6402. [Google Scholar] [CrossRef]
- Li, S.; Luo, J.; Wu, D.; Xu, Y.J. Carbon and nutrients as indictors of daily fluctuations of pCO2 and CO2 flux in a river draining a rapidly urbanizing area. Ecol. Indic. 2020, 109, 105821. [Google Scholar] [CrossRef]
- Davidson, T.A.; Audet, J.; Svenning, J.C.; Lauridsen, T.L.; Søndergaard, M.; Landkildehus, F.; Larsen, S.E.; Jeppesen, E. Eutrophication effects on greenhouse gas fluxes from shallow-lake mesocosms override those of climate warming. Glob. Chang. Biol. 2015, 21, 4449–4463. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, M.; Hu, Z.; Gao, Y.; Hu, C.; Liu, C.; Liu, S.; Zhang, Z.; Zhao, J.; Xiao, W. Spatial variations of methane emission in a large shallow eutrophic lake in subtropical climate. J. Geophys. Res. Biogeosci. 2017, 122, 1597–1614. [Google Scholar] [CrossRef]
- Zhang, L.; He, K.; Wang, T.; Liu, C.; An, Y.; Zhong, J. Frequent algal blooms dramatically increase methane while decrease carbon dioxide in a shallow lake bay. Environ. Pollut. 2022, 312, 120061. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, S.; Xiao, S.; Yu, K.; Fang, X.; Xia, L.; Wang, J.; Liu, S.; Freeman, C.; Zou, J. Global methane and nitrous oxide emissions from inland waters and estuaries. Glob. Chang. Biol. 2022, 28, 4713–4725. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Liu, C.; Li, Y.; Liu, X.; Hao, X. Discussion on water cycle mechanism of interconnected river system network. J. Nat. Resour. 2011, 26, 523–529. [Google Scholar]
- Junkai, Z.; Lixian, L.; Aishe, Z.; Jiufa, L.; Qiuxia, G. A new approach for the health assessment of river systems based on interconnected water system networks. J. Resour. Ecol. 2017, 8, 251–257. [Google Scholar] [CrossRef]
- Havens, K.; Fukushima, T.; Xie, P.; Iwakuma, T.; James, R.; Takamura, N.; Hanazato, T.; Yamamoto, T. Nutrient dynamics and the eutrophication of shallow lakes Kasumigaura (Japan), Donghu (PR China), and Okeechobee (USA). Environ. Pollut. 2001, 111, 263–272. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s Law Constants for Inorganic and Organic Species of Potential Importance in Environmental Chemistry. 1999. Available online: http://www.henrys-law.org (accessed on 15 December 2022).
- Zheng, D.Q.; Guo, T.M.; Knapp, H. Experimental and modeling studies on the solubility of CO2, CHClF2, CHF3, C2H2F4 and C2H4F2 in water and aqueous NaCl solutions under low pressures. Fluid Phase Equilibria 1997, 129, 197–209. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 80th ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Cole, J.J.; Caraco, N.F. Emissions of nitrous oxide (N2O) from a tidal, freshwater river, the Hudson River, New York. Environ. Sci. Technol. 2001, 35, 991–996. [Google Scholar] [CrossRef]
- Wanninkhof, R. Relationship between wind speed and gas exchange over the ocean. J. Geophys. Res. Ocean. 1992, 97, 7373–7382. [Google Scholar] [CrossRef]
- Borges, A.V.; Vanderborght, J.-P.; Schiettecatte, L.-S.; Gazeau, F.; Ferrón-Smith, S.; Delille, B.; Frankignoulle, M.J.E. Variability of the gas transfer velocity of CO2 in a macrotidal estuary (the Scheldt). Estuaries 2004, 27, 593–603. [Google Scholar] [CrossRef]
- Clough, T.J.; Buckthought, L.E.; Kelliher, F.M.; Sherlock, R.R. Diurnal fluctuations of dissolved nitrous oxide (N2O) concentrations and estimates of N2O emissions from a spring-fed river: Implications for IPCC methodology. Glob. Chang. Biol. 2007, 13, 1016–1027. [Google Scholar] [CrossRef]
- Raymond, P.A.; Cole, J. Gas exchange in rivers and estuaries: Choosing a gas transfer velocity. Estuaries 2001, 24, 312–317. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.; Yu, Q.; Yan, W.; Li, X.; Lv, S. Dominance of nitrous oxide production by nitrification and denitrification in the shallow Chaohu Lake, Eastern China: Insight from isotopic characteristics of dissolved nitrous oxide. Environ. Pollut. 2019, 255, 113212. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Xu, Y.J.; Ma, Y.; Maher, D.T.; Li, S. Hot spot of CH4 production and diffusive flux in rivers with high urbanization. Water Res. 2021, 204, 117624. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, L.; Liu, T.; He, Y.; Wu, S.; Chen, H.; Yuan, X.; Wang, J.; Li, X.; Li, H. Methane and nitrous oxide concentrations and fluxes from heavily polluted urban streams: Comprehensive influence of pollution and restoration. Environ. Pollut. 2022, 313, 120098. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Ren, C.; Shi, Y.; Chen, G.; Xie, J.; Ng, E. Modeling spatiotemporal carbon emissions for two mega-urban regions in China using urban form and panel data analysis. Sci. Total Environ. 2023, 857, 159612. [Google Scholar] [CrossRef]
- Liang, X.; Xing, T.; Li, J.; Wang, B.; Wang, F.; He, C.; Hou, L.; Li, S. Control of the hydraulic load on nitrous oxide emissions from cascade reservoirs. Environ. Sci. Technol. 2019, 53, 11745–11754. [Google Scholar] [CrossRef]
- Niu, Y.; Ye, Q.; Liu, Q.; Yu, H.; Tao, Y.; Wang, H.; Niu, Y.; Luo, M. Effect of river–lake connectivity on ecological stoichiometry of lake and carbon storage status in Eastern Plain, China. Environ. Geochem. Health 2022, 45, 1905–1917. [Google Scholar] [CrossRef]
- Marandi, A.; Polikarpus, M.; Jõeleht, A. A new approach for describing the relationship between electrical conductivity and major anion concentration in natural waters. Appl. Geochem. 2013, 38, 103–109. [Google Scholar] [CrossRef]
- Li, L.; Xue, B.; Yao, S.; Tao, Y.; Yan, R. Spatial–temporal patterns of methane dynamics in Lake Taihu. Hydrobiologia 2018, 822, 143–156. [Google Scholar] [CrossRef]
- Perga, M.E.; Maberly, S.C.; Jenny, J.P.; Alric, B.; Pignol, C.; Naffrechoux, E. A century of human-driven changes in the carbon dioxide concentration of lakes. Glob. Biogeochem. Cycles 2016, 30, 93–104. [Google Scholar] [CrossRef]
- Xiao, Q.; Xu, X.; Duan, H.; Qi, T.; Qin, B.; Lee, X.; Hu, Z.; Wang, W.; Xiao, W.; Zhang, M. Eutrophic Lake Taihu as a significant CO2 source during 2000–2015. Water Res. 2020, 170, 115331. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.S.; Park, J.-H.; Yang, L.; Shin, K.H.; Hur, J. Optical and molecular indices of dissolved organic matter for estimating biodegradability and resulting carbon dioxide production in inland waters: A review. Water Res. 2023, 228, 119362. [Google Scholar] [CrossRef] [PubMed]
- Caniani, D.; Caivano, M.; Pascale, R.; Bianco, G.; Mancini, I.; Masi, S.; Mazzone, G.; Firouzian, M.; Rosso, D. CO2 and N2O from water resource recovery facilities: Evaluation of emissions from biological treatment, settling, disinfection, and receiving water body. Sci. Total Environ. 2019, 648, 1130–1140. [Google Scholar] [CrossRef]
- Cotovicz, L.C., Jr.; Knoppers, B.A.; Brandini, N.; Poirier, D.; Costa Santos, S.J.; Abril, G. Spatio-temporal variability of methane (CH4) concentrations and diffusive fluxes from a tropical coastal embayment surrounded by a large urban area (Guanabara Bay, Rio de Janeiro, Brazil). Limnol. Oceanogr. 2016, 61, S238–S252. [Google Scholar] [CrossRef]
- Yang, L.; Lei, K. Effects of land use on the concentration and emission of nitrous oxide in nitrogen-enriched rivers. Environ. Pollut. 2018, 238, 379–388. [Google Scholar] [CrossRef]
- Thalasso, F.; Sepulveda-Jauregui, A.; Cabrol, L.; Lavergne, C.; Olgun, N.; Martinez-Cruz, K.; Aguilar-Muñoz, P.; Calle, N.; Mansilla, A.; Astorga-España, M.S. Methane and carbon dioxide cycles in lakes of the King George Island, maritime Antarctica. Sci. Total Environ. 2022, 848, 157485. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, Y.J. Dissolved carbon transport in a river-lake continuum: A case study in a subtropical watershed, USA. Sci. Total Environ. 2018, 643, 640–650. [Google Scholar] [CrossRef]
- Sepulveda-Jauregui, A.; Hoyos-Santillan, J.; Martinez-Cruz, K.; Anthony, K.M.W.; Casper, P.; Belmonte-Izquierdo, Y.; Thalasso, F. Eutrophication exacerbates the impact of climate warming on lake methane emission. Sci. Total Environ. 2018, 636, 411–419. [Google Scholar] [CrossRef]
- Atkins, M.L.; Santos, I.R.; Maher, D.T. Seasonal exports and drivers of dissolved inorganic and organic carbon, carbon dioxide, methane and δ13C signatures in a subtropical river network. Sci. Total Environ. 2017, 575, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Whitman, W.; Wiegel, J.; Maier, R.; Adams, M. Incredible Anaerobes: From Physiology to Genomics to Fuels; Wiegel, J., Maier, R.J., Adams, M.W.W., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 171–189. [Google Scholar]
- Yu, Z.; Wang, D.; Li, Y.; Deng, H.; Hu, B.; Ye, M.; Zhou, X.; Da, L.; Chen, Z.; Xu, S. Carbon dioxide and methane dynamics in a human-dominated lowland coastal river network (Shanghai, China). J. Geophys. Res. Biogeosci. 2017, 122, 1738–1758. [Google Scholar] [CrossRef]
- Yoon, T.K.; Jin, H.; Begum, M.S.; Kang, N.; Park, J.-H. CO2 outgassing from an urbanized river system fueled by wastewater treatment plant effluents. Environ. Sci. Technol. 2017, 51, 10459–10467. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Berggren, M.; Ask, J.; Byström, P.; Jonsson, A.; Laudon, H.; Jansson, M. Terrestrial organic matter support of lake food webs: Evidence from lake metabolism and stable hydrogen isotopes of consumers. Limnol. Oceanogr. 2012, 57, 1042–1048. [Google Scholar] [CrossRef]
- Wen, Z.; Song, K.; Zhao, Y.; Jin, X. Carbon dioxide and methane supersaturation in lakes of semi-humid/semi-arid region, Northeastern China. Atmos. Environ. 2016, 138, 65–73. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, Y.; Yang, H.; Zhang, Y.; Xu, J.; Tan, L.; Tong, C.; Lai, D.Y. Large fine-scale spatiotemporal variations of CH4 diffusive fluxes from shrimp aquaculture ponds affected by organic matter supply and aeration in Southeast China. J. Geophys. Res. Biogeosci. 2019, 124, 1290–1307. [Google Scholar] [CrossRef]
- Kryvenda, A.; Tischner, R.; Steudel, B.; Griehl, C.; Armon, R.; Friedl, T. Testing for terrestrial and freshwater microalgae productivity under elevated CO2 conditions and nutrient limitation. BMC Plant Biol. 2023, 23, 27. [Google Scholar] [CrossRef]
- Solano, V.; Duvert, C.; Birkel, C.; Maher, D.T.; García, E.A.; Hutley, L.B. Stream respiration exceeds CO2 evasion in a low-energy, oligotrophic tropical stream. Limnol. Oceanogr. 2023. Limnol. Oceanogr. 2023. early view. [Google Scholar] [CrossRef]
- Jin, H.; Yoon, T.K.; Begum, M.S.; Lee, E.-J.; Oh, N.-H.; Kang, N.; Park, J.-H. Longitudinal discontinuities in riverine greenhouse gas dynamics generated by dams and urban wastewater. Biogeosciences 2018, 15, 6349–6369. [Google Scholar] [CrossRef]
- Finlay, K.; Leavitt, P.; Wissel, B.; Prairie, Y. Regulation of spatial and temporal variability of carbon flux in six hard-water lakes of the northern Great Plains. Limnol. Oceanogr. 2009, 54, 2553–2564. [Google Scholar] [CrossRef]
- Xiao, Q.; Hu, Z.; Hu, C.; Islam, A.T.; Bian, H.; Chen, S.; Liu, C.; Lee, X. A highly agricultural river network in Jurong Reservoir watershed as significant CO2 and CH4 sources. Sci. Total Environ. 2021, 769, 144558. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Schelske, C.L.; Coveney, M.F. Low carbon dioxide partial pressure in a productive subtropical lake. Aquat. Sci. 2011, 73, 317–330. [Google Scholar] [CrossRef]
- Larsen, S.; Andersen, T.; Hessen, D.O. The pCO2 in boreal lakes: Organic carbon as a universal predictor? Glob. Biogeochem. Cycles 2011, 25, GB2012. [Google Scholar] [CrossRef]
- Tang, W.; Xu, Y.J.; Ni, M.; Li, S. Land use and hydrological factors control concentrations and diffusive fluxes of riverine dissolved carbon dioxide and methane in low-order streams. Water Res. 2023, 231, 119615. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Allen, A.P.; Bastviken, D.; Conrad, R.; Gudasz, C.; St-Pierre, A.; Thanh-Duc, N.; Del Giorgio, P.A. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 2014, 507, 488–491. [Google Scholar] [CrossRef]
- Que, Z.; Wang, X.; Liu, T.; Wu, S.; He, Y.; Zhou, T.; Yu, L.; Qing, Z.; Chen, H.; Yuan, X. Watershed land use change indirectly dominated the spatial variations of CH4 and N2O emissions from two small suburban rivers. J. Hydrol. 2023, 619, 129357. [Google Scholar] [CrossRef]
- Alshboul, Z.; Encinas-Fernandez, J.; Hofmann, H.; Lorke, A. Export of dissolved methane and carbon dioxide with effluents from municipal wastewater treatment plants. Environ. Sci. Technol. 2016, 50, 5555–5563. [Google Scholar] [CrossRef]
- Van der Nat, F.; De Brouwer, J.; Middelburg, J.J.; Laanbroek, H.J. Spatial distribution and inhibition by ammonium of methane oxidation in intertidal freshwater marshes. Appl. Environ. Microbiol. 1997, 63, 4734–4740. [Google Scholar] [CrossRef]
- Roth, F.; Broman, E.; Sun, X.; Bonaglia, S.; Nascimento, F.; Prytherch, J.; Brüchert, V.; Lundevall Zara, M.; Brunberg, M.; Geibel, M.C. Methane emissions offset atmospheric carbon dioxide uptake in coastal macroalgae, mixed vegetation and sediment ecosystems. Nat. Commun. 2023, 14, 42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).